Abstract
Arthropods have evolved advanced metabolic detoxification pathways that have helped them adapt to complex stresses induced by plant secondary metabolites and synthetic pesticides. Nonetheless, how xenobiotics induce the suite of intracellular metabolic changes important for detoxification in arthropods remains unexplored. In this study, we found that the ancient oxidative stress signaling pathway has adapted to transmit the detoxification signal in T. cinnabarinus, where accumulation of reactive oxygen species (ROS) plays a crucial role in initiating detoxification metabolism. Acaricides exposure led to ROS accumulation, resulting in the activation of Forkhead box O (FOXO) that in turn upregulates the expression of downstream xenobiotic sensors, including CncC, AhR, and HR96. These xenobiotic sensors then upregulate the expression of downstream detoxification genes, helping mites metabolically detoxify acaricides. This study reveals a cascade-amplifying mechanism for broad-spectrum detoxification in T. cinnabarinus that is initiated through ROS–FOXO triggering broad-spectrum detoxification of xenobiotics. These findings further elucidate the adaptive evolution of arthropods and suggest new avenues for novel pesticides and pest control.
Similar content being viewed by others
Introduction
Tetranychus cinnabarinus, commonly referred to as the carmine spider mite, is an acarid species within the family Tetranychidae, subclass Acari, of the class Arachnida1. This mite is widely distributed and recognized for its remarkable adaptability to a broad spectrum of host plants, including over 150 crop species. T. cinnabarinus shares numerous ecological characteristics with its closely related counterpart, Tetranychus urticae. Both species are notorious for their ability to develop resistance rapidly to various classes of acaricides. The extensive host range of carmine spider mite, coupled with its rapid adaptation to chemical control methods, makes it a key model organism for investigating the evolutionary mechanisms of detoxification in arthropods2. The induction of detoxification enzymes, such as cytochrome P450s, glutathione-S-transferase (GSTs), and choline/carboxylesterases (CCEs), represents an adaptive response to both natural and synthetic xenobiotics3,4,5. This induction not only facilitates xenobiotic detoxification but also contributes to the development of metabolic resistance3,4. Additionally, different selective pressures have resulted in an expansion of gene families related to digestion, detoxification and xenobiotic transport in spider mites compared to other arthropods6.
The expression of detoxification genes is regulated by several transcription factor superfamilies known as xenobiotic sensors, including the basic leucine zipper (bZIP) family, the basic helix–loop–helix family of Per/ARNT (aryl hydrocarbon receptor nuclear translocator)/Sim, and the nuclear receptor superfamily7,8,9,10. The roles of the aryl hydrocarbon receptor (AhR) and ARNT as transcriptional regulators of detoxification enzymes have been validated on the promoters of the CYP6B subfamily11. Cap “n” collar isoform C (CncC), is a member of bZIP family, shows that loss of CncC function in adult Drosophila melanogaster led to the attenuation of induction in CYPs, GSTs, and UGTs by xenobiotics12,13. Hormone receptor 96 (HR96) has been shown to be directly activated by the acaricide cyflumetofen in T. cinnabarinus, subsequently regulating the expression of the detoxification gene TcGSTm29.
Oxidative stress results from an imbalance between cellular oxidants and antioxidants, leading to the generation of large amounts of oxygen intermediates, such as superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl free radicals (HO•), collectively known as reactive oxygen species (ROS)14,15. Excess ROS production is typically the result of various environmental stresses, such as abnormal temperature, ultraviolet light, pathogen infection, or insecticide exposure. Organisms have evolved adaptive mechanisms to cope with the harmful effects of excessive ROS16. Forkhead box O (FOXO), a transcription factor in the forkhead superfamily, is pivotal to the oxidative stress response17. FOXO responds to intracellular and extracellular stimuli, including oxidative stress, as its direct downstream targets include antioxidant enzymes. For instance, in D. melanogaster neurons, FOXO upregulates Jafrac1, the homolog of human peroxiredoxin 2, to significantly reduce ROS levels and restore mitochondrial function in response to paraquat-induced oxidative stress18. FOXO also responds to ROS through phosphorylation. For example, treatment of rat cells and human kidney-derived HEK293T cells with H2O2 results in low levels of ROS, which stimulates the c-Jun N-terminal kinase signaling pathway to phosphorylate FOXO. This process enhances the nuclear localization of FOXO in these cells, increasing its transcriptional activity on downstream genes19. However, much remains unknown about how the ROS–FOXO pathway responds to xenobiotic stress to trigger detoxification metabolism in arthropods.
In this study, we found that acaricides induce ROS accumulation in T. cinnabarinus. The accumulation of ROS further activates the transcription factor FOXO, which in turn promotes the expression of downstream xenobiotic sensors induced by FOXO. By investigating the detoxification metabolism function of downstream genes regulated by the xenobiotic sensors CncC, AhR, and HR96 in T. cinnabarinus, we delineated the molecular regulation pathway for broad-spectrum detoxification in response to acaricide stress. In summary, our research presents a framework that allows a deep understanding of the mechanisms by which arthropods initiate broad-spectrum inducible expression of detoxification genes.
Results
Acaricide-induced stress triggered ROS accumulation and increased antioxidant enzyme activity in T. cinnabarinus
To assess oxidative stress following acaricide exposure, we measured the content of O2•− and the activity of antioxidant enzymes in T. cinnabarinus. The results showed significant increases in O2•− content at 12 and 24 h following treatment with fenpropathrin, cyflumetofen, and chlorpyrifos, respectively. At 24 h post-treatment, the content of O2•− exhibited increases of 1.70-, 2.10-, and 1.97-fold over the control, respectively (Fig. 1a). The O2•− content in female adult mites treated with fenpropathrin, cyflumetofen, and chlorpyrifos, was also directly observed through confocal microscopy following dihydroethidium (DHE) staining. The fluorescence intensity of DHE after 24 h of treatment with the three acaricides was significantly higher than that of the control (Fig. 1b, c). Moreover, the two-way ANOVA results indicate that both treatment time and acaricide type significantly affect the accumulation of O2•− content in T. cinnabarinus, and there is a significant interaction between the two factors (Fig. 1a, b). As an indirect measure of ROS levels, we characterized the activity of antioxidant enzymes, including catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD), in T. cinnabarinus. Significant increases of CAT, POD and SOD activities were detected after treatment with fenpropathrin, cyflumetofen, and chlorpyrifos, respectively (Supplementary Fig. 1). These results indicate that exposure to different acaricides can lead to in vivo ROS accumulation in T. cinnabarinus.
a The O2•− content after fenpropathrin, cyflumetofen and chlorpyrifos treatment in T. cinnabarinus. Acetone was used for the control treatment, and the data was normalized accordingly. Data was expressed as the mean, n = 3. b DHE fluorescence signal intensity in T. cinnabarinus after DHE staining. Acetone was used for the control treatment. Data was expressed as the mean, n = 17–34. In a, b lowercase letters indicate statistically significant differences at different time points following treatment with the same acaricide. In contrast, uppercase letters represent significant differences between different acaricides, as analyzed by two-way ANOVA (Tukey’s HSD), p < 0.05. The F value represents the result of the Fisher test. c T. cinnabarinus were sectioned after DHE staining, and images were obtained using a laser confocal microscope. The scale bar represents 50 μm. The O2•−-specific fluorescent probe is shown in red. In the figure, the deep red signal indicates a higher ROS level.
Changes in ROS levels altered acaricide sensitivity in T. cinnabarinus
To investigate the role of ROS in acaricide detoxification in T. cinnabarinus, we evaluated the viability of adult female mites under acaricide stress with ROS scavenging. We found that treatment with 2% N-acetylcysteine (NAC), a specific ROS scavenger, significantly reduced in vivo ROS levels with or without acaricide exposure (Fig. 2a, b). Furthermore, pretreatment with 2% NAC significantly reduced the LC50 of fenpropathrin from 2110 mg/L (95% CI, 1532–3399 mg/L) to 289 mg/L (95% CI, 188–493 mg/L) (Table 1). Similarly, the LC50 of cyflumetofen significantly decreased from 3.79 mg/L (95% CI, 3.29–4.32 mg/L) to 1.08 mg/L (95% CI, 0.96–1.21 mg/L), and that of chlorpyrifos significantly decreased from 305 mg/L (95% CI, 238–401 mg/L) to 83 mg/L (95% CI, 40–122 mg/L) (Table 1).
a ROS levels in T. cinnabarinus treated with sprayed NAC. b ROS levels in T. cinnabarinus when treated with cyflumetofen followed by sprayed NAC. In a,b the left figure indicates the measurement time point. P450s (c), GSTs (d), and CCEs (e) activities in T. cinnabarinus after fenpropathrin treatment. P450s (f), GSTs (g), and CCEs (h) activities in T. cinnabarinus after cyflumetofen treatment. P450s (i), GSTs (j), and CCEs (k) activities in T. cinnabarinus after chlorpyrifos treatment. In c–k tween 80 in water as a control and NAC as a treatment were used to pretreat adult female mites for 24 h before dividing the mites into two groups: one treated with acetone for 24 h and the other treated with acaricide for 24 h. l ROS levels in T. cinnabarinus after paraquat treatment. The control group was treated with acetone and used for normalization. Error bars represent the mean ± SE, n = 3. Lowercase letters represent statistically significant differences. One-way ANOVA (Tukey’s HSD) was used for data analysis, p < 0.05. m Acaricide sensitivity in T. cinnabarinus after 6 h of paraquat pretreatment. The Y-axis represents mortality rate. In a−k, m error bars represent mean ± SE, n = 3. Asterisks indicate a statistically significant difference analyzed by Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Next, we measured the activities of detoxification enzymes (i.e., P450s, GSTs, and CCEs), in the presence and absence of ROS scavenging. Direct treatment with NAC did not significantly affect the basal activities of P450s. However, fenpropathrin treatment significantly induced P450s activity when pretreated with Tween 80 in water (control), whereas the acaricide-induced P450s activation was significantly inhibited after pretreatment with 2% NAC (Fig. 2c). GSTs and CCEs activities followed trends similar to P450s (Fig. 2d, e). The activities of P450s, GSTs, and CCEs after cyflumetofen and chlorpyrifos treatments were consistent with those after fenpropathrin treatment (cyflumetofen: Fig. 2f–h; chlorpyrifos: Fig. 2i–k).
Conversely, we assessed changes in acaricide sensitivity in response to increased ROS levels using the ROS inducer paraquat, which significantly increased ROS levels after paraquat treatment in T. cinnabarinus (Fig. 2l). Following 6-hour pretreatment with paraquat, the mortality rate after acaricide treatment at LC30 and LC50 was significantly lower than in the control (pretreated with Tween-80 in water) (Fig. 2m). High temperatures similarly caused in vivo ROS accumulation. Pretreatment of T. cinnabarinus at 34 °C for 6 h significantly increased ROS levels to 1.51-fold compared to those pretreated at 26 °C (Supplementary Fig. 2). Following 34 °C pretreatment, the LC50 of fenpropathrin significantly increased from 2128 mg/L (95% CI, 1677–2639 mg/L) to 5508 mg/L (95% CI, 3806–8367 mg/L) (Table 2). Similarly, the LC50 for cyflumetofen significantly increased from 3.19 mg/L (95% CI, 2.71–3.77 mg/L) to 5.95 mg/L (95% CI, 4.94–7.75 mg/L), and that of chlorpyrifos significantly increased from 176 mg/L (95% CI, 116–232 mg/L) to 284 mg/L (95% CI, 209–356 mg/L) (Table 2). Our findings indicate that in vivo acaricide sensitivity is negatively correlated with ROS levels and that ROS mediates the activity of detoxification enzymes, altering acaricide sensitivity in T. cinnabarinus.
ROS accumulation stiulates FOXO activity
Studies in mammals have shown that ROS accumulation leads to FOXO overexpression and activation17, prompting us to explore the relationship between ROS accumulation and FOXO in T. cinnabarinus. Analysis of the T. cinnabarinus FOXO protein sequence revealed a full-length sequence with 616 amino acids, a molecular weight of 67.56 kDa and an isoelectric point of 6.28 (Supplementary Table 2). The protein sequence indicated the presence of a putative nuclear localization signal (NLS, K161–K188) immediately following the fork head domain (Fig. 3a). Phylogenetic tree analysis revealed that the FOXO protein is evolutionarily conserved and clustered with Arachnida (Supplementary Fig. 3).
a Structural analysis of the T. cinnabarinus FOXO protein. Forkhead denotes the DNA-binding domain, NLS represents a putative nuclear localization signal, and TAD refers to the transactivation domain. b qPCR analysis of FOXO mRNA expression in T. cinnabarinus pretreated with NAC after fenpropathrin treatment. c qPCR analysis of FOXO mRNA expression in T. cinnabarinus pretreated with NAC after cyflumetofen treatment. d qPCR analysis of FOXO mRNA expression in T. cinnabarinus pretreated with NAC after chlorpyrifos treatment. In b–d tween 80 in water as a control and NAC as a treatment were used to pretreat adult female mites for 24 h before dividing the mites into two groups: one treated with acetone for 24 h and the other treated with acaricide for 24 h. Error bars represent the mean ± SE, n = 3. Asterisks indicate a statistically significant difference analyzed by Student’s t-test. *p < 0.05. e qPCR analysis of FOXO mRNA expression in T. cinnabarinus after paraquat treatment. The control was treated with acetone and used for normalization. Error bars represent the mean ± SE, n = 3. Lowercase letters represent statistically significant differences, analyzed by one-way ANOVA (Tukey’s HSD), p < 0.05. f qPCR analysis of FOXO mRNA expression in T. cinnabarinus pretreated with NAC after paraquat treatment. Error bars represent the mean ± SE, n = 3. Asterisks indicate a statistically significant difference, as analyzed by Student’s t-test. *p < 0.05. g Western blot analysis of FOXO protein expression after paraquat treatment. α-tubulin was used as the loading control. h Western blot analysis of FOXO protein distribution after paraquat treatment. CK1 indicates the untreated group, CK2 indicates the 0.1% Tween 80 water treatment for 24 h, and paraquat indicates the paraquat treatment for 24 h. α-tubulin and histone H3 were used as cytoplasmic and nuclear loading controls, respectively. The values in g, h represent the ratio of the TcFOXO protein band density relative to tubulin or histone H3.
To investigate the relationship between ROS accumulation and FOXO expression, acaricide induction was executed in T. cinnabarinus. QPCR results showed that under normal conditions (without NAC pretreatment), the three acaricides significantly upregulated FOXO mRNA levels. In contrast, NAC pretreatment suppressed ROS levels and acaricides no longer induced FOXO mRNA expression (Fig. 3b–d). Similarly, FOXO mRNA expression was significantly upregulated in T. cinnabarinus after paraquat induction, and this upregulation was influenced by ROS levels (Fig. 3e, f). The result of Western blot analysis further indicated that FOXO protein levels in T. cinnabarinus increased after ROS accumulation (Fig. 3g). Aggregation of transcription factors in the cell nucleus is indicative of their activation20. Analysis of FOXO levels in the cytoplasm and nucleus of T. cinnabarinus before and after paraquat treatment showed that FOXO protein levels significantly increased in the nucleus after paraquat treatment (Fig. 3h), indicating that increased expression of FOXO following ROS accumulation as well as increase of FOXO protein level in the nucleus.
FOXO regulates the expression of xenobiotic sensors
To further study the role of FOXO in the detoxification pathways of T. cinnabarinus, we used RNAi to inhibit FOXO mRNA expression in the mites and determined the changes in their in vivo sensitivity to the acaricides fenpropathrin, cyflumetofen, and chlorpyrifos. After feeding with dsFOXO, the FOXO silencing efficiency in the SS strain was 52% ± 10% compared with the dsGFP control (Fig. 4a). Silencing FOXO FOXO significantly decreased the fenpropathrin LC50 from 2708 mg/L (95% CI, 1772–5715 mg/L) to 1274 mg/L (95% CI, 838–1958 mg/L) (Table 3). Similarly, the LC50 of cyflumetofen significantly decreased from 2.18 mg/L (95% CI, 1.84–2.53 mg/L) to 1.16 mg/L (95% CI, 0.99–1.34 mg/L), and that of chlorpyrifos significantly decreased from 227 mg/L (95% CI, 156–308 mg/L) to 57 mg/L (95% CI, 43–70 mg/L) (Table 3). These RNAi results suggest that decreased FOXO expression increased acaricide sensitivity in T. cinnabarinus, suggesting that FOXO may be involved in acaricide detoxification metabolism in T. cinnabarinus.
a qPCR and Western Blotting analysis of FOXO mRNA and protein expression after dsFOXO feeding. b qPCR analysis of CncC, AhR, and HR96 mRNA expression after FOXO silencing in T. cinnabarinus. In a and b, controls were fed dsGFP and used for normalization. Error bars represent the mean ± SE, n = 3. Asterisks indicate a statistically significant difference analyzed by Student’s t-test. *p < 0.05, **p < 0.01. c FOXO regulates CncC, AhR, and HR96 promoter activities. Fluc/Rluc represents firefly luciferase activity/Renilla luciferase activity. Error bars represent the mean ± SE, n = 3. Lowercase letters represent statistically significant differences, as analyzed by one-way ANOVA (Tukey’s HSD), p < 0.05. d The yeast one-hybrid assay was used to test FOXO binding to the promoters of CncC, AhR, and HR96.
FOXO functions primarily through transcription factor regulation21. Therefore, we investigated the effects of FOXO silencing on the expression of transcription factors CncC, AhR, and HR96 in T. cinnabarinus. The results indicated a simultaneous decrease in FOXO expression alongside reduced expression of CncC and AhR as well as five HR96 genes (TcHR96a, TcHR96b, TcHR96d, TcHR96f, and TcHR96g) (Fig. 4a, b). To determine whether FOXO directly regulates the expression of CncC, AhR, and five HR96 genes, we performed the dual luciferase reporter assay. The results indicated that FOXO overexpression significantly increased the promoter activities of CncC, AhR, TcHR96b, TcHR96d, TcHR96f, and TcHR96g, but had no significant effects on the promoter activity of TcHR96a (Fig. 4c). Additionally, a yeast one-hybrid (Y1H) assay demonstrated that FOXO interacts with the promoters CncC, AhR, and TcHR96f (TcHR96f was selected as the representative for HR96 gene due to its maximum promoter activity with FOXO) (Fig. 4d). These findings indicate that FOXO directly regulates the expression of xenobiotic sensor transcription factors, thereby mediating acaricide detoxification in T. cinnabarinus.
DAP-seq elucidates detoxification genes regulated by xenobiotic sensors
A growing body of evidence indicates that the xenobiotic sensors CncC, AhR, and HR96 play crucial roles in detoxification metabolism and the development of insecticide resistance in arthropods5. However, the specific detoxification genes regulated by these sensors remain unclear. To address this, we employed RNA interference (RNAi) to investigate the role of CncC, AhR, and TcHR96f in detoxification in T. cinnabarinus. The results showed that decreased expression of CncC and AhR (to 42% ± 5% and 71% ± 16%, respectively) (Fig. 5a, b) significantly increased the sensitivity of T. cinnabarinus to the three acaricides (Fig. 5d). However, RNAi-mediated silencing of TcHR96f (53% ± 13%) (Fig. 5c) significantly increased the sensitivity of T. cinnabarinus to cyflumetofen and chlorpyrifos. At the same time, no significant difference in mortality was observed with fenpropathrin compared to the control group (Fig. 5d).
a qPCR analysis of CncC mRNA expression after dsCncC feeding. b qPCR analysis of AhR mRNA expression after dsAhR feeding. c qPCR analysis of TcHR96f mRNA expression after dsTcHR96f feeding. A down arrow indicates expression silencing after interference, with silencing efficiency shown as a percentage. d Measurement of T. cinnabarinus sensitivity toward fenpropathrin, cyflumetofen, and chlorpyrifos after CncC, AhR, and TcHR96f silencing. The Y-axis represents mortality rate. In a–d error bars represent the mean mortality rate ± SE, n = 3. Asterisks indicate a statistically significant difference (dsCncC, dsAhR, or dsTcHR96f vs. dsGFP), as analyzed by Student’s t-test. *p < 0.05, **p < 0.01. e Distribution of CncC peaks in functional genomic elements. f Promoter binding sequence motifs of the T. cinnabarinus CncC predicted by DAP-seq. g Distribution of AhR peaks in functional genomic elements. h Promoter binding sequence motifs of T. cinnabarinus AhR predicted by DAP-seq. i Distribution of TcHR96f peaks in functional genomic elements. j Promoter binding sequence motifs of TcHR96f predicted by DAP-seq.
DNA affinity purification sequencing (DAP-seq) is a method for in vitro high-throughput discovery of transcription factor binding sites using genomic DNA22. We deployed DAP-seq to examine the detoxification genes directly regulated by transcription factors. Specifically, 3269 peaks for CncC were identified (Supplementary Table 4), with the DNA binding sites predominantly near the TSS (Supplementary Fig. 4a). Approximately 755 peaks (734 genes, 23.10%) were situated within promoter regions (–2 kb to TSS) (Fig. 5e), enriching pathways such as KO05020 (Prion disease), KO05165 (Human papillomavirus infection), KO04810 (Regulation of actin cytoskeleton), KO04976 (Bile secretion), and KO04270 (Vascular smooth muscle contraction) (Supplementary Figs. 5a, 6a). Notably, 23 detoxification genes were identified from these 734 genes (Supplementary Table 4). Discriminative Regular Expression Motif Elicitation (DREME) identified a significant enrichment in the “AMCAG” motif (E value = 2.8e−4) (Fig. 5f). For AhR, 4493 peaks were identified (Supplementary Table 4), and the DNA binding sites distributed near the TSS (Supplementary Fig. 4b). Approximately 656 peaks (642 genes, 14.6%) were within promoter regions (−2 kb to TSS) (Fig. 5g), enriching in pathways such as KO04510 (Focal adhesion), KO00970 (Aminoacyl-tRNA biosynthesis), KO04361 (Axon regeneration), and KO04072 (Phospholipase D signaling pathway) (Supplementary Figs. 5b, 6b). Notably, 12 detoxification genes were identified from these 642 genes (Supplementary Table 4) and DREME analysis revealed a significant enrichment in the “AYCADCA” motif (E value = 2.4e−5) (Fig. 5h). TcHR96f yielded 7831 peaks (Supplementary Table 4), with the DNA binding sites near the TSS (Supplementary Fig. 4c). Approximately 1087 peaks (1042 genes, 13.9%) were located in promoter regions (−2 kb to TSS) (Fig. 5i), enriching pathways such as KO05203 (Viral carcinogenesis), KO04010 (MAPK signaling pathway), KO05163 (Human papillomavirus infection), KO00970 (Aminoacyl-tRNA biosynthesis), and KO04550 (Signaling pathways regulating pluripotency of stem cells) (Supplementary Figs. 5c, 6c). Notably, 27 detoxification genes were identified (Supplementary Table 4), with DREME highlighting enrichment of the “DGTTGR” motif (E value = 1.2e−7) (Fig. 5j).
P450s, GSTs, and CCEs are the primary detoxification enzymes in xenobiotic metabolism3,4. Upon integrating our DAP-seq data with published datasets, we found that among the identified detoxification genes, CncC regulated one GST gene and two CCE genes, while TcHR96f regulated three P450 genes, one GST gene, and one CCE gene (Supplementary Tables 5, 7). However, no AhR was found to regulate these identified genes. (Supplementary Table 6)6. Notably, the functions of TcGSTm2 and TcCCE06 in the metabolic detoxification of acaricides were verified in our previous work23,24. Using the dual luciferase reporter assay, we validated the role of CncC-Maf heterodimer in regulating the expression of TcGSTm8, TcCCE34 and TcCCE51 (Supplementary Fig. 7a−c) and the role of TcHR96f-TcRXR2 heterodimer in regulating the expression of CYP388A1、CYP392A3、CYP392E3、TcGSTm5 and TcCCE14 (Supplementary Fig. 7d−h).
In vitro acaricide metabolism by recombinant detoxification protein
The expression patterns of the above eight detoxification genes were analyzed following exposure to three different acaricides. Fenpropathrin treatment significantly upregulated the expressions of CYP392E3, CYP388A1, TcGSTm5, TcGSTm8, and TcCCE34 (Fig. 6a); cyflumetofen significantly upregulated the expressions of CYP392E3, CYP388A1, TcGSTm8, TcCCE34, and TcCCE51 (Fig. 6b); and chlorpyrifos significantly upregulated the expression of CYP392A3, CYP388A1, and TcCCE34 (Fig. 6c). We further knocked down the expression of CYP392E3, CYP388A1, TcGSTm8, and TcCCE34, and observed increased mortality rates in these genes knockdown individuals following exposure to acaricides (Fig. 6d−h). These findings suggested that these four detoxification genes may play important roles in the in vivo metabolic detoxification of acaricides in T. cinnabarinus.
a qPCR analysis of the expression of eight detoxification genes after fenpropathrin treatment. b qPCR analysis of the expression of eight detoxification genes after cyflumetofen treatment. c qPCR analysis of the expression of the eight genes after chlorpyrifos treatment. In a–c, controls were treated with acetone and used for normalization. Data was expressed as the mean, n = 3. Asterisks indicate a statistically significant difference, analyzed by Student’s t-test, *p < 0.05. d qPCR analysis of CYP392E3 mRNA expression after dsCYP392E3 feeding. e qPCR analysis of CYP388A1 mRNA expression after dsCYP388A1 feeding. f qPCR analysis of TcGSTm8 mRNA expression after dsTcGSTm8 feeding. g qPCR analysis of TcCCE34 mRNA expression after dsTcCCE34 feeding. A down arrow indicates expression silencing after interference, with silencing efficiency shown as a percentage. h The sensitivity of T. cinnabarinus to fenpropathrin, cyflumetofen, and chlorpyrifos after CYP392E3, CYP388A1, TcGSTm8, and TcCCE34 silencing. The Y-axis represents mortality rate. In d–h, error bars represent the mean ± SE, n = 3. Asterisks indicate a statistically significant difference, analyzed by Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001. i Recombinant TcGSTm8 metabolizes cyflumetofen. CK1: recombinant protein + 10 mg L−1 cyflumetofen, reaction for 0 h; CK2: inactivated recombinant protein + 10 mg L−1 cyflumetofen, reaction for 1 h; CK3: PBS without recombinant protein + 10 mg L−1 cyflumetofen, reaction for 1 h. j Recombinant CYP388A1 metabolizes fenpropathrin. CK is fenpropathrin + recombinant CYP388A1 – NADPH. k Recombinant CYP388A1 metabolizes cyflumetofen. CK1: recombinant CYP388A1 + NADPH + 10 mg L−1 cyflumetofen, reaction for 0 h; CK2: inactivated recombinant CYP388A1 + NADPH + 10 mg L−1 cyflumetofen, reaction for 3 h; CK3: PBS without recombinant CYP388A1 + 10 mg L−1 cyflumetofen, reaction for 3 h. l Recombinant CYP388A1 degrades chlorpyrifos. CK is chlorpyrifos + recombinant CYP388A1 – NADPH. m Recombinant TcCCE34 metabolizes fenpropathrin. CK is fenpropathrin + recombinant inactivated TcCCE34, reaction for 3 h. In i–m The Y-axis represents the acaricide concentration, and error bars represent the mean ± SE, n = 3. Asterisks indicate a statistically significant difference, analyzed by Student’s t-test. **p < 0.01, ***p < 0.001. A down arrow indicates that chlorpyrifos is metabolized, with catabolic efficiency expressed as a percentage.
To further support these findings, we employed exogenous expression combined with chromatography to demonstrate the metabolic capacity of these four enzymes toward acaricides in vitro. We successfully obtained recombinant proteins of these four genes (Supplementary Fig. 8a−d), and found that the IC50 of fenpropathrin, cyflumetofen, and chlorpyrifos against TcGSTm8 were 1090 ± 50 μM, 1570 ± 110 μM, and 1180 ± 90 μM, respectively (Supplementary Table 8). Additionally, TcGSTm8 metabolized cyflumetofen with a metabolic rate of 48% ± 4% in 1 h (Fig. 6i; Supplementary Fig. 10a−c). The reduced carbon monoxide-difference spectrum indicated that CYP388A1 was predominantly expressed in its P450 form, which is indicative of a high-quality functional enzyme (Supplementary Fig. 8e). The IC50 against CYP388A1 for fenpropathrin, cyflumetofen, and chlorpyrifos were 0.46 ± 0.11 μM, 0.047 ± 0.001 μM, and 0.11 ± 0.02 μM, respectively (Supplementary Table 8). CYP388A1 metabolized all three acaricides with different metabolic rates (35% ± 3% for fenpropathrin; 9% ± 1% for cyflumetofen, and 24% ± 11% for chlorpyrifos) (Fig. 6j−l; Supplementary Fig. 10d–l). We further conducted liquid chromatography-mass spectrometry (LC-MS) analysis to investigate the metabolism of fenpropathrin and chlorpyrifos by CYP388A1. However, due to the low metabolic rate of cyflumetofen, it was excluded from the analysis. In the CYP388A1+ chlorpyrifos + NADPH treatment group, the primary mass spectrum revealed a newly formed metabolite, with a stable ion detected at m/z = 333.96. This ion is presumed to correspond to chlorpyrifos-oxon (M1), likely formed through the desulfuration of chlorpyrifos (Supplementary Fig. 11a, b). To confirm the identity of this metabolite, a secondary mass spectrometry analysis was performed, revealing three major fragment ions: A (m/z = 197.93), B (m/z = 277.89), and C (m/z = 305.93) (Supplementary Fig. 11c). LC-MS analysis did not detect any newly formed metabolites from the metabolism of fenpropathrin by CYP388A1. CYP392E3 is predominantly expressed as P420, suggesting that the protein is inactivated (Supplementary Fig. 8f). TcCCE34 was active toward α-naphthol, and the IC50 of fenpropathrin, cyflumetofen, and chlorpyrifos against TcCCE34 were 42.51 ± 8.44 μM, 629.76 ± 39.76 μM, and 57.20 ± 7.42 μM, respectively (Supplementary Table 8). In vitro metabolism experiments showed that TcCCE34 metabolized fenpropathrin with metabolic rate of 39% ± 3% in 3 h (Fig. 6m; Supplementary Fig. 10m–o). In the TcCCE34 + fenpropathrin treatment group, LC-MS analysis identified a newly formed metabolite with a stable ion at m/z = 226, which is presumed to correspond to C14H11NO2 (M2), likely produced through the hydrolysis of fenpropathrin (Supplementary Fig. 11d, e). Secondary mass spectrometry analysis confirmed four major fragment ions: A (m/z = 93), B (m/z = 133), C (m/z = 171), and D (m/z = 198) (Supplementary Fig. 11f). These findings indicate that CYP392E3, CYP388A1, TcGSTm8, and TcCCE34 could metabolize acaricides and are functionally important for detoxification.
Discussion
Insect-plant interactions have spawned the development of robust defense systems in host plants, including the production of a variety of toxic secondary metabolites, also known as secondary plant substances, to defend against insects and other herbivores25. As an adaptive mechanism to combat these secondary plant substances, herbivorous insects have also evolved corresponding defense mechanisms such as a variety of detoxification enzymes to metabolize these toxic compounds3,4,26. Oxidative stress occurs when external stimuli, such as secondary plant substance, results in the overproduction of ROS and activate the protective function of the organism27,28. Given that both secondary plant substances and insecticides are chemical toxins, it is possible that these toxins trigger similar oxidative stress pathway for metabolic detoxification, but because of the wide variety of secondary plant substances and synthetic insecticides, it is unlikely that each xenobiotic corresponds to a specific detoxification metabolic pathway in arthropods. Instead, developing a shared, broad-spectrum detoxification mechanism would be more efficient and less energy-consuming from an evolutionary perspective29,30. Interestingly, studies have shown that when mites adapt to a new host plant, acaricide resistance is promoted. For example, when T. urticae were transferred from a legume to a tomato seedling, the effectiveness of acaricides significantly decreased, with similar expression patterns of many detoxification enzymes between host transfer lines and insecticide resistance lines31. This suggests that detoxification pathways in mites, shaped by long-term co-evolution with plant secondary metabolites, may have been evolved through conserved initiation and signal transduction mechanisms to defend against both ancestral plant toxins and synthetic insecticides/acaricides.
Extensive studies have revealed significant ROS accumulation in arthropods following insecticide exposure27,32. In D. melanogaster and Spodoptera litura, researchers have characterized ROS accumulation after exposure to neonicotinoid or pyrethroid insecticides33,34. Similarly, this study showed that ROS levels of T. cinnabarinus significantly increased after exposure to the pyrethroid insecticide fenpropathrin, the mitochondrial inhibitor acaricide cyflumetofen, the organophosphate insecticide chlorpyrifos, and the herbicide paraquat. Compared with the control group, the activities of CAT, POD, and SOD in T. cinnabarinus were also significantly increased, further demonstrating that acaricide exposure causes ROS accumulation at the biochemical level. The accumulation of ROS in arthropods following insecticide exposure is a well-documented phenomenon, in which dual oxidase, NADPH oxidases (NOx), and cytochrome P450 enzymes play critical roles35,36,37. Specifically, treatment with organochlorine pesticides promotes the phosphorylation of the catalytic subunit p47phox of NOx and its translocation to the cell membrane, leading to a significant increasing the intracellular superoxide levels38. This mechanism may also account for the accumulation of ROS in T. cinnabarinus after acaricide exposure. Furthermore, the production of ROS is potentially associated with mitochondrial damage. For instance, cyflumetofen specifically targets mitochondrial complex II of spider mites, resulting in mitochondrial damage and subsequent ROS accumulation39. The precise mechanisms underlying ROS accumulation in T. cinnabarinus under acaricide stress require further investigation.
Previously, ROS were considered inevitable toxic byproduct of aerobic metabolism. However, growing evidence indicates that low ROS levels serve as “secondary messengers” in cells40. In plants, ROS act as signaling molecules involved in development and stress adaptation41. In insects, ROS as a signaling molecule regulate AMPK activity and the activation of the transcription factor CncC42,43. CncC is recognized as a key regulator of insect responses to xenobiotics and oxidative stress. Under normal conditions, it is inhibited by Kelch-like ECH-associated protein 1 (Keap1) and retained in the cytoplasm. However, during oxidative stress, ROS interact with the cysteine thiol groups of Keap1, triggering the release of CncC, which is swiftly translocated to the nucleus. Once in the nucleus, CncC forms a heterodimer with small muscle aponeurosis fibromatosis (Maf, a bZIP transcription factor), thereby regulating both the basal and induced expression of Antioxidant Response Element (ARE)-responsive genes5. In arthropods, CncC is well documented for its role in oxidative stress responses and has recently been shown to contribute to the constitutive activation of detoxification pathways associated with metabolic resistance34,44,45. In this study, we found that decreasing or increasing ROS levels led to a reverse corresponding increase or decrease in sensitivity toward three acaricides, indicating that ROS participates in acaricide detoxification in T. cinnabarinus. Assessment of enzyme activity has also shown that decreases in ROS levels significantly inhibited acaricide-induced upregulation of detoxification enzyme activities. These ROS changes led to a decrease in acaricide susceptibility in T. cinnabarinus, suggesting that ROS play a role as signaling molecules in the detoxification metabolism of acaricides.
In addition to roles in cell cycle, apoptosis, energy metabolism, autophagy, and insulin/insulin-like growth factor signal transduction, the transcription factor FOXO plays an important role in the cellular responses to oxidative stress17,46. In our study, we assessed FOXO mRNA expression after inhibiting ROS with NAC in T. cinnabarinus. The results showed that FOXO mRNA expression was suppressed when ROS levels decreased, whereas increased ROS levels after paraquat treatment significantly increased FOXO protein expression. This indicates that FOXO expression is positively correlated with ROS levels. Additionally, observing FOXO nuclear localization in T. cinnabarinus after paraquat treatment increased nuclear FOXO protein in cells, indicating FOXO’s activation by ROS20,47. These findings suggest that FOXO plays a critical role in responding to ROS in T. cinnabarinus. Studies have shown that under oxidative stress conditions, Mammalian Ste20-like Kinase (MST1)/Hippo and c-Jun kinase (JNK) in mammals and nematodes phosphorylate FOXO, leading to its nuclear translocation48,49. Recent research has found that ROS activates FOXO through the AMPK signaling pathway in Tribolium castaneum50. However, the specific pathways through which ROS activate FOXO still require further investigation in T. cinnabarinus.
FOXO can regulate antioxidant enzymes in response to oxidative stress in vivo, as well as act as a key factor in insulin signaling to regulate insect growth or play a role in the development of insecticide resistance46,51,52,53,54. For instance, FOXO participated in deltamethrin resistance in T. castaneum through regulating CncC expression55. In Plutella xylostella, FOXO mediated Bt resistance development by regulating MAPK expression56. We performed experiments using RNA interference (RNAi) with a single RNAi construct to elucidate the regulatory pathways of FOXO. The results indicated a decrease in the expression levels of the CncC, AhR, and five HR96 genes following FOXO suppression. However, since the RNAi experiment employed only one construct targeting the FOXO gene, the off-target effects cannot be ruled out. Therefore, we conducted dual-luciferase reporter assays and Y1H experiments using FOXO transfection alone, as its ligand partner remains unidentified in T. cinnabarinus. These results also suggest that FOXO regulates the promoter activity of various xenobiotic sensors, including CncC, AhR, and HR96. In addition, the regulation of CncC, AhR, and TcHR96f by FOXO is due to the ability of FOXO to bind to the promoters of CncC, AhR, and TcHR96f, acting as an activator to upregulate the expression of these xenobiotic sensors. In recent years, many FOXO target genes, including cytochrome P450 genes, have been identified and characterized, indicating that FOXO can directly regulate the expression of detoxification genes57. However, compared to well-studied xenobiotic receptors-such as the transcription factors CncC, AhR, and HR96-the diversity and quantity of detoxification genes directly regulated by FOXO remain relatively limited. Therefore, it is essential to explain a more efficient and comprehensive pathway that links FOXO with the expression of lots of detoxification genes. This study elucidates how FOXO regulates detoxification genes through a cascade signaling pathway involving the regulation of xenobiotic receptors. This pathway demonstrates greater intensity and a broader scope, enabling FOXO to regulate a wider variety and greater number of detoxification genes. Such a mechanism is particularly advantageous for arthropods in initiating broad-spectrum detoxification in response to plant secondary metabolites and insecticides.
Recent studies increasingly indicate that the trans-upregulation of detoxification genes is mediated by key transcription factors essential for insect detoxification and the development of insecticide resistance5,7,58,59,60. CncC, AhR, and HR96 have been implicated in detoxifying various acaricides, including but not limited to pyrethroids, organophosphates, carbamates, and mitochondrial inhibitors12,44,58,61,62. In spider mites, Ji et al. suggest that trans-regulation is the primary mechanism by which resistance gene expressions are regulated in T. urticae63. Our research also indicates that silencing the expression of CncC and AhR using a single RNAi construct increases the toxicity of the three acaricides to T. cinnabarinus to varying degrees. This process silences TcHR96f expression with a single RNAi construct and enhances the toxicity of cyflumetofen and chlorpyrifos to T. cinnabarinus, while the toxicity of fenpropathrin remains unchanged. This may be due to TcHR96f not being the primary regulatory factor in the detoxification process of fenpropathrin45. Additionally, the regulation of detoxification genes by these transcription factors is not a simple point-to-point regulation, but rather a complex network involving multiple interactions. For example, CncC, HR96, and AhR require obligate partners, such as small Maf for CncC, RXR for HR96 homo/heterodimers, and ARNT for AhR, to activate gene transcription5. These transcription factors regulate the expression of various detoxification genes, including P450, GST, CCE, ABC transporters, and UGT. The DAP-seq data further suggests that the same transcription factors can regulate the expression of phase I, II, and III detoxification genes. For example, the TcHR96f-RXR complex can simultaneously modulate the expression of P450, GST, and CCE. Together, these intricate regulatory networks enhance the ability of T. cinnabarinus to detoxify xenobiotic compounds efficiently.
The interaction between detoxification enzymes and xenobiotics is critical to detoxification3. In arthropods, the detoxification of secondary plant substances and synthetic insecticides by P450s, GSTs, and CCEs has been extensively researched. The P450 superfamily detoxifies xenobiotics by increasing their water solubility through oxidation, epoxidation, dehydrogenation, hydrolysis, and reduction26. A classic example is CYP389C16, which metabolizes cyflumetofen and pyridaben, thereby mediating the cross-resistance to these acaricides in T. cinnabarinus64. GSTs are important Phase II metabolic enzymes that metabolize and detoxify xenobiotics by increasing the water solubility, providing sterical hindrance, scavenging oxidative damage, and facilitating dehydrogenation65. In T. cinnabarinus, TcGSTm2 detoxifies cyflumetofen through direct binding23. As Phase I metabolic enzymes, CCEs similarly detoxify xenobiotics through hydrolysis or binding66. Interestingly, hydrolysis by CCEs may sometimes produce more toxic substances. For example, cyflumetofen is metabolized to the more toxic AB-1 after CCE hydrolysis39. In this study, we found that the induced expression of four detoxification genes identified from DAP-seq can detoxify one or more acaricides in vivo through RNAi. Furthermore, in vitro metabolism experiments provided evidence that CYP388A1, TcGSTm8 and TcCCE34 can directly degrade one or more acaricides. Although differences exist in RNAi bioassay results and in vitro metabolic capacity, we speculate that these discrepancies may be related to the role of isoenzymes in forming more complex metabolic networks in vivo—for example, CYP388A1 metabolizes cyflumetofen, whereas TcCCE34 metabolizes fenpropathrin64,67. Specifically, the data presented in this study suggest that although CYP388A1 can metabolize cyflumetofen in vitro (9%, low metabolism rate), its role in vivo detoxification (as evidenced by RNAi results) may be modulated by compensatory mechanisms in the metabolic network (e.g., functional redundancy of other P450 isoenzymes). This implies that the metabolic pathway mediated by CYP388A1 is not the primary route controlling the toxicity of cyflumetofen. Similarly, the results in this study suggest that TcCCE34 can metabolize fenpropathrin in vitro. However, its contribution to overall detoxification may be influenced by compensatory mechanisms within the in vivo metabolic network. Previous studies have shown that silencing a single CCE gene does not significantly affect fenpropathrin lethality in T. cinnabarinus. In contrast, silencing multiple CCE genes simultaneously leads to a significant increase in fenpropathrin toxicity to the mites67. Notably, CYP388A1 activates chlorpyrifos into chlorpyrifos-oxon (a more toxic metabolite); paradoxically, silencing CYP388A1 increases mortality in T. cinnabarinus. This seemingly contradictory result may reflect a dual function of CYP388A1: while it facilitates the bioactivation of chlorpyrifos, it may also contribute to the detoxification of chlorpyrifos-oxon. This interpretation is supported by the significant upregulation of CYP388A1 expression under acaricide-induced stress (Fig. 6a–c). In conclusion, our findings highlight the crucial role of these enzymes in acaricide detoxification.
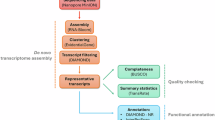
Despite extensive research on how detoxification enzymes detoxify xenobiotics, the activation of the entire detoxifying metabolic pathway remains poorly understood, particularly how detoxification signal transduction is initiated following insecticide exposure. Our current study reveals for the first time a comprehensive model for the mechanism of broad-spectrum induction of detoxification genes (Fig. 7). Upon exposure to external stimulus, such as acaricides and other xenobiotics, oxidative stress responses will cause in vivo ROS accumulation at low levels, converting chemical signals into cellular physiological signals. ROS act as second messenger, activating FOXO signaling cascades and causing FOXO nuclear translocation. The activated nuclear FOXO then regulates the expression of different xenobiotic sensors (CncC, AhR, and HR96), which ultimately upregulate numerous detoxification genes. These genes are translated into detoxification proteins that degrade acaricides, converting them into non-toxic or less toxic substances that are then expelled. This broad-spectrum induction model for detoxification metabolism provides a comprehensive framework for understanding how the arthropods make use of ancient signal transduction pathways to deal with exogenous compound stress in a simple and efficient manner. It is concerning that, in the context of global warming, it is reasonable to infer that rising temperatures will result in increased ROS accumulation in arthropods. This, in turn, will upregulate detoxification gene expression via the ROS-FOXO pathway. Such a phenomenon is likely to pose significant challenges for pesticide control and resistance management in pests. In conclusion, our study advances the understanding of mechanisms for detoxification metabolism and adaptive evolution in arthropods. Additionally, our results also suggest new strategies for the design of novel insecticides against pest.
The model is based on the integration of data from this study, with validated relationships depicted by solid lines and hypothetical relationships represented by dashed lines. The model was created with the help of BioRender.com.
Methods
Mite strain
The susceptible strain (SS), collected in 1998, from Beibei District, Chongqing, China, was maintained on fresh cowpea (Vigna sinensis) leaves for >20 years, and has since been reared in the laboratory at a temperature of 26 ± 1 °C and relative humidity of 55–70% under a 16 h/8 h light/dark cycle in the absence of pesticide exposure.
Determination of O2•− content
In this study, the content of O2•− was measured as an indicator of ROS. Two hundred 3–5-day-old adult female mites were homogenized in precooled PBS, followed by the addition of O2•− probe dihydroethidium (DHE) (Yeasen Biotechnology Co., Ltd., Shanghai, China) to achieve a final concentration of 30 μM33. The mixture was then incubated in the dark at 37 °C for 60 min. A fluorescence spectrophotometer (F97 pro, Shanghai Lengguang Technology Co., Ltd., China) was then used to measure fluorescence intensity using excitation wavelengths of 488–535 nm and an emission wavelength of 610 nm. An identical volume of PBS and DHE was used for blank correction on the device. The results were normalized by protein content which measured using the Bradford assay68. To visualize ROS levels, DHE was used to stain adult female mites. In brief, female adult mites were treated with acaricide at LC30 prior to incubation with the ROS fluorescence probe DHE at a final concentration of 30 μM for 60 minutes. The concentrations were set to the LC30 values for fenpropathrin(CAS number: 64257-84-7), cyflumetofen (CAS number: 400882-07-7), and chlorpyrifos (CAS number: 2921-88-2), which were 1200 mg/L, 2.40 mg/L, and 75 mg/L, respectively. Details of the determination method are provided in the acaricide bioassay section. Mites were then fixed overnight with 4% paraformaldehyde at 4 °C, followed by embedding in optimal cutting temperature compound (Sakura Finetek USA, lnc.) in an embedding box. After removing paraformaldehyde, about 30 adult female mites were carefully placed in the embedding agent. The embedding box was snap-frozen in liquid nitrogen for 1 min and then transferred to −80 °C for 12 h. The embedded sample was sectioned using a precooled microtome, and sections were placed on treated slides and air-dried for 40 min before being covered with a fluorescence quencher (Beyotime, Shanghai, China). A cover slip was used to cover the tissue before mounting it with nail polish. The sections were observed under a confocal microscope (Carl Zeiss SAS, Germany) using the Z-stack mode, with approximately 10 consecutive images captured within a range of 20 μm. The imaging conditions included an excitation wavelength of 514 nm, and an emission wavelength of 519–600 nm. The fluorescence signal intensity was analyzed and calculated using Image J (version 1.52a) software.
Measurement of antioxidant enzyme activity
The LC30 of fenpropathrin, cyflumetofen, and chlorpyrifos were applied for acaricide exposure using the residual coated vial method (details provided in the bioassay section). The concentrations were set to the LC30 values for fenpropathrin, cyflumetofen and chlorpyrifos, which were 1200 mg/L, 2.40 mg/L, and 75 mg/L, respectively. The control group was treated with acetone (purity 99.5%) following the same protocol. Samples of approximately 200 adult mites were collected after 6, 12, and 24 h of stress exposure, with three biological replicates set for each treatment. The collected mite samples were homogenized using corresponding pre-cooled antioxidant enzyme extraction buffers. Specifically, the CAT extraction buffer was Tris-HCl (pH 7.5), while the SOD and POD extraction buffers were included in their respective commercial kits. Each sample was homogenized on ice using a motorized tissue homogenizer, followed by centrifugation at 4 °C and 12,000 × g for 15 min. The supernatant was collected for further assays. The homogenates were divided into two parts: one part was used to measure protein concentration using the BCA protein assay kit (Biomed, China), and the other part was used for antioxidant enzyme activity assays.
For catalase (CAT) activity measurement, the assay was performed following the protocol described by Aebi69, with minor modifications. Specifically, CAT activity was determined by monitoring the reduction in absorbance at 240 nm as H2O2 was decomposed. The reaction mixture contained 100 μL of mite protein extract, 1 mL of 15 mM H2O2, and 1.8 mL of 67 mM Tris-HCl (pH 7.5). The reaction was initiated by adding the H2O2 solution, and the decrease in absorbance was recorded every 30 s for 3 minutes using a UV spectrophotometer. CAT activity was calculated using the following formula. E240 is the change in absorbance every minute (ΔOD) at 240 nm, and EW is the protein content in 100 μl of sample solution (mg), 3 represents the reaction volume (mL), and 0.0436 indicates the molar extinction coefficient of hydrogen peroxide (μmol·mL-1).
For SOD and POD activity assays, the manufacturer’s instructions provided in the respective commercial kits were followed. SOD activity was measured using a superoxide dismutase assay kit (Solarbio, Beijing, China), which determines the enzyme’s ability to inhibit the reduction of nitroblue tetrazolium (NBT) by the superoxide anion. POD activity was measured using the peroxidase assay kit (Solarbio, Beijing, China), which quantifies guaiacol oxidation catalyzed by peroxidase in the presence of H2O2.
N-Acetylcysteine (NAC) treatment
NAC (CAS number: 616-91-1) is a specific ROS scavenger that can reduce ROS levels in vivo. The leaf disc spraying method was used to determine the toxicity of NAC toward T. cinnabarinus (Supplementary Table 1). NAC was dissolved in water with 0.1% Tween-80 (v/v) as a surfactant to enhance solubility, and a concentration of 20,000 mg/L (2%) NAC was used to evaluate its ability to scavenge ROS in T. cinnabarinus in the presence and absence of acaricides. The role of Tween-80 was to ensure uniform NAC application on the leaf discs and mite bodies. Although 0.1% Tween-80 is higher than the typical concentrations used for surfactants, it was necessary to achieve full solubilization of NAC. No significant toxic effects of 0.1% Tween-80 alone were observed in preliminary trials; therefore, it was used as a control for NAC treatments. The mites were sprayed with 1 mL of 2% NAC or 0.1% Tween 80 for 24 h for ROS scavenging studies. Then the mites were divided into two groups and transferred to prepared residual coated vials. One group was treated with acetone, whereas the other group was treated with acaricides (details provided in the “acaricide exposure” section) for 24 h. Pretreated and treated adult female mites were collected for bioassay, detoxification enzyme activity measurement, and FOXO level measurement.
Measurement of detoxification enzyme activity
Two hundred female adult mites were collected to measure detoxification enzyme activity. The Bradford method was used to measure protein concentrations in the enzyme solution68.
P450 activity was measured using the method by Shang et al.70. Briefly, approximately 200 female adult mites were homogenized in 1 mL of PBS (0.2 mol/L, pH 7.8) on ice. The homogenate was centrifuged (10,000 × g for 15 min, 4 °C), and the supernatant was used as the enzyme source. The enzyme solution and NADPH were added to 0.1 mol/L p-nitroanisole (in acetone) and incubated at 37 °C for 30 min. The reaction was stopped with 1 mol/L HCl, then extracted with chloroform and 0.5 mol/L NaOH. The absorbance was read at 400 nm using a microplate reader (EON, BioTek Instruments Inc., Winooski, Vermont, USA). Specific activity was calculated from the nitrophenol standard curve (0.3–2.7 nmol) and protein concentration.
The GST activity was measured according to the method71: approximately 200 female adult mites were homogenized in 1 mL PBS (0.04 mol/L, pH 7.5) on ice and then centrifuged (10,000 × g for 10 min at 4 °C). The reaction used CDNB (1-Chloro-2,4-dinitrobenzene, 0.6 mmol/L) and GSH (6 mmol/L) as substrates. A total of 100 μL of each substrate was incubated at 37 °C for 20 min, followed by the addition of 100 μL mite extract. Enzyme activity was measured in a microplate reader (EON, BioTek Instruments Inc., Winooski, Vermont, USA) at 340 nm and 37 °C, with an interval of 30 s for 5 min. The GST activity (μM·min−1 mg−1 protein) = (ΔA340 × V)/(ε × T × L × E) was used to calculate enzyme activity, where ΔA340 is the absolute value of A340 in total reaction time, V is the volume of the reaction system in milliliters (0.2 mL), ε represents the extinction coefficient of the CDNB-GSH conjugated (9.6 mmol·L−1·cm−1), T is the total reaction time (5 min), L is the cuvette path in centimeters (1 cm), and E indicates the amount of protein in each reaction.
Esterase activity was measured using the method by Vanasperen72. Briefly, approximately 200 female adult mites were homogenized in 1 mL PBS (0.04 mol/L, pH 7.5) on ice and then centrifuged (10,000 × g for 10 min at 4 °C). The supernatant was kept on ice for testing. The reaction used α-naphthyl acetate (3 × 10−4 mol/L) as the substrate and was incubated at 37 °C for 10 min. The color-developing agent (5% SDS:1% Fast Blue B Salt = 5:2, v/v) was added, and the OD at 600 nm was recorded using a microplate reader (EON, BioTek Instruments Inc., Winooski, Vermont, USA). Esterase activity was calculated from the α-naphthol standard curve (2.5–40 nmol) and protein concentration.
High temperatures treatment, acaricides exposure and bioassay
3–5-day adult female mites were gently placed on leaf discs using a brush before the discs were preincubated in a 26 °C (room temperature) or 34 °C (high temperature) incubator for 6 h. After preincubation at room temperature or high temperature, the mites were used to measure O2•− content and bioassay for the three acaricides.
Acaricide exposure studies were conducted using the residual coated vial method at the LC30 of each acaricide. The concentrations were set to the LC30 values for fenpropathrin, cyflumetofen, and chlorpyrifos, which were 1200 mg/L, 2.40 mg/L, and 75 mg/L, respectively. Acetone was used as the control, and mites were treated for 6, 12, and 24 h. Each treatment was performed with three biological replicates, with each replicate using about 200 female adult mites. Paraquat treatment (3000 mg/L) was administered using the leaf disc spraying method. Prior to treatment studies, leaf disc spraying was used to assess the toxicity of paraquat to T. cinnabarinus (Supplementary Table 1).
Bioassays were conducted using the residual-coated vial method73. First, fenpropathrin, cyflumetofen, and chlorpyrifos were dissolved in acetone to obtain a high-concentration stock solution, which was then diluted to the desired concentration. Then, 1.5 mL of the acaricide solution was transferred into a 2-mL centrifuge tube, vortexed thoroughly, alternately placed upright, and inverted for 15 min. Afterward, the solutions were discarded, and the tubes were air dried for 24 h in a fume hood. Five concentrations were set up for each pesticide in triplicate, and about 30 female adult mites were used for each biological replicate. The number of live and dead individuals was recorded, and mortality rates, defined as the ratio of dead individuals to the total number of live and dead individuals, were calculated after 24 h. The LC50 and 95% confidence intervals were calculated using POLO Plus (version 2.0).
Because paraquat (CAS number: 4685-14-7) is water-soluble but insoluble in acetone, and the NAC is highly soluble in water, the spray method was selected for the bioassay of NAC and paraquat. Briefly, a high-concentration stock solution of each reagent was prepared using 0.1% Tween 80 water, followed by dilution with 0.1% Tween 80 water to create 4–6 concentration gradients. Thirty female mites, aged 3–5 days, were placed on fresh leaf discs and sprayed with 1 mL of acaricide solution. A 0.1% Tween 80 aqueous solution without acaricides served as the control. The number of live and dead individuals was recorded, and mortality rates were evaluated after 24 h. The experiment was performed in triplicate with three biological replicates. The LC50 and 95% confidence intervals were calculated using POLO Plus (version 2.0).
DNA and RNA extraction, cDNA synthesis, and RT-qPCR analysis
Genomic DNA and total RNA were extracted from about 200 3–5-day-old female adult mites. A DNA extraction reagent named Phenol/Chloroform/Isoamyl alcohol (25:24:1) (Solarbio, Beijing, China) was used for genomic DNA extraction according to the instruction manual, and TRIzol (Thermo Fisher Scientific Inc., USA) was used to determine total RNA extraction in T. cinnabarinus following the manufacturer’s instructions. RNA quality was assessed using 1% agarose gel electrophoresis and the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). The Prime ScriptTM RT reagent Kit with gDNA Eraser (Takara Biotechnology Dalian Co., Ltd., Dalian, China) was used for first-strand cDNA synthesis, and the products were stored at −20 °C. Primers used for qPCR were designed using Primer Premier 5.0 (PREMIER Biosoft International, Palo Alto, California, USA). Ribosome S18 (RP18S, FJ608659) and alpha tubulin (α-Tub, FJ526336) were used as references (Supplementary Data 2). After gradient concentration template testing of qPCR primers, primers with high amplification efficiency (90–110%) and with a single peak in the melt curve were selected. The reaction system consisted of 1-μL diluted cDNA, 10-μL GoTaq qPCR Master Mix, 7-μL nuclease-free water, and 0.2 mM primers. The thermal cycling conditions were as follows: 95°C × 2 min, 95°C × 15 s, 60°C × 30 s, and melt curve analysis at 60 °C–95 °C. Steps 2 to 4 were repeated 40 times. Biological triplicates and technical duplicates were used. The 2−ΔΔCt method was used to analyze expression. SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used to analyze statistically significant differences (p < 0.05).
Molecular cloning and bioinformatics analysis
The sequence information for T. cinnabarinus FOXO (Supplementary Data 3) was obtained from the T. cinnabarinus genome (BioProject number: PRJCA1227279). The bioinformatics tools in NCBI were used to determine conserved protein domains in FOXO (https://www.ncbi.nlm.nih.gov/Structure/cdd/). The pI/Mw tool (https://web.expasy.org/compute_pi/) was used to calculate the theoretical isoelectric point (pI) and molecular weight (MW). Supplementary Table 2 shows the detailed information for FOXO. MEGA 7.0 (maximum likelihood LG + G method) was used to construct the phylogenetic tree based on amino acid sequence alignment performed with MUSCLE. The analysis was conducted with 1000 bootstrap replicates. Different FOXO amino acid sequences in different insect species were downloaded from the NCBI database (Supplementary Table 3).
Total protein extraction and nuclear translocation of FOXO
T. cinnabarinus were treated with paraquat at LC30 using the spraying method. Forty milligrams of surviving female adult mites were collected for each treatment for total protein extraction. The Minute Total Protein Extraction Kit for Insects (Invent) was used to extract total protein following the manufacturer’s recommendations. The nuclear protein extraction kit (Solarbio, Beijing, China) was used to extract cytoplasmic protein and nuclear protein from adult female mites, strictly following the manufacturer’s protocol. Protein concentration was measured using a BCA protein concentration assay kit (Biomed, China). Subsequently, 40 μL of total protein solution was mixed with loading buffer and heated in a water bath at 100 °C for 10 min prior to Western blot analysis.
A rabbit polyclonal antibody against recombinant FOXO (1.3 mg/mL) of T. cinnabarinus was generated by Zoonbio Biotechnology Co., Ltd. (Zoonbio Biotechnology, Nanjing, China) and diluted with Tris-buffered saline–Tween solution at a ratio of 1: 8000 before use. Antibodies against α-Tubulin and histone H3, purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China), were used as endogenous loading controls in Western blot analysis. Proteins were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto 0.45 μm polyvinylidene fluoride (PVDF) membranes (Millipore, USA). Membranes were blocked with 5% skim milk for 1 h and incubated overnight at 4 °C with the primary antibody (1: 8000 dilution). After washing with TBST, the membranes were incubated with a secondary antibody (1:20,000 dilution), purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China), for 1 h at room temperature. Specific protein bands were visualized using enhanced chemiluminescence (ECL) reagents (Coolaber, China).
dsRNA feeding
Specific T7 sequence-containing primers (Supplementary Data 2) were used to PCR amplify the partial sequences of FOXO, CncC, AhR, TcHR96f, CYP388A1, CYP392E3, TcGSTm8, TcCCE34 (Supplementary Data 3) and GFP (NCBI: ACY56286, negative control, sequence fragment: 435 bp). The Transcript Aid T7 High Yield Transcription Kit (Thermo Scientific, Vilnius, Lithuania) was used to synthesize the dsRNA of the corresponding gene. The concentrations of dsRNA were adjusted to ~1000 ng/μL and then stored at −80 °C. The leaf disc feeding method was used for dsRNA delivery. Cowpea leaves were cut into 4 cm2, placed in a 60 °C oven for 3 min, and soaked in 40-μl dsRNA until the dsRNA was completely absorbed. Eighty female adult mites were placed on each leaf for 72 h of feeding. The feeding temperature was 26 °C ± 1 °C, relative humidity was 55%, and the light/dark cycle was 16/8 h. After being fed with dsRNA for 72 h, female adult mites were used for RT-qPCR and bioassay. Biological triplicates were used for the experiment.
Cell culture, vector construction, and dual luciferase activity measurement
Chinese hamster ovary (CHO) cells were cultured in 90% DMEM/F-12 (Gibco BRL, Gaithersburg, MD, USA), 10% fetal bovine serum (FBS; Gibco, USA), and antibiotics. The culture conditions were 37 °C and 5% CO2. The promoter information of CncC (-1892 — +22), AhR (-1976 — +22), TcHR96 (a (-1898 — +97), b (-2036 — +162), d (-2069 — +81), f (-1087 — +110), and g (-1212 — +98)), CYP388A1(-1922 — +44), CYP392A3 (-615 — +66), CYP392E3 (-1903 — +63), TcGSTm5 (-994 — +37), TcGSTm8 (-1939 — +55), TcCCE14 (-1934 — +20), TcCCE34 (-1137 — +65) and TcCCE51 (-1971 — +23) was obtained from the T. cinnabarinus genome. The promoter sequences of corresponding genes were amplified from the genomic DNA of T. cinnabarinus (Supplementary Data 3). Supplementary Data 2 shows the corresponding primers. The promoter sequences were subcloned into the pGL 3.0 Basic vector (Tsingke Biotechnology Co., Ltd.). The open reading frame of FOXO, CncC, Maf, TcHR96f, TcRXR1 or TcRXR2 was cloned into the expression vector pcDNA3.1(+) (Supplementary Data 3).
The dual luciferase reporter gene assay used CHO cells in a six-well cell culture plate. A total of 100 μL of cells (1 × 106 cells/mL) were transferred to single wells and cultured in DMEM/F-12 culture medium (10% FBS, 1% penicillin, and 1% streptomycin) at 37 °C and 5% CO2 for 24 h. To test the regulatory activity of transcription factor on promoters, different transfection combinations and Attractene transfection reagents (QIAGEN NV, Germany) were added to 500 μL of FBS-free and antibiotic-free DMEM/F-12 culture medium. After being left to stand for 15 min, the culture medium was added to cell culture plates. The samples were incubated for 6 h. Then, the DMEM/F-12 medium was removed and 2 mL of fresh DMEM/F-12 medium, with 10% FBS and antibiotics, was added to each well. The cells were harvested by centrifugation at 10,000 × g for 5 min after 48 h of transfection. Then, the dual-luciferase activity was measured using Dual Luciferase Assay System E2920 (Promega, Madison, WI, USA). The results are expressed as the ratio of firefly luciferase activity/Renilla luciferase activity. Three biological replicates in each setup for each transfection group and three technical replicates for the luciferase activity assay were used.
Yeast one-hybrid (Y1H) assay
The Y1H Gold yeast one-hybrid system (Coolaber, China) was used for Y1H assay. In brief, CncC, AhR, and TcHR96f promoters were ligated to a linearized pAbAi to create the bait vector. Supplementary Data 2 shows the primer information. The BstBl restriction enzyme was used to linearize the pAbAi-bait vector before integration into the Y1HGold yeast genome. The SD/-Ura culture medium was used to screen for positive bait strains. pGADT7-FOXO was introduced to bait strains to screen for interactions between prey and bait. Various combinations of single colonies with interactions were selected for expansion culture until OD600 ≈ 0.2. Thereafter, serial dilution from 10 to 1000 fold was conducted. The colonies were inoculated onto SD/-Leu deficient culture medium containing 150 ng/mL aureobasidin A (AbA), cultured at 30 °C for 3 days, and observed for colony growth. pGADT7-p53 and p53-pAbAi vectors were used as positive controls, and the pGADT7 and p53-pAbAi vectors were used as negative controls.
DAP-seq
DAP gDNA library construction and DAP reaction were performed22. Genomic DNA was extracted from from whole mites T. cinnabarinus. A DNA extraction reagent named Phenol/Chloroform/Isoamyl alcohol (25:24:1) (Solarbio, Beijing, China) was used for genomic DNA extraction according to the instruction manual. A Covaris M220 Focused-ultrasonicator (Covaris, Massachusetts, USA) was used to fragment enriched gDNA fragments into 200-bp fragments, the ends of which were repaired and a 3′A was added. Then, aptamers were ligated to the gDNA fragment. The full-length open reading frames of CncC, AhR, and TcHR96f were recombined separately with the pFN19K HaloTag® T7 SP6 Flexi® vector to construct the Halo-TcCncC, Halo-TcAhR, and Halo-TcHR96f vectors. Supplementary Data 2 shows the primer information. The Halo-CncC, Halo-AhR, and Halo-TcHR96f recombinant proteins were expressed according to the manufacturer’s instructions for the TNT SP6-coupled wheat germ extraction system (Promega Inc., Fitchburg, USA). Magne HaloTag beads (Promega Inc., Fitchburg, USA) were used to purify the recombinant proteins, and Western blotting was used to confirm protein actually expression. Then, the DAP gDNA libraries were separately incubated with Halo-TcCncC, Halo-TcAhR, or Halo-TcHR96f recombinant proteins at room temperature for 1 h. The eluted DNA was used for PCR amplification sequencing using Illumina Novaseq 6000 (Genetic Engineering Biotechnology Co. Ltd., Guangzhou, China). After Illumina sequencing, Bowtie2 (v2.2.5) was used to align cleaned DAP-seq data with the T. cinnabarinus genome (NCBI BioProject number PRJNA1227279)74. All reads 2 kb upstream and downstream of TSSs and transcription end sites (TES) were calculated using Deep Tools (v3.2.0). MACS2 (v2.1.2) was used to identify peaks with q < 0.05. The ChIP Seeker R software package was used to annotate peak value-related genes and analyze the distribution status of peak values in different genomic regions. MEME Suite (https://meme-suite.org/) was used to test the corresponding genes combined with motifs. The raw sequence data of DAP-seq have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/ Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA020103).
Expression of CYP388A1, CYP392E3, TcGSTm8, and TcCCE34
The TcGSTm8 protein was expressed using the Escherichia coli prokaryotic expression system23. The full-length open reading frame sequence of TcGSTm8 (Supplementary File 10) was cloned to the pCold II vector. The recombinant vector was then transformed into BL21 (DE3)-competent cells. A single colony was cultured overnight at 37 °C with shaking, after which the cells were diluted 1:100 in LB-ampicillin medium containing 100 mg L−1 ampicillin and cultured with shaking at 37 °C until the OD600 reached 0.6–0.8. IPTG, at a final concentration of 0.1 mM, was added to the cell suspension, and the culture was incubated at 15 °C for 12–16 h at 180 rpm. The cells were harvested by centrifugation at 4000 g for 30 min at 4 °C. The cell pellets were resuspended in 20 mL of PBS (0.04 M, pH 7.4) and lysed by sonication (8-s pulses, 150 W) on ice for 30 min. The cell lysate was centrifuged at 9000 g for 30 min at 4 °C. The resulting supernatant was purified using a Ni-NTA preloaded gravity column (Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. Buffer I (0.04 M PBS, pH 7.4, 0.5 M NaCl, 40 mM imidazole) was used to remove impurities, and the target protein was eluted using Buffer II (0.04 M PBS, pH 7.4, 0.5 M NaCl, 500 mM imidazole). The activity and enzyme kinetic parameters of the expressed TcGSTm8 protein was measured as described23. The potential interaction between recombinant TcGSTm8 proteins and acaricides was assessed by measuring the inhibition of activity toward CDNB. Briefly, recombinant TcGSTm8 protein was incubated at 25 °C for 10 min with varying concentrations of acaricides (0–5 mM) in 5% acetone. The percentage inhibition of TcGSTm8 activity was determined in the presence of 6 mM GSH and 0.6 mM CDNB. The IC50 value was calculated using GraphPad Prism 9.5.0.
CYP388A1 and CYP392E3 were expressed using the E. coli prokaryotic expression system75,76. The 17α N-terminal modification strategy was applied to the CYP388A1 and CYP392E3 sequences (Supplementary Data 3) to optimize the expression of the P450 genes in E. coli. This procedure involved replacing the first eight amino acid residues with a specific sequence (MALLLAVF: ATGGCTCTGTTATTAGCAGTTTTT). The modified full-length open reading frame sequences of CYP388A1 and CYP392E3, excluding their signal peptides, were cloned into the pCold II vector. Supplementary Data 2 shows the primer information. The recombinant vector was transformed into BL21 (DE3)-competent cells. A single colony was cultured overnight at 37 °C with shaking. The cells were diluted 1:100 in terrific broth medium containing 100 mg/L ampicillin, 1 M vitamin B1, and trace microelements, and the culture was incubated at 37 °C with shaking until the OD600 reached 0.6–0.8. IPTG (1 mM) and α-ALA (0.5 mM) were added to the suspension, and the culture was incubated at 15 °C for 12–16 h at 180 rpm. The cells were harvested by centrifugation at 4000 g for 30 min at 4 °C. The spheroplasts were resuspended in 2× TSE buffer (0.1 M Tris-acetate, pH 7.8; 0.5 M sucrose; 0.5 mM EDTA) containing lysozyme (0.25 mg/mL) and gently mixed for 30 min at 4 °C. The mixture was centrifuged at 2800 g for 25 min at 4 °C, and the resulting pellet was resuspended in SP buffer (0.1 M potassium phosphate buffer, pH 7.8; 6 mM magnesium acetate; 20% glycerol) containing 0.5 mM dithiothreitol and 1 mM phenylmethanesulfonyl fluoride (PMSF). The suspension was disrupted by sonication (8-s pulses, 150 W) on ice for 30 min, followed by centrifugation at 9000 g for 30 min at 4 °C. The supernatant was further centrifuged at 100,000 g for 60 min. The membrane fractions were diluted in 1× TSE buffer and stored at −80 °C77. The protein content, activity and enzyme kinetic parameters of the expressed P450 protein were measured as described64,78. The potential interaction between recombinant proteins (CYP388A1/CPR = 1:2 protein/protein) and acaricides were evaluated by measuring the inhibition of activity toward p-nitroanisole. The expression and purification of the NADPH-cytochrome P450 reductase (CPR) protein from T.cinnabarinus were conducted according to a previously published study64. Briefly, recombinant proteins were incubated at 30 °C for 10 min with varying concentrations of acaricides (0–0.8 mM) in 5% acetone. The percentage inhibition of CYP388A1 activity was determined using 0.1 M p-nitroanisole as the substrate. The IC50 value was calculated using GraphPad Prism version 9.5.
The TcCCE34 protein was expressed using the Pichia pastoris eukaryotic expression system79. The open reading frame sequences of TcCCE34 (Supplementary File 11) without their signaling peptides were cloned to the PPIC9k vector. The recombinant plasmid was linearized with SacI and transformed into the P. pastoris GS115 strain using a chemical transformation method (Coolaber, China). Positive transformants were selected on minimal dextrose (MD) agar plates and screened for protein expression. For protein induction, a single positive colony was cultured in 25 mL of buffered glycerol-complex medium (BMGY) at 30 °C with shaking at 250 rpm until the optical density at 600 nm reached 2.0–6.0. The cells were harvested by centrifugation at 3000 g for 5 min at room temperature and resuspended in 50 mL of buffered methanol-complex medium (BMMY) to induce protein expression. Methanol was added to a final concentration of 1% (v/v) every 24 h, and the culture was maintained at 30 °C with shaking at 250 rpm for 96 h. The cells were harvested by centrifugation at 4000 g for 30 min at 4 °C. The cell pellets were resuspended in 20 mL of PBS (0.04 M, pH 7.4) and lysed by sonication (8-s pulses, 150 W) on ice for 30 min. The cell lysate was centrifuged at 9000 g for 30 min at 4 °C. The resulting supernatant was purified using a Ni-NTA preloaded gravity column (Sangon Biotech Co., Ltd., Shanghai, China). Buffer I (0.04 M PBS, pH 7.4, 0.5 M NaCl, 40 mM imidazole) was used to remove impurities, and the target protein was eluted using Buffer II (0.04 M PBS, pH 7.4, 0.5 M NaCl, 500 mM imidazole). The activity and enzyme kinetic parameters of the expressed TcCCE34 protein was measured as described80. The potential interaction between recombinant TcCCE34 protein and acaricides was assessed by measuring the inhibition of enzymatic activity toward α-naphthyl acetate. Briefly, recombinant proteins were incubated at 30 °C for 10 min with varying concentrations of acaricides (0.1–10 mM) in 5% acetone. The percentage inhibition of TcCCE34 activity was determined using 3 × 10−4 mol/L α-naphthyl acetate as the substrate. The IC50 value was calculated using GraphPad Prism version 9.5.
In vitro acaricide metabolism by recombinant detoxification protein
HPLC analysis was conducted using an Agilent 1260 system with a G1322A degasser, G1311B quaternary pump, G1329B autosampler, G1316A column compartment, and G1315D diode array detector. Samples were transferred to HPLC vials and injected (10 μL) onto a C18 reverse-phase analytical column (4.6 × 250 mm, 5 μm; Agilent Technologies, Santa Clara, CA, USA). Data acquisition was performed using Agilent ChemStation software. The detailed protocol for the decomposition of acaricides by recombinant proteins is described below.
High-performance liquid chromatography (HPLC) analysis was used to measure the degradation capacity of the TcGSTm8 protein for fenpropathrin, cyflumetofen, and chlorpyrifos23. To plot a standard curve, the gradient concentration of acaricide reference standard was used as the X-axis and the peak area was used as the Y-axis (Supplementary Fig. 9a–c). The standard curve was then used to calculate the acaricide concentration. Three replicates were performed for treatment and controls. In brief, the 1-mL reaction system is composed of 10 mg L−1 acaricide (final concentration); 10, 20, or 40 µg recombinant protein; 2.5 mM GSH; and PBS (0.04 M, pH 6.5). This mixture was incubated at 30 °C and 250 rpm for 1 h before 750-µL acetonitrile or ethyl acetate was added to stop the reaction. The mixture was then centrifuged at 4 °C and 16,000 g for 10 min. Thereafter, 500-µL supernatant was transferred to a HPLC vial, and 10 µL was injected into a C18 (4.6 × 250 mm, 5 µm) reverse phase analysis column (Agilent Technologies, CA, USA). As cyflumetofen is unstable in the aqueous phase due to auto-degradation, three control groups were used in this study, namely CK1: recombinant protein + 10 mg L−1 cyflumetofen, 0-h reaction; CK2: inactivated recombinant protein + 10 mg L−1 cyflumetofen, 1-h reaction; and CK3: PBS without recombinant protein + 10 mg L−1 cyflumetofen, 1-h reaction.
Similarly, HPLC analysis was used to measure the degradation capacity of the CYP388A1 protein for fenpropathrin, cyflumetofen, and chlorpyrifos64. The 1-mL reaction system is composed of a 1:2 ratios of P450 and CPR, 0.25 mM MgCl2, 1 mM glucose-6-phosphate, 1 U glucose-6-phosphate dehydrogenase, 0.1 mM NADPH, and 10 mg L−1 fenpropathrin, cyflumetofen, or chlorpyrifos. The NADPH-free reaction system was used as the control. The cyflumetofen control was set up identically to the method used for TcGSTm8 metabolism, and the reaction conditions and extraction process were the same as above.
HPLC analysis was used to measure the degradation capacity of the TcCCE34 protein for fenpropathrin, cyflumetofen, and chlorpyrifos80. The 1-mL reaction system is composed of 10 mg L−1 acaricides and 10, 20, or 40 µg recombinant TcCCE34 protein. The inactivated-protein reaction system was used as the control. The cyflumetofen control was set up identically to the method used for TcGSTm8 metabolism, and the reaction conditions and extraction process were the same as above. Under 40°C and a flow rate of 0.8 mL min−1, the mobile phase consisting of 90% A:10% B (A: acetonitrile, B: water) was used to separate fenpropathrin and the sample. Monitoring of fenpropathrin elution was conducted using absorbance changes at wavelengths of 210 and 254 nm. Then, 80% A:20% B (A: acetonitrile, B: water) was used to separate cyflumetofen and the sample, with a flow rate of 0.8 mL/min−1 and a detection temperature of 40 °C. Cyflumetofen elution was monitored by observing changes in absorbance at 210 and 230 nm. Finally, 80% A:20% B (A: acetonitrile, B: water) was used to separate chlorpyrifos and the sample, with a flow rate of 0.8 mL/min−1 and a detection temperature of 30 °C. Changes in absorbance at 235 and 290 nm were used to monitor chlorpyrifos elution.
Analysis of acaricide metabolites by LC-MS
The enzymatic activity of CYP388A1 with fenpropathrin and chlorpyrifos (with or without an NADPH-generating system), as well as the activity of TcCCE34 with fenpropathrin, was further investigated using a high-resolution HPLC-MS/MS system. Chromatographic separation was performed on a Vanquish Flex UHPLC system coupled to an Orbitrap Exploris 120 mass spectrometer (Thermo Scientific, USA), equipped with a 1.7 μm ACQUITY BEH C18 (100 × 2.1 mm) reverse-phase analytical column (Waters, USA). The reactions were analyzed using an isocratic mobile phase of 0.1% formic acid water and methanol, flowing at 0.3 mL/min for 20 min. Detection was conducted using heated electrospray ionization (HESI) in positive ion mode for both fenpropathrin and chlorpyrifos. Mass spectrometric detection employed full-scan and extracted ion monitoring for each acaricide. Data analysis were conducted using Xcalibur (v.4.0) software (Thermo Scientific, USA). Spectrometer parameters included a spray voltage of 3800 V, sheath gas flow rate was set at 40 mL/min psi, auxiliary gas was set at 8 mL/min, ion transfer capillary temperature maintained at 320 °C, and the source collision induced dissociation at 30 eV.
Statistics and reproducibility
Student’s t-test, analysis of variance (ANOVA), analysis of Two-way ANOVA, and Tukey’s HSD were used for statistical analysis of intersample differences. All quantitative data was expressed as the mean ± standard error (SE). SPSS v. 22.0 was used for statistical analysis in this study. The sample size “n” refers to biologically independent samples or experiments. In Fig. 1b, the specific “n” values range from 17 to 34, while the remaining experiments are set to three biological replicates. * P < 0.05, ** P < 0.01, *** P < 0.001.
Data availability
The raw sequence data of DAP-seq reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/ Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA020103). The genome of T. cinnabarinus is available under NCBI BioProject number PRJNA1227279. All source data used to produce the graphs in this work are provided in Supplementary Data 1. The DNA sequence information in this study is provided in Supplementary Data 3. Uncropped blots/gels are provided in Supplementary Figs. 12–15. All other data are available from the corresponding author (or other sources, as applicable) on reasonable request.
Change history
16 September 2025
A Correction to this paper has been published: https://doi.org/10.1038/s42003-025-08860-9
References
Gerson, U. & Weintraub, P. G. Mites (Acari) as a factor in greenhouse management. Annu. Rev. Entomol. 57, 229–247 (2012).
Auger, P., Migeon, A., Ueckermann, E. A., Tiedt, L. & Navajas, M. Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): review and new data. Acarologia 53, 383–415 (2013).
Li, X. C., Schuler, M. A. & Berenbaum, M. R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231–253 (2007).
Van Leeuwen, T. & Dermauw, W. The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu. Rev. Entomol. 61, 475–498 (2016).
Amezian, D., Nauen, R. & Le Goff, G. Transcriptional regulation of xenobiotic detoxification genes in insects-An overview. Pest. Biochem. Physiol. 174, 10 (2021).
Grbic, M. et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479, 487–492 (2011).
Li, X. C., Deng, Z. Y. & Chen, X. W. Regulation of insect P450s in response to phytochemicals. Curr. Opin. Insect Sci. 43, 108–116 (2021).
Brown, R. P., McDonnell, C. M., Berenbaum, M. R. & Schuler, M. A. Regulation of an insect cytochrome P450 monooxygenase gene (CYP6B1) by aryl hydrocarbon and xanthotoxin response cascades. Gene 358, 39–52 (2005).
Wen, X. et al. A detoxification pathway initiated by a nuclear receptor TcHR96h in Tetranychus cinnabarinus (Boisduval). PLoS Genet. 19, 27 (2023).
Guo, Z. J., Qin, J. Y., Zhou, X. M. & Zhang, Y. J. Insect transcription factors: a landscape of their structures and biological functions in Drosophila and beyond. Int. J. Mol. Sci. 19, 33 (2018).
Hung, C. F., Holzmacher, R., Connolly, E., Berenbaum, M. R. & Schuler, M. A. Conserved promoter elements in the CYP6B gene family suggest common ancestry for cytochrome P450 monooxygenases mediating furanocoumarin detoxification. Proc. Natl. Acad. Sci. USA93, 12200–12205 (1996).
Wilding, C. S. Regulating resistance: CncC:Maf, antioxidant response elements and the overexpression of detoxification genes in insecticide resistance. Curr. Opin. Insect Sci. 27, 89–96 (2018).
Misra, J. R., Horner, M. A., Lam, G. & Thummel, C. S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Faseb J. 26, 1 (2012).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Dowling, D. K. & Simmons, L. W. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B-Biol. Sci. 276, 1737–1745 (2009).
Mittler, R., Vanderauwera, S., Gollery, M. & Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498 (2004).
Eijkelenboom, A. & Burgering, B. M. T. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Lee, K. S. et al. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J. Biol. Chem. 284, 29454–29461 (2009).
Essers, M. A. G. et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 23, 4802–4812 (2004).
van der Heide, L. P., Hoekman, M. F. M. & Smidt, M. P. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem. J. 380, 297–309 (2004).
Klotz, L. O. & Steinbrenner, H. Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 13, 646–654 (2017).
Bartlett, A. et al. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat. Protoc. 12, 1659–1672 (2017).
Feng, K. Y. et al. Stability of cyflumetofen resistance in Tetranychus cinnabarinus and its correlation with glutathione-S-transferase gene expression. Pest Manag. Sci. 75, 2802–2809 (2019).
Feng, K. Y. et al. The dynamic changes of genes revealed that persistently overexpressed genes drive the evolution of cyflumetofen resistance in Tetranychus cinnabarinus. Insect Sci. 20, 1129–1148 (2022).
War, A. R. et al. Plant defence against herbivory and insect adaptations. Aob Plants 10, 19 (2018).
Feyereisen, R. Insect CYP Genes and P450 Enzymes. (Elsevier Academic Press Inc, 2012).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 (2014).
Lu, K. et al. Phytochemical flavone confers broad-spectrum tolerance to insecticides in Spodoptera litura by activating ROS/CncC-mediated xenobiotic detoxification pathways. J. Agric. Food Chem. 69, 7429–7445 (2021).
Fujii, T. et al. Energy reserve compensating for trade-off between metabolic resistance and life history traits in the brown planthopper (Hemiptera: Delphacidae). J. Econ. Entomol. 113, 1963–1971 (2020).
Heidel-Fischer, H. M. & Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 8, 8–14 (2015).
Dermauw, W. et al. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc. Natl. Acad. Sci. USA110, E113–E122 (2013).
Wang, X. et al. Mechanism of neonicotinoid toxicity: impact on oxidative stress and metabolism. Annu. Rev. Pharmacol. Toxicol. 58, 471–507 (2018).
Martelli, F. et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA117, 25840–25850 (2020).
Lu, K. et al. Activation of CncC pathway by ROS burst regulates cytochrome P450 CYP6AB12 responsible for lambda-cyhalothrin tolerance in Spodoptera litura. J. Hazard. Mater. 387, 10 (2020).
Zhang, Y. H. et al. Dual oxidase-dependent reactive oxygen species are involved in the regulation of UGT overexpression-mediated clothianidin resistance in the brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 77, 4159–4167 (2021).
Yao, Z. C. et al. The dual oxidase gene BdDuox regulates the intestinal bacterial community homeostasis of Bactrocera dorsalis. ISME J. 10, 1037–1050 (2016).
Hanukoglu, I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab. Rev. 38, 171–196 (2006).
Mangum, L. C. et al. Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2 arachidonic acid. Chem. Res. Toxicol. 28, 570–584 (2015).
Hayashi, N., Sasama, Y., Takahashi, N. & Ikemi, N. Cyflumetofen, a novel acaricide - its mode of action and selectivity. Pest Manag. Sci. 69, 1080–1084 (2013).
Mittler, R. ROS are good. Trends Plant Sci. 22, 11–19 (2017).
Foyer, C. H. & Noctor, G. Redox signaling in plants. Antioxid. Redox Sign. 18, 2087–2090 (2013).
Jiang, H. et al. The pleiotropic AMPK-CncC signaling pathway regulates the trade-off between detoxification and reproduction. Proc. Natl. Acad. Sci. USA120, 9 (2023).
Deng, H. & Kerppola, T. K. Visualization of the Drosophila dKeap1-CncC interaction on chromatin illumines cooperative, xenobiotic-specific gene activation. Development 141, 3277–3288 (2014).
Kalsi, M. & Palli, S. R. Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 65, 47–56 (2015).
Shi, L. et al. The expression of P450 genes mediating fenpropathrin resistance is regulated by CncC and Maf in Tetranychus cinnabarinus (Boisduval). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 198, 28–36 (2017).
Klotz, L. O. et al. Redox regulation of FoxO transcription factors. Redox Biol. 6, 51–72 (2015).
Storz, P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid. Redox Sign. 14, 593–605 (2011).
Lehtinen, M. K. et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125, 987–1001 (2006).
Oh, S. W. et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 102, 4494–4499 (2005).
Zhang, N. et al. Restoration of energy homeostasis under oxidative stress: duo synergistic AMPK pathways regulating arginine kinases. PLoS Genet. 19, 33 (2023).
de Keizer, P. L. J., Burgering, B. M. T. & Dansen, T. B. Forkhead box O as a sensor, mediator, and regulator of redox signaling. Antioxid. Redox Sign. 14, 1093–1106 (2011).
Tian, Z. Q. et al. FoxO-promoted peroxiredoxin1 expression induced by Helicoverpa armigera single nucleopolyhedrovirus infection mediates host development and defensive responses. Ecotoxicol. Environ. Safe. 234, 9 (2022).
Chen, J. et al. The function of LmPrx6 in diapause regulation in Locusta migratoria through the insulin signaling pathway. Insects 11, 17 (2020).
Schwenke, R. A., Lazzaro, B. P. & Wolfner, M. F. Reproduction-immunity trade-offs in insects. Annu. Rev. Entomol. 61, 239–256 (2016).
Xu, X. et al. FOXO acts as a positive regulator of CncC and deltamethrin tolerance in the red flour beetle, Tribolium castaneum. Pest Manag. Sci. 78, 1938–1945 (2022).
Guo, Z. J. et al. Retrotransposon-mediated evolutionary rewiring of a pathogen response orchestrates a resistance phenotype in an insect host. Proc. Natl. Acad. Sci. USA 120, 10 (2023).
Zhang, H. H., Lin, X. M., Yang, B. J., Zhang, L. C. & Liu, Z. W. Two point mutations in CYP4CE1 promoter contributed to the differential regulation of CYP4CE1 expression by foxo between susceptible and nitenpyram-resistant Nilaparvata lugens. J. Agric. Food Chem. 72, 1779–1786 (2024).
Lu, K., Song, Y. Y. & Zeng, R. S. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 43, 103–107 (2021).
Guo, Z. J. et al. A single transcription factor facilitates an insect host combating Bacillus thuringiensis infection while maintaining fitness. Nat. Commun. 13, 15 (2022).
Guo, L. et al. MAPK-mediated transcription factor GATAd contributes to Cry1Ac resistance in diamondback moth by reducing PxmALP expression. PLoS Genet. 18, 22 (2022).
Hu, B. et al. Transcription factors CncC/Maf and AhR/ARNT coordinately regulate the expression of multiple GSTs conferring resistance to chlorpyrifos and cypermethrin in Spodoptera exigua. Pest Manag. Sci. 75, 2009–2019 (2019).
Kim, I. Y. et al. Nuclear receptor HR96 up-regulates cytochrome P450 for insecticide detoxification in Tribolium castaneum. Pest Manag. Sci. 78, 230–239 (2022).
Ji, M. Y. et al. A nuclear receptor HR96-related gene underlies large trans-driven differences in detoxification gene expression in a generalist herbivore. Nat. Commun. 14, 17 (2023).
Feng, K. Y. et al. The cytochrome P450 CYP389C16 contributes to the cross-resistance between cyflumetofen and pyridaben in Tetranychus cinnabarinus (Boisduval). Pest Manag. Sci. 76, 665–675 (2020).
Pavlidi, N., Vontas, J. & Van Leeuwen, T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Curr. Opin. Insect Sci. 27, 97–102 (2018).
Satoh, T. & Hosokawa, M. The mammalian carboxylesterases: From molecules to functions. Annu. Rev. Pharmacol. Toxicol. 38, 257–288 (1998).
Wei, P. et al. Functional analysis of four upregulated carboxylesterase genes associated with fenpropathrin resistance in Tetranychus cinnabarinus (Boisduval). Pest Manag. Sci. 75, 252–261 (2019).
Bradford, M. M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Aebi, H. Catalase in vitro. Methods enzymol. 105, 121–126 (1984).
Shang, C. C. & Soderlund, D. M. Monooxygenase activity of tobacco budworm (Heliothis-virescens F) larvae - tissue distribution and optimal assay conditions for the gut activity. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 79, 407–411 (1984).
Clark, A. G., Dick, G. L. & Smith, J. N. Kinetic-studies on a glutathione S-transferase from the larvae of Costelytra-zealandica. Biochem. J. 217, 51–58 (1984).
Vanasperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 8, 401–414 (1962).
Feng, Y.-N. et al. The sodium channel gene in Tetranychus cinnabarinus (Boisduval): identification and expression analysis of a mutation associated with pyrethroid resistance. Pest Manag. Sci. 67, 904–912 (2011).
Feng, K., Wen, X. & Lin, H. Chromosome-level genome assembly of the spider mite Tetranychus cinnabarinus. bioRxiv https://doi.org/10.1101/2025.06.24.661196 (2025).
Tian, K., Liu, D., Yuan, Y. Y., Li, M. & Qiu, X. H. CYP6B6 is involved in esfenvalerate detoxification in the polyphagous lepidopteran pest, Helicoverpa armigera. Pest. Biochem. Physiol. 138, 51–56 (2017).
Vontas, J. et al. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA 115, 4619–4624 (2018).
Cheesman, M. J. et al. Soluble and membrane-bound Drosophila melanogaster CYP6G1 expressed in Escherichia coli: Purification, activity, and binding properties toward multiple pesticides. Insect Biochem. Mol. Biol. 43, 455–465 (2013).
Guengerich, F. P., Martin, M. V., Sohl, C. D. & Cheng, Q. Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 4, 1245–1251 (2009).
Tolner, B., Smith, L., Begent, R. H. J. & Chester, K. A. Production of recombinant protein in Pichia pastoris by fermentation. Nat. Protoc. 1, 1006–1021 (2006).
Wei, P. et al. Downregulation of carboxylesterase contributes to cyflumetofen resistance in Tetranychus cinnabarinus (Boisduval). Pest Manag. Sci. 75, 2166–2173 (2019).
Acknowledgements
The work is supported by National Natural Science Foundation of China (32472597 and U2202202 to Lin He, 32202337 to Kaiyang Feng), the National Key Research and Development Program of China (2023YFD1700702 to Lin He). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: X.W., K.F., and L.H.; Investigation: X.W., K.F., P.W., and J.L. Methodology: X.W. and K.F.; Visualization: X.W. and J.L.; Writing – Original Draft Preparation: X.W., K.F., and L.H.; Writing – Review & Editing: X.W., P.W., Z.G., M.L., W.D., Y.Z., and L.H.; Funding Acquisition: K.F. and L.H.; Supervision: L.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Yutao Xiao, Yongqiang Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wen, X., Feng, K., Wei, P. et al. The ROS–FOXO pathway mediates broad-spectrum detoxification of acaricides in Tetranychus cinnabarinus. Commun Biol 8, 1274 (2025). https://doi.org/10.1038/s42003-025-08726-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08726-0