Abstract
Dental pulp regeneration is significantly aided by human dental pulp stem cells (hDPSCs). An increasing number of studies have demonstrated that circular RNAs (circRNAs) are crucial in the multidirectional differentiation of many mesenchymal stem cells, but their specific functions and mechanisms remain unknown. This work aimed at elucidating the molecular mechanism by which hsa_circ_0001599 works in hDPSCs during odontogenic differentiation. The expression of hsa_circ_0001599 in hDPSCs and dental pulp tissue was determined by using quantitative real-time PCR (qRT‒PCR). The role of hsa_circ_0001599 in the odontogenic differentiation of hDPSCs and its mechanism were studied using a variety of in vivo and in vitro assessments. The odontogenic differentiation of hDPSCs was facilitated by the overexpression of hsa_circ_0001599, which activated the PI3K/AKT signalling pathway in vitro. In vivo, hsa_circ_0001599 can promote the formation of new dentin-like structures. Mechanistically, hsa_circ_0001599 enhanced ITGA2 expression by sponging miR-889-3p. Furthermore, hsa_circ_0001599 interacts with the methylation reader hnRNPA2B1, promoting hnRNPA2B1 translocation from the nucleus to the cytoplasm and increasing ITGA2 mRNA stability. This research revealed the important role of hsa_circ_0001599 in odontogenic differentiation. Thus, hDPSCs engineered with hsa_circ_0001599 have the potential to be effective therapeutic targets for dental pulp repair and regeneration.
Similar content being viewed by others
Introduction
Pulpitis and periapical periodontitis are common oral diseases that not only cause severe pain and swelling in the maxillofacial region but also may affect a patient’s chewing function and facial aesthetics1. The preferred option is root canal therapy, but the lack of blood supply and nerve innervation after root canal therapy can result in a reduction in tooth stress and a greater risk of root fracture2. In addition, the failure of root canal therapy can lead to serious problems, including root canal absorption, foreign body reactions, and apical cyst formation. Therefore, the repair and regeneration of dental pulp tissue are vital for protecting teeth, especially in the treatment of infected pulp, and are highly important.
The ideal repair and regeneration of dental pulp tissue depends largely on the processes of proliferation, migration and differentiation of human dental pulp stem cells (hDPSCs) into odontoblast-like cells and the formation of restorative dentin3. Although many scholars have studied how to achieve odontogenic differentiation from hDPSCs, regeneration of pulp has not been achieved4,5,6. Therefore, clarifying the molecular regulatory function of odontogenic differentiation in hDPSCs will help scholars across the world to better understand tissue regeneration engineering. This research can also provide a theoretical foundation for the application of stem cell therapy in clinical practice.
Circular RNAs (circRNAs) are important regulatory factors of gene expression and participate in many biological processes by serving as transcription regulators, RNA binding protein (RBP)-binding molecules, microRNA (miRNA) sponges, and templates for protein translation7. In particular, research has demonstrated that circRNAs are related to the regulation of multidirectional differentiation in various mesenchymal stem cells. For example, under inflammatory conditions, downregulation of circBIRC6 inhibits PTEN and activates the PI3K/AKT/mTOR signalling pathway, thereby affecting the osteogenic differentiation of human periodontal ligament stem cells8. By sequestering miR-188-3p, hsa_circ_0026827 facilitates the osteogenic differentiation of hDPSCs via the Beclin1 and RUNX1 signalling pathways9. Through the miR-708-5p/GIT2 axis, circFKBP5 is capable of protecting hDPSCs from LPS-induced apoptosis, inflammation, and the inhibition of osteogenic differentiation10. The above studies have revealed that circRNAs are critical for osteogenic differentiation of dental-derived mesenchymal stem cells. Nevertheless, despite much of the research remaining inconclusive regarding the ability of circRNAs to promote restorative dentin formation, several circRNAs have been certified and reported. It was found that circRNAs can work as “molecular sponges” to adsorb miRNA, and hsa_circ_0005044 can promote the osteogenic/odontogenic differentiation of hDPSCs by modulating miR-296-3p/FOSL111. Moreover, there is a paucity of studies that have reported on the mechanism related to the regulatory impact of circRNAs on odontogenic differentiation in hDPSCs.
According to previous gene chip screening by our research group, hsa_circRNA_104101 (termed hsa_circ_0001599 in this study)12, which was significantly and stably upregulated during the process of mineralization in hDPSCs, was chosen for further study. We discovered that hsa_circ_0001599 was significantly upregulated in dental pulp tissues with deep caries and in hDPSCs. Mechanistically, hsa_circ_0001599 may upregulate ITGA2 expression to promote odontogenic differentiation by sponging miR-889-3p. Moreover, hsa_circ_0001599 directly interacted with the RBP hnRNPA2B1 and improved ITGA2 mRNA stability, thereby promoting the odontogenic differentiation of hDPSCs. This work revealed the important role of hsa_circ_0001599 in the odontogenic differentiation of hDPSCs.
Results
hDPSCs exhibit greater odontogenic differentiation under mineralization induction
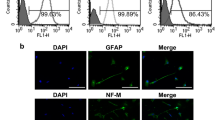
hDPSCs were successfully isolated from dental pulp tissue and then passaged (Supplementary Fig. 1a, b). A staining test demonstrated the differential capability of hDPSCs (Supplementary Fig. 1c-h). The results of the flow cytometry analysis revealed that hDPSCs were negative for haemopoietic cell markers, such as CD45 and CD34, but positive for the stem cell markers CD44, CD90, CD105 and CD29 (Supplementary Fig. 1i). Mineralization-related genes were subsequently detected via quantitative real-time PCR (qRT‒PCR). As indicated in Supplementary Fig. 1j, k, hDPSCs exhibited greater expression of odontogenic marker genes in the induced group than in the control group.
hsa_circ_0001599 is upregulated during odontogenic differentiation in hDPSCs
To explore the functions of hsa_circ_0001599 in the odontogenic differentiation of hDPSCs, we evaluated the expression of hsa_circ_0001599 in hDPSCs and dental pulp tissues. Expression of hsa_circ_0001599 was markedly upregulated in hDPSCs (Fig. 1a). The expression of hsa_circ_0001599 at the RNA level was then confirmed by qRT‒PCR in pulp tissues from patients with deep caries and normal pulp tissues. The results revealed that, in deep caries pulp tissue, the expression of hsa_circ_0001599 was elevated (Fig. 1b). Then, we constructed lentiviral overexpression vectors, and qRT‒PCR analyses revealed their overexpression efficiency (Fig. 1c). In hDPSCs, the mRNA expression levels of odontogenic marker genes, including dentin sialophosphoprotein (DSPP), dentin matrix acidic phosphoprotein 1 (DMP1), Runt-related transcription factor 2 (RUNX2) and alkaline phosphatase (ALP), were markedly increased by LV- circ_0001599 (Fig. 1d, e). Western blot analysis of the protein expression levels of DSPP, DMP1, RUNX2, and ALP revealed a consistent trend (Fig. 1f). ALP and alizarin red S (ARS) staining demonstrated that LV-circ_0001599 increased ALP activity and calcium deposition (Fig. 1g). Moreover, according to the results of cellular immunofluorescence, more RUNX2- and ALP-positive immune cells were detected in LV-circ_0001599-transfected hDPSCs than in LV-NC-transfected hDPSCs (Fig. 1h). Thus, our data suggest that hsa_circ_0001599 enhances odontogenic differentiation in hDPSCs.
a The expression of hsa_circ_0001599 in hDPSCs. b The expression of hsa_circ_0001599 in dental pulp tissue. c Overexpression efficiency of hsa_circ_0001599 in hDPSCs. d, e The expression level of DSPP, DMP1, RUNX2 and ALP at 7 days and 14 days after odontogenic differentiation using qRT‒PCR. f Following odontogenic differentiation for 7 and 14 days, the expression level of genes related to mineralization can be detected by Western blot analysis. g ALP and ARS staining. h Immunocytochemistry analysis of RUNX2 and ALP expression levels in hDPSCs. a–g All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, a n = 5 for each group, b n = 4 for each group, c–g n = 3 for each group. g Scale bar, 200 μm, h Scale bar, 50 μm.
hsa_circ_0001599 promotes the odontogenic differentiation of hDPSCs in vivo
The hDPSCs stably overexpressing hsa_circ_0001599 or control cells (LV-NC, hDPSCs or PBS) were loaded onto HA/TCP scaffolds and implanted into the subcutaneous tissues of BALB/c nude mice (5 BALB/c nude mice in each group) for 4 weeks of growth (Fig. 2a). haematoxylin and eosin (H&E) staining revealed significantly more newly formed dentinal-like tissues in the hsa_circ_0001599 overexpression group than in the PBS, LV-NC and simple hDPSC transplantation groups. These results indicated that hsa_circ_0001599 overexpression in hDPSCs promoted dentin regeneration (Fig. 2b). Masson’s trichrome staining revealed greater collagen deposition in the hDPSCs of the hsa_circ_0001599-overexpressing group than in those of the control group (Fig. 2c). To identify the newly generated tissues, immunohistochemistry (IHC) staining for dentin-related markers (DSPPs) was performed. Signals were detected in the LV-circ_0001599 group, and DSPP immune response cells were mainly distributed in newly formed dentinal-like tissues (Fig. 2d). In summary, these data show that hsa_circ_0001599 also promotes the odontogenic differentiation of hDPSCs in vivo.
a Subcutaneous transplantation was performed in BALB/c nude mice at 5-week-old for 4 weeks in control group and experimental group. b The regeneration of dentinal-like tissues was assessed in each group using H&E staining. c Masson staining detection of collagen fiber regeneration in each group. d Immunohistochemical staining of DSPP in each group. b–d Scale bar, 50 μm.
hsa_circ_0001599 promotes the odontogenic differentiation of hDPSCs by activating the PI3K/AKT signalling pathway
To investigate the processes underlying the impact of hsa_circ_0001599 on hDPSC odontogenic differentiation, we utilized RNA sequencing (RNA‒seq) technology to determine the potential mechanism of action of hsa_circ_0001599. Compared with control cells, LV-circ_0001599 cells presented 580 upregulated genes and 1160 downregulated genes (Fig. 3a, b). The differentially expressed mRNAs were shown to be abundant in signal transduction, multicellular organism development, regulation of transcription, DNA‒templated, positive regulation of transcriptional by RNA polymerase II, cell differentiation and cell adhesion, according to GO analysis (Fig. 3c). For hsa_circ_0001599, KEGG pathway analysis revealed the differential expression of genes and enrichment of the PI3K/AKT signalling pathway. (Fig. 3d). Since the PI3K/AKT signalling pathway is vital for the odontogenic differentiation of hDPSCs, we investigated the possibility that hsa_circ_0001599 influences this signalling pathway. After hsa_circ_0001599 was overexpressed in hDPSCs, Western blot revealed that the levels of p-AKT and p-PI3K were significantly increased (Fig. 3e). The experiment was further conducted by treating cells with the PI3K/AKT pathway inhibitor LY294002. The results demonstrated that treatment with the inhibitor LY294002 partially rescued p-AKT and p-PI3K overexpression in hDPSCs (Fig. 3f). In addition, treatment with LY294002 partially rescued the ability of hsa_circ_0001599 to promote odontogenic differentiation in hDPSCs (Fig. 3g). These findings suggest that hsa_circ_0001599 is a positive regulator of the PI3K/AKT signalling pathway.
a Heatmap showing the genes in LV-NC-treated cells and LV-circ_0001599-treated cells. b Volcano plot of differentially expressed mRNAs in LV-NC- and LV-circ_0001599-treated cells. c The GO enrichment analysis. d The enriched KEGG functional pathways. e Western blot results showed that the expression levels of p-AKT and p-PI3K were increased in the LV-circ_0001599 group. f Treated with PI3K/AKT inhibitor LY294002. g The impact of LY294002 on the odontogenic differentiation of hDPSCs was examined using Western blot. e–g All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns: not significant. e–g n = 3 for each group.
Overexpression of miR-889-3p inhibits odontogenic differentiation
The function of circRNAs is related to their localization in cells. In this study, Fluorescence in situ hybridization (FISH) was used to confirm the localization of hsa_circ_0001599 in cells and dental pulp tissue. hsa_circ_0001599 was located in the nucleus and cytoplasm, as shown in Fig. 4a. CircRNAs located in the cytoplasm are usually regarded as competing endogenous RNAs (ceRNAs). Therefore, it is hypothesized that hsa_circ_0001599 can regulate target genes by competitively adsorbing miRNAs and promoting the odontogenic differentiation of hDPSCs. The starBase, miRanda, CircInteractome and NCBI GEO: GSE138180 databases13 were used to predict that hsa_circ_0001599 has binding sites for miR-889-3p (Fig. 4b). In contrast to the results of the control group, miR-889-3p mimics group greatly decreased hsa_circ_0001599 luciferase activity, whereas binding site mutation had no effect on miR-889-3p luciferase activity (Fig. 4c). The results demonstrated that hsa_circ_0001599 can combine with miR-889-3p. Other studies have shown that circRNAs can bind to the AGO2 protein to adsorb miRNAs and act as “miRNA sponges”. After binding to the AGO2 protein, miRNA forms an RNA-induced silencing complex to inhibit mRNA. Ago2- RNA immunoprecipitation (RIP) assays can be used to identify the target transcripts of miRNAs14,15. The AGO2-RIP results revealed that the AGO2 protein could simultaneously enrich hsa_circ_0001599 and miR-889-3p (Fig. 4d). These results also suggest that hsa_circ_0001599 is one of the miRNA targets of the AGO2 protein complex and can act as a sponge of miRNA. qRT‒PCR revealed that the miR-889-3p levels tended to decrease gradually during induced hDPSCs odontogenic differentiation. In addition, qRT‒PCR was also used to detect the efficiency of overexpression of miR-889-3p (Supplementary Fig. 2a). The expression of odontogenic genes was inhibited by the miR-889-3p mimics, as indicated by qRT‒PCR and Western blot (Supplementary Fig. 2b, c), on Days 7 and 14 of odontogenesis, which was consistent with the increase in the number of RUNX2- and ALP-positive cells (Supplementary Fig. 2d). ALP and ARS staining revealed similar patterns (Supplementary Fig. 2e), suggesting that miR-889-3p negatively modulates hDPSCs odontogenic differentiation.
a RNA FISH was performed to identify the cellular localization of hsa_circ_0001599 in hDPSCs and dental pulp tissue. b Databases consistently predicted that miR-889-3p interacts with hsa_circ_0001599. c Dual luciferase assay. d AGO2-RIP assay. c, d All data were presented as the mean ± SD. **P < 0.01, ***P < 0.001, ****P < 0.0001, c, d n = 3 for each group. a Scale bar, 50 μm.
hsa_circ_0001599 upregulates ITGA2 levels through miR-889-3p
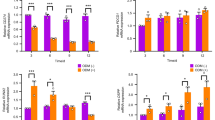
We also investigated the miR-889-3p target genes that are essential for hDPSC odontogenic differentiation. Firstly, we predicted the target in starBase and TargetScan. Secondly, we analyzed the differential gene expression in the cells (LV-circ_0001599 vs. LV-NC) by RNA-seq. According to the prediction of target genes and the results of RNA-seq, we identified 59 common target genes, of which 11 were molecules related to mineralization (CCND1, ITGA2, FGF5, TNC, COL10A1, SPRY2, HMGCS1, LRP8, NUSAP1, PDE11A and DLGAP5) (Fig. 5a). Following the induction of hDPSCs, the mRNA expression of candidate genes was assessed, revealing a significant increase in the mRNA levels of ITGA2 and TNC within the cells. (Fig. 5b). A literature review revealed that ITGA2 is related to the PI3K/AKT signalling pathway, so we chose ITGA2 for further research. Double luciferase assays and AGO2-RIP assays confirmed that miR-889-3p can bind ITGA2 (Fig. 5c, d). Therefore, we hypothesized that hsa_circ_0001599 improved odontogenic differentiation by protecting ITGA2 from a decrease in miR-889-3p. First, qRT‒PCR revealed that hsa_circ_0001599 upregulates ITGA2 levels through miR-889-3p (Fig. 5e). To further address the relationships among hsa_circ_0001599, miR-889-3p, and ITGA2, hDPSCs were cotransfected with these factors. First, hDPSCs were cotransfected with LV-circ_0001599 and miR-889-3p mimics. qRT‒PCR analysis revealed that LV-circ_0001599 clearly increased the expression of ITGA2 and genes related to odontogenic differentiation. When LV-circ_0001599 was transfected with miR-889-3p mimics, the high expression levels of odontogenic genes induced by LV-circ_0001599 were partially rescued (Fig. 5f). Western blot and staining analyses revealed a similar trend (Fig. 5g, h). Collectively, the above results indicate that hsa_circ_0001599 regulates hDPSCs partially through miR-889-3p, so we hypothesized that hsa_circ_0001599 may also affect the odontogenic differentiation ability of hDPSCs in other ways.
a Intersection of the predicted results in the data plot. b qRT‒PCR analysis of the expression of predicted target genes after 7 days and 14 days of odontogenic differentiation. c Dual luciferase assay. d AGO2-RIP assay. e hsa_circ_0001599 regulates the target gene ITGA2 through miR-889-3p. f Relative mRNA expression of mineralization-related genes in cells cotransfected with LV-circ_0001599 and miR-889-3p mimics after odontogenic differentiation for 7 days or 14 days. g Relative protein expression of mineralization-related genes in the cotransfection experiment after odontogenic differentiation. h ALP and ARS staining results in cotransfection experiments. b–h All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, b–h n = 3 for each group. h Scale bar, 200 μm.
hsa_circ_0001599 binds to hnRNPA2B1
CircRNAs are of paramount importance in regulating the expression of genes and are involved in numerous biological processes by serving as templates for protein translation, RBP-binding molecules, and miRNA sponges. Preliminary study results demonstrated that miR-889-3p only partially rescued the ability of hsa_circ_0001599 to promote the odontogenic differentiation of hDPSCs. The odontogenic differentiation of hDPSCs is thought to be regulated by hsa_circ_0001599 via additional pathways. The results of the circRNADb database coding potential analysis revealed that the hsa_circ_0001599 possibility of encoding protein is relatively low (Supplementary Fig. 3).
Therefore, we investigated the possibility of hsa_circ_0001599 interacting with RBPs and then used RBPmap and starBase to identify proteins that interact with hsa_circ_0001599 (Fig. 6a). Then, 24 selected proteins were analysed and validated using ChIRP-PRM, among which 9 proteins could be detected (hnRNPK, hnRNPL, hnRNPU, hnRNPA2B1, SRSF1, KHDRBS1, LIN28A, RBM47, TIA1). The extracted ion chromatogram (XIC) revealed a correlation between the ionic strength and retention time of each fragment and prospective peptides of RNA splice-related proteins in the hsa_circ_0001599 group. Additionally, the histogram provided a quantitative comparison of prospective peptides among the sample groups (Fig. 6b). The specific peptide segment of hnRNPA2B1 was quantitatively compared between the LV-circ_0001599 and LV-NC groups, and there was a maximum difference of 15 times between the groups (Fig. 6c). Furthermore, we analysed the nucleotide sequence of hsa_circ_0001599 with catRAPID and found that multiple nucleotides can bind to hnRNPA2B1 (Fig. 6d). Figure 6e illustrates the potential combination of hsa_circ_0001599 and hnRNPA2B1. Therefore, hnRNPA2B1 was selected for our next study. RIP experiments revealed that the enrichment of hsa_circ_0001599 precipitated by hnRNPA2B1 antibodies further confirmed the interaction between hsa_circ_0001599 and hnRNPA2B1 (Fig. 6f). To identify the exact fragment that bound to hnRNPA2B1, crosslinking immunoprecipitation (CLIP) ‒qPCR was performed. As shown in Fig. 6g, 15 RT-qPCR primers were designed for every region of hsa_circ_0001599 (primers used in CLIP-qPCR assay are listed in Supplementary Table 4), and the CLIP-qPCR results revealed that hnRNPA2B1 and hsa_circ_0001599 have a binding interaction, with particularly strong binding observed at the 98--176 bp region.
a Venn diagram of RBPs that bind to hsa_circ_0001599, as predicted using the starBase and RBPmap databases. b Ion chromatograph. c Histogram of quantitative comparison of peptides between sample groups. d The binding site of hsa_circ_0001599 to hnRNPA2B1 was predicted using the catRAPID database. e The RPISeq database was used to predict the binding ability of hsa_circ_0001599 to hnRNPA2B1. f RIP assay results. g CLIP‒qPCR analysis of the enrichment of different regions of hsa_circ_0001599. h The expression of hnRNPA2B1 in hDPSCs. i Efficiency of hnRNPA2B1 overexpression and knockdown in hDPSCs. j, k Effects of hnRNPA2B1 overexpression or knockdown on the expression of odontogenic differentiation-related proteins in hDPSCs. l ALP and ARS staining results. f, h–l All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 3 for each group. l Scale bar, 200 μm.
We then tested the relative levels of hnRNPA2B1 during odontogenic differentiation and found that hnRNPA2B1 was overexpressed (Fig. 6h). To clarify whether hnRNPA2B1 participates in hDPSCs odontogenic differentiation, hnRNPA2B1 expression was knocked down using sh-hnRNPA2B1, and hnRNPA2B1 lentiviruses were used to overexpress hnRNPA2B1 in hDPSCs (Fig. 6i). Western blot analysis revealed that hnRNPA2B1 facilitated hDPSCs odontogenic differentiation and that knockdown of hnRNPA2B1 reduced DSPP, DMP1, RUNX2, and ALP expression levels, whereas hnRNPA2B1 overexpression increased the expression levels of these markers (Fig. 6j, k). The staining experiment results were mostly consistent with those of the Western blot analysis (Fig. 6l).
Interaction between hsa_circ_0001599 and hnRNPA2B1
To further examine the role of hnRNPA2B1 and hsa_circ_0001599 in regulating odontogenic differentiation in hDPSCs, we investigated whether hsa_circ_0001599 can regulate hnRNPA2B1 expression through its interaction. Western blot results revealed that the total protein level of hnRNPA2B1 in hDPSCs was not affected by the overexpression or knockdown of hsa_circ_0001599 (Fig. 7a). According to previous reports, some hnRNPs shuttle between the nucleus and the cytoplasm, thereby promoting their binding to target RNA16,17. Our results indicated that hsa_circ_0001599 overexpression enhanced the enrichment of hnRNPA2B1 in the cytoplasm, whereas hsa_circ_0001599 knockdown reduced its cytoplasmic levels (Fig. 7b). In addition, immunofluorescence data confirmed that hsa_circ_0001599 overexpression increased hnRNPA2B1 protein levels in the cytoplasm (Fig. 7c). In summary, these data demonstrated that hsa_circ_0001599 can interact with hnRNPA2B1 and promote its translocation from the nucleus to the cytoplasm. Considering that hnRNPA2B1 is an m6A reader, we used SRAMP software to predict the m6A modification sites of hsa_circ_0001599. Multiple m6A modification sites were found in hsa_circ_0001599, including 1 high confidence site (Position. 291), 3 moderate confidence sites (Position. 17, 23, 108) and 1 low confidence site (Position. 45) (Fig. 7d). These sites were then validated using m6A single-base site PCR (MazF) to determine whether there were m6A modification sites on hsa_circ_0001599. The selection of positions 108 and 291 for verification was based on the utilization of MazF, which targets the core ACA sequence within a conserved motif region in single-base PCR. The results confirmed the presence of m6A at position 108 in hsa_circ_0001599. (Fig. 7e). Subsequently, after knocking down hnRNPA2B1, the m6A modification level of hsa_circ_0001599 decreased, indicating that the binding of hnRNPA2B1 to hsa_circ_0001599 may be mediated by m6A modification (Fig. 7f).
a Western blot analysis of the total hnRNPA2B1 levels in each groups. b Effect of hsa_circ_0001599 on hnRNPA2B1 protein levels in the nucleus and cytoplasm. c Immunofluorescence results. d The SRAMP database was used to predict the m6A site of hsa_circ_0001599. e MazF assay was used to analyse the m6A modification level of hsa_circ_0001599 after mineralization. f MazF assay was used to analyse the m6A modification level of hsa_circ_0001599 after hnRNPA2B1 knockdown. a, b, e, f All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns: not significant, a, b, f n = 3 for each group, e n = 9, n = 7 for each group. c Scale bar, 50 μm.
The hsa_circ_0001599 and hnRNPA2B1 complex promotes hDPSCs odontogenic differentiation by stabilizing ITGA2 mRNA
hnRNPs are known regulators of RNA stability, and hnRNPA2B1 enhances mRNA stability by recognizing mRNA methylation sites. Based on this function, we predicted that hnRNPA2B1 may bind to mRNA (Fig. 8a). Since our previous research indicating the association of hsa_circ_0001599 with the PI3K/AKT signalling pathway and ITGA2 with this pathway, we selected ITGA2 for further investigation. RPIseq predicted that hnRNPA2B1 and ITGA2 have higher binding affinity, and RIP experiments verified that these two can interact (Fig. 8b). SRAMP website tools were used to reveal the m6A modification sites in ITGA2 (Fig. 8c). The MazF assay was used to verify the occurrence of m6A modification at site 2393 (Fig. 8d). It is highly likely that hnRNPA2B1 binds to the methylation site on ITGA2, but further verification is needed to confirm this result.
a mRNA binding to hnRNPA2B1 was predicted using the starBase database and transcriptomic sequencing. b The binding ability of ITGA2 to hnRNPA2B1 was predicted using the RPISeq database and RIP assay results. c The m6A site of ITGA2 was predicted using the SRAMP database. d MazF assay was used to analyse the m6A modification level of ITGA2 after mineralization. e Western blot and qRT‒PCR analysis of ITGA2 expression after 7 and 14 days of odontogenic differentiation. f qRT-PCR was used to determine the ITGA2 expression levels. g, h Expression of odontogenic differentiation-related mRNAs and proteins 7 days and 14 days after ITGA2 knockdown. i ALP and ARS staining results. j Western blot analysis revealed that the sh-ITGA2 group had lower levels of p-AKT and p-PI3K expression. k SC79 increased the expression of p-AKT and p-PI3K, according to Western blot analysis. l The impact of SC79 on the odontogenic differentiation of hDPSCs was detected using Western blot. b, d–l All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns: not significant, b, e–l n = 3 for each group, d n = 9, n = 6 for each group. i Scale bar, 200 μm.
Next, we evaluated whether ITGA2 can enhance odontogenic differentiation in hDPSCs. ITGA2 was significantly upregulated in induced hDPSCs. As demonstrated by qRT-PCR, Western blot and staining experiments, downregulation of ITGA2 markedly reduced the odontogenic differentiation of hDPSCs. (Fig. 8e-i). Furthermore, Western blot analysis revealed that a reduction in ITGA2 significantly reduced p-AKT and p-PI3K protein levels (Fig. 8j). This experiment was further conducted by treating cells with the PI3K/AKT pathway agonist SC79. The results showed that the SC79 agonist partially reversed the decreases in p-AKT and p-PI3K in hDPSCs (Fig. 8k). Additionally, the inhibitory effect of sh-ITGA2 on the odontogenic differentiation of hDPSCs was partially rescued by treatment with SC79 (Fig. 8l). These findings suggest that ITGA2 positively regulates the PI3K/AKT signalling pathway.
Next, the correlations among hsa_circ_0001599, hnRNPA2B1 and ITGA2 were explored. As shown in Fig. 9a, b, overexpression of hsa_circ_0001599 or hnRNPA2B1 increased ITGA2 protein expression in hDPSCs, whereas hsa_circ_0001599 or hnRNPA2B1 knockdown reduced ITGA2 protein levels in hDPSCs. A rescue experiment revealed that knocking down hnRNPA2B1 reversed the increase in ITGA2 expression after the overexpression of hsa_circ_0001599 (Fig. 9c). In addition, staining experiments showed that knockdown of ITGA2 partially reversed the promoting effect of overexpression of hsa_circ_0001599 on the odontogenic differentiation of hDPSCs (Fig. 9d). hnRNPA2B1 may enhance mRNA stability by binding mRNA or identifying mRNA methylation sites18. These results indicate that hnRNPA2B1 can bind to ITGA2 and that ITGA2 may contain m6A modification sites. We speculated that hsa_circ_0001599 and hnRNPA2B1 modulate ITGA2 mRNA stability, so we treated cells with actinomycin D to prevent new RNA synthesis. Our findings revealed that hsa_circ_0001599 and hnRNPA2B1 silencing significantly decreased ITGA2 stability in hDPSCs. (Fig. 9e). Moreover, the overexpression of hsa_circ_0001599 and hnRNPA2B1 extended the half-life of ITGA2 mRNA in hDPSCs (Fig. 9f). Moreover, knockdown of hnRNPA2B1 reversed hsa_circ_0001599-induced ITGA2 mRNA stabilization (Fig. 9g). These results indicate that hsa_circ_0001599/hnRNPA2B1 promotes odontogenic differentiation in hDPSCs by enhancing the stability of ITGA2 mRNA through the formation of an RNA-protein complex.
a Effect of hsa_circ_0001599 overexpression/knockdown on ITGA2 expression. b Effect of hnRNPA2B1 overexpression/knockdown on ITGA2 expression. c Knockdown of hnRNPA2B1 rescued ITGA2 expression after overexpression of hsa_circ_0001599. d Knocking down ITGA2 partially rescued the ability of hsa_circ_0001599 overexpression to promote odontogenic differentiation in hDPSCs. e, f The influence of hsa_circ_0001599 and hnRNPA2B1 on ITGA2 stability. g Knocking down hnRNPA2B1 rescued the ability of hsa_circ_0001599 to promote ITGA2 stability. a–d All data were presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 3 for each group. d Scale bar, 200 μm.
Discussion
circRNAs are novel noncoding RNAs with stable closed-loop structures, and they originate from exons, introns, or intergenic regions19. circRNAs are resistant to external RNA nucleases or RNase R and are mostly found in the cytoplasm of cells and partially in the nucleus. An increasing amount of evidence shows that circRNAs play an important role in the occurrence and development of many diseases in humans20,21,22. Importantly, emerging literature illustrates the link between circRNAs and odontogenic differentiation11. Our findings reveal the function of hsa_circ_0001599 in hDPSCs during odontogenic differentiation. The odontogenic differentiation of hDPSCs was improved by overexpressing hsa_circ_0001599. At present, no relevant studies have confirmed that circRNAs can regulate the formation of restorative dentin in hDPSCs in vivo. In recent years, with the continuous development of tissue engineering medicine, numerous studies have shown that a subcutaneous ectopic model of nude mice can be used to verify the mineralization induction ability of biological materials and can also be used to detect the mineralization performance of mesenchymal stem cells in vivo23,24,25. This study revealed that HA/ТCP dental root segments loaded with LV-circ_0001599-hDPSCs enhanced dentin-like tissue formation in vivo. Thus, the role of hsa_circ_0001599 in promoting odontogenic differentiation was revealed. However, it may be difficult to simulate the process of pulp regeneration in the subcutaneous ectopic regeneration model of the nude mice used in this study. Therefore, in situ regeneration models such as rats, beagle dogs or miniature pigs need to be employed for further verification of these results.
The majority of circRNA research to date has demonstrated that translation, binding to RBPs, and binding to ceRNAs are the three primary methods by which circRNAs carry out their biological tasks. ceRNAs are mainly noncoding RNAs that compete for binding to the same miRNAs via miRNA response elements (MREs), which can weaken their regulatory functions26. miR-889-3p plays a significant role in numerous biological processes27,28,29. Nevertheless, the role of miR-889-3p in the odontogenic differentiation of hDPSCs and its related mechanisms remain unclear. In this study, our results confirmed that miR-889-3p is a downregulated miRNA that inhibits odontogenic differentiation. A series of experiments revealed that hsa_circ_0001599 can act as a miRNA sponge to regulate miR-889-3p, but further confirmation through the combination of site mutation experiments to observe phenotypes is needed. In addition, it has been reported that noncoding RNAs not only act as ceRNAs of miRNAs but also regulate the same miRNA by destabilizing pri-miRNAs30. However, this topic needs to be further explored. The data of this study revealed that hsa circ_0001599 regulates the expression of ITGA2 through miR-889-3p and promotes the odontogenic differentiation of hDPSCs, thus forming the hsa_circ_0001599/miR-889-3p/ITGA2 axis. Interestingly, the upregulation of hsa_circ_0001599 only partially reversed the inhibitory effect of miR-889-3p on the odontogenic differentiation of hDPSCs. These findings prompted us to hypothesize that hsa_circ_0001599 may regulate the odontogenic differentiation of hDPSCs through other means. RBPs are essential regulatory factors in transcription and translation, and they reportedly participate in downstream gene regulation by interacting with circRNAs31,32. In addition, circRNAs with dual-faceted regulatory pathways (ceRNAs and RBPs) have been discovered in recent years. For example, by sponging miR-154-3p, circ_0000009 increases the expression of PDZD2. Moreover, circ_0000009 recruits IGF2BP2 to stabilize PDZD233. CircTHBS1 promotes the progression of gastric cancer by increasing the expression and stability of INHBA mRNA in a ceRNA- and RBP-dependent manner34. Our research revealed that the hnRNPA2B1 protein and hsa_circ_0001599 physically interact. As a conserved mRNA stability regulator35, hnRNPA2B1 has been reported to shuttle between the nucleus and the cytoplasm36 and consequently facilitate its binding to target RNA37. Liu et al. reported that circMYH9 regulates the stability of p53 premrRNA through hnRNPA2B138. Nevertheless, it is still unknown whether hnRNPA2B1 contributes to the odontogenic differentiation of hDPSCs. In this study, we found that hsa_circ_0001599 binds to hnRNPA2B1 and promotes its accumulation in the cytoplasm, subsequently increasing the stability of ITGA2 mRNA after binding to ITGA2, which is in line with how hnRNPA2B1 functions, as described in previous studies. However, the mechanism by which hnRNPA2B1 affects the stability of ITGA2 mRNA has not been fully elucidated. The regulation of mRNA stability depends to a certain extent on diverse cis-acting elements and trans-acting factors39. hnRNPA2B1 reportedly acts as an m6A reader40, recognizing the m6A site on TCF7L2 mRNA to stabilize the poly(A) tail and maintain its mRNA stability41. Moreover, hnRNPA2B1 proteins are involved in the regulation of mRNA deadenylation through their interaction with the CCR4-NOT deadenylation complex. They accomplish this by binding to the UAASUUAU sequence located in the 3’ untranslated region (3’UTR) of the mRNA, which in turn affects the degradation process of the mRNA transcript42. Therefore, the specific mechanisms still require further exploration.
ITGA2 is a biologically multifunctional member of the integrin family, and most studies have shown that it can promote tumour progression, inhibit DNA repair, and increase cancer sensitivity to radiotherapy43,44. Recent studies have shown that ITGA2 regulates the osteogenic differentiation of periodontal ligament stem cells by activating downstream proteins of the FAK/AKT/PI3K signalling pathway45. Moreover, amphiregulin can induce the odontogenic differentiation of hDPSCs through the PI3K/AKT signalling pathway5. Some scholars have also reported that after ETV2 overexpression in hDPSCs, the ERK/MAPK and PI3K/AKT signalling pathways are activated, significantly increasing the mRNA and protein expression levels of osteogenic markers46. The above studies indicate that the PI3K/AKT signalling pathway may be involved in the regulation of the osteogenic/odontogenic differentiation of mesenchymal stem cells, which is consistent with the results of this study. However, studies have also shown that the odontogenic differentiation of hDPSCs can be blocked by the PI3K/AKT/mTOR signalling pathway47. These differences may be caused by different factors stimulating hDPSCs or different modelling methods. Considering these differences, the role of the PI3K/AKT signalling pathway in the odontogenic differentiation of hDPSCs needs further study. Moreover, few studies have investigated the molecular mechanism by which ITGA2 regulates the odontogenic differentiation of hDPSCs. Our study confirmed that ITGA2 participates in hsa_circ_0001599-mediated regulation of the odontogenic differentiation of hDPSCs. Additionally, elevated ITGA2 expression caused by hsa_circ_0001599 overexpression also activated PI3K/AKT. We also found that hsa_circ_0001599 upregulates ITGA2 expression and stability by sponging miR-889-3p and recruiting hnRNPA2B1.
Conclusions
Our research demonstrates that hsa_circ_0001599 promotes the odontogenic differentiation of hDPSCs by increasing ITGA2 mRNA expression and stability in a ceRNA- and RBP-dependent manner (Fig. 10). This study provides a new therapeutic target for pulp tissue regeneration.
Materials and methods
Isolation and culture of hDPSCs
The tooth samples used in this study were obtained from healthy premolars that required extraction for orthodontic treatment and third molars without caries from patients aged 18 –25 years. This study has been approved by the Ethics Committee of Southern Medical University NanFang Hospital (Ethics Approval Number: NFEC-2022-173). All ethical regulations relevant to human research participants were followed. Tooth surfaces were rinsed with phosphate buffer saline (PBS) buffer and cut the tooth by using sterilized dental burs to reveal the pulp chamber. The pulp tissue was gently extracted from the tooth and then digested in a solution of 3 mg/mL collagenase type I for 10 min at 37°C under 5% CO2 in air. Fourteen healthy dental pulp tissue samples were harvested and examined in various experiments. The hDPSCs were seeded into 25-cm2 culture flasks and cultured in 4 mL of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1% penicillin/streptomycin and 10% foetal bovine serum (FBS) in a 5% CO2 atmosphere at 37 °C. Every 3 days, the medium was replaced until the cells reached 80% confluence. The hDPSCs were subsequently cultured using the limited dilution method, and those from passages 3 to 5 were used in each of the experiments.
hDPSCs characterization
The hDPSCs phenotypes were identified by using flow cytometry, which was used to screen for surface markers against CD45, CD34, CD44, CD29, CD105 and CD90. hDPSCs were differentiated into odontoblasts, adipocytes, and chondrocytes using odontogenic media (contained with 10% FBS, 50 mg/mL ascorbic acid, 100 nmol/L dexamethasone, and 10 mmol/L β-glycerophosphate), adipogenic media (contained with 10% FBS, 0.5 mM isobutyl-methylxanthine (IBMX), 10 − 6 M dexamethasone, 10 μg/mL insulin, and 200 μM indomethacin), and chondrogenic media (contained with 10% FBS, 1% ITS+Premix, 0.1 μM dexamethasone, 0.2 mM L-ascorbic acid-2-phosphate, 1% pen/strep, and 10 ng/mL TGF-β3) respectively. After 21 days of induction, the hDPSCs were stained with ARS, Oil Red O and alcian blue48,49.
qRT‒PCR
In this study, an EZ-press RNA Purification Kit (EZB, USA), a Colour Reverse Transcription Kit (with gDNA Remover), and 2 × Colour SYBR Green qPCR Master Mix (ROX2 plus) were used following the manufacturer’s instructions. Three kits were used for RNA extraction, reverse transcription and qRT‒PCR. The sequences of the primers are presented in Supplementary Table 1 and Table 2.
Western blot analysis
RIPA lysis buffer (Epizyme, China) was added to the cells, and the concentration of the subsequent protein was measured using an Enhanced BCA Protein Assay Kit (Beyotime, China). The protein was then separated via a 10% gel prepared with the Colour PAGE Gel Rapid Preparation Kit (Epizyme, China) and transferred to a PVDF membrane. After the membranes were blocked in protein-free rapid blocking buffer (1×) (Epizyme, China), the primary antibodies were allowed to bind to the membranes overnight at 4 °C. The membranes were then incubated for 1 hour with secondary antibodies. Finally, an enhanced chemiluminescence kit (Epizyme, China) was used to detect the protein concentration. Nuclear and cytoplasmic RNA were separated using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, China) following the manufacturer’s instructions. The levels of the specified genes were normalized to those of GAPDH, which served as the endogenous reference. Laminb was used as a nuclear control, and GAPDH expression was used as a cytoplasmic control to identify protein in various cellular fractions. Supplementary Table 3 contains information on the primary and secondary antibodies.
Transcriptomics
circRNAs from the LV- circ_0001599 groups and control groups were collected for sequencing analysis. Total RNA was isolated from hDPSCs and subjected to library preparation in accordance with the Illumina standard protocol utilizing the VAHTS Universal V6 RNA-seq Library Prep Kit for Illumina®. The concentration and size distribution of the cDNA library was assessed using an Agilent 4200 Bioanalyzer prior to sequencing on an Illumina NovaSeq 6000 platform, following the manufacturer’s guidelines (Illumina). Raw sequencing reads were preprocessed with Seqtk and aligned to the reference genome using Hisat2 (version 2.0.4). Gene fragment counts were determined using StringTie (version 1.3.3b), followed by normalization with the Trimmed Mean of M values method. Differentially expressed genes (DEGs) were considered significant if they exhibited a False Discovery Rate (FDR) below the threshold (Q < 0.05) and a fold-change greater than 1.5, as determined by edgeR software.
ChIRP-PRM analysis
For each ChIRP experiment, 4×109 cells were transfected with a biotin-labelled probe. The enzymatic hydrolysis products were separated using a nano UPLC liquid phase system (EASY-nLC1200) for mass spectrometry50. A 5 μL of tryptic peptides per sample were solubilized in 0.1% formic acid with acetonitrile. Samples were directly loaded onto a 100 μm ID x 15 cm reversed phase chromatographic column for separation. The flow rate was set at 300 nanoliters per minute, with a gradient duration of 120 minutes. Mobile phase B was programmed as follows: held at 6-28% for 92 minutes, increased to 40% over the next 20 minutes, ramped up to 100% for 2 minutes, maintained at 100% for another 2 minutes, then decreased back to 2% over 2 minutes, and finally held at 2% for the last 2 minutes. Following nano-UPLC, PRM data were acquired via MS and uploaded to Skyline for transition analysis51. The above experiments were conducted at Kangchen Biotechnology Co., Ltd. (China).
CLIP-qPCR
CLIP experiments were conducted using a CLIP kit (BersinBio™ CLIP-qPCR Kit, Bes3014). The cells were cultured overnight in medium containing 100 μM 4-thiouracil and then exposed to 365 nm UV light the following day to induce covalent bonding between RNA fragments and proteins. After the cells were harvested with NP-40 lysis buffer, RNase was applied to digest the unbound RNA fragments, and the specific binding sites between proteins hnRNPA2B1 and hsa_circ_0001599 were identified.
Single-base PCR (MazF)
The conserved motif regions of the core ACA sequence (m6A-ACA site) on hsa_circ_0001599 and ITGA2 were verified. The extracted RNA sample was split into two parts: one part was not treated with MazF, and the other part was treated with MazF. The m6A methylation level of specific ACA sites in each sample was subsequently measured using qRT‒PCR52,53.
RIP assay
RIP assays were carried out in accordance with the manufacturer’s protocol using Dynabeads® Protein G (Thermo Fisher Scientific, USA). IgG or the indicated antibody was added to whole-cell lysates, which were then incubated overnight at 4 °C. Proteinase K buffer treatment was followed by extraction of the immunoprecipitated RNAs. Next, qRT‒PCR was used to measure the relative expression and normalize it to the input sample levels.
Dual-luciferase reporter assay
The 3’ untranslated region (UTR) sequence of ITGA2, the full-length sequence of hsa_circ_0001599, and the corresponding mutant sequences were synthesized and introduced to the luciferase reporter vector psiCHECK-2. The day before transfection, HEK293T cells were seeded at a density of 5×104 cells per well into 24-well plates. The Renilla and firefly luciferase activities were assessed independently 48 hours following transfection using a dual-luciferase reporter assay system (Promega, USA).
FISH and Immunofluorescence assay
FISH was used to locate hsa_circ_0001599 utilizing probes (Probe Sequence: CTTTTAAG + TAGAGAACT + TTCTACTG + TTGCTC) that were tagged with Fam. The signals were analysed using a FISH kit (Genepharma, China). The cells were seeded onto sterile slides. After the cells reached 80% confluence, they were fixed for 20 min with 4% paraformaldehyde. The antigens were blocked with 10% goat serum for 30 min at room temperature following membrane permeabilization with 0.1% Triton X-100 for 10 min. The cells were then treated with an antibody at a 1:100 dilution overnight at 4 °C. Next, they were treated with a goat anti-mouse or rabbit IgG secondary antibody for an additional hour at room temperature. Finally, the DNA was labelled with DAPI for 15 min.
RNA stability analysis
RNA transcription in hDPSCs was inhibited by treatment with 5 μg/mL actinomycin D, as described in previous studies analysing mRNA decay rates54. After 0, 4, 8 and 12 hours, the mRNA was isolated, and qRT‒PCR was performed.
In vivo odontogenic differentiation assay
To characterize the odontogenic differentiation ability of hsa_circ_0001599, hDPSCs or PBS mixed with HA/TCP ceramic powder (20 mg per sample) were placed into human tooth root segments and then transplanted subcutaneously into 5-week-old BALB/c nude mice. Nude mice (male, 4-5 weeks) were purchased from BesTest Bio-Tech (Zhuhai, China). All animal experimental procedures were approved by the Laboratory Animal Center of South China Agricultural University (Ethics number: 2023D076). We have complied with all relevant ethical regulations for animal use. Twenty mice were randomly assigned to four groups: the HA/TCP + PBS group, HA/TCP+hDPSC group, HA/TCP + LV-NC group, and HA/TCP + LV-circ_0001599 group. The grafts were obtained at 4 weeks post-transplantation, preserved with 4% paraformaldehyde, decalcified for 4 weeks using buffered 10% EDTA, embedded in paraffin for further studies, and subjected to H&E staining, Masson’s trichrome staining and IHC.
Statistics and reproducibility
Student t test or one-way ANOVA were used to evaluate statistical differences between two groups or multiple groups. Experiments were independently repeated three times to confirm reproducibility and all values are expressed as the mean ± standard deviations (SDs). GraphPad Prism 9.5 software was used to create the figures. Experiments for Dual-luciferase reporter assay, qRT-PCR, and Western blot analysis were repeated in hDPSCs. P value < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Uncropped western blots can be found in Supplementary Information. The source data for the graphs can be found in Supplementary Data. RNA-seq data is available through Gene-Expression omnibus (GEO) accession number GSE284122. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbott, P. V. Present status and future directions: Managing endodontic emergencies. Int. Endod. J. 55, 778–803 (2022).
Brodzikowska, A., Ciechanowska, M., Kopka, M., Stachura, A. & Włodarski, P. K. Role of lipopolysaccharide, derived from various bacterial species, in pulpitis-a systematic review. Biomolecules 12, 138 (2022).
Wen, B. et al. Reparative dentin formation by dentin matrix proteins and small extracellular vesicles. J. Endod. 47, 253–262 (2021).
Han, B. et al. Injectable double-network hydrogel-based three-dimensional cell culture systems for regenerating dental pulp. ACS Appl. Mater. Interfaces 15, 7821–7832 (2023).
Li, J. et al. Amphiregulin regulates odontogenic differentiation of dental pulp stem cells by activation of mitogen-activated protein kinase and the phosphatidylinositol 3-kinase signaling pathways. Stem Cell Res Ther. 13, 304 (2022).
Liang, C. et al. Bone morphogenetic protein 7 mediates stem cells migration and angiogenesis: therapeutic potential for endogenous pulp regeneration. Int. J. Oral. Sci. 14, 38 (2022).
Yu, Y.-Z. et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol. Cancer 21, 12 (2022).
Zhao, X., Sun, W., Guo, B. & Cui, L. Circular RNA BIRC6 depletion promotes osteogenic differentiation of periodontal ligament stem cells via the miR-543/PTEN/PI3K/AKT/mTOR signaling pathway in the inflammatory microenvironment. Stem Cell Res. Ther. 13, 417 (2022).
Ji, F. et al. hsa_circ_0026827 promotes osteoblast differentiation of human dental pulp stem cells through the Beclin1 and RUNX1 signaling pathways by sponging miR-188-3p. Front Cell Dev. Biol. 8, 470 (2020).
Liang, C., Li, W., Huang, Q. & Wen, Q. CircFKBP5 suppresses apoptosis and inflammation and promotes osteogenic differentiation. Int. Dent. J. 73, 377–386 (2023).
Liu, Z. et al. Hsa_Circ_0005044 Promotes Osteo/Odontogenic Differentiation of Dental Pulp Stem Cell Via Modulating miR-296-3p/FOSL1. DNA Cell Biol. 42, 14–26 (2023).
Chen, M., Yang, Y., Zeng, J., Deng, Z. & Wu, B. circRNA expression profile in dental pulp stem cells during odontogenic differentiation. Stem Cells Int. 2020, 5405931 (2020).
Chen, Z. et al. Genome-wide identification of long noncoding RNAs and their competing endogenous RNA networks involved in the odontogenic differentiation of human dental pulp stem cells. Stem Cell Res Ther. 11, 114 (2020).
Xu, S., Song, Y., Shao, Y. & Zhou, H. Hsa_circ_0060927 Is a Novel Tumor Biomarker by Sponging miR-195-5p in the Malignant Transformation of OLK to OSCC. Front Oncol. 11, 747086 (2021).
Hansen, T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
van der Houven van Oordt, W. et al. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149, 307–316 (2000).
Jiang, H. et al. Long noncoding RNA CRNDE stabilized by hnRNPUL2 accelerates cell proliferation and migration in colorectal carcinoma via activating Ras/MAPK signaling pathways. Cell Death Dis. 8, e2862 (2017).
Goodarzi, H. et al. Systematic discovery of structural elements governing stability of mammalian messenger RNAs. Nature 485, 264–268 (2012).
Yu, L. et al. circ_0003204 regulates the osteogenic differentiation of human adipose-derived stem cells via miR-370-3p/HDAC4 axis. Int. J. Oral. Sci. 14, 30 (2022).
Xu, C. et al. A circulating panel of circRNA biomarkers for the noninvasive and early detection of pancreatic ductal adenocarcinoma. Gastroenterology 166, 178–190.e16 (2024).
Ngo, L. H. et al. Nuclear export of circular RNA. Nature 627, 212–220 (2024).
Nemeth, K., Bayraktar, R., Ferracin, M. & Calin, G. A. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat. Rev. Genet 25, 211–232 (2024).
Lu, Y.-F. et al. Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-β1 signaling. FASEB J. 31, 954–964 (2017).
Liu, L. et al. Carbon dots enhance extracellular matrix secretion for dentin-pulp complex regeneration through PI3K/Akt/mTOR pathway-mediated activation of autophagy. Mater. Today Bio 16, 100344 (2022).
Wang, F. et al. Adipose-derived stem cells with miR-150-5p inhibition laden in hydroxyapatite/tricalcium phosphate ceramic powders promote osteogenesis via regulating Notch3 and activating FAK/ERK and RhoA. Acta Biomater. 155, 644–653 (2023).
Wang, Y., Wang, B., Cao, W. & Xu, X. TGF-β-activated circRYK drives glioblastoma progression by increasing VLDLR mRNA expression and stability in a ceRNA- and RBP-dependent manner. J. Exp. Clin. Cancer Res 43, 73 (2024).
Sun, W. et al. miR-889-3p Facilitates the Browning Process of White Adipocyte Precursors by Targeting the SON Gene. Int J. Mol. Sci. 24, 17580 (2023).
Xu, G., Ding, Z. & Shi, H.-F. The mechanism of miR-889 regulates osteogenesis in human bone marrow mesenchymal stem cells. J. Orthop. Surg. Res 14, 366 (2019).
Liu, H. et al. RUNX3-mediated circDYRK1A inhibits glutamine metabolism in gastric cancer by up-regulating microRNA-889-3p-dependent FBXO4. J. Transl. Med 20, 120 (2022).
Zheng, Z.-N. et al. NF-κB-mediated lncRNA AC007271.3 promotes carcinogenesis of oral squamous cell carcinoma by regulating miR-125b-2-3p/Slug. Cell Death Dis. 11, 1055 (2020).
Hentze, M. W., Castello, A., Schwarzl, T. & Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 19, 327–341 (2018).
Schlundt, A., Tants, J.-N. & Sattler, M. Integrated structural biology to unravel molecular mechanisms of protein-RNA recognition. Methods 118–119, 119–136 (2017).
Tan, T., Ma, M. & Xing, S. Effect of circ_0000009 on lung adenocarcinoma progression by regulating PDZD2 in a ceRNA- and RBP- dependent manner. Gene 877, 147555 (2023).
Qiu, S. et al. CircTHBS1 drives gastric cancer progression by increasing INHBA mRNA expression and stability in a ceRNA- and RBP-dependent manner. Cell Death Dis. 13, 266 (2022).
Brennan, C. M. & Steitz, J. A. HuR and mRNA stability. Cell Mol. Life Sci. 58, 266–277 (2001).
Martínez–Chantar, M. L. et al. S–adenosylmethionine regulates cytoplasmic HuR Via AMP–activated kinase. Gastroenterology 131, 223–232 (2006).
Kotta-Loizou, I., Giaginis, C. & Theocharis, S. Clinical significance of HuR expression in human malignancy. Med. Oncol. 31, 161 (2014).
Liu, X. et al. CircMYH9 drives colorectal cancer growth by regulating serine metabolism and redox homeostasis in a p53-dependent manner. Mol. Cancer 20, 114 (2021).
Boo, S. H. & Kim, Y. K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp. Mol. Med. 52, 400–408 (2020).
Alarcón, C. R. et al. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308 (2015).
Liu, H. et al. Interaction of lncRNA MIR100HG with hnRNPA2B1 facilitates m6A-dependent stabilization of TCF7L2 mRNA and colorectal cancer progression. Mol. Cancer 21, 74 (2022).
Geissler, R. & Grimson, A. A position-specific 3’UTR sequence that accelerates mRNA decay. RNA Biol. 13, 1075–1077 (2016).
Zhou, C. et al. ITGA2 overexpression inhibits DNA repair and confers sensitivity to radiotherapies in pancreatic cancer. Cancer Lett. 547, 215855 (2022).
Zhou, P. et al. ATF4-mediated circTDRD3 promotes gastric cancer cell proliferation and metastasis by regulating the miR-891b/ITGA2 axis and AKT signaling pathway. Gastric Cancer 26, 565–579 (2023).
Qu, H.-L. et al. Long non-coding RNA AC018926.2 regulates palmitic acid exposure-compromised osteogenic potential of periodontal ligament stem cells via the ITGA2/FAK/AKT pathway. Cell Prolif. 56, e13411 (2023).
Li, J. et al. ETV2 promotes osteogenic differentiation of human dental pulp stem cells through the ERK/MAPK and PI3K-Akt signaling pathways. Stem Cell Res Ther. 13, 495 (2022).
Park, S. Y. et al. Inactivation of PI3K/Akt promotes the odontoblastic differentiation and suppresses the stemness with autophagic flux in dental pulp cells. J. Dent. Sci. 17, 145–154 (2022).
Liu, W. et al. Rapamycin-induced autophagy promotes the chondrogenic differentiation of synovium-derived mesenchymal stem cells in the temporomandibular joint in response to IL-1β. Biomed. Res Int 2020, 4035306 (2020).
Farahzadi, R., Fathi, E. & Vietor, I. Mesenchymal stem cells could be considered as a candidate for further studies in cell-based therapy of Alzheimer’s disease via targeting the signaling pathways. ACS Chem. Neurosci. 11, 1424–1435 (2020).
Chu, C., Quinn, J. & Chang, H. Y. Chromatin isolation by RNA purification (ChIRP). J. Vis. Exp. https://doi.org/10.3791/3912 (2012).
Peterson, A. C., Russell, J. D., Bailey, D. J., Westphall, M. S. & Coon, J. J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell Proteom. 11, 1475–1488 (2012).
Garcia-Campos, M. A. et al. Deciphering the ‘m6A Code’ via antibody-independent quantitative profiling. Cell 178, 731–747.e16 (2019).
Zhang, Z. et al. Single-base mapping of m6A by an antibody-independent method. Sci Adv 5, eaax0250 (2019).
Huang, H. et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295 (2018).
Acknowledgements
This work was supported by the General Program of National Natural Scientific Foundation of China (No. 82170942), Science Research Cultivation Program of Stomatological Hospital, Southern Medical University (PY2021021), Teaching Reform Research Project of Clinical Teaching Bases in Guangdong Province (2021JD144).
Author information
Authors and Affiliations
Contributions
Y.Q.Y., C.J. and J.K.Z. have given the conception and wrote the main manuscript. X.L.G., Y.Q.Y. and J.K.Z. had contributed to acquisition, analysis, and interpretation of the data. M.C. and B.L.W. have designed this study and revised it critically. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Xiao Cen and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Kaliya Georgieva.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Jiang, C., Zeng, J. et al. hsa_circ_0001599 promotes odontogenic differentiation of human dental pulp stem cells by increasing ITGA2 expression and stability. Commun Biol 8, 74 (2025). https://doi.org/10.1038/s42003-025-07488-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07488-z