Abstract
Bats as the only flying mammals incur a high metabolic cost during extended powered flight, which results in febrile-like temperatures without injury. Herein, we investigate the in vivo heat shock response (HSR) in the cave nectar bat Eonycteris spelaea. We demonstrate that E. spelaea exhibits enhanced physiological heat resistance, marked by reduced lethality, tissue damage and serum corticosterone levels in comparison to mice upon heat challenge. Additionally, E. spelaea did not exhibit an acute transcriptional response observed in heat stressed mice. Instead, bats displayed a delayed and non-canonical HSR that did not involve the activation of classical heat shock related genes and pathways. This altered response in E. spelaea is attributed to the elevated basal expression of heat shock proteins, which we demonstrate to be a common characteristic exhibited by bats from diverse sub-orders, families and diets. Taken together, we demonstrate a distinct HSR in E. spelaea relative to the conventional model organism, mouse, which may provide insights to understand novel regulatory targets and effector proteins that underlie the mammalian heat shock response.
Similar content being viewed by others
Introduction
Bats (order Chiroptera) are unique mammals with notable phenotypes such as increased longevity, enhanced viral tolerance and the distinction of being the only mammal capable of self-powered flight1. Flight, as a mode of locomotion, incurs a high absolute energetic cost2,3. During flight, bats experience a sharp increase in metabolic rate and presumably oxidative stress, with heart rates reaching up to 1000 bpm4. Furthermore, field studies highlight that in-flight bats maintain elevated febrile-like temperature with some species of bats reaching extreme temperatures of 42 °C or more without injury3,5,6,7,8,9,10,11,12,13,14,15,16,17. This overarching observation of bats sustaining high core temperatures during flight without injury serves the foundational basis to elucidate the biological nuances of bats during heat stress.
The heat shock response (HSR) is a highly conserved defence mechanism that is essential for preventing protein misfolding and aggregation during stress, inhibiting the activation of cell death pathways18 and is thus vital for organismal protection. This response can be triggered by a variety of stresses such as high temperature, oxidative stress and protein aggregation. Activation of the HSR fosters a protective phenotype, enhancing cellular resilience to these stresses19. In mammals, HSR is controlled by multiple heat shock factors (HSFs), of which HSF1 is the master regulator of the heat shock response which orchestrates a classical response through activation of molecular chaperones20,21. In resting conditions, HSF1 exists as an inactive monomer. During stress, HSF1 unfolds, trimerizes, translocates to the nucleus, undergoes post-translational modification and functions as a transcription factor by binding heat shock elements to activate transcription of heat shock proteins (HSPs)18. HSPs are a group of evolutionarily-conserved molecular chaperones that confer thermotolerance by facilitating protein stabilization, translocation, re-folding and degradation19 and play a role in myriads of biological processes such as immunity, inflammation and aging.
Previously, our group demonstrated that bat cell lines have an elevated basal expression of HSPs compared to other mammals22 which protected bat cells during prolonged in vitro heat treatment in a HSP-dependent manner. Bats are postulated to have developed evolutionary adaptations enabling them to withstand the thermal challenges associated with flight. Overall, this motivates the overarching hypothesis that bats have an atypical heat shock response compared to other mammals with potentially altered temporal kinetics, magnitude of induction, and activation of unique gene pathways. To the best of our knowledge, there are no studies that characterized the in vivo heat shock response of a bat.
In this study, cave nectar bat (Eonycteris spelaea) and mice (Mus musculus) were subjected to conscious animal in vivo heat shock challenge to compare their systems-level heat shock response. Herein, we report that bats have enhanced physiological heat resistance when compared to mice. Post-heat shocked mice was characterized to have increased plasma corticosterone, pathological renal tubular degeneration and a mortality rate of 20%, however these outcomes were not observed in bats. RNA sequencing of various organs from mice and bats across multiple time points revealed that bats exhibited a non-canonical heat shock response that is transcriptionally delayed and do not induce classical heat shock related genes and pathways. Differential gene analysis comparing bats and mice at baseline across multiple tissues revealed that major stress inducible HSP isoforms (HSPA1A, HSPA1B, HSP90AA1 and DNAJA1) were the most upregulated HSP and were not further transcriptionally activated after heat stress. The enhanced physiological protection coupled with the non-canonical heat shock response in bats are presumably attributed to the elevated basal expression of heat shock proteins. We further demonstrate using CRISPR-KO bat cell lines that HSF1, the master regulator of heat shock, is required for the induction of HSR target genes but not the maintenance of high HSP baseline in vitro. Lastly, this altered response in E. spelaea is attributed to the elevated basal expression of heat shock proteins, which we demonstrate to be a common characteristic exhibited by bats from diverse sub-orders, families and diets.
Results
Establishment of the in vivo heat shock system
To validate the meta-observation of bats as unique mammals that can tolerate extreme heat stress, we searched through primary literature for studies that characterized various core temperature of mammals during exercise or strenuous activity (Supplementary Table 1)3,8,9,23,24,25,26,27,28,29. In this analysis, we prioritized studies that were designed for (i) a voluntary mode of exercise and (ii) rectal or internal telemetry mode of sampling core temperature. To expand the scope of our observations, horses were included in the analysis as they are an appropriate proxy for a non-sedentary, active animal. Notably, amongst the species compared (humans, mice, horses and bats), bats were the only species that were observed to have a core temperature of >40 °C during exercise with the upper limit reaching to approximately 42 °C (Fig. 1A). Furthermore, existing literature supports the observation that bats are highly tolerant of extreme extrinsic temperatures as well30,31.
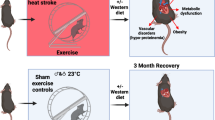
A Bats exhibit extreme core temperatures ( >40 °C) (illustrated in red dotted line) during flight as compared to mice, human and horses during exercise. Each bar indicates an independent study (Supplementary Table 1). Black square symbols indicate the average core temperatures while the lines indicate the standard deviation. The numbers indicate the n size in each study from which the temperature was derived. B The animals are subjected to an in vivo heat challenge at 42 °C for 1 h. Three terminal time points were chosen – Animals in the baseline group are not subjected to heat challenge (Ctl), immediately post heat shock (HS) and heat shock followed by 4 h of rest (HS + R). C The bat species used in this study is E. spelaea (Es), a pteropodid bat from the suborder Yinpterochiroptera, from which our group has established an active breeding colony. The mammalian comparator species used in this heat shock challenge is M. musculus (Mm), C57BL/6 J strain, a mammalian model animal used in HS studies. D Core temperature of animals assayed throughout experimental conditions (n = 4) (Supplementary Table 2). Figures were created with elements from BioRender (2025). https://BioRender.com/0fcvmt1.
To compare the dynamic heat shock response of bats and other mammals, an in vivo heat shock model was established with three terminal time points: Ctl – baseline at the start of the experiments, HS - immediately after 1 h of heat shock, HS + R – 1 h of heat shock followed by 4 h of recovery (Fig. 1B). Since no prior studies, to the best of our knowledge, have characterized transcriptional heat shock response time points in bats, we based our approach on widely used methodologies in mouse studies, where a 4 h recovery is frequently employed to assay in vivo heat shock response32,33,34. The bat species used in this study is E. spelaea (Es), a nectarivorous pteropodid bat from the suborder Yinpterochiroptera, which is geographically located in Asia from which our group has established an active breeding colony35 (Fig. 1C). Our established bat colony ensures a consistent biological baseline in terms of health and allowing for age-matched comparisons with mammalian controls35. The mammalian counterpart species used in this heat shock challenge is M. musculus (Mm), C57BL/6 J strain, which is a common mammalian model system and widely used in experimental heat shock studies36,37,38 (Fig. 1C). We subjected both species to a conscious heat shock stress challenge at 42 °C for 1 h using a heating incubator. From the heat shock challenge, the core temperature of both species increased gradually throughout the heating duration (Supplementary Fig. 1) and was significantly raised from a baseline temperature of 38.4 °C in mice and 37.9 °C in bats to a similar post-HS temperature of 41 °C in mice and bats (Fig. 1D, Supplementary Table 2). Following recovery of 4 h, both animals core body temperature returned back to similar homeostatic baseline levels (Fig. 1D). Overall, our in vivo heat shock system provides robust heat stress stimulus to raise the core temperature of both bats and mice significantly and serves as an appropriate method to induce the animal’s heat shock response.
Hormonal, physiological and histological evidence of enhanced heat resistance in bats
To characterize the stress response of both animals from the heat challenge, the serum corticosterone level was assayed. Corticosterone is a glucocorticoid steroid hormone that is secreted via the hypothalamus-pituitary-adrenal axis during stressful conditions that challenge an animal’s homeostasis. This method has been shown to be species independent39,40,41. In this study, mice had an approximate 3-fold increase in plasma corticosterone from the baseline 90 ng/mL to 260 ng/mL when subjected to HS which returned to baseline after recovery (HS + R) (Fig. 2A). However, in bats, there was no change in corticosterone concentration with an average at 40 ng/mL throughout the experimental time points. This highlights that at an autonomic level, bats do not perceive the exogenous heat stress which significantly raised their core temperature to critical levels of approximately 41 °C to be a stressful event.
APlasma corticosterone of mice (Mm) and bats (Es) detected using immunoassay (n = 3) (Supplementary Data 5). B Survival of mice (n = 10) and bats (n = 8) during the heat challenge (Supplementary Data 5). C Representative H&E staining of kidney from animals subjected to varying conditions (Ctl, HS, HS + R, n = 3). Scale bar = 200 μm. Black arrows flank areas of renal tubular degeneration, characterized by cytoplasmic tinctorial changes (hypereosinophilia) and nuclear pyknosis. D Scoring of renal tubular necrosis in mice (Mm) and bats (Es) kidneys (n = 3 at each time point) by an independent pathologist. The number indicates the severity of the renal tubular necrosis in increasing severity (0–2). Figures were created with elements from BioRender (2025). https://BioRender.com/0fcvmt1.
Physiologically, there was 20% lethality observed in mice (n = 2/10) but none in bats (n = 0/8). (Fig. 2B). Notably, mice subjected to HS challenge displayed symptoms such as lethargy, limited mobility and aberrant behaviour such as clearing out the bedding to cool themselves. Bats did not show any change in behaviour throughout the experimental heat stress challenge.
To characterise histological damage from both animals across HS treatment, we performed hematoxylin and Eosin (H&E) staining on kidney, a key heat sensitive organ consistently demonstrated in several reports42,43. The severity of the HS damage was assessed in an unbiased manner, with the staining graded by an independent pathologist using a scoring scale of 0 to 2 (0: none; 1: moderate; 2: severe) for renal tubular necrosis. The result shows that renal tubular degeneration was observed in mice kidney as characterized by cytoplasmic tinctorial changes (hypereosinophilia) and nuclear pyknosis (Fig. 2C). In mice and bat controls, both species score 0, indicating the healthy status of both animals prior heat challenge. Remarkably, 2 out of 3 mice HS kidney samples showed acute kidney damage with severity score of 2. (Fig. 2D). In contrast, bat kidney samples were graded to be overall healthy and suffered no acute damage from heat stress in all conditions (score of 0 across all conditions).
Taken together, the hormonal, physiological and histological evidence demonstrates that bats have an enhanced physiological heat stress resistance when compared to mice when subjected to the same heat stress stimulus that raised both their core temperatures to a similar level.
Acute classical heat shock response elicited in mice but absent in bats
To compare the systems-level heat shock response of both animals, we performed bulk RNA sequencing of lung, blood and muscle of both bats and mice on all time points – Ctl, HS and HS + R. The muscle was chosen as it is the dominant organ of heat generation during flight-associated metabolism. The corresponding muscle chosen for each species was based on the dominant muscle required for locomotion. In this case, pectoral muscle was used in bat and bicep femoris was used in mice. Furthermore, the lung was chosen as they are heat sensitive, metabolically distinct and the primary site of immune infection in bats44. Additionally, the blood was selected as it is a systemic readout of the animal. While we previously observed no significant kidney damage in bats, unlike in mice, the kidney plays a more peripheral role in physiology compared to the muscle, lung, and blood, which are more directly involved in heat generation, metabolic regulation and systemic stress responses.
A classical global stress transcriptional response was observed in mice, defined by a robust induction of differentially expressed genes (DEG) that are both significantly up- and down- regulated across all tissues acutely upon heat stress followed by a general trend of returning to baseline after recovery (Fig. 3A). Unexpectedly, bats had an opposite global transcriptional response with a weaker response immediately after acute heat stress, followed by an induction of DEGs that occur predominantly only after recovery phase (Fig. 3B). In the HS condition, the magnitude of the total number of DEG in bats for all organs is strikingly lower than mice, 31.5-fold (441 in mice vs 14 in bats) and 7-fold (1985 in mice vs 283 in bats) for up- and down- regulated DEG respectively (Fig. 3C). In contrary, for HS + R condition, there is a reverse trend in which bats demonstrated higher magnitude in total numbers of DEGs, 4.75-fold (214 in bats vs 45 in mice) and 30.8-fold (616 in bats vs 20 in mice) for both up- and down regulated DEGs, respectively. Notably, the majority of the responses in bat only occurred after recovery phase (Figs. 3A and 3B). This transcriptional dynamics is atypical of a classical heat shock response.
A Global differentially up- and down- regulated DEG ( >2 FC and 0.05 < FDR) of HS and HS + R conditions when compared against Ctl group in mice (Mm) (B) and bats (Es) (Supplementary Data 1). C Bar graph comparing the number of DEG found in mice (Mm) and bats (Es) upon different conditions, the number indicates the total DEG involved in all organs. Comparisons made were against Ctl group. D Venn diagram of overlapping DEG found across organ in mice (Mm) (E) and bats (Es) acutely in HS condition. F Intersecting upregulated DEG of all mice (Mm) HS organs and filtering genes belonging to inducible HSP family genes and co-chaperones. G Filtered GSEA against GOBP pathways comparing chaperone mediated protein folding between mice (Mm) and bats (Es) of different condition and organs (Supplementary Data 3). H Transcription factor enrichment analysis of DEG set list defined in (A) for HS timepoint in mice (Mm) organs, analysis was done using ChEA3 against ENCODE database (Supplementary Data 5). Figures were created with elements from BioRender (2025). https://BioRender.com/0fcvmt1.
The core responsive heat shock signature were defined by intersecting the upregulated DEG of each animals from all organs during the HS condition. In accordance with existing literature, we observe classical HSP inducible genes and co-chaperones (HSP90AA1, HSP90AB1,HSPA1A, HSPA1B, DNAJB1, AHA1,STIP1) being induced in mice within the core heat shock responsive signature genes (Figs. 3D, 3F and Supplementary Data 1). Importantly, this highlights that our heat shock system is robust and valid in administrating a reproducible stimulus to the animals similar to literature in the field. Nonetheless, bats upon receiving this stimulus did not produce any core responsive heat shock signature and had no common DEGs from the different organs during HS (Fig. 3E).
Gene set enrichment analysis (GSEA) was performed to characterize genes that are differentially induced in both animals from varying conditions (HS, HS + R) and organs (blood, lung, muscle) against gene ontology biological processes (GOBP) heat responsive pathways (Fig. 3G). De novo protein folding, chaperone mediated protein folding and cellular response to heat pathways were positively enriched in mice organs indicating a bona fide heat shock systemic response. Interestingly, these protein folding pathways were not significantly enriched in bats. Moreover, various protein folding pathways were unexpectedly negatively enriched in HS + R condition in bats across all organs below homeostatic baseline levels (Fig. 3G). Transcription factor enrichment analysis (TFEA) was performed on DEG from mice organs at the HS timepoint revealed that HSF1 played a significant role in modulating the overall response from the heat challenge. Furthermore, other transcription factors (TFs) such as MYOG, CTCF, JUND which were previously reported to be implicated in heat shock45,46 were similarly detected in our analysis (Fig. 3H).
The heat shock response of bats in the acute phase (HS) of lung and muscle resulted in the up-regulation of a strikingly few number of genes that were not majorly associated with the heat shock response regulon. The exception being in the bat lung, whereby the only heat shock gene being induced was Dnajb1, this gene encodes a protein from the heat shock protein 40 kD containing the “J-domain” that stimulates the ATPase activity of Hsp70 heat-shock protein and promotes protein folding (Supplementary Data 2). Although Dnajb1 is upregulated, the induction magnitude in bats is approximately 4-fold lower than that of mice in the corresponding tissue (Supplementary Data 1-2). Notably, there are no up- or down-regulated DEGs in the blood of bats in HS. Furthermore, the limited DEGs that are upregulated in the lung and muscle are not predominantly associated with canonical heat stress response (Supplementary Data 2). Amongst these upregulated genes, TP63 (a p53-related transcription factor) and PRIMPOL (an enzyme involved in DNA replication and repair), have been previously demonstrated in literature to have an association with a stress response in the aspect of DNA damage and oxidative stress47,48.
Taken together, the heat stimulus elicited by our experimental system successfully resulted in a classical acute heat shock response in mice evidenced by the induction of classical HSP genes, pathways and transcription factors in the lung, muscle and blood. However, bats that receive the same stimulus had strikingly few genes upregulated in solid organs and none in blood. Acutely enriched bat DEGs had no known association with the classical heat shock response with the exception of Dnajb1 in lung.
Delayed non-canonical heat shock response in bats
To further characterize the genes and pathways as a response to heat challenge in bats, we narrowed in on the condition of HS + R as it contains the predominant transcriptional signal. We first attempted to identify DEGs that were commonly upregulated in all tissues; however, there was no overlapping genes (Supplementary Fig. 2), suggesting a tissue independent transcriptional module response to heat stress in bats.
To identify stress responsive signatures in bats, DEG analysis, pathway analysis with GSEA and TF enrichment analysis was performed. The resultant genes and pathways were not positively enriched for classical HSP related family genes or chaperone related biological pathways. Nonetheless, from DEG analysis, there are certain genes that might play a related role to stress response but are non-canonical, which are the top upregulated hits illustrated in the volcano plots (Fig. 4A–C). IL37 (interleukin 37), a member of the IL-1 family with anti-inflammatory properties is implicated in immune stress response, was upregulated in the lung. NEDD4 (Neural Precursor Cell Expressed, Developmentally Down-Regulated 4), involved in ubiquitination by targeting proteins for degradation, was upregulated in the muscle (Supplementary Data 2). Pathway analysis (Fig. 4D and Supplementary Data 3), it revealed atypical bat specific enrichment in biological pathways which comparatively had a corresponding lack of or negative enrichment in mice. Subsequently, ChEA3 TF analysis on upregulated DEGs in bats (Fig. 4E) revealed that there is a tissue-specific transcriptional programme and a lack of common TF which contrasts with the TF enrichment of mice response to heat (Fig. 3H).
A Volcano plot of up- and down- regulated DEG ( >2 FC and 0.05 < FDR) of HS + R conditions in blood, (B) lung and (C) muscle in bat (Es) (Supplementary Data 2). D Filtered GSEA against GOBP pathways that are enriched in bats (Es) with comparative mice (Mm) enrichment of different condition and organs (Supplementary Data 3). E Transcription factor enrichment analysis of DEG set list upregulated or (F) downregulated in for HS + R timepoint in bat (Es) organs, analysis was done using ChEA3 against ENCODE database (Supplementary Data 5). Figures were created with elements from BioRender (2025). https://BioRender.com/0fcvmt1.
Intriguingly, HSP family related genes were among the most downregulated DEGs in bats in muscle and lung at HS + R condition. At FDR cut-off < 0.1, HSPA1A (inducible heat shock protein family A member 1A), was the only commonly downregulated amongst all the tissues in HS + R in bat. HSPA8, DNAJB1, DNAJA1, CHORDC1 and STIP1 were also among top DEGs negatively enriched in various tissues of bat in HS + R condition (Fig. 4A–C, Supplementary Data 2). From ChEA3 TF analysis, which ranks TF whose target genes are over-represented, HSF1 was identified as the top transcription factor associated with downregulated DEGs whereby target genes were negatively enriched. (Fig. 4F). This suggest a stress-induced response from bat resulting in the downregulation of HSF1 in recovery phase.
Overall, bats exhibited a stress response to heat challenge characterized by a delayed activation of non-classical stress genes and pathways. Notably, there is a consistent systems-level downregulation of heat shock proteins, indicating a distinctive nuanced stress response in bats.
High basal HSP expression in bats is not further transcriptionally induced during heat shock
To elucidate the differences in the heat shock response in bats compared to mice, we conducted a DEG analysis and focused on HSP family genes and regulators across muscle and lung tissues at the control (Ctl) timepoint, excluding blood due to its inherent heterogeneity in both species. There is high basal expression of HSP in bats across tissues at baseline relative to mice (Fig. 5A, B). Specifically, at the baseline (Ctl), there was an upregulation of 18/54 and 33/54 HSPs in bat lung and muscle tissues, respectively, as opposed to 5/54 and 3/54 upregulated in mice (Fig. 5A, B). Notably, the most significantly upregulated HSP in bats (HSPA1A, HSPA1B, HSP90AA1 and DNAJA1) correspond with the major stress inducible isoform associated with each HSP family (HSP90, HSP70 and HSP40) (Fig. 5A, B). Additionally, we included other stress response pathways, such as hypoxia, unfolded protein response (UPR), and oxidative stress, in our analysis. We visualized the key inducible genes in these pathways using our RNA-seq transcriptional data which comparatively contrasted top DEG within the Ctl group of mice and bats (Supplementary Fig. 3). Our results indicate that only target genes in the heat shock response pathway, and not those in other stress pathways, are basally upregulated in bats.
A Volcano plot of DEG of mice (Mm) and bats (Es) at baseline (Ctl) in lung and in (B) muscle (Supplementary Data 5). Coloured dots indicate different types of genes that are differentially expressed, Red – HSPs and grey – non HSP related genes. The number of DE upregulated HSP summarizes the total upregulated number of HSP in each species (Supplementary Data 5). C Heatmap of HSP family and co-chaperones baseline relative expression in each species. Z-score is derived via the scaled value of log(nCounts+1) normalized against each gene and organ (Supplementary Data 5). D Module signature of each HSP class across treatment conditions for mice (Mm) and bats (Es). The module signature is calculated from the arithmetic mean of log(nCounts+1) for all genes within the HSP family class listed in (C) Vertical line denotes standard error (Supplementary Data 5). E qPCR validation of HSPA1A and HSP90AA1 and (F) western blot of HSP70 and HSP90 in muscle tissue of animals that were subjected to in vivo heat shock (Supplementary Data 5). Figures were created with elements from BioRender (2025). https://BioRender.com/0fcvmt1.
HSPs are predominantly controlled by master regulators HSF1 and HSF2. Interestingly, the master regulator of heat shock response, HSF1 was downregulated in bats (Supplementary Fig. 4A). Furthermore, HSF2, an alternative regulator of heat shock response, was not differentially expressed (Supplementary Fig. 4B). A parallel analysis was performed on all HSP family genes and co-chaperones in mice and bats by comparing the constitutive expression difference via a z-score (Fig. 5C). Similarly, the heatmap plot visualizes trend of overall higher HSP expression in bats for every HSP class compared to mice. Furthermore, this analysis qualitatively highlights a tissue specific effect, whereby the elevated constitutive expression of HSP is more evident in muscle (Fig. 5C). Additionally, the HSP genes, which exhibited a strong induction in mice following heat shock, showed a moderate correlation with the baseline elevation of HSP genes in bats within lung and muscle tissues, respectively, as evidenced by correlation coefficients of R² = 0.512 and R² = 0.578 (Supplementary Fig. 5). Consistently, the major inducible HSPs (HSP90AA1, HSPA1A, HSPA1B and DNAJA1), are strikingly higher in bats at baseline. This highlights that bats have an elevated levels of total HSPs (inducible and constitutive) relative to mice at baseline, with a pronounced effect on major stress-inducible HSP isoforms.
To compare the magnitude of effect and dynamics of the heat shock response in both animals, a heat shock module score which quantifies the expression of all genes within each HSP family class across treatment was analysed (Fig. 5D). This analysis visualizes two general trends: Firstly, at baseline, bats have a higher module score for each class of HSP family and related genes (HSP90, HSP70, sHSPs, chaperonin and co-chaperones). Secondly, the induction of these HSP family classes in both animals plateaus at a similar peak (close to the baseline levels of bats), indicating a ceiling effect for the total amount of HSPs (Fig. 5D). To validate these observations, we performed qPCR on the two inducible major HSP gene from HSP90 and HSP70 class - HSP90AA1 and HSPA1A. qPCR of the relative gene expression of these major HSP genes in the muscle were in line with the earlier observations: a high baseline in bats and the induction in mice does not surpass the relative expression levels (Fig. 5E). Assay with western blot recapitulated a similar theme- even as a function of heat shock, the HSP levels in mice are not higher than the levels in bats. (Fig. 5F). Additionally, we demonstrate that in vivo bat tissues express significantly higher levels of HPSs when compared to in vitro bat cell lines (Supplementary Fig. 6). Of note, when the high baseline of HSP expression is lowered in vitro, bats can upregulate inducible HSPs, such as HSPA1A and HSP90AA1, under heat shock conditions (Supplementary Fig. 6). Importantly, even with the ‘reduced’ levels of HSPs observed in in vitro bat cell lines it is still higher than the levels observed in vivo mice, with higher relative gene expression of approximately 10-fold in HSPA1A (0.01 in mice compared to 0.1 in bats) and 100-fold in HSP90AA1 (0.01 in mice compared to 1.5 in bats) (Fig. 5E, Supplementary Fig. 6).
Taken together, the constitutively elevated baseline of HSPs in bats negates the need for the further upregulation and likely contributes to the non-canonical HSR highlighted in bats.
HSF1 is responsible for the induction but not the maintenance of high HSP baseline in vitro
HSF1 is recognized as the master regulator of the heat shock response. We hypothesize that the elevated basal levels of heat shock proteins (HSPs) observed in bats may be due to: (1) adaptations in the genomic promoter regions involving the duplication of heat shock elements HSEs/TF binding sites or (2) unique adaptations in HSF1 specific to bats that lead to a rewiring of the regulatory network governing basal HSP expression.
To investigate the presence of unique genomic features, such as intact or duplicated HSE binding sites, or additional motifs that may provide binding sites for other transcription factors, we conducted a comparative promoter analysis. We obtained the promoter regions (defined as 1500 bp upstream of the transcription start site) of key inducible heat shock proteins (HSPA1A, HSPA1B, HSP90AA1, DNAJA1, and DNAJB1) from humans (Hs), mice (Mm), and cave nectar bat (Es). Our comparative promoter qualitative analysis revealed that the HSEs across all studied genes are intact, with no evidence of promoter duplication (Supplementary Fig. 7). Additionally, analysis indicated no distinct differences within the cis-regulatory promoter regions among the species (Supplementary Fig. 7). This suggests that the observed high basal HSP expression in bats is not due to alterations in the cis-regulator canonical promoter elements.
Furthermore, in our attempt to directly assay the master regulator of heat shock response - HSF1, we found no suitable cross-reactive commercial antibodies for bat HSF1. To overcome this limitation and elucidate HSF1’s role in contributing to the elevated basal expression of heat shock-related genes in an antibody-independent manner, we generated CRISPR knockout (KO) bat cell lines lacking HSF1 (Supplementary Fig. 8A-B). This approach aimed to address two questions: (1) Does HSF1 drive the heat-induced expression of HSPs in bat cells as a master regulator? (2) Is HSF1 responsible for the elevated baseline expression of HSPs in bat cells? Our CRISPR HSF1-KO results indicate that heat shock (HS) induces the expression of HSPA1A and HSP90AA1 in wild-type (WT) cells, but not in HSF1-KO cells, confirming HSF1’s role as the master regulator of the heat shock response (Supplementary Fig. 8C, D). However, both HSF1-KO and WT cells displayed similar baseline expression levels of HSPA1A and HSP90AA1, suggesting that HSF1 is not responsible for the elevated basal levels of HSPs observed in bat cells in vitro (Supplementary Fig. 8 C-D).
High basal HSP expression is a shared characteristic in bats
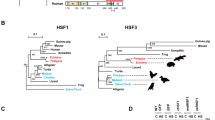
The Chiroptera order is highly diverse, encompassing nearly 1,400 species with varied classifications and ecological adaptations. This study focused on E. spelaea, a nectarivorous bat from the Pteropodidae family within the Yinpterochiroptera sub-order. To bolster the robustness of our findings, we expanded our analysis to include a wide range of bat species spanning across both sub-orders (Yinpterochiroptera and Yangochiroptera), multiple families (Pteropodidae, Rhinolophidae, Megadermatidae, and Vespertilionidae) and varied diets (nectarivorous, frugivorous, and insectivorous) (Fig. 6A). The additional bats that were sampled exhibited considerable variation in their morphology and characteristics (Fig. 6B). The enhanced physiological protection coupled with the non-canonical heat shock response in bats are presumably attributed to the elevated basal expression of heat shock proteins. To test this hypothesis, we measured the in vivo baseline protein levels of HSP70 and HSP90 using western blot analysis of tissues from the sampled bats. Our findings demonstrate that high basal expression of these heat shock proteins is not limited to a single species or family but is indeed a shared characteristic in bats, spanning diverse taxonomic classifications and dietary adaptations (Fig. 6C) in the Chiroptera order.
A Taxonomic tree generated from phyloTv2 with supplemented information of diet, family, sub-order and order from varying species of bats - Eonycteris spelaea, Penthetor lucasi, Cynopterus brachyotis, Hipposideros bicolor, Rhinolophus refulgens, Rhinolophus trifoliatus, Megaderma spasma, Pipistrellus javanicus, Tylonycteris sp. and Myotis horsfieldii. The species of bat used in experimental heating study, Eonycteris spelaea, is highlighted in red. B Photos taken of bats in the phylogenetic tree in (A) highlighting diverse morphological variation in Chiroptera order. Eonycteris spelaea photo was taken during a routine health check and other bat photos were taken post-mortem. C Western blot of HSP70 and HSP90 in in vivo muscle tissue of bats with mice samples used in the study. The species of bat used in experimental heating study, Eonycteris spelaea, is highlighted in red.
Discussion
The heat shock response is an ancient and highly conserved defence mechanism that results in the rapid expression of molecular chaperones - HSPs, to buffer proteomic stress. Prior observations in the literature of bats being able to tolerate elevated temperatures3,5,6,7,8,9,10,11,12,13,14,15,16,17 prompted a further investigation of the biological response of bats to heat stress. We hypothesized that bats have evolved adaptations to the heat shock response to withstand this thermogenic insult during flight. In this study, we challenged in vivo colony bred cave nectar bats (E. spelaea) to heat stress to elucidate the comparative heat shock response against mice (M. musculus) which is a model organism that elicits a conserved HSR similar to other species. To our knowledge, this is the first study that characterized the dynamic physiological and molecular response to heat stimulus in bats.
Consistent with other studies42,43,45,49 our heat shock system elicited a canonical response to extreme heat in mice evidenced by increased corticosterone levels, histopathological signs of kidney damage, and rapid expression of HSPs related genes and pathways across several tissues. Classical inducible HSP genes and related co-chaperones (HSP90AA1, HSP90AB1,HSPA1A, HSPA1B, DNAJB1, AHA1,STIP1) were highly upregulated in mice muscle and lung tissue. In contrast, E. spelaea bats subjected to the same heat shock system demonstrated a remarkably stability in physiological and molecular responses. Transcriptional analysis revealed induction of HSP genes in HS mice results in activation of HSP to a peak similar to the relative expression found in baseline level in bats, and does not surpass this ‘ceiling’. Additionally, specific HSPs that are strongly induced in mice upon HS were observed to be already basally upregulated in bats. This highlights that the baseline HSPs of in vivo bats are constitutively activated at levels of a ‘heat-shocked’ state, negating the need for further activation. Our data also demonstrated that HSP genes can be induced in bats if the constitutive baseline is not elevated, as evidenced by the induction of DNAJB1 in bat lung during in vivo HS a major inducible isoform HSP that was not significantly upregulated at baseline in bats. Similarly, major stress inducible HSPs, HSPA1A and HSP90AA1 were observed to be induced by HS, when the high baseline expression level is reduced in primary lung fibroblast lines.
A key observation in this study is the limited systemic transcriptional response observed in E. spelaea when subjected to heat stress. Interestingly, heat stress has been linked to global downregulation of transcription via multiple mechanisms such as (1) chromatin histone modification50 (2) inhibition of Pol II paused-release into elongation50 (3) ncRNA which directly or indirectly function in post-transcriptional gene silencing51 and (4) transcription factor modulation whereby non-essential TFs are degraded and modified52. The resultant effect of this multifaceted phenomenon is observed in mice evidenced by the vast downregulated DEGs in the HS condition. Intriguingly, bats appear to be resistant to this transcriptional shutdown and continue to preserve their native transcriptional programme. Of note, extended analysis of other stress response pathways (hypoxia, UPR and oxidative stress) within our RNA-seq data indicated that only the target genes in the heat shock response pathway, and not those in other stress pathways, are baseline upregulated in bats. Taken together, constitutively ‘activated’ state of HSPs in bats might be sufficient to buffer severe heat stress, allowing the bat to preserve their transcriptional programme even during heat challenge.
The heat profile used in this heat shock in our study was sufficient to challenge the bat response to heat. Conventionally, a 1 h exposure to 42 °C is recognized as a standard heat shock challenge for in vivo studies32,34,53. In our experiments, this level of stimulus proved to be severe to mice, evidenced by stress hormone induction and pathological damage. Furthermore, the heating profile successfully elevated the core temperature of bats to levels comparable with those in mice.
The unique transcriptional HSR signature observed in bats is plausibly due to an evolutionary adaptation and not an artifact from heat acclimatisation in bats. This is observed from multiple studies that characterized the in vivo inducibility of HSPs between heat acclimatised and control unacclimatised rats. Heat acclimatisation of Rattus norvegicus for 30 days did increase basal HSP72 levels (corresponding to HSPA1A)54,55. Importantly, the inducibility of HSP72 (HSPA1A) from in vivo HS was preserved after heat acclimatisation and was in fact rapidly enhanced. This is unlike the case in bats whereby the baseline of bat HSPs are constitutively activated to a level similar to post-HS mice. Additionally, our findings demonstrate that high basal expression of these heat shock proteins is not limited to a single species or family but is indeed a shared phenotype in bats, spanning diverse taxonomic classifications and dietary adaptations, suggesting that enhanced physiological protection via elevated basal HSP expression is a shared characteristic across the entire Chiroptera order. The trait of elevated basal HSP likely evolved early in the evolutionary history in bats, that is possibly due to the metabolic stress from temperature induced changes from flight or metabolic stress1,2,3,4 that elicits a selection pressure56 that is hypothesized to result in a rewiring of the HSR as highlighted in this study. The constitutive activation of HSPs in bats could result in distinct regulatory dynamics post-heat shock, differing from conventional mammalian models. As part of this non-canonical response, the post-recovery drop in inducible HSP (HSPA1A, HSP90AA1) levels may reflect an alternative regulatory mechanism in bats as part of a negative feedback loop to prevent overactivation of HSP network, however more work is needed to validate this functional significance. Overall, our work highlights the underappreciated diversity of the mammalian HSR, and it is plausible that other mammals with high environmental or metabolically derived heat exposure also exhibit non-canonical HSRs, and thus more systems levels studies on the HSR in non-model esoteric species could provide a glimpse into the rich diversity of the HSR wiring.
In the “canonical” HSR, HSF1 is inactive under baseline conditions and only activates heat shock proteins (HSPs) in response to stress18,19. In bats, however, we observe a ‘non-canonical’ HSR characterized by constitutive upregulation of HSPs, enhanced transcriptional stability under stress and delayed transcriptional activity post-stress. We attempted to dissect the mechanism through in silico genomic promoter analysis and HSF1-KO CRISPR lines. However, the promoter analysis did not indicate an enrichment of HSE/TF binding elements in cis-promoter regions. Using HSF1-KO CRISPR bat lines, we demonstrate that HSF1 is required for the induction of HSP target genes but not for the baseline upregulation of HSPs. Elevated basal levels of HSPs may be potentiated from upstream modifications beyond the cis-promoter regions, trans-promoter elements enhancing HSP expression, or hyperactive transcription factors other than HSF1. Systemic soluble factors, or complex tissue-specific interactions may also contribute to epigenomic reprogramming in bats, leading to high basal HSP expression.
Our previous work demonstrated that pteropodid bat cell lines exhibit elevated basal levels of heat shock proteins (HSPs), conferring protective effects against stress in vitro22. In the current study, we demonstrate that in vivo bat tissues express significantly higher levels of HSPs even compared to in vitro cell lines. We further demonstrate that this upregulation of HSPs in bat tissues applies to a larger cohort of HSPs – particularly major inducible HSP isoforms HSP90AA1 and HSPA1A - than was previously identified, via improvements made to the assembly and annotation of the E. spelaea genome by our group. Lastly, we provide novel insights into the dynamic in vivo heat shock response to heat stress, revealing distinct differences in the response of E. spelaea compared to the canonical heat stress response observed in the model organism M. musculus.
This study is limited in its ability to generalize the distinct heat shock response observed in E. spelaea to all species of bats. Expanding this study to other bat species remains logistically challenging due to the scarcity of captive colonies and the diversity of bats in the Chiroptera order. However, we demonstrate that high basal expression of HSPs in bat tissues is observed in representative bats from diverse lineages within the Chiroptera clade.
Recent studies have highlighted the remarkable biology of bats, particularly in their immune responses, healthy aging and resistance to cancer57,58,59,60,61,62,63. These traits are hypothesized to have evolved under the unique selective pressures of flight. Our current findings demonstrate a distinctive heat shock response in E. spelaea bats with a ‘rewired’ non-canonical heat shock response when compared with the conventional model organism, M. musculus mouse. This work also emphasizes the unique biology of bats, suggesting their potential as valuable model organisms in the heat shock field. Notably, the heat shock response is a highly conserved defence mechanism, implicated in numerous biological processes and human diseases. Further research will broaden our understanding of mammalian heat shock response diversity and may inform the development of innovative therapeutic strategies in biomedical research.
Methods
In vivo animal heat shock
Eonytceris spelaea (Es), cave nectar bats were obtained from a local captive-breeding colony and handled according to protocols approved by the SingHealth Institutional Animal Use and Ethics Committee (IACUC number 2015/SHS/1088 and 2020/SHS/1582). Mus musculus, house mice were either purchased from InVivos or bred in-house (IACUC number 2022/SHS/1707) and acclimatised for a week prior to experiment. All experiments were performed in accordance with relevant guidelines and regulations. Both animals were age matched when the species are characterized to be sexually mature, 6–10 weeks for mice and 12–18 months for bats in at least biological triplicates with a mix of both genders (Supplementary Table 2). Experiments were performed strictly from 0800 – 1300 h to control for diurnal rhythm in both nocturnal animals. Animals were randomly selected for each experimental group. Prior to heat stress, the animal are individually placed in a cage and acclimatised for an hour with ad libitum food and water. Subsequently, the animal was transferred to a preheated cage and placed in a heating chamber for specified heating duration (15, 30, 45 or 60 mins) at 42 °C without food and water. During the heat shock procedure, the carbon dioxide and oxygen levels were constantly monitored to be at a safe level with RS PRO DT-326 and SMART SENSOR Oxygen Meter respectively. At the end of the heat shock treatment, the animals were rested for 4 h at RT with ad libitum food and water. Throughout the experiment, animals were monitored periodically for lethality or severe clinical signs. Animals were scarified at 3 time points: the baseline of animal after acclimatisation (Ctl), after 1 h of heat shock challenge (HS) or 1 h of heat shock followed by rest (H + R). The core temperature of the animals were measured via a rectal probe BIO-TK8851 (BIO-BRET3) with at least 2 cm depth. At indicated end-points, animals were sedated with isoflurane (4-5% dosage, inhalation via nose cone for 3–5 min), bled via cardiac puncture, and then euthanized by intracardiac delivery of sodium pentobarbitone (85 mg/kg, Valabarb Euthanasia Solution). Animal sacrifice was performed by a trained SEMC/NLARF Veterinarian. Tissues were stored in either TRIzol, Neutral Buffered Formalin or snap frozen at -80 °C for RNA, histology and protein work respectively. Plasma was obtained from total blood by centrifuging at 500 g for 5 min, followed by an additional centrifugation of the cell free fraction at 3000 g for 5 min to obtain the cell free supernatant and stored at −80 °C. All work was done with the ethics approval by the SingHealth Institutional Animal Use and Ethics Committee (IACUC number 2022/SHS/1747). We have complied with all relevant ethical regulations for animal use.
Histology and glucocorticoid characterization
Kidneys that were formalin fixed were embedded in paraffin. Slices were stained with hematoxylin and eosin (H&E). Area of renal tubular degeneration is characterized as cytoplasmic tinctorial changes (hypereosinophilia) and nuclear pyknosis by an independent veterinarian pathologist. Glucocorticoid levels were assayed on plasma samples from animals. Plasma corticosterone levels were analysed using a species independent corticosterone chemiluminescent immunoassay kit in accordance with the manufacturer’s instructions (Arbor AssaysTM K014-H1, Ann Arbor, MI, USA).
Immunoblot
Bat samples from diverse species were a kind donation from National Parks Board (NParks) (Singapore). Snap frozen tissues were placed in RIPA buffer (ThermoScientific™ 89900) with protease inhibitors (Roche) and homogenized with lysing matrix Y tubes (MP 6960500) using tissue homogenizer (FastPrep-24TM, M.P. Biomedical, LLC, Santa Ana California, USA). Proteins were solubilized in 1% SDS and separated on 10% SDS-PAGE gels and transferred onto PVDF membranes (Milipore). Protein loading were normalized with BCA assay (ThermoScientific™). Membranes were blocked with 5% BSA and probed with anti-Hsp90 (1:1000)(F-8, #sc-13119; Santacruz), anti-Hsp70 (1:5000) (3a3, #ab5439; Abcam) or anti-GAPDH (1:1000) (MA5-15738; Invitrogen) for 1 h at room temperature. After washing, the membrane was incubated with goat anti-mouse IgG-HRP (HAF007 ; R&D Systems) for 1 h. Membranes were developed using Amersham ECL Prime Western blotting detection reagent (GE Healthcare) and signals were detected with a ChemiDoc MP Imaging System (Bio-Rad).
Real-time qPCR
Organs previously stored in TRIzol were lysed with lysing matrix Y tubes (MP 6960500) using a tissue homogenizer (FastPrep-24TM, M.P. Biomedical, LLC). Subsequently, the TRIzol was processed as per manufacturer’s protocol (Invitrogen). Briefly, chloroform was added and the RNA in the aqueous phase was precipitated using isopropanol. The resultant RNA was washed with 70% ethanol and quantified using nanodrop. Complementary DNA (cDNA) from tissue and cells was made using QuantiTect® Reverse Transcription Kit (QIAGEN). Quantitative PCR (qPCR) was performed to determine mRNA levels of different HSP genes. Reactions were setup using the SensiFASTTM SYBR No-ROX Kit (Bioline) and assays were run on the CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad) under the following cycling condition: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 57 °C for 15 s and 72 °C for 30 s, and ending with a melt profile analysis. Relative expression of the targeted gene was determined by 2(−Δ Ct) and fold change was determined by 2(−ΔΔ Ct) relative to housekeeping gene SNRPD3. qPCR primers for HSP90AA1, HSPA1A and SNRPD3 genes are listed in Supplementary Table 3. SNRPD3 has been shown to be an appropriate housekeeping gene in multiple species and across tissue and treatment.
Cell culture and in vitro heat shock protocol
Es primary bat cell lines were generated by digestion of minced tissues with 800 U/mL collagenase IV (Worthington) for an hour at 37 °C, explanted on plate and cultured with Dulbecco’s modified Eagle medium (DMEM), low glucose. All media were supplemented with 10% (v/v) foetal bovine serum (FBS) and 1X Antibiotic-Antimycotic. Cells were maintained at 37 °C and 5% CO2 and 90% humidity. Cells were routinely passaged every 4 days, or when cells were confluent. In vitro heat shock was performed by seeding 1.5 ×105 cells on 24 well plate overnight and subsequently submerging the plate in a water bath set to 42 °C for 1 h. The temperature of the water bath was independently measured with an external thermometer.
Es HSF1 KO CRISPR
Es HSF1 sequences were extracted from in-house Es genome, visualized using integrative genome browser (IGV) and the conserved exon across all isoforms was selected for CRISPR targeting. Guide RNA (gRNA) targets were identified using geneious prime programme. Three gRNA sequence was selected based on the metric of score, specificity and localization in close proximity to generate a multibase in-del large deletion (Supplementary Table 3, Supplementary Fig. 8A-B). The pSpCas9(BB)-2A-GFP (PX458) plasmid was used as a vector for the current study following previously published protocol64. EsFib, which are SV40 immortalized nasal fibroblast cell lines from E. spelaea are seeded on 6-well plates at a density of 2.5 × 105 and was transfected with 2.5 μg of plasmid using FuGENE® 6 (Promega). 48 h after transfection, live GFP+ cells were sorted using FACSAria III (BD Biosciences, San Jose, CA) into a 96-well plate at a concentration of 1 cell/well. Two weeks later, the single colony cells were processed using the QuickExtract solution (Epicentre, Madison, WI, USA) and validated via genomic DNA sanger sequencing. Primers used for the PCR was designed to span across the location spanning gRNA. PCR reaction was performed using reagent GoTaq® (Promega).
RNA sequencing and bioinformatics analysis
RNA from samples was extracted from TRIzol per manufacturer’s recommendation (Invitrogen). rRNA and library preparation was done by NovogeneAIT Genomics Singapore Pte Ltd. Briefly, ribosomal RNA was removed by TIANSeq rRNA Depletion Kit (Cat.No.NR101-T1) and sequencing libraries were generated using Fast RNA-seq Lib Prep Kit V2 (RK20306) following manufacturer’s recommendations. They were then sequenced with NovaSeq 6000. FastQC was used to determine the quality of the samples. Alignment was done using STAR (version 2.7)65 to mice genome of GRCm39 or our in-house bat genome (https://doi.org/10.5281/zenodo.15099109) respectively. DEG Counts were generated using featureCounts from Rsubread (version 2.8.2). filterByExpr was used to filter out genes with low counts based on each organ with min.count = 4 and min.total.count = 10. Differentially expressed genes (DEG) analysis was performed using EdgeR (version 3.42.4)66 pipeline and defined as significantly enriched with the cutoff of fold-change greater than 2 and Benjamini-Hochberg corrected p-value less than 0.05. DEG analysis between species was done by re-aligning reads to a mice-centric genome annotation that prioritises mice gene isoforms, prefiltering and dropping genes that have less than minimum 10 total count and inner-join of dataframe to keep the intersect of common genes between both species followed by the aforementioned EdgeR pipeline. ncRNA was omitted from further analysis. Global volcano plots, bar plots, dot plots and heatmaps were constructed with the ggplot2 (version 3.4.4). Venn diagram was constructed using ggvenn (version 0.1.10). Individual volcano plots were constructed with EnhancedVolcano (version 1.18.0). GSEA analysis was done using clusterProfiler (version 4.8.3) against msigdbr with a FDR-cutoff of 0.1. Transcription factor analysis was performed via ChEA3 using the ENCODE database67 and plotted with using ggplot2. Heatmap signatures of HSP was plotted the mean of log(nCounts+1) obtained from Deseq2 (version 1.40.2)68. The module signature is calculated from the arithmetic mean of log(nCounts+1) for all genes within the HSP family class. HSP family classification of genes derived from GeneCards or NCBI literature search. Phylogenetic tree of bats was constructed using taxonomic information using phylotV2 - https://phylot.biobyte.de/.
Promoter analysis
Promoter sequences were downloaded from National Center for Biotechnology Information (NCBI) by obtaining 1500 bp upstream of the transcriptional start site (TSS) from the genes of HSPA1A, HSPA1B, HSP90AA1, DNAJA1 and DNAJB1 from Homo sapiens (Hs) and Mus musculus (Mm). Additionally, promoter sequences for the corresponding genes from Eonycteris spelaea (Es) were retrieved from our in-house genome database. To identify potential transcription factor binding sites (TFBS), motif scanning was performed using the MEME Suite. The promoter sequences were analyzed using the MEME function from MEME Suite69, with the following parameters: -dna -revcomp -nmotifs 10 -evt 1 -mod anr -objfun classic. Subsequent identification and annotation of transcription factor binding sites (TFBS) were conducted using TOMTOM, a motif comparison tool. The MEME-derived motifs were compared against the TFBSshape_JASPAR database using default parameters: -verbosity 1 -min-overlap 5 -dist pearson -evalue -thresh 10.0.
Statistics and reproducibility
Statistical testing for temperature measurement and corticosterone assay was carried out using matched and unmatched one-way ANOVA with Tukey’s multiple comparison test respectively. qPCR statistical testing was carried out using unpaired two-tailed t test. In vivo replicates are defined by biological replicates from independent animals. In vitro replicates are defined by cell lines from different passage. Statistical analysis done in bioinformatic analysis are described further in figure legends. All statistics were calculated using GraphPad Prism 9 or ggsignif in R (“ * ”indicates p < 0.05, “ ** “ indicates p < 0.01, “ *** “ indicates p < 0.001).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data generated in this study has been deposited in NCBI’s Gene Expression Omnibus and accessible through GEO Series accession number GSE261939. The E. spelaea genome assembly and annotation used in this study is available via the Sequence Read Archive (SRA) BioSample ID: SAMN4835912 and has been uploaded to: https://doi.org/10.5281/zenodo.15099109. This paper does not report original code. All source data is listed in figure legends and can be found in either indicated Supplementary Table or Supplementary Data 1-5.
References
Irving, A. T., Ahn, M., Goh, G., Anderson, D. E. & Wang, L. F. Lessons from the host defences of bats, a unique viral reservoir. Nature 589, 363–370 (2021).
Alexander, R. M. The merits and implications of travel by swimming, flight and running for animals of different sizes. Integr. Comp. Biol. 42, 1060–1064 (2002).
Thomas, S. P. & Suthers, R. A. The physiology and energetics of bat flight. J. Exp. Biol. 57, 317–335 (1972).
O’Mara, M. T., et al (2017). Cyclic bouts of extreme bradycardia counteract the high metabolism of frugivorous bats. Elife 6. https://doi.org/10.7554/eLife.26686.
Carpenter, R. E. Flight Physiology of Intermediate-Sized Fruit Bats (Pteropodidae). J. Exp. Biol. 120, 79–103 (1986).
Burbank, R. C. & Young, J. Z. Temperature changes and winter sleep of bats. J. Physiol. 82, 459–467 (1934).
Morrison, P. Body Temperatures in Some Australian Mammals. I. Chiroptera. Biol. Bull. 116, 484–497 (1959).
Voigt, C. C. & Lewanzik, D. Trapped in the darkness of the night: thermal and energetic constraints of daylight flight in bats. Proc. Biol. Sci. 278, 2311–2317 (2011).
Morrison, P. & McNab, B. K. Temperature regulation in some Brazilian phyllostomid bats. Comp. Biochem. Physiol. 21, 207–221 (1967).
Roverud, R. C. & Chappell, M. A. Energetic and thermoregulatory aspects of clustering behavior in the neotropical bat Noctilio albiventris. Physiological Zool. 64, 1527–1541 (1991).
Reeder, W. G. & Cowles, R. B. Aspects of thermoregulation in bats. J. Mammal. 32, 389–403 (1951).
Willis, C. & Brigham, R. Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J. Comp. Physiol. B 173, 379–389 (2003).
Bronrier, G. N., Maloney, S. K. & Buffenstein, R. Survival tactics within thermally-challenging roosts: heat tolerance and cold sensitivity in the Angolan free-tailed bat, Mops condylurus. South Afr. J. Zool. 34, 1–10 (1999).
Herreid, C. Temperature regulation and metabolism in Mexican freetail bats. Science 142, 1573–1574 (1963).
Leitner, P. Body temperature, oxygen consumption, heart rate and shivering in the California mastiff bat, Eumops perotis. Comp. Biochem. Physiol. 19, 431–443 (1966).
O’Farrell, M. J. & Bradley, W. G. Comparative thermal relationships of flight for some bats in the southwestern United States. Comp. Biochem. Physiol. Part A: Physiol. 58, 223–227 (1977).
Rissmann, M., et al. Baseline of Physiological Body Temperature and Hematological Parameters in Captive Rousettus aegyptiacus and Eidolon helvum Fruit Bats. Frontiers in Physiology 13. https://doi.org/10.3389/fphys.2022.910157. (2022).
Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: life on the verge of death. Mol. Cell 40, 253–266 (2010).
De Maio, A. The heat-shock response. N. Horiz. 3, 198–207 (1995).
Akerfelt, M., Morimoto, R. I. & Sistonen, L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Gomez-Pastor, R., Burchfiel, E. T. & Thiele, D. J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 4–19 (2018).
Chionh, Y. T. et al. High basal heat-shock protein expression in bats confers resistance to cellular heat/oxidative stress. Cell Stress Chaperones 24, 835–849 (2019).
Maughan, R. J., Leiper, J. B. & Thompson, J. Rectal temperature after marathon running. Br. J. Sports Med. 19, 192–195 (1985).
Saltin, B. & Hermansen, L. Esophageal, rectal, and muscle temperature during exercise. J. Appl. Physiol. 21, 1757–1762 (1966).
Buono, M. J., Holloway, B., Levine, A., Rasmussen, C. & Kolkhorst, F. W. Effect of air temperature on the rectal temperature gradient at rest and during exercise. Int J. Physiol. Pathophysiol. Pharm. 6, 61–65 (2014).
Morris, C., Atkinson, G., Drust, B., Marrin, K. & Gregson, W. Human Core Temperature Responses during Exercise and Subsequent Recovery: An Important Interaction between Diurnal Variation and Measurement Site. Chronobiol. Int. 26, 560–575 (2009).
Scott, C. M., Marlin, D. J., and Schroter, R. C. Thermoregulatory strategies during short-term exercise at different intensities. Equine Vet J Suppl, 356-361. (1999).
Rhodes, J. S., Koteja, P., Swallow, J. G., Carter, P. A. & Garland, T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. J. Therm. Biol. 25, 391–400 (2000).
Yasumoto, Y., Nakao, R. & Oishi, K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PloS one 10, e0116476 (2015).
Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment (Cambridge University Press). (1997).
Bondarenco, A., Kortner, G. & Geiser, F. Hot bats: extreme thermal tolerance in a desert heat wave. Naturwissenschaften 101, 679–685 (2014).
Shen, K. H., Lin, C. H., Chang, H. K., Chen, W. C. & Chen, S. H. Premarin can act via estrogen receptors to rescue mice from heatstroke-induced lethality. Shock 30, 668–674 (2008).
Chatterjee, S. et al. Arginine metabolic pathways determine its therapeutic benefit in experimental heatstroke: Role of Th1/Th2 cytokine balance. Nitric Oxide 15, 408–416 (2006).
Tai, P. A. et al. Reduction of ischemic and oxidative damage to the hypothalamus by hyperbaric oxygen in heatstroke mice. J. Biomed. Biotechnol. 2010, 609526 (2010).
Foo, R. et al. Establishment of a Captive Cave Nectar Bat (Eonycteris spelaea) Breeding Colony in Singapore. J. Am. Assoc. Lab Anim. Sci. 61, 344–352 (2022).
Rizzoto, G., Boe-Hansen, G., Klein, C., Thundathil, J. C. & Kastelic, J. P. Acute mild heat stress alters gene expression in testes and reduces sperm quality in mice. Theriogenology 158, 375–381 (2020).
Leon, L. R., DuBose, D. A. & Mason, C. W. Heat stress induces a biphasic thermoregulatory response in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R197–R204 (2005).
Islam, A. et al. Heat exposure induces tissue stress in heat-intolerant, but not heat-tolerant, mice. Stress 16, 244–253 (2013).
Gong, S. et al. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10, e0117503 (2015).
Kelm, D. H., Popa-Lisseanu, A. G., Dehnhard, M. & Ibáñez, C. Non-invasive monitoring of stress hormones in the bat Eptesicus isabellinus – Do fecal glucocorticoid metabolite concentrations correlate with survival?. Gen. Comp. Endocrinol. 226, 27–35 (2016).
Klose, S. M., Smith, C. L., Denzel, A. J. & Kalko, E. K. V. Reproduction elevates the corticosterone stress response in common fruit bats. J. Comp. Physiol. A 192, 341–350 (2006).
Goto, H. et al. Heat acclimation ameliorated heat stress-induced acute kidney injury and prevented changes in kidney macrophages and fibrosis. Am. J. Physiol. Ren. Physiol. 323, F243–F254 (2022).
Leon, L. R., Blaha, M. D. & DuBose, D. A. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J. Appl Physiol. (1985) 100, 1400–1409 (2006).
Bittar, C. et al. Alphacoronavirus Detection in Lungs, Liver, and Intestines of Bats from Brazil. Microb. Ecol. 79, 203–212 (2020).
Neueder, A. et al. HSF1-dependent and -independent regulation of the mammalian in vivo heat shock response and its impairment in Huntington’s disease mouse models. Sci. Rep. 7, 12556 (2017).
Sawai, M., Ishikawa, Y., Ota, A. & Sakurai, H. The proto-oncogene JUN is a target of the heat shock transcription factor HSF1. FEBS J. 280, 6672–6680 (2013).
Petitjean, A., Hainaut, P. & Caron de Fromentel, C. TP63 gene in stress response and carcinogenesis: a broader role than expected. Bull. Cancer 93, E126–E135 (2006).
Jacobs, K. et al. Stress-triggered hematopoietic stem cell proliferation relies on PrimPol-mediated repriming. Mol. Cell 82, 4176–4188.e4178 (2022).
Miyamoto, K. et al. A novel mouse model of heatstroke accounting for ambient temperature and relative humidity. J. Intensive Care 9, 35 (2021).
Vihervaara, A., Duarte, F. M. & Lis, J. T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet 19, 385–397 (2018).
Malecová, B. & Morris, K. V. Transcriptional gene silencing through epigenetic changes mediated by non-coding RNAs. Curr. Opin. Mol. Ther. 12, 214–222 (2010).
Guerra, D., et al. Post-transcriptional and post-translational regulations of drought and heat response in plants: a spider’s web of mechanisms. Frontiers in Plant Science 6. https://doi.org/10.3389/fpls.2015.00057. (2015).
Chen, Z.-C., Wu, W.-S., Lin, M.-T. & Hsu, C.-C. Protective effect of transgenic expression of porcine heat shock protein 70 on hypothalamic ischemic and oxidative damage in a mouse model of heatstroke. BMC Neurosci. 10, 111 (2009).
Maloyan, A. & Horowitz, M. beta-Adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. J. Appl Physiol. (1985) 93, 107–115 (2002).
Maloyan, A., Palmon, A. & Horowitz, M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am. J. Physiol. 276, R1506–R1515 (1999).
Matsuda, Y. & Makino, T. Comparative genomics reveals convergent signals associated with the high metabolism and longevity in birds and bats. Proc. R. Soc. B: Biol. Sci. 291, 20241068 (2024).
Ahn, M. et al. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 4, 789–799 (2019).
Banerjee, A., Rapin, N., Bollinger, T. & Misra, V. Lack of inflammatory gene expression in bats: a unique role for a transcription repressor. Sci. Rep. 7, 2232 (2017).
Gorbunova, V., Seluanov, A. & Kennedy, B. K. The World Goes Bats: Living Longer and Tolerating Viruses. Cell Metab. 32, 31–43 (2020).
Koh, J. et al. ABCB1 protects bat cells from DNA damage induced by genotoxic compounds. Nat. Commun. 10, 2820 (2019).
Wilkinson, G. S. & Adams, D. M. Recurrent evolution of extreme longevity in bats. Biol. Lett. 15, 20180860 (2019).
Hua, R. et al. Experimental evidence for cancer resistance in a bat species. Nat. Commun. 15, 1401 (2024).
Gamage, A. M. et al. Single-cell transcriptome analysis of the in vivo response to viral infection in the cave nectar bat Eonycteris spelaea. Immunity 55, 2187–2205.e2185 (2022).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Keenan, A. B. et al. ChEA3: transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 47, W212–W224 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Bailey, T. L., Johnson, J., Grant, C. E. & Noble, W. S. The MEME Suite. Nucleic Acids Res. 43, W39–W49 (2015).
Acknowledgements
We thank Professor Michael Hiller and team for the E. spelaea genome assembly and annotation. We thank Dr. Edgar M. Pena, Rommel E. Yroy., Ranjit Kumar S/O Patmanathan and the rest of the veterinary team at SingHealth Experimental Medicine Centre (SEMC) for technical expertise and assistance in maintaining the E. spelaea colony and in vivo experiments. We would also like to thank SingHealth Duke-NUS Advanced Molecular Pathology Laboratory for histology services performed. Finally, we would like to thank Dr Malcolm Soh and the rest of the team at National Parks Board (NParks) Singapore for capturing and processing of additional bat species used in the study. This study was funded by a grant from Singapore Ministry of Education (MOE2019-T2-2-130).
Author information
Authors and Affiliations
Contributions
L.J.W.T., A.M.G. and L-F.W. conceptualized the project. L.J.W.T., A.M.G., W.L.N, W.R.S, R.F. performed the experiments. L.J.W.T. performed bioinformatics analysis. L.J.W.T., A.M.G., R.F., N.Y.S., W.O.Y.C., S.C., V.C.W.C., L.B.L., T.C.W. and L-F.W contributed to the experimental planning and methodology. L.J.W.T. and L-F.W. analysed the data. L.J.W.T. and L-F.W. wrote the manuscript. All authors contributed to reviewing and editing the manuscript. L-F.W. acquired funding and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, L.J.W., Gamage, A.M., Ng, W.L. et al. Heat stress response in the cave nectar bat Eonycteris spelaea differs from that of Mus musculus. Commun Biol 8, 811 (2025). https://doi.org/10.1038/s42003-025-08224-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08224-3