Abstract
WYL-domain containing transcription factors regulate fundamental processes in bacterial physiology, yet how these proteins sense cellular cues to elicit an allosteric response is not well understood. Here we describe the allosteric activation mechanism of DriD, a Caulobacter crescentus homodimeric WYL-domain containing transcription regulator that activates a non-canonical DNA damage pathway. DriD senses ssDNA, produced upon DNA damage via interaction with its WYL domain. This stimulates DriD target DNA binding. However, its DNA-binding domains (DNABDs) are 50 Å from the WYL-domains and linked by a three-helix bundle domain (3HB). Using a combination of crystallography, biochemistry, and HDX-MS we unveil an allosteric mechanism whereby an inhibitory interaction, formed between the DriD DNABD and 3HB in the apo form, is freed upon ssDNA binding, allowing target DNA binding. These findings may serve as a model for understanding activation by the large family of homodimeric WYL activators, including those in pathogenic bacteria.

Similar content being viewed by others
Introduction
The maintenance of genomic integrity during DNA-damaging events is critical for the viability of all organisms1. In the presence of DNA damage, bacteria utilize a conserved, canonical DNA repair pathway known as the SOS response2,3,4,5,6. Single-stranded DNA (ssDNA) accumulates during DNA damage due to repair of double-stranded breaks and serves as a genotoxic signal in the cell7,8,9,10,11. In the SOS response, ssDNA binds to RecA, which acts as a coprotease to facilitate the self-cleavage of the LexA repressor thus allowing expression of the LexA regulon. The LexA regulon encodes a range of factors, including those involved in DNA damage repair, antibiotic resistance, and cell division checkpoints12,13,14,15,16,17,18,19,20,21. The generation of checkpoint proteins is essential to pause cell division and allow time for the repair of damaged DNA that would otherwise be transmitted to daughter cells.
Despite the generally conserved nature of the SOS response in bacteria, studies have begun to identify alternative DNA damage response systems22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. One such SOS-independent pathway is present in Deinococcus, which is one of the most damage-tolerant bacteria32,33,34. This system involves cleavage of a transcriptional repressor, the DdrO repressor, by a protease called IrrE (also called PprI), which activates DNA repair33,34. The CBASS immune system in E. coli employs a similar metallopeptidase/repressor pair called CapP/CapH, with CapP serving as the protease35. The growing number of SOS-independent DNA damage repair pathways is of significant interest to the DNA repair and microbiology community, yet the detailed structural mechanisms mediated and sensed by SOS-independent DNA damage repair regulators are still in its early stages. Here, we focus on the recently delineated SOS-independent DNA damage pathway in Caulobacter crescentus. Data revealed that this system is mediated by a transcription activator, not a repressor. The activator protein, called DriD, was shown to regulate the transcription of genes encoding proteins involved in both DNA repair and the cell cycle36.
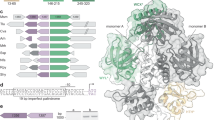
DriD regulates transcription by binding specifically to a 20 bp pseudo-palindromic target DNA site, ATACGAC(X)7GTCGTAT (underlined nucleotides are conserved), within regulated promoters36,37,38. Interestingly, studies also revealed a DriD binding site within the recA promoter, and cells lacking driD exhibited slower induction of the recA gene36,37. These analyses suggest there could be cross-talk/overlap between the SOS and DriD responses. In addition, recent analyses showed that DriD may be involved in regulating the process of horizontal gene transfer39. Thus, these data indicate that DriD plays an important role in general C. crescentus DNA physiology. DriD is a 327-residue protein that contains a predicted WYL motif. WYL domain containing proteins are a recently characterized group of proteins named for the presence of a conserved Trp-Tyr-Leu sequence40,41,42. Sequence analyses indicate these proteins are predominately found in bacteria43. Studies on DriD uncovered that its WYL domain binds ssDNA and subsequent analyses confirmed that ssDNA binding to DriD facilitates its ability to function as a transcription activator37,38. Hence, ssDNA has emerged as a generally utilized signal for the control of DNA damage responses. But interestingly, it does so by binding a diverse range of proteins involved in repair pathways, including proteases and transcription factors. Structural studies revealed that DriD functions as a homodimer with each subunit comprised of a winged helix-turn-helix (wHTH) DNA binding domain (DNABD), linker region, three-helix bundle (3HB), WYL domain and WYL C-terminal extension (WCX) dimerization domain37,38.
While WYL TFs are categorized into nine classes, the majority of proteins belong to Class A, of which DriD is a member41,43. Like DriD, class A WYL TFs contain a wHTH DNABD, followed by WYL and WCX domains. Structures of WYL TFs solved thus far have revealed two main categories, those represented by DriD, which are activators and those represented by BrxR and CapW, which are repressors29,37,38,43,44,45,46,47,48. BrxR, is a repressor of the BREX phage defense system, and CapW is a repressor of the antiviral CBASS system, which initiates cell death44,45,46. While both repressor and activator WYL proteins have wHTH and WYL domains, the remainder of their structures are distinct and they even employ different dimerization modules. Not surprisingly, given these structural differences the repressor proteins bind DNA target sites in a manner distinct from DriD. These differences also likely reflect the dissimilar forms of allosterism in each; in DriD, effector binding to the WYL-domain stimulates DNA binding in WYL-activators but in WYL-repressors, interaction of signaling molecules induces or removes it from its operator DNA. Although nucleic acids are suggested to be the signaling molecule for WYL-repressors, the specific identities of most of the effectors are currently not well understood.
Bioinformatic analyses showed that DriD is representative of a large subclass of WYL-activators and hence it can serve as a model system to understand the molecular details behind the activation of these regulators. The recent structure of the DriD-ssDNA-target DNA complex revealed that ssDNA binds with the WYL domain and that DriD recognizes its target DNA by binding in an asymmetrical manner, imparting an ∼30° bend to the target DNA38. DriD recognizes its target DNA site with high specificity primarily through the simultaneous formation of two hydrogen bonds from conserved arginine residues to guanine bases in its consensus DNA site38. The structure also revealed that residues in the linker region between the DNABD and 3HB also make key stabilizing interactions that connect the 3HB with the DNABDs38.
Despite the knowledge gained from recent DriD structures, it is currently unclear how ssDNA triggers the allosteric mechanism to achieve the active state, as an apo structure of DriD lacking ssDNA in both subunits of the homodimer has not been obtained. And to date, there are no structures available for an apo homodimeric WYL activator. Thus, to investigate this mechanism, here we describe a battery of studies, including crystallographic, biochemical, and HDX-MS experiments, aimed at elucidating the allosteric mechanism of ssDNA-mediated target DNA binding by DriD. A structure of the fully apo truncated DriD, DriD(78-327), shows that the 3HB region is highly dynamic and mostly not visible in the crystal structure. This is explained by HDX-MS analyses carried out on all DriD states. These data show that the presence of ssDNA leads to exposure of parts of the 3HB and DNABD compared to the apo form and that removal or truncation of the DNABD leaves the 3HB unprotected and unrestrained. Collectively, these analyses support that in the apo form, the DriD DNABDs contact the 3HB domain, thus precluding the interaction of the DNABDs with target DNA. Combined with our analyses on the ssDNA and target DNA-bound structures of DriD, these data reveal the mechanisms of allosteric and target DNA binding for a WYL activator.
Results
Structure determination of apo DriD(78-327)
We previously solved the structure of full-length (FL) DriD bound to ssDNA and target DNA (PDB: 8TP8) (Fig. 1A–D). The structure revealed that DriD consists of an N-terminal DNABD (residues 1-70), linker (71-78), three helix bundle domain (3HB) (residues 79-133), WYL-domain (residues 134-245), and WYL C-terminal extension dimerization domain (WCX) (residues 246-327). To understand the allosteric mechanism by which ssDNA stimulates DriD to bind target DNA requires an apo structure. In addition to our DriD-ssDNA-target DNA complex, we previously solved structures of truncated forms of DriD, DriD(73-327) and DriD(126-327) (PDB codes: 7U02 and 7TZV, respectively)37 (Supplementary Fig. 1A, B). The DriD(73-327) structure, which was solved in the absence of added nucleotide, revealed ssDNA bound in the WYL pocket of one subunit, which was fortuitously crystalized with the protein, while the other subunit was in the apo state. DriD(126-327) was solved in the presence of 9mer ssDNA and revealed ssDNA bound in both subunits. Based on these structures, we hypothesized that ssDNA confers the active state conformation of DriD by binding residues in the region between the WYL and WCX domains (residues 236–244), anchoring the WYL to the WCX domain, and stabilizing the WYL domain. However, while the DriD(73-327) structure contained one apo subunit, we could not draw a definitive conclusion regarding the mechanism by which ssDNA induces the active state structure of DriD. The presence of ssDNA in one subunit of DriD(73-327) could influence the structure of the other apo subunit in the dimer (see below). Also, in both the DriD(73-327) and DriD(126-327) structures, residues in the 3HB were not observed.
A Schematic showing the 2-D domain organization of DriD and the constructs used in this study. The residue numbers encompassed within each domain is included with secondary structural elements shown over the schematics, where arrows represent ß sheets and coils represent α helices. The DNABD is in green (residues 1–70), the linker is red (residues 71–78), the 3HB is gray (residues 79–133), the WYL domain is purple (residues 134–245), and the WCX domain is blue (residues 246–327). Key residues that make contacts to target DNA and ssDNA are indicated with lines within the top schematic. The secondary structural elements above the domain schematics were generated in SSDraw67. B Cartoon diagram of FL DriD (PDB: 8TP8) with domains colored according to Fig. 1A. Target double stranded DNA is shown in orange, and ssDNA is shown in black spheres. C A close-up view of the DriD-ssDNA interaction is shown. Residues that interact with ssDNA are shown in light purple sticks. ssDNA is shown in black sticks. D A close-up view of the DriD DNABD interacting with target DNA is shown. Key residues that contact the target DNA are shown in green sticks.
Thus, we aimed to obtain the crystal structure of FL apo DriD to understand the allosteric mechanism by which ssDNA influences the structure of downstream domains to permit target DNA binding. However, crystallization trials of the FL apo DriD protein failed. We, therefore, tested another truncated form of DriD, DriD(78-327), for crystallization trials (Fig. 1A). The FL DriD-ssDNA-target DNA structure revealed the first ordered residue of the 3HB as residue 79. Thus, to enhance our chances of obtaining well-ordered crystals, we removed N-terminal residues 73–77, resulting in DriD(78–327) (Supplementary Fig. 1A, B). DriD(78-327) lacks only the DNABD and most of the linker, hence because it contains the ssDNA binding WYL domain and all the residues in the 3HB, we hypothesized that its apo structure would provide insight into the conformational effects of ssDNA binding. Indeed, the truncated form, DriD(78-327), binds ssDNA with essentially the same affinity as the FL protein (Supplementary Fig. 2)38. Crystals were obtained of apo DriD(78-327), and data collected to 1.90 Å resolution. The structure was solved by Molecular Replacement (MR) (Materials and Methods). The structure required significant rebuilding as the WYL domains were displaced relative to the WCX domains compared to previous crystal structures. The final apo DriD(78-327) structure has Rwork/Rfree values of 20.2%/23.2% to 1.90 Å resolution (Fig. 2A, Table 1).
A Cartoon diagram of the 1.9 Å apo DriD(78-327) structure. Chain 1 is represented in slate and chain 2, in magenta. B SEC analysis of apo DriD(78-327) reveals the protein elutes at 46 kDa (pink square), consistent with a dimer. The protein standards used for the calculation of the standard curve are indicated by slate circles. These protein standards are aprotinin (6.5 kDa), cytochrome c (12.4 kDa), carbonic anhydrase (29.0 kDa), and bovine serum albumin (66.0 kDa). The x axis represents the log10(MW) in Da, and the y axis represents the elution parameter Kav which is calculated as Kav = (elution volume for the standard-void volume)/(column volume-void volume). C Close up of the 2mFo-DFc electron density map around the WYL domains of each apo DriD(78-327) subunit. The location of the bound ssDNA in DriD-ssDNA complexes is shown. Notably, no electron density for ssDNA is observed in either domain in the apo structure. D ssDNA binding mediated conformational changes. Shown is an overlay of the WCX domains of the apo (cyan) and ssDNA and target DNA bound forms of DriD (red, PDB: 8TP8). Included is the ssDNA. Residues that interact with ssDNA or are involved in conformational changes are shown as sticks. Note the large shift of the 3HB regions upon ssDNA binding (emphasized by the arrow).
The structure of apo DriD(78-327) revealed the same WCX dimer arrangement as observed in the ssDNA-bound and ssDNA-target DNA-bound forms (Supplementary Fig. 3)37,38. This dimeric conformation was confirmed by size exclusion chromatography (SEC) analysis, which showed that the DriD(78-327) protein eluted at a volume corresponding to a molecular weight (MW) of 46 kDa, consistent with a dimer (theoretical MW of the dimer is 59 kDa) (Fig. 2B, Supplementary Fig. 4). These data confirm that DriD is a dimer in its apo as well as ligand bound forms. PISA analyses revealed that the buried surface area (BSA) in the dimer is significant, 3039.7 Å2 (1519 Å2 per subunit). Consistent with the structure representing the apo form, no electron density was observed for ssDNA within the ssDNA binding pocket located between the WYL and WCX domains in either subunit of the DriD homodimer (Fig. 2C). Notably, in the structure, only 9 residues of the C-terminal helix of one of the 3HB are visible.
Comparison of the apo DriD(78-327) structure with the ssDNA-bound and target DNA bound state underscores that ssDNA binds at the nexus where three domains converge, the 3HB, WYL and WCX domains (Fig. 2D). These comparisons showed that ssDNA binding by the WYL motif causes local and global shifts in residues in this region relative to the apo form (Fig. 2D). Conformational changes upon ssDNA binding occur in residues 235–240, which are located between the WYL and WCX domains and residues in the C-terminal region of the 3HB. In the apo state, residues I239 and Y240 pack against the WYL region and would clash with bound ssDNA. ssDNA binding results in a relocation of these residues. Upon relocation, the Y240 side chain makes key stacking interactions with a ssDNA base. The shift in the positions of the R189 side chain and residues I239-Y240 results in a concomitant movement of amino acids 134-138 in both subunits of the dimer. Importantly, residues 134–138 connect the WYL and 3HB and thus, shifting these regions leads to the large-scale movements of the entire 3HB (Fig. 2D). The importance of residues 235-240 in allostery was further underscored by overlaying the WCX domain of a subunit of our apo DriD(78-327) crystal structure, in which both domains lack bound ssDNA, with the apo subunit of the DriD(73-327) structure (PDB code: 7U02). This comparison indicates that large changes in residues 235-240 caused by ssDNA binding in one of the DriD(73-327) WYL domains result in a shift of the WYL domain in the adjacent subunit (Supplementary Fig. 5). This suggests that ssDNA bound in one WYL pocket in one subunit influences the orientation of the WYL domain in the other subunit.
Because most of the 3HB residues are not visible in the apo DriD(78-327) structure we cannot determine the location of the remainder of this domain. However, these findings indicate that ssDNA binding at the three-domain intersection sets off a cascading effect that ultimately leads to relocation of 3HB residues. This is shown by the relocation of the 3HB residues that are present in the structure. Multiple sequence alignments of DriD homologs, all of which contain conserved target DNA binding residues, reveal that residues that contact ssDNA are strongly conserved as are key signaling residues, such as Y240 (Supplementary Fig. 6). In homologs where the Y240 is not conserved, it is replaced by a phenylalanine which could play the same role in the allosteric mechanism as a tyrosine.
The lack of electron density for most of the 3HB in the DriD(78-327) structure is consistent with our previous structures of truncated forms of DriD (DriD(73-327) PDB: 7U02 and DriD(126-327) PDB: 7TZV) because as noted, only the C-terminal 9 residues of the last helix of one of the 3HB was visualized in our crystal structure. This indicated that the 3HB might be unfolded or dynamic in the apo form, lacking ssDNA bound to both subunits. Interestingly, the altered positions of the WYL domains in the apo DriD(78-327) structure relative to the DNA-bound state would preclude the formation of the same 3HB dimer as observed in the ssDNA-target DNA bound state. This suggests that the 3HB adopts distinct states in the apo compared to the ssDNA/target DNA bound states. Hence, we next sought to further investigate how ssDNA binding might impact the folding of the 3HB.
Investigating the effect of ssDNA binding on DriD folding
Our apo DriD(78-327) structure revealed a lack of electron density for most of the 3HB domain, indicating it was either unfolded or flexible. As this domain is nearly entirely helical in the DriD-ssDNA-target DNA complex38, and accounts for 17% of the overall structure, we should be able to delineate between the two possibilities using circular dichroism (CD), which reveals helical content of proteins. For CD experiments we titrated 2 μM and 5 μM of 7mer ssDNA (5´-TGACTAT-3´) into samples of either 0.5 μM DriD(78-327) or FL DriD and analyzed the CD spectra. We did not observe a major significant shift in the spectra upon addition of ssDNA that would be indicative of changed helicity in either the FL DriD or DriD(78-327) (Fig. 3A, B) (Supplementary Fig. 7A, B). Hence, these data indicate that the 3HB is not unfolded in the apo state of DriD. This was also supported by thermal shift experiments, which revealed that the addition of ssDNA did not significantly affect the melting temperature of either DriD(78-327) (Tm = 46.5 °C) or FL DriD (Tm = 45 °C) (Supplementary Fig. 8A, B).
Circular Dichroism (CD) experiments for FL DriD and DriD(78-327). A 0.5 μM of DriD(78-327) and (B) 0.5 μM of FL DriD were used, and 2 μM and 5 μM of the 7-mer ssDNA were titrated into the apo protein, and CD spectra for protein plus each concentration of ssDNA were obtained. Wavelength in nm is plotted on the x axis, and mean residue ellipticity calculated as ((CD signal - corresponding blank signal) * 100) / (protein concentration in mM * path length in cm * number of residues in a monomer) is plotted on the y axis. Data points with dynode voltage >500 V are excluded. C Chymotrypsin proteolysis on DriD(78-327) in the presence and absence of ssDNA. Peptide fragments are color-coded by domain using the same color scheme in Fig. 1A. The Y105 cleavage site is highlighted in green. D Gel quantification for the DriD(78-327) proteolysis experiment is shown. E Chymotrypsin proteolysis of FL DriD in the presence and absence of ssDNA. F Gel quantification for the FL DriD proteolysis experiment is shown. For gel quantification, intensity was obtained by selecting bands in ImageJ, and then % FL DriD remaining was calculated by dividing the band intensity of DriD for each time point by the band intensity for DriD in the corresponding control lane, then multiplying by 100. Two independent experiments are plotted for DriD(78-327) and FL DriD proteolysis experiments with GraphPad, and the bar represents the median. All data points are shown.
Proteolysis studies reveal differences between DriD(78-327) and FL DriD upon ssDNA binding
To further probe the conformation of DriD in the presence and absence of ssDNA, we performed proteolysis assays. For these experiments, chymotrypsin, which cleaves C-terminal to solvent-exposed tryptophan, tyrosine, and phenylalanine residues, was employed49. These experiments showed that in the absence of ssDNA, the DriD(78-327) protein degraded more quickly than in the presence of 7mer ssDNA (5´-TGACTAT-3´) (Fig. 3C, Supplementary Fig. 9). After incubating DriD(78-327) with chymotrypsin for 5 minutes in the absence of ssDNA, 29.7% of DriD remained. However, after 5 minutes in the presence of ssDNA, 66.5% DriD remained. Upon longer treatment (15 minutes), in the absence of ssDNA, DriD(78-327) appeared to be mostly proteolyzed. By contrast, ssDNA afforded some protection from cleavage. LC-MS/MS sequencing revealed the main proteolysis site upon chymotrypsin cleavage for DriD(78-327) was after Y105 (Fig. 3C, Supplementary Figs. 9, 10). Y105 is located in the middle of the DriD 3HB, indicating the region around Y105 is more accessible and flexible or disordered in apo DriD(78-327) (Supplementary Fig. 11A, B).
We next carried out proteolysis studies on apo FL DriD, FL DriD with ssDNA and FL DriD with ssDNA and target DNA (Fig. 3D, Supplementary Fig. 12A, B, Supplementary Fig. 13). These experiments reveal different degradation rates compared to DriD(78-327). In both the absence and presence of the 7mer ssDNA, the FL DriD protein was proteolyzed to a major degradation product after 30 minutes. The impact of ssDNA protection from chymotrypsin degradation was not as prominent as observed for the DriD(78-327) structure. After incubating the FL DriD protein with chymotrypsin for 5 minutes, in the absence of ssDNA, 77.3% of DriD remained while in the presence of ssDNA, 80.8% DriD remained (Fig. 3D). Proteolysis experiments on the FL DriD protein in the presence of target DNA with and without ssDNA revealed that the presence of target DNA increased overall protection of the FL DriD protein from chymotrypsin as FL DriD in the presence of target DNA and absence of ssDNA did not degrade to < 20% until the 60 minutes incubation time point (Supplementary Fig. 12A, B, Supplementary Fig. 13). However, the protection afforded by ssDNA was also not significant, consistent with what is observed for FL DriD in the absence of target DNA (Fig. 3D, Supplementary Fig. 12A, B, Supplementary Fig. 13). After incubating the FL DriD protein plus target DNA with chymotrypsin for 5 minutes, in the absence of ssDNA, 77.3% of DriD remained while in the presence of ssDNA, 83.8% DriD remained (Supplementary Fig. 12A, B, Supplementary Fig. 13). As ssDNA affords greater protection to the DriD(78-327) protein compared to the FL DriD protein with and without target DNA, these experiments revealed that the presence of the DNABD imparts a difference to the susceptibility of DriD to proteolysis.
HDX-MS experiments on DriD
Our crystal structure of DriD(78-327) revealed density for few residues of the 3HB suggesting this domain is either disordered or highly dynamic. CD data, however, suggested that the 3HB domain is still folded. Interestingly, our proteolysis experiments show different rates of proteolysis of the truncated versus FL protein. Two mechanisms could explain these data. In the first, the 3HB is dynamic in the DriD apo form, preventing orientation of the DNABD onto the target DNA. In a second mechanism, the DNABD is somehow involved in the conformational and allosteric transition of the 3HB and WYL domains. Thus, to further delineate the molecular mechanism we employed HDX-MS experiments on the FL protein in its apo, ssDNA bound and ssDNA-bound/target DNA-bound forms as well as the apo form of DriD(78-327).
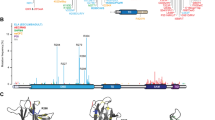
HDX-MS probes protein backbone dynamics and accessibility by differentially labeling backbone amide hydrogens with deuterium; stably hydrogen-bonded and solvent-protected amide hydrogens incorporate deuterium at a slower rate than those backbone positions that have high solvent exposure and that are weakly or not hydrogen-bonded. With greater than 90% coverage of the FL DriD backbone, we were able to use overlapping peptides (all peptides followed in the HDX-MS experiments are included in Supplementary Data 1) in our comparative analyses to localize changes in HDX to specific regions throughout the protein. HDX-MS analyses comparing the apo FL DriD protein to the ssDNA-bound form showed, as expected, that the addition of ssDNA resulted in protection of residues 166-186 and 233-239, which our structures show are involved in ssDNA binding. As noted, residues 233-239 also undergo conformational changes associated with ssDNA binding (Supplementary Fig. 5). Strikingly, the binding of ssDNA to DriD resulted in increases in deuterium uptake at regions that correspond to the 3rd helix of the 3HB and the N-terminal region of the WYL (residues 116-128 and 133-151) reflective of an induced structural change (Fig. 4A, B, Supplementary Fig. 14, Supplementary Data 1). This exposure is observed in both structures DriD with ssDNA and with ssDNA and target DNA. The addition of ssDNA also led to deprotection of residues 41-42 and 71-72, which correspond to residues in DNABD and two residues of the linker, respectively (Figs. 4A, 5A, Supplementary Fig. 14, Supplementary Data 1).
A HDX-MS data for curated peptides. Peptides that are coincident across all the constructs of DriD FL studied (DriD FL, DriD FL + ssDNA, DriD FL + ssDNA + target DNA are shown. The relative difference data are calculated by subtracting the relative deuterium incorporation of the DriD-FL alone from the other constructs, and the differences are colored according to the scale shown. B Deuterium incorporation curves of selected peptides (indicated with a gray box in A labeled a–f). C The selected peptides are mapped on DriD FL (PDB ID: 8TP8) with respective color code labeled a–f. All HDX-MS data used to make the graphs as well as the complete HDX-MS difference data are found in Supplementary Data 1.
Regions showing difference in deuterium exchange with respect to the DriD FL at 1 min time point for selected peptides are mapped onto the structure following the color scale as shown below. The ssDNA and the dsDNA are shown in black (A) the binding effects of ssDNA (B) the binding effects of both ssDNA and dsDNA. All HDX-MS difference data used to make these figures are found in Supplementary Data 1.
Addition of the target DNA to the ssDNA-bound form of DriD had a similar effect, showing an increased deuterium uptake at residues 116–128 and 133–151 compared to apo but resulted in protection of the DNABD, residues 8–78 (Fig. 4A, B, Supplementary Data 1). The latter protection is consistent with the interaction of DNABD domain residues 8–70, with target DNA that we observe in our DriD-ssDNA-target DNA crystal structure38. In addition, the protection of residues including the linker (residues 55–71) is also consonant with the DriD-ssDNA-target DNA crystal structure as the structure revealed this region makes contacts that link the DNABD to the 3HB that stabilizes the unusual conformation of the DriD DNA-bound state (Fig. 5B, Supplementary Fig. 14, Supplementary Data 1). Finally, there is some protection of residues 83–104 upon target DNA binding. These residues are largely buried within the 3HB. The increased protection may reflect stabilization of this region upon target DNA interaction.
The finding that residues 116-128 in the 3HB (comprised of residues 79–133) were deprotected in the ssDNA-bound FL DriD compared to the apo form was counter to our apo DriD(78-327) structure, which showed essentially no electron density for this domain. However, the structural and biochemical data would be consistent with a mechanism in which the DNABD, which was also deprotected, is involved in interactions with or protection of the C-terminal region of the 3HB. Such an interaction would create an autoinhibited form of DriD in regard to target DNA binding as it would lead to the sequestration of the DNABDs. The prominent exposure of residues 133-151, which contain the connection between the 3HB and WYL domains and the N-terminal region of the WYL domain due to ssDNA binding might suggest the potential for creating a relaxed and dynamic environment for DNABD to bind to the target DNA. According to this mechanism, the DriD(78-327) structure would be expected to reveal a dynamic 3HB because it lacks the DNABD. This is in fact, what we observed in our DriD(78–327) structure. To test this hypothesis, we next analyzed apo DriD(78-327) by HDX-MS. Consistent with our hypothesis, in this case, residues 78–130 (as well as regions localized to 133–148, 167–180, 208–214, 259–279, and 318–319) are deprotected in the apo DriD(78-327) form compared to the apo FL DriD protein (Fig. 6A–C, Supplementary Fig. 14). As the only difference in apo DriD(78-327) and the apo FL DriD proteins is the presence of the DNABDs, these data suggest that the DNABD plays a role in stabilizing the 3HB region of DriD, as well as several regions of the WYL domain, possibly via an interaction between the regions.
A HDX difference data for truncated DriD. Cartoons on top of each panel show the difference in deuterium calculated between the states in Da. Color scale and peptide/time course arrangement are the same as in Fig. 4. B Deuterium uptake plots from peptides indicated by grey boxes in panel A, [labeled c –i]. C Deuterium difference at the 1 min time point from panel A for selected peptides plotted onto the structure DriD [PDB ID: 8TP8]. DNABD when truncated, is shown as surface in grey and is shown as cartoon green when added in trans. All HDX-MS data used to make the graphs as well as the complete HDX-MS difference data, are found in the Supplementary Data 1.
The DriD DNABD interacts with truncated DriD in the apo state
Deprotection of DNABD residues upon ssDNA binding is notable (Supplementary Fig. 14). However, the protection of these residues in the apo form is lower than observed for the 3HB. DriD is dimeric, and hence it is possible that only one of the DNABD regions is protected or interacts with the 3HB. Nonetheless, to form an active DNA-bound state, both DNABDs must dock within the DNA target major grooves, so the sequestration of just one would prevent effective cognate DNA binding. To test the hypothesis that the DNABD interacts with the 3HB (and hence truncated DriD), we generated a construct encoding the DriD DNABD, residues 1–70 (Supplemental Fig. 1A), and analyzed the ability of this domain to interact with DriD(78-327) using both HDX-MS and microscale thermophoresis (MST)50,51. The purified DriD DNABD eluted as a monomer on SEC and did not copurify with excess DNA (Supplementary Fig. 15).
HDX-MS analyses comparing the apo DriD(78-327) protein to the apo DriD(78-327) protein with the addition of excess DNABD protein showed increased protection in residues 83–104 and 112–130, which directly correspond to the 3HB (Fig. 6A-C, Supplementary Fig. 14, Supplementary Data 1). Increased protection was also observed in residues 165–186, 194–214, and 232–245, which correspond to the WYL domain and includes residues involved in ssDNA binding. To further probe for the possible interaction between apo DriD(78-327) and the DriD DNABD we employed microscale thermophoresis (MST). In these experiments an interaction between the apo DriD(78-327) protein and the DNABD was revealed with a Kd of ~15 µM; however, in the presence of ssDNA, this interaction was significantly weakened (Supplementary Fig. 16). Collectively, these data are consistent with an autoinhibitory interaction between DriD(78-327) and the DNABD, which would impair DNABD binding to target DNA (Fig. 7). Such an interaction would need to be relatively weak, as we observe in MST, because too tight an interaction would lock in a permanently inhibited state.
Shown is a cartoon schematic of the proposed mechanism of ssDNA-mediated activation of DriD (colored according to Fig. 1). In this mechanism, apo DriD is held in an inhibited state by interactions between the DNABD and 3HB regions. Upon DNA damage, represented by a lightning bolt, ssDNA is created and binds DriD within the WYL domain. This interaction leads to large-scale conformational changes in the 3HB that disable the DNABD-3HB contacts, thus freeing the DNABDs to interact with target DNA within DriD-regulated promoters.
HDX-MS informs on changes in exposure in backbone atoms, and we have no 3-D structural details as to the specific residues that are involved in contacts between the 3HB and the DNABD. However, the HDX-MS data implicated the 3HB as interacting with the DNABD. Thus, we made substitutions on the outward face of the C-terminal helix of the 3HB, generating DriD(R121E-R122E-P125E-E128R), to test the effects of these mutations on the DNABD interaction. First, we showed that this mutant protein was folded via CD experiments (Supplementary Fig. 17A, B). We then performed HDX-MS experiments on the mutant in ssDNA-bound and ssDNA and target DNA-bound states (Supplementary Fig. 18). Comparative HDX analysis of apo FL DriD(R121E-R122E-P125E-E128R) with that of the wild type DriD showed extensive increases in deuterium incorporation in the WCX, WYL, and the N-terminus of the 3HB-linker region. Conversely, there were two regions that showed an increase in protection from HDX: the wHTH DNABD (residues 12–54), and the second helix in the 3HB region (residues 85–104). Interestingly, the decreased HDX observed at the DNABD in DriD(R121E-R122E-P125E-E128R) mutant protein is similar in location to that of the HDX difference profile of FL DriD in the ssDNA and target DNA-bound state (Fig. 4A). Whereas the protection in HDX seen for the second helix in the 3HB shares a similar HDX difference profile to that of DriD(78-327) when the DNABD is added in trans (comparing Fig. 6A and Supplementary Fig. 18–20). Taken together, these data support an intramolecular domain-domain interaction between the DNABD and the 3HB, thereby stabilizing the DNABD in a retracted conformation by folding back onto the 3HB. Further, comparisons of the HDX-MS data for DriD(R121E-R122E-P125E-E128R) in the ssDNA-bound and ssDNA and target DNA-bound states to the apo form show only a mild protection from HDX in the DNABD upon ssDNA and target DNA binding (comparing Fig. 4A and Supplementary Figs. 18–20). This combined with HDX-MS deprotection at the second helix in the 3HB (residues 85–104), reveals that ssDNA binding might induce a release of the DNABD from the 3HB, facilitating target DNA binding. Overall, these results suggest that any structural alteration in the 3HB perturbs the crosstalk between the 3HB and DNABD, potentially impairing the conformational dynamics of the DNABD that are required for efficient DNA binding in the presence of ssDNA.
Discussion
Recent data have revealed that WYL proteins comprise an important group of bacterial regulators that control key biological processes ranging from host/pathogen defense to DNA damage responses34,40,41,42,43,47. Understanding the molecular cues that are recognized by the WYL motifs of these regulators and how these ligand-based cues impact the protein’s function, however, is still in its early stages. The C. crescentus DriD regulator, one of the best studied WYL regulators, belongs to the largest class of WYL proteins, class A41. These WYL proteins appear to function primarily as transcriptional regulators. Indeed, recent work showed that DriD is an activator of multiple genes involved in a non-SOS mediated, DNA damage response in C. crescentus36,37. A key step in understanding DriD function came from studies that revealed DriD binds ssDNA, which is produced during the DNA damage response37. Cellular and genetic studies confirmed that ssDNA acts as an allosteric regulator of DriD37,38. Structures of DriD bound to ssDNA and both ssDNA and target DNA revealed the atomic details of the DriD-ssDNA interaction as well as how DriD recognizes its target DNA site37,38. The structures show that DriD is composed of an N-terminal wHTH DNABD, linker, 3HB, WYL domain and WCX domain. The DNA bound form showed that the DriD dimer binds target DNA asymmetrically (whereby the DNABDs-DNA are oriented in a different plane compared to the 3HB-WYL-WCX dimer module). In the structure, the linker region between the 3HB and DNABD plays a key role in proper DNA docking.
To elucidate the ssDNA-mediated allosteric mechanism of DriD, here we performed a combination of structural, biochemical and HDX-MS experiments. Our studies show that binding of ssDNA to DriD causes large movements within the WYL domains with concomitant shifts in the 3HB domain. Our structure of apo DriD(78-327), which is missing the N-terminal DNABD, lacked density for most of the 3HB domain despite containing the ordered residues in the 3HB revealed in the FL DriD structure bound to ssDNA and target DNA38. This suggested that the 3HB region may be unfolded when both subunits are not bound to ssDNA. However, HDX-MS experiments on FL DriD revealed that regions of the 3HB and DNABD are simultaneously deprotected upon the addition of ssDNA, suggesting they may affect each other’s conformations. As the DNABD was absent in the DriD(78-327) structure, the 3HB was destabilized, which explains its dynamic state. MST and HDX-MS experiments using the DriD DNABD and DriD(78-327) protein supported that the DNABD can contact the DriD C-domain and showed the interaction was favored in the absence of ssDNA.
These data combined with our previous structural, biochemical, and genetic studies on DriD provide a full mechanism for its ssDNA-mediated DNA binding stimulation (Fig. 7). Whether other WYL regulators employ a similar mechanism is unclear as there is still a dearth of structural information for WYL regulators. The structural information that has been obtained for the WYL repressors, BrxR and the CapW44,45,46, would suggest that such a mechanism is not likely for these regulators. Indeed, while these repressors both contain wHTH and WYL domains, they harbor distinct C-terminal domains, and most critically in terms of the allosteric mechanism, the key signaling region connecting the wHTH and WYL is a helix-β-hairpin in these repressors. As would be expected given these differences, the structure of the WYL repressor BrxR bound to DNA46 revealed a symmetric and thus very different complex compared to the DriD-ssDNA-target DNA structure. The fact that WYL repressors bind target DNA differently and also lack the 3HB suggests that they will likely use a distinct mechanism for effector signaling. In particular, effector binding is expected to remove the repressors from the DNA rather than activate them to bind DNA.
Though there are no structures available for WYL homodimeric activators, aside from DriD, there is structural information for the heterodimeric WYL activator, PafBC, which is composed of PafB and PafC subunits29,30,43,52. Similar to DriD, data has shown that the Mycobacterial PafBC protein functions in DNA damage, activating transcription of ∼150 genes29,52. Also like DriD, Mycobacterial PafBC is stimulated by ssDNA binding to its WYL motif52. However, unlike DriD, which binds a palindromic DNA site with high affinity and specificity in the absence of RNA Polymerase (RNAP), PafBC interacts with a nonpalindromic DNA site and cannot bind DNA in the absence of RNAP37,38,43,52. The reason for this is suggested by a cryo-EM structure of PafBC with RNAP and promoter52 DNA, which showed that the DNABD of PafB binds DNA; the PafC DNABD instead interacts with domain 4 of σ70.
Despite showing significant functional differences from DriD, the structure of apo A. aurescens PafBC, which is a covalent fusion of PafB and PafC-like subunits, revealed the Paf subunits have similar overall domain organizations as DriD, with N-terminal wHTH domains connected to helical bundle domain regions, followed by a WYL and WCX domains43. The main structural difference between DriD and PafBC lies in the region corresponding to the DriD 3HB, which is the very domain our studies revealed as key for the ssDNA binding signaling mechanism. The PafC subunit harbors a 3HB, while in PafB, this region is composed of one helix. In the apo A. aurescens PafBC structure, helix 1 of the DNABDs interacts with the lone helix in PafB and the first helix of the helical bundle in PafC, thus also suggesting an autoinhibitory mechanism for PafBC DNA binding activation43.
The use of autoinhibition as an allosteric mechanism in DNA binding activation has been observed in other DNA binding protein families53,54,55,56,57,58. One example is the utilization of negatively charged/DNA mimic regions to sequester basic DNABDs. This mechanism has been demonstrated for the eukaryotic zinc finger transcription regulator ZNF41058. Similarly, the phosphorylation and creation of a negatively charged region within the bZIP TF, c-Jun, has been shown to lead to DNA binding inhibition53. Autoinhibitory mechanisms have also been observed in bacterial DNA-binding proteins. For example, the Gram-positive master regulator of nitrogen homeostasis, GlnR, harbors a C-terminal tail that interacts with its N-terminal DNA binding domain to prevent DNA contacts54,55,56. Glutamine synthetase binds the GlnR C-tail, thus relieving this autoinhibition54,55,56. Recent data for the TipA bacterial transcription activator, SkgA, showed that in its apo state, its ligand binding domain interacts directly with its HTH DNABD to prevent DNA interactions, and that ligand binding induces conformational changes to free the DNABDs57. These latter mechanisms are similar to our proposed mechanism for DriD. However, in DriD, the signal of ssDNA binding is transmitted through an intermediate domain, the 3HB. Nonetheless, these combined data indicate that autoinhibition may be a widely used mechanism in DNA binding allosterism and that it can be implemented using multiple strategies. In conclusion, our data has revealed that DriD employs a unique allosteric mechanism of ligand-mediated DNA binding activation that involves a trigger domain between the WYL ligand binding domain and the DNABD, which is the 3HB. These data combined with our previous structural, biochemical, and genetic analyses, provide mechanisms for allosterism and DNA binding recognition by this WYL regulator.
Materials and Methods
Purification of C. crescentus DriD proteins
For protein expression and purification, the genes encoding FL DriD, DriD(78-327), FL DriD(R121E-R122E-P125E-E128R) and DriD(1-70) (DNABD) were purchased from Genscript Corporation (Piscataway, NJ, USA: http://www.genscript.com) and subcloned into pET15b such that a His-tag was expressed at the N-terminus of the proteins for purification. E. coli C41(DE3) competent cells (Fisher Scientific) were transformed with the vectors. Cells with each expression vector were grown at 37°C in LB with 0.1 mg/ml ampicillin to an OD600 of 0.6, then induced with 0.5 mM isopropyl β-d-thiogalactopyranoside (IPTG) at 15 °C overnight. Cells were harvested by centrifugation (4000 rpm, 4 °C, 15 min) and resuspended in buffer A (25 mM Tris-HCl pH 7.5, 300 mM NaCl, 5% (v/v) glycerol, 1 mM β-mercaptoethanol (βME)), then 1X protease inhibitor cocktail and 0.1 mg/mL DNase I were added. The resuspended cells were lysed by sonication and cell debris removed by centrifugation (15,000 rpm, 4 °C, 45 min). The supernatant was loaded onto a cobalt NTA column prepared in buffer A. To remove contaminating DNA copurifying with DriD, the column was washed slowly overnight with a buffer composed of 25 mM Tris-HCl pH 7.5, 800 mM NaCl, 2 M Urea, 10 mM imidazole, 5% (v/v) glycerol, and 1 mM βME. The next day, the column was washed with 300 mL of high salt buffer (25 mM Tris-HCl pH 7.5, 800 mM NaCl, 5% (v/v) glycerol, and 1 mM βME). The protein was eluted in steps of increasing imidazole concentration (20 mM, 30 mM, 50 mM, 100 mM, 200 mM, 500 mM, 1 M, and 2 M imidazole) in buffer A. Fractions were analyzed by SDS-PAGE and those containing the protein were combined and concentrated in centricons with a 30 kDa MW cutoff. The protein was further purified by size exclusion using a HiLoadTM 26/600 SuperdexTM 75 pg column. The fractions containing the protein were combined and concentrated using centricons with a 30 kDa MW cutoff. Proteins with A260/A280 ratios <1, which indicate low quantities of contaminating DNA, were used for experiments. The samples used for subsequent studies had A260/A280 values of 0.69 and 0.64 for apo DriD(78-327) and FL DriD, respectively. The DNABD had an A260/A280 of 0.72. The FL DriD(R121E-R122E-P125E-E128R) protein had a A260/A280 of 0.76.
Size Exclusion Chromatography (SEC)
We performed SEC with a HiLoad 26/600 SUPERDEX™ 75 pg column that was connected to an AKTA Prime Plus. For these experiments apo DriD(78-327) and DriD(1-70) were buffer exchanged into buffer B (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 5% (v/v) glycerol and 1 mM βME). The buffer change was carried out using sigma-Millipore concentrators (Amicon). 2 mg/mL of protein (34 μM for a DriD(78-327) subunit) was loaded. The elution volume of the proteins were compared with a protein standard set to assess the molecular weights. The protein standards utilized were aprotinin (6.5 kDa), cytochrome c (12.4 kDa), carbonic anhydrase (29.0 kDa), and bovine serum albumin (66.0 kDa). The data were plotted to obtain a standard curve. The elution parameter Kav was calculated by Kav = (elution volume for the standard-void volume)/(column volume-void volume).
Crystallization and structure determination of apo C. crescentus DriD(78-327)
Crystallization trials of purified apo DriD(78-327) were performed with the Wizard Classic screens I to IV at room temperature (rt) using the hanging drop vapor diffusion method. Crystals were obtained with DriD(78-327) at 12 mg/mL by mixing the protein at a 1:1 ratio with a crystallization reagent consisting of 1260 mM ammonium sulfate dibasic and 100 mM sodium cacodylate trihydrate/HCl pH 6.5. Crystals were cryopreserved by dipping them in a solution composed of the crystallization condition supplemented with 20% (v/v) glycerol prior to plunging them in liquid nitrogen. Data were collected at the Advanced Light Source (ALS) beamline 5.0.2 with a wavelength of 1.03 Å at 100 K and processed with XDS59. The crystals take the P3121 space group and contain a DriD(78-327) homodimer in the crystallographic asymmetric unit (ASU). The structure was solved in Phenix by Molecular Replacement (MR)60. For MR, individual WCX and WYL domains from the DriD(126-327) PDB code 7U02 structure were used37. There were two solutions for WCX domains and one for a WYL domain. After several rounds of refinement, density for the second WYL domain was observed and was built into the electron density61. After multiple rounds of refitting and refinement in Coot and Phenix60,61, the Rwork/Rfree converged to 20.2%/23.2% to 1.90 Å resolution (Table 1). The Ramachandran analyses showed that 98.7%, 1.27%, and 0.00% were in the most favored, favored, and disallowed regions. There are only 0.94% rotamer outliers.
Proteolysis experiments
For proteolysis experiments, chymotrypsin (Promega) was resuspended in 1 mL of reaction buffer (10 mM Tris-HCl pH 7.4, 145 mM NaCl, 5 mM CaCl2)62. 0.1 mg/mL of protein was proteolyzed with 0.002 mg/mL chymotrypsin for 5 min, 10 min, 15 min, 30 min, and 60 min. The experiments were done in the presence and absence of 500 μM 7mer ssDNA (5´-TGACTAT-3´) for DriD(78-32) and FL DriD. 200 µM ssDNA and 20 µM target DNA (Top strand: 5′-ATACGACAGTTACTGTCGTAT-3′) were used for experiments with FL DriD in the presence of target DNA. Controls were done with no chymotrypsin. Tubes were transferred from the ice to 37°C, then heated for 5 min, 10 min, 15 min, 30 min, and 60 min. 10 µL of each sample was analyzed via SDS-PAGE (4-12% acrylamide). Gel images were taken on an Azure Biosystems c300, and band intensities were obtained with ImageJ. The % Remaining DriD was calculated by dividing the band intensity of DriD for each time point by the band intensity for DriD in the control lane, then multiplying by 100. Two technical replicates of % Remaining DriD for DriD(78-327) and FL DriD, and three technical replicates for FL DriD plus target DNA are plotted in GraphPad Prism version 10.0.0 for Windows, GraphPad Software, Boston, Massachusetts, USA, www.graphpad.com.
LC-MS/MS data analysis
For LC-MS/MS analyses of proteolysis products, one band for DriD(78-327) in the absence of ssDNA - chymotrypsin and another band of DriD(78-327) in the absence of ssDNA cleaved + chymotrypsin were excised from the SDS PAGE gel and submitted to the Duke Proteomics and Metabolomics Core Facility. Gel bands were subjected to in-gel reduction with 10 mM DTT, alkylation with 25 mM iodoacetamide, and proteolytic digestion with 100 ng/µL of sequencing grade modified trypsin (Promega). Extracted peptides were analyzed by LC-MS/MS using a Thermo Fusion Lumos Orbitrap coupled with a Thermo Neo Vanquish UPLC chromatography system. Peptides were chromatographically separated over 30 minutes on a 75 µm 15 cm C18 UPLC column using a linear gradient of 5%-40% acetonitrile at a flow rate of 300 nL/min. The mass spectrometer was operated in a data-dependent acquisition mode with a precursor from m/z 375–1500 in the Orbitrap at a r = 120,000, an accumulation time of 50 ms, and an AGC target of 4e5. MS2 scans in the ion-trap had a max injection time of 100 ms with an AGC target of 1e4. A stepped HCD collision energy was set to 28.5, 30, 31.5 with a dynamic exclusion limit of 20 seconds. One LC-MS/MS sequencing experiment was performed per band. DriD was identified with 51-92 unique peptides within each sample, which equates to a 0.0% false discovery rate with nearly 100% confidence in identification. Data was collected with 2 ppm mass accuracy on the precursor scan and all data were scored with a maximum of 1% false discovery rate across the entire dataset. The raw LC-MS/MS data were analyzed using Proteome Discoverer 3.0 (Thermo) and the SEQUEST HT database search algorithm. The peptides were searched using a fixed Carbamidomethyl modification on cysteine, variable oxidation on methionines, and the digestion enzyme was set as full trypsin rules with an allowance of 2 missed cleavages.
Fully tryptic peptides from both the DriD(78-327) + chymotrypsin and DriD(78-327) – chymotrypsin samples, including their respective spectral counts were exported from Scaffold (version 5.3.3). Fully tryptic peptides were then further filtered for those that contained a single chymotrypsin cleavage site (i.e. those that contain either a W, Y, or F residue). Peptides that were not assayed in both data sets were also excluded. Alcohol dehydrogenase (ADH) was used as an internal standard for each experiment. The number of spectral counts for each quantified peptide was normalized by the spectral counts for the ADH protein (the sum of all the identified ADH peptide spectral counts). Ratios were generated by dividing the normalized spectral counts of the peptides in the DriD(78-327) + chymotrypsin sample by the normalized spectral counts of their matched peptide in the DriD(78-327) - chymotrypsin sample. A smaller ratio indicates that there is more of the peptide in the DriD(78-327) – chymotrypsin sample relative to the DriD(78-327) + chymotrypsin sample, meaning that this peptide region is more susceptible to chymotrypsin cleavage. The major cleavage site of DriD(78-327) was determined to be C-terminal to Y105 by LC-MS/MS sequencing. This is represented by plotting the absolute value of the log2 fold change of the normalized spectral counts of the peptides in the DriD(78-327) + chymotrypsin sample / the normalized spectral counts of their matched peptide in the DriD(78-327) - chymotrypsin sample.
Hydrogen Deuterium Exchange-Mass Spectrometry (HDX-MS) experiments
Apo DriD(78-327) and FL DriD were buffer exchanged into buffer B using 30 kDa cutoff Sigma-Millipore concentrators (Amicon) prior to HDX-MS analyses. The following samples were prepared for HDX-MS: 20 μM of apo DriD(78-327), 20 μM of apo FL DriD, 20 μM of FL DriD plus 800 μM 7-mer ssDNA (5´-TGACTAT-3´), 20 μM of FL DriD plus 800 μM 7-mer ssDNA (5´-TGACTAT-3´) plus 40 μM double stranded target DNA (top strand: 5´-ATACGACAGTAACTGTCGTAT-3´). Deuterium labeling of each condition was initiated with a 19-fold dilution into D2O buffer (25 mM Tris, 150 mM NaCl, pD 7.5, 5% (v/v) glycerol, 5 mM DTT, 99% D2O). After each labeling time point (10 s, 1 min, 10 min, 1 hr, and 4 hr) at 20 °C, the labeling reaction was quenched with the addition of an equal volume of ice-cold quench buffer (3.5 M guanidium chloride, 0.8% (v/v) formic acid, pH 2.3, H2O). Online protein digestion was performed using an Affipro pepsin column held at 15 °C and resultant peptides were trapped and desalted on a VanGuard Pre-column trap for 3 min at 100 μL/min. Peptides were then eluted from the trap and separated using a 5–35% gradient of acetonitrile over 6 min at a flow rate of 100 μL/min using an ACQUITY UPLC HSS T3 1.8 μm, 1.0 mm × 50 mm reversed-phase column. Both the desalting and separation columns were housed in a Waters HDX cooling chamber that was held at 0°C. Mass spectra were analyzed using a Waters- SELECT SERIES Cyclic IMS operated in HDMSE mode. Peptide identification was performed from replicates of undeuterated control samples using PLGS 3.0.3 (Waters Corporation) as described in Supplementary Data 1. The peptides identified in PLGS were then analyzed in DynamX 3.0 (Waters Corporation) with a minimum products per amino acid cutoff of 0.25 and at least two consecutive product ions (see Supplementary Data 1). Those peptides meeting initial filtering criteria were further processed by DynamX 3.0. The relative deuterium incorporation of each peptide was calculated for each time point and condition tested by subtracting the centroid mass of the undeuterated from the deuterated form. The resulting values were utilized to produce the graphs of deuterium uptake and the difference maps. The average deuterium incorporation error for each peptide was at or below ±0.25 Da. The deuterium levels were not corrected for back exchange and therefore indicated as relative63.
Fluorescence Polarization (FP) experiments
Fluorescence polarization was used to determine binding of DriD(78-327) to ssDNA. For these experiments, 5′ fluorescein tagged ssDNA (5′-TGTCTAT-3′) was used. Increasing concentrations of DriD(78-327) were titrated into the tube containing FP buffer, 0 nM and 1 nM 5′ fluorescein-tagged ssDNA. Three technical replicates were performed for each protein sample. Normalized change in millipolarization (mP) was plotted against increasing protein concentration, and binding affinities were calculated by fitting the curves with GraphPad Prism. Change in mP units was normalized by (A – A0)/(Amax- A0) where A is change in mP reading, A0 is the initial mP value before addition, and Amax is the maximal mP reading upon binding saturation. The error in Kd was determined as the standard deviation between the calculated Kds for three runs.
Circular Dichroism experiments
Apo FL DriD, apo DriD(78-327), and apo FL DriD mutant (R121E-R122E-P125E-E128R) at 0.5 µM in CD buffer containing 10 mM Na2HPO4 pH 7, 50 mM NaF, and 1 mM βME were used in CD experiments, in the absence and presence of 7mer ssDNA (5′-TGTCTAT-3′). For each experiment, samples were measured in a quartz cuvette with a path length of 1 mm. Ellipticity was collected for wavelengths from 280–180 nm for protein in the presence of 0 µM, 2 µM, and 5 µM ssDNA as well as for the corresponding blanks, which contained CD buffer with and without 0 µM, 2 µM, and 5 µM ssDNA. Three technical replicates were performed for each sample and then averaged. The corresponding blank signal average was subtracted from each protein sample average. Data points with dynode voltage >500 V were excluded. Mean residue ellipticity is calculated as (CD signal – corresponding blank signal)*100)/(protein concentration in mM * path length in cm * number of residues in a monomer). Mean residue ellipticity is plotted as a function of wavelength in nm in GraphPad Prism version 10.0.0 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com.
Thermal Melt Assay
Apo FL DriD and DriD(78-327) proteins at 0.5 mg/mL were used for Thermal shift assays in 96-well plates. Three wells contained protein with no ssDNA, three wells contained 50 μM ssDNA 7-mer DNA and three wells contained 100 μM ssDNA for each protein were measured. Three (control) wells also contained the buffer A blank, buffer blank plus 50 μM ssDNA, and buffer blank plus 100 μM ssDNA. 2 µL of 10X GlowMeltTM Dye (biotium) was added to bring the final volume in each well to 24 μL. Fluorescence was detected using a Biorad CFX Connect Real-Time System. An average of three technical replicates for each sample was taken, then the average for each protein sample was subtracted by the average of the corresponding blank. Data was normalized on a scale of 0-1. The normalized -dF/DT is plotted on the y axis and the temperature in °C is plotted on the x axis using GraphPad Prism (version 10.0.0 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com). The melting temperature (Tm) is the temperature at which the -dF/dT value is at its minimum.
Microscale thermophoresis (MST)
For MST experiments, 20 μL of 4 mM His-tagged DNABD was added to well 1 in a 384-well plate. 10 μL of buffer (12.5 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) pH 7.5, 100 mM NaCl, 5 mM MgCl2, 5% (v/v) glycerol, and 1 mM ßME) was added to wells 2-16. A serial 1:1 dilution was performed from wells 1-16. 10 μL of 50 nM RED-tris-NTA 2nd Generation His-tag labeling dye (Nanotemper) was added to wells 1-16, then allowed to incubate at rt for 30 min. Premium capillaries were loaded, and then the samples were measured at 40% excitation and medium MST power at 25°C on the Nanotemper Monolith X MST. The Kd of the protein-label was determined to be 3.03 nM by fitting the dose response curve (Ratio 670 nm/650 nm vs. ligand concentration [M]). As the Kd was less than 10 nM, the DNABD was labeled by mixing a 1:1 ratio of 100 nM RED-tris-NTA 2nd Generation dye with 200 nM of His-tagged DNABD.
Using a thrombin cleavage capture kit (Millipore 69022), the His-tag was cleaved from DriD(78-327). His-tag cleaved DriD(78-327) was run over a reverse cobalt column, and the flow-through collected, which contained the His-tag free DriD(78-327). This protein was used for subsequent MST experiments. For each experiment, 20 μL of a 597 μM stock solution of DriD(78-327) with or without 800 μM 7-mer ssDNA 5´-TGACTAT-3´ plus 0.05% (v/v) TWEEN 20 was added to well 1 of a 384-well plate. 10 μL of MST buffer (12.5 mM HEPES pH 7.5, 50 mM NaCl, 5 mM MgCl2, 5% (v/v) glycerol, 1 mM βME, and 0.05% (v/v) TWEEN 20) was added to wells 2–16, then a serial 1:1 dilution was performed. 10 μL of 20 nM of the red His-tag labeled DNABD protein plus 0.05% (v/v) TWEEN 20 was added to each well 1–16. Premium capillaries were loaded, and then the samples were measured at 100% excitation and medium MST power at 25 °C. The Kd was determined by fitting the dose response curve (Ratio 670 nm/650 nm vs. DriD(78-327) concentration) of four technical replicates for no ssDNA and three technical replicates for in the presence of ssDNA. Outliers were removed via the two-sided IQR method. IQR was calculated by subtracting the first and third quartiles for each trial. The upper bound was calculated as the third quartile plus 1.5 times the IQR. The lower bound was calculated as the first quartile minus 1.5 times the IQR. If the data point was greater than the upper bound or less than the lower bound, it was identified as an outlier.
Statistics and reproducibility
Independent experiments are defined as technical replicates in this study. Circular dichroism data plotted represent three independent experiments. Three trials of each blank were collected for 0 µM ssDNA, 2 µM ssDNA, and 5 µM ssDNA. For each blank, the three trials were averaged. The corresponding blank average was then used to subtract from the average of three independent trials of DriD(78-327) and FL DriD in the presence of 0 µM ssDNA, 2 µM ssDNA, and 5 µM ssDNA. The ratio of A222/A208 was taken for each of the three independent trials of DriD(78-327) or FL DriD in the presence of 0 µM ssDNA, 2 µM ssDNA, and 5 µM ssDNA. Error bars represent the mean with SD. A two-tailed t test was performed to identify if the difference between the A222/A208 0 µM ssDNA ratio and the 2 µM or 5 µM ssDNA A222/A208 ratios are statistically significant. Proteolysis data for DriD(78-327) and FL DriD are plotted representing two independent experiments. The median value is plotted. Proteolysis data for three independent experiments of FL DriD plus the target DNA are plotted. Error bars represent the mean with SD. One independent experiment was performed for LC-MS/MS sequencing, yet DriD was identified with 51-92 unique peptides within each sample, which equates to a 0.0% false discovery rate with nearly 100% confidence in identification. No statistical tests were applied to the HDX-MS measurements. Rather, based on measurements of mean methodological error [+/− 0.14 Da]64 we chose a value (+/− 0.25 Da) well above that as the threshold for calling differences in relative deuterium incorporation measurements meaningful. See also explanations of this methodology in Engen & Wales65. Four independent MST experiments were performed in the absence of ssDNA and three independent MST experiments were performed in the presence of excess ssDNA (800 µM). Outliers were removed via the two-sided IQR method in which the interquartile range (IQR) was calculated by subtracting the first and third quartile for each trial. The upper bound was calculated as the third quartile plus 1.5 times the IQR. The lower bound was calculated as the first quartile minus 1.5 times the IQR. Data points greater than the upper bound or less than the lower bound were identified as outliers.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
HDX-MS and LC-MS/MS data have been deposited to the ProteomeXchange Consortium via the PRIDE66 partner repository with dataset identifier: PXD056334 for HDX-MS data and with dataset identifier: PXD062430 for LC-MS/MS data. Coordinates and structure factor amplitudes have been deposited in the Protein Data Bank under the accession code 9CU3. The raw HDX-MS data can be found under Supplementary Data 1. The raw data used to generate plots for CD, proteolysis, thermal melt, LC-MS/MS sequencing, and MST experiments can be found in Supplementary Data 2. New plasmids in this study were deposited to Addgene. The plasmid for DriD(1-70) was deposited with the identifier 237851, the plasmid for FL DriD(R121E-R122E-P125E-E128R) was deposited with the identifier 237852, and the plasmid for DriD(78-327) was deposited with the identifier 237853. Uncropped gels are shown in the Supplementary Information. All other data are available from the corresponding author (or other sources, as applicable) on reasonable request.
References
Chatterjee, N. & Walker, G. C. Mechanisms of DNA damage, repair and mutagenesis. Environ. Mol. Mutage. 58, 235–263 (2017).
Bridges, B. A. Error-prone DNA repair and translesion DNA synthesis: II: The inducible SOS hypothesis. DNA Repair 4, 725–739 (2005).
Baharoglu, Z. & Mazel, D. S. O. S. The formidable strategy of bacteria against aggressions. FEMS Microbiol. Rev. 38, 1126–1145 (2014).
Kreuzer, K. N. DNA damage responses in prokaryotes: regulating gene expression, modulating growth patterns, and manipulating replication forks. Cold Spring Harb. Perspect. Biol. 5, a012674 (2013).
Maslowska, K. H., Makiela-Dzbenska, K. & Fijalkowska, I. J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen 60, 368–384 (2019).
Lima-Noronha, M. A. et al. Sending out an SOS - the bacterial DNA damage response. Genet. Mol. Biol. 45, e20220107 (2022).
Little, J. W. & Mount, D. W. The SOS regulatory system of Escherichia coli. Cell 29, 11–22 (1982).
Salles, B. & Defais, M. Signal of induction of recA protein in E coli. Mutat. Res. 131, 53–59 (1984).
Sassanfar, M. & Roberts, J. W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J. Mol. Biol. 212, 79–96 (1990).
Pennington, J. M. & Rosenberg, S. M. Spontaneous DNA breakage in single living Escherichia coli cells. Nat. Genet. 39, 797–802 (2007).
Symington, L. S. & Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 45, 247–271 (2011).
Huisman, O. & D’Ari, R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature 290, 797–799 (1981).
Little, J. W. & Gellert, M. The SOS regulatory system: control of its state by the level of RecA protease. J. Mol. Biol. 167, 791–808 (1983).
Little, J. W. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie 73, 411–421 (1991).
Lewis, L. K., Harlow, G. R., Gregg-Jolly, L. A. & Mount, D. W. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 241, 507–523 (1994).
Ohmori, H., Hatada, E., Qiao, Y., Tsuji, M. & Fukuda, R. dinP, a new gene in Escherichia coli, whose product shows similarities to UmuC and its homologues. Mutat. Res. 347, 1–7 (1995).
Trusca, D., Scott, S., Thompson, C. & Bramhill, D. Bacterial SOS checkpoint protein SulA inhibits polymerization of purified FtsZ cell division protein. J. Bacteriol. 180, 3946–3953 (1998).
Giese, K. C., Michalowski, C. B. & Little, J. W. RecA-dependent cleavage of LexA dimers. J. Mol. Biol. 377, 48–161 (2008).
Charpentier, X., Polard, P. & Claverys, J.-P. Induction of competence for genetic transformation by antibiotics: convergent evolution of stress responses in distant bacteria lacking SOS?. Curr. Opin. Microbiol. 15, 570–576 (2012).
Qin, T.-T. et al. SOS response and its regulation on the fluoroquinolone resistance. Ann. Transl. Med. 3, 358 (2015).
Crane, J. K., Alvarado, C. L. & Sutton, M. D. Role of the SOS response in the generation of antibiotic resistance in vivo. Antimicrob. Agents Chemother. 65, e0001321 (2021).
Howe, W. E. & Mount, D. W. Production of cells without deoxyribonucleic acid during thymidine starvation of lexA- cultures of Escherichia coli K-12. J. Bacteriol. 124, 1113–1121 (1975).
Huisman, O., D’Ari, R. & George, J. Inducible sfi dependent division inhibition in Escherichia coli. Mol. Gen. Genet. MGG 177, 629–636 (1980).
McGinness, T. & Wake, R. G. A fixed amount of chromosome replication needed for premature division septation in Bacillus subtilis. J. Mol. Biol. 146, 173–177 (1981).
Hill, T. M., Sharma, B., Valjavec-Gratian, M. & Smith, J. sfi-independent filamentation in Escherichia coli Is lexA dependent and requires DNA damage for induction. J. Bacteriol. 179, 1931–1939 (1997).
Liu, G., Begg, K., Geddes, A. & Donachie, W. D. Transcription of essential cell division genes is linked to chromosome replication in Escherichia coli. Mol. Microbiol. 40, 909–916 (2001).
Goranov, A. I., Katz, L., Breier, A. M., Burge, C. B. & Grossman, A. D. A transcriptional response to replication status mediated by the conserved bacterial replication protein DnaA. Proc. Natl Acad. Sci. USA 102, 12932–12937 (2005).
Mielecki, D. & Grzesiuk, E. Ada response- a strategy for repair of alkylated DNA in bacteria. FEMS Microbiol. Lett. 355, 1–11 (2014).
Olivencia, B. F. et al. Mycobacterium smegmatis PafBC is involved in regulation of DNA damage response. Sci. Rep. 7, 13987 (2017).
Müller, A. U., Imkamp, F. & Weber-Ban, E. The mycobacterial LexA/RecA-independent DNA damage response is controlled by PafBC and the pup-proteosome system. Cell Rep. 23, 3551–3564 (2018).
Kamat, A. & Badrinarayanan, A. SOS-independent bacterial DNA damage responses: diverse mechanisms, unifying function. Curr. Opin. Microbiol. 73, 102323 (2023).
Blanchard, L. & de Groot, A. Coexistence of SOS-dependent and SOS-independent regulation of DNA repair genes in radiation-resistant Deinococcus bacteria. Cells 16, 10.3390 (2021).
Ludanyi, M. et al. Radiation response in Deinococcus deserti: IrrE is a metalloprotease that cleaves repressor protein DdrO. Mol. Microbiol. 94, 434–449 (2014).
Lu, H. et al. The Deinococcus protease PprI senses DNA damage by directly interacting with single-stranded DNA. Nat. Commun. 29, 1892 (2024).
Lau, R. K., Enustun, E., Gu, Y., Nguyen, J. V. & Corbett, K. D. A conserved signaling pathway activates bacterial CBASS immune signaling in response to DNA damage. EMBO J. 41, e111540 (2022).
Modell, J. W., Kambara, T. K., Perchuck, B. S. & Laub, M. T. A DNA damage-induced, SOS-independent checkpoint regulates cell division in Caulobacter crescentus. PLoS Biol. 12, e1001977 (2014).
Gozzi, K., Salinas, R., Nguyen, V. D., Laub, M. T. & Schumacher, M. A. ssDNA is an allosteric regulator of the C. crescentus SOS-independent DNA damage response transcription activator. DriD. Genes Dev. 36, 618–633 (2022).
Schumacher, M. A. et al. Structure of the WYL-domain containing transcription activator, DriD, in complex with ssDNA effector and DNA target site. Nucleic Acids Res. 52, 1435–1449 (2023).
Gozzi, K., Tran, N. T., Modell, J. W., Le, T. B. K. & Laub, M. T. Prophage-like gene transfer agents promote Caulobacter crescentus survival and DNA repair during stationary phage. PLoS Biol. 20, e3001790 (2022).
Makarova, K. S., Anantharaman, V., Grishin, N. V., Koonin, E. V. & Aravind, L. CARF and WYL domains: ligand-binding regulators of prokaryotic defense systems. Front. Genet. 5, 102 (2014).
Keller, L. M. & Weber-Ban, E. An emerging class of nucleic acid-sensing regulators in bacteria: WYL domain-containing proteins. Curr. Opin. Microbiol. 74, 102296 (2023).
Keller, L. M. L., Flattich, K. & Weber-Ban, E. Novel WYL domain-containing transcriptional activator acts in response to genotoxic stress in rapidly growing mycobacteria. Commun. Biol. 6, 1222 (2023).
Müller, A. U., Leibundgut, M., Ban, N. & Weber-Ban, E. Structure and functional implications of WYL domain-containing bacterial DNA damage response regulator PafBC. Nat. Commun. 10, 4653 (2019).
Beck, I. N., Picton, D. M. & Blower, T. R. Crystal structure of the BREX phage defence protein BrxA. Curr. Res. Struct. Biol. 4, 211–219 (2022).
Blankenchip, C. L. et al. Control of bacterial immune signaling by a WYL domain transcription factor. Nucleic Acids Res. 50, 5239–5250 (2022).
Luyten, Y. A. et al. Identification and characterization of the WYL BrxR protein and its gene as separable regulatory elements of a BREX phage restriction system. Nucleic Acids Res. 50, 5171–5190 (2022).
Picton, D. M. et al. A widespread family of WYL-domain transcriptional regulators co-localizes with diverse phage defence systems and islands. Nucleic Acids Res. 50, 5191–5207 (2022).
Cohen, D. et al. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature 574, 691–695 (2019).
Koth, C. M., Orlicky, S. M., Larson, S. M. & Edwards, A. M. Use of limited proteolysis to identify protein domains suitable for structural analysis. Methods Enzymol. 368, 77–84 (2003).
El Deeb, S. et al. Microscale thermophoresis as a powerful growing analytical technique for the investigation of biomolecular interaction and the determination of binding parameters. Methods Appl. Fluoresc. 10, 042001 (2022).
Zhang, H., Dong, C., Li, L., Wasney, G. A. & Min, J. Structural insights into the modulatory role of the accessory protein WYL1 in the Type VI-D CRISPR-Cas system. Nucleic Acids Res. 47, 5420–5428 (2019).
Schilling, C. M. et al. Single-stranded DNA binding to the transcription factor PafBC triggers the mycobacterial DNA damage response. Sci. Adv. 11, 10.1126 (2025).
Lin, A. et al. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell 70, 777–789 (1992).
Fisher, S. H. & Wray, L. V. Jr Bacillus subtilis glutamine synthetase regulates its own synthesis by acting as a chaperon to stabilize GlnR-DNA complexes. Proc. Natl Acad. Sci. USA 105, 1014–1019 (2008).
Schumacher, M. A., Chinnam, N.-G., Cuthbert, B., Tonthat, N.-K. & Whitfill, T. Structures of regulatory machinery reveal novel molecular mechanisms controlling B. subtilis nitrogen homeostasis. Genes Dev. 29, 451–464 (2015).
Travis, B. et al. Molecular dissection of the glutamine synthetase-GlnR nitrogen regulatory circuitry in Gram-positive bacteria. Nat. Commun. 13, 3793 (2022).
Jiang, X., Zhang, L., Teng, M. & Xu, L. Antibiotic binding releases autoinhibition of the TipA multidrug-resistance transcriptional regulator. J. Biol. Chem. 295, 17865–17876 (2020).
Kaur, G. et al. Allosteric autoregulation of DNA binding via a DNA-mimicking protein domain: a biophysical study of ZNF410-DNA interaction using small angle X-ray scattering. Nucleic Acids Res. 51, 1674–1686 (2023).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66,125-132 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. Sect. Struct. Biol. 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501 (2010).
Acquasaliente, L., Pelc, L. A. & Di Cera, E. Probing prothrombin structure by limited proteolysis. Sci. Rep. 9, 6125 (2019).
Wales, T. E. & Engen, J. R. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 25, 158–170 (2006).
Houde, D., Berkowitz, S. A. & Engen, J. R. The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies. J. Pharm. Sci. 100, 2071–2086 (2011).
Engen, J. R. & Wales, T. E. Analytical aspects of Hydrogen Exchange Mass Spectrometry. Annu. Rev. Anal. Chem. 8, 127–148 (2015).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Chen, E. A. & Porter, L. L. SSDraw: software for generating comparative protein secondary structure diagrams. Protein Sci. 32, 10.1002 (2023).
Acknowledgements
We acknowledge beamline 5.0.2 for X-ray diffraction data collection. The ALS (Berkeley, CA) is a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the US Department of Energy under Contract DE-AC02-05CH11231, Office of Basic Energy Sciences. Beamline 5.0.2 of the ALS, a US Department of Energy Office of Science User Facility under Contract DE-AC02-05CH11231, is supported in part by the ALS-ENABLE program funded by the NIH, National Institute of General Medical Sciences, Grant P30 GM124169-01. We also acknowledge Dr. Erik Soderblom for assistance with mass spec analyses of proteolytic fragments. This research was supported by a Nanaline H Duke Endowed Chair and National Institutes of Health grants (R35GM130290 to M.A.S.), and the work of TEW is supported by National Institutes of Health grant 5R01CA233978.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.A.S., T.E.W.; Methodology: M.A.S., T.E.W., E.C., B.Y.S.; Analysis: M.A.S., E.C., B.Y.S., J.C.O., K.G., T.E.W.; Resources: M.A.S., T.E.W.; Data curation: T.E.W., B.Y.S., M.A.S., E.C.; Writing – original draft: E.C., M.A.S.; Writing & Editing: M.A.S., E.C., T.E.W., B.Y.S., Funding acquisition: M.A.S., T.E.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Joanna Timmins and Laura Rodríguez Pérez.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cannistraci, E., Srinivasu, B.Y., Chavez Orozco, J. et al. Allosteric activation mechanism of DriD, a WYL-domain containing transcription regulator. Commun Biol 8, 679 (2025). https://doi.org/10.1038/s42003-025-08111-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08111-x