Abstract
Lysine β-hydroxybutyrylation (Kbhb) is a post-translational modification induced by the ketogenic diet (KD), a diet showing therapeutic effects on multiple human diseases. Little is known how cellular processes are regulated by Kbhb. Here we show that protein Kbhb is strongly affected by the KD through a multi-omics analysis of mouse livers. Using a small training dataset with known functions, we developed a bioinformatics method for the prediction of functionally important lysine modification sites (pFunK), which revealed functionally relevant Kbhb sites on various proteins, including aldolase B (ALDOB) Lys108. KD consumption or β-hydroxybutyrate supplementation in hepatocellular carcinoma cells increases ALDOB Lys108bhb and inhibits the enzymatic activity of ALDOB. A Kbhb-mimicking mutation (p.Lys108Gln) attenuates ALDOB activity and its binding to substrate fructose-1,6-bisphosphate, inhibits mammalian target of rapamycin signalling and glycolysis, and markedly suppresses cancer cell proliferation. Our study reveals a critical role of Kbhb in regulating cancer cell metabolism and provides a generally applicable algorithm for predicting functionally important lysine modification sites.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw RNA-seq data that support the findings of this study are deposited in the Gene Expression Omnibus (GEO) under accession code GSE238099. The raw MS/MS data of proteomics, Kbhb and metabolomics profiles have been deposited to the integrated proteome resources (iProX, https://www.iprox.org/), under dataset identifier PXD044027. Previously reported Kbhb, Kla, Kac and Kcr sites used for model training were taken from the database CPLM 4.0, accessible at http://cplm.biocuckoo.cn/. Source data are provided with this paper. All other relevant data supporting the findings of this study are included within the article and its Supplementary Information.
Code availability
The source code of pFunK and other custom code is available on GitHub at https://github.com/biocuckooHXH/pFunK/.
References
Ułamek-Kozioł, M., Czuczwar, S. J., Januszewski, S. & Pluta, R. Ketogenic diet and epilepsy. Nutrients 11, 2510 (2019).
Dowis, K. & Banga, S. The potential health benefits of the ketogenic diet: a narrative review. Nutrients 13, 1654 (2021).
Zhu, H. et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 7, 11 (2022).
Westerterp-Plantenga, M. S., Nieuwenhuizen, A., Tomé, D., Soenen, S. & Westerterp, K. R. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr. 29, 21–41 (2009).
Veldhorst, M. et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol. Behav. 94, 300–307 (2008).
Ma, S., Huang, Q., Tominaga, T., Liu, C. & Suzuki, K. An 8-week ketogenic diet alternated interleukin-6, ketolytic and lipolytic gene expression, and enhanced exercise capacity in mice. Nutrients 10, 1696 (2018).
Fine, E. J. & Feinman, R. D. Thermodynamics of weight loss diets. Nutr. Metab. 1, 15 (2004).
Feinman, R. D. & Fine, E. J. Nonequilibrium thermodynamics and energy efficiency in weight loss diets. Theor. Biol. Med. Model. 4, 27 (2007).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Barry, D. et al. The ketogenic diet in disease and development. Int. J. Dev. Neurosci. 68, 53–58 (2018).
Miyamoto, J. et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl Acad. Sci. USA 116, 23813–23821 (2019).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Koronowski, K. B. et al. Ketogenesis impact on liver metabolism revealed by proteomics of lysine β-hydroxybutyrylation. Cell Rep. 36, 109487 (2021).
Zheng, Y. et al. β-hydroxybutyrate inhibits ferroptosis-mediated pancreatic damage in acute liver failure through the increase of H3K9bhb. Cell Rep. 41, 111847 (2022).
Terranova, C. J. et al. Reprogramming of H3K9bhb at regulatory elements is a key feature of fasting in the small intestine. Cell Rep. 37, 110044 (2021).
Zhang, H. et al. Ketogenesis-generated beta-hydroxybutyrate is an epigenetic regulator of CD8+ T-cell memory development. Nat. Cell Biol. 22, 18–25 (2020).
Liu, K. et al. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. 10, 243 (2019).
Li, H. et al. β-hydroxybutyrate reduces reinstatement of cocaine conditioned place preference through hippocampal CaMKII-α β-hydroxybutyrylation. Cell Rep. 41, 111724 (2022).
Nasser, S. et al. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol. Metab. 65, 101578 (2022).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 (2017).
Yang, Z. et al. Effects of low-carbohydrate diet and ketogenic diet on glucose and lipid metabolism in type 2 diabetic mice. Nutrition 89, 111230 (2021).
Martínez-Reyes, I. & Chandel, N. S. Cancer metabolism: looking forward. Nat. Rev. Cancer 21, 669–680 (2021).
You, M. et al. Signaling pathways in cancer metabolism: mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 8, 196 (2023).
Hoy, A. J., Nagarajan, S. R. & Butler, L. M. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat. Rev. Cancer 21, 753–766 (2021).
Ochoa, D. et al. The functional landscape of the human phosphoproteome. Nat. Biotechnol. 38, 365–373 (2020).
Vaswani, A. et al. Attention is all you need. Adv. Neural Inf. Process. Syst. 30, 5998–6008 (2017).
Finn, C., Abbeel, P. & Levine, S. Model-agnostic meta-learning for fast adaptation of deep networks. Proc. 34th International Conference on Machine Learning 70, 1126–1135 (2017).
Lundberg, S. M. & Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 30, 4768–4777 (2017).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Rutter, W. J., Rajkumar, T., Penhoet, E., Kochman, M. & Valentine, R. Aldolase variants: structure and physiological significance. Ann. N. Y. Acad. Sci. 151, 102–117 (1968).
Chang, Y. C., Yang, Y. C., Tien, C. P., Yang, C. J. & Hsiao, M. Roles of aldolase family genes in human cancers and diseases. Trends Endocrinol. Metab. 29, 549–559 (2018).
Shafqat, N., Turnbull, A., Zschocke, J., Oppermann, U. & Yue, W. W. Crystal structures of human HMG-CoA synthase isoforms provide insights into inherited ketogenesis disorders and inhibitor design. J. Mol. Biol. 398, 497–506 (2010).
Zhang, Z., Bi, X., Lian, X. & Niu, Z. BDH1 promotes lung cancer cell proliferation and metastases by PARP1-mediated autophagy. J. Cell. Mol. Med. 27, 939–949 (2023).
Zhang, C. S. et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116 (2017).
Li, M. et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 31, 478–481 (2021).
Ren, C. et al. Expanding the scope of genetically encoded lysine post-translational modifications with lactylation, beta-hydroxybutyrylation and lipoylation. ChemBioChem 23, e202200302 (2022).
Huang, H. et al. The regulatory enzymes and protein substrates for the lysine beta-hydroxybutyrylation pathway. Sci. Adv. 7, eabe2771 (2021).
Oughtred, R. et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 47, D529–D541 (2019).
You, Z. et al. Requirement for p62 acetylation in the aggregation of ubiquitylated proteins under nutrient stress. Nat. Commun. 10, 5792 (2019).
Roberts, M. N. et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 26, 539–546 (2017).
Wang, Y. H., Liu, C. L., Chiu, W. C., Twu, Y. C. & Liao, Y. J. HMGCS2 mediates ketone production and regulates the proliferation and metastasis of hepatocellular carcinoma. Cancers 11, 1876 (2019).
Wang, Y. H., Suk, F. M. & Liao, Y. J. Loss of HMGCS2 enhances lipogenesis and attenuates the protective effect of the ketogenic diet in liver cancer. Cancers 12, 1797 (2020).
Dmitrieva-Posocco, O. et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 605, 160–165 (2022).
Shang, S., Wang, L., Zhang, Y., Lu, H. & Lu, X. The beta-hydroxybutyrate suppresses the migration of glioma cells by inhibition of NLRP3 inflammasome. Cell Mol. Neurobiol. 38, 1479–1489 (2018).
Shukla, S. K. et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2, 18 (2014).
Sabatini, D. M. Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc. Natl Acad. Sci. USA 114, 11818–11825 (2017).
Shim, H. et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl Acad. Sci. USA 94, 6658–6663 (1997).
Huang, K. et al. A novel allosteric inhibitor of phosphoglycerate mutase 1 suppresses growth and metastasis of non-small-cell lung cancer. Cell Metab. 30, 1107–1119 (2019).
Qin, Y. J. et al. Loss of PDK4 expression promotes proliferation, tumorigenicity, motility and invasion of hepatocellular carcinoma cells. J. Cancer 11, 4397–4405 (2020).
Qie, S. & Diehl, J. A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 94, 1313–1326 (2016).
Dang, C. V. MYC on the path to cancer. Cell 149, 22–35 (2012).
Kim, H., Jung, W., Kim, A., Kim, H. K. & Kim, B. H. High Paip1 expression as a potential prognostic marker in hepatocellular carcinoma. In Vivo 34, 2491–2497 (2020).
UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Yang, Z. et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 5, 61–79 (2023).
Yu, K. et al. Deep learning based prediction of reversible HAT/HDAC-specific lysine acetylation. Brief. Bioinform. 21, 1798–1805 (2020).
Popa, F. I. et al. 3-hydroxyacyl-coenzyme a dehydrogenase deficiency: identification of a new mutation causing hyperinsulinemic hypoketotic hypoglycemia, altered organic acids and acylcarnitines concentrations. JIMD Rep. 2, 71–77 (2012).
Koronowski, K. B. et al. Ketogenesis impact on liver metabolism revealed by proteomics of lysine beta-hydroxybutyrylation. Cell Rep. 36, 109487 (2021).
McDaniel, S. S., Rensing, N. R., Thio, L. L., Yamada, K. A. & Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 52, e7–e11 (2011).
Kusakabe, T., Motoki, K. & Hori, K. Mode of interactions of human aldolase isozymes with cytoskeletons. Arch. Biochem. Biophys. 344, 184–193 (1997).
Yao, H., Wei, Y., Huang, J. & Li, Z. Hierarchically structured meta-learning. International Conference on Machine Learning 97, 7045–7054 (2019).
Zheng, Y., Fan, J., Zhang, J. & Gao, X. Discriminative fast hierarchical learning for multiclass image classification. IEEE Trans. Neural Netw. Learn. Syst. 31, 2779–2790 (2020).
Li, Z., Gong, D., Li, X. & Tao, D. Aging face recognition: a hierarchical learning model based on local patterns selection. IEEE Trans. Image Process. 25, 2146–2154 (2016).
Xu, L. C. et al. Towards data-driven design of asymmetric hydrogenation of olefins: database and hierarchical learning. Angew. Chem. Int. Ed. Engl. 60, 22804–22811 (2021).
Fazeli, N. et al. See, feel, act: hierarchical learning for complex manipulation skills with multisensory fusion. Sci. Robot 4, eaav3123 (2019).
Gene Ontology Consortium. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 49, D325–D334 (2021).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Zhang, W. et al. CPLM 4.0: an updated database with rich annotations for protein lysine modifications. Nucleic Acids Res. 50, D451–D459 (2022).
Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Wang, C. et al. GPS 5.0: an update on the prediction of kinase-specific phosphorylation sites in proteins. Genomics Proteom. Bioinformatics 18, 72–80 (2020).
Ning, W. et al. GPS-Palm: a deep learning-based graphic presentation system for the prediction of S-palmitoylation sites in proteins. Brief. Bioinform. 22, 1836–1847 (2021).
Ning, W. et al. HybridSucc: a hybrid-learning architecture for general and species-specific succinylation site prediction. Genomics Proteom. Bioinformatics 18, 194–207 (2020).
Chen, M. et al. GPS 6.0: an updated server for prediction of kinase-specific phosphorylation sites in proteins. Nucleic Acids Res. 51, W243–W250 (2023).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Yang, Y. et al. SPIDER2: a package to predict secondary structure, accessible surface area, and main-chain torsional angles by deep neural networks. Methods Mol. Biol. 1484, 55–63 (2017).
Galloway, C. D. et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol. 4, 428–436 (2019).
Li, W., Xu, Z., Xu, D., Dai, D. & Van Gool, L. Domain generalization and adaptation using low rank exemplar SVMs. IEEE Trans. Pattern Anal. Mach. Intell. 40, 1114–1127 (2018).
Zhao, X., Wu, S., Fang, N., Sun, X. & Fan, J. Evaluation of single-cell classifiers for single-cell RNA sequencing data sets. Brief. Bioinform. 21, 1581–1595 (2020).
Pei, S., Chen, H., Nie, F., Wang, R. & Li, X. Centerless clustering. IEEE Trans. Pattern Anal. Mach. Intell. 45, 167–181 (2023).
Yu, Y. & Tran, H. An XGBoost-based fitted Q iteration for finding the optimal STI strategies for HIV patients. IEEE Trans. Neural Netw. Learn. Syst. 35, 648–656 (2022).
Schwartz, D. & Gygi, S. P. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat. Biotechnol. 23, 1391–1398 (2005).
Kumar, M. et al. ELM—the Eukaryotic Linear Motif resource—2024 update. Nucleic Acids Res. 52, D442–D455 (2024).
Shen, X. et al. A thiazole-derived oridonin analogue exhibits antitumor activity by directly and allosterically inhibiting STAT3. J. Biol. Chem. 294, 17471–17486 (2019).
Han, Z. et al. Model-based analysis uncovers mutations altering autophagy selectivity in human cancer. Nat. Commun. 12, 3258 (2021).
Tu, Y. et al. TBC1D23 mediates Golgi-specific LKB1 signaling. Nat. Commun. 15, 1785 (2024).
Zhao, L. et al. FAM91A1–TBC1D23 complex structure reveals human genetic variations susceptible for PCH. Proc. Natl Acad. Sci. USA 120, e2309910120 (2023).
Webster, J. & Oxley, D. Protein identification by MALDI-TOF mass spectrometry. Methods Mol. Biol. 800, 227–240 (2012).
Takanashi, M. & Saito, T. Characterization of two 3-hydroxybutyrate dehydrogenases in poly(3-hydroxybutyrate)-degradable bacterium, Ralstonia pickettii T1. J. Biosci. Bioeng. 101, 501–507 (2006).
Shimazu, T. et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 12, 654–661 (2010).
Andrew Skaff, D. & Miziorko, H. M. A visible wavelength spectrophotometric assay suitable for high-throughput screening of 3-hydroxy-3-methylglutaryl-CoA synthase. Anal. Biochem. 396, 96–102 (2010).
Rardin, M. J. et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 18, 920–933 (2013).
Zhang, S. et al. FSP1 oxidizes NADPH to suppress ferroptosis. Cell Res. 33, 967–970 (2023).
Robinson, A. D., Eich, M. L. & Varambally, S. Dysregulation of de novo nucleotide biosynthetic pathway enzymes in cancer and targeting opportunities. Cancer Lett. 470, 134–140 (2020).
Kotlyar, M., Pastrello, C., Malik, Z. & Jurisica, I. IID 2018 update: context-specific physical protein–protein interactions in human, model organisms and domesticated species. Nucleic Acids Res. 47, D581–D589 (2019).
Li, T. et al. A scored human protein–protein interaction network to catalyze genomic interpretation. Nat. Methods 14, 61–64 (2017).
Calderone, A., Castagnoli, L. & Cesareni, G. mentha: a resource for browsing integrated protein-interaction networks. Nat. Methods 10, 690–691 (2013).
Das, J. & Yu, H. HINT: high-quality protein interactomes and their applications in understanding human disease. BMC Syst. Biol. 6, 92 (2012).
Razick, S., Magklaras, G. & Donaldson, I. M. iRefIndex: a consolidated protein interaction database with provenance. BMC Bioinformatics 9, 405 (2008).
Cowley, M. J. et al. PINA v2.0: mining interactome modules. Nucleic Acids Res. 40, D862–D865 (2012).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
We thank Y. Zhao (The University of Chicago), H. Huang (Chinese Academy of Sciences), J. Cheng (Shanghai Jiao Tong University), Y. Zhang, Y. Gong, S. He, Y. Luo (Sichuan University), S. Tan (Chongqing Medical University), D. Peng, D. Tang (Huazhong University of Science and Technology), F. Tang, J. Li (Peking University) and Z. Fang (Dalian Institute of Chemical Physics) for helpful discussions. We thank PTM Biolabs for kbhb proteomics analysis. We thank J. Zhu and Z. Lin and colleagues (PTM Biolabs) for the MS analysis of β-hydroxybutyrylation. We thank Y. Hu (Sichuan University) for providing plasmids, X. Shen (Chengdu University of Traditional Chinese Medicine), Q. Wei and Z. Liu (Sichuan University) for providing cells. We also thank D. Du, X. Wu and R. Wang (Advanced Mass Spectrometry Center, Research Core Facility, Frontiers Science Center for Disease-related Molecular Network, West China Hospital) for the help with the MS. This work was supported by the National Key R&D Program of China (2022YFA1105200, 2021YFF0702000 and 2022YFC2704304), Natural Science Foundation of China (92254302, 32341020, 32341021 and 31930021), National Science Fund for Distinguished Young Scholars (32125012), Hubei Innovation Group Project (2021CFA005) and Research Core Facilities for Life Science (HUST).
Author information
Authors and Affiliations
Contributions
Y.X. and D.J. conceived and designed the research. J.Q. performed animal studies with assistance from H.C. J.Q., S.G. and Q.Z. performed cellular studies and enzyme activity assays with assistance from S.Z., M.T., S.N., L.Z. and Y.T. S.Z. and J.Q. generated ALDOB Lys108bhb and ALDOB Lys147bhb with assistance from L.S. and W.X. X.H. carried out multi-omics analysis, pFunK model training and the other computational work with the help and discussion of J.Q., M.C., C.H., Y.G., D.L, Z.F., Z.L. and L.D. Y.X., D.J., J.Q. and X.H. wrote the manuscript with input from all the authors. All authors reviewed and approved the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Di Zhang, Jiangning Song, Xiang Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 KD significantly increases Kbhb modification.
Quantification of immunoblotting for PTMs from liver whole-cell lysates (n = 3 per group). The graph showed the fold change quantified by densitometry using Image J software. Statistical data are presented as mean ± SEM, p values were determined by unpaired two-tailed t test. ns (not significant). Ctrl Kbhb vs KD Kbhb: p value: 4.4e − 7.
Extended Data Fig. 2 Additional analyses of mouse liver multi-omic data.
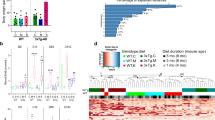
a The distribution of Kbhb sites on β-hydroxybutyrated proteins. b The localization probability scores of Kbhb sites. c Comparison of reported Kbhb sites and Kbhb sites identified in this study. d The word cloud analysis of functional descriptions for proteins containing at least one DRK. e The numbers of clean reads sequenced from Ctrl and KD. f The distribution of numbers of peptides in mappable proteins. g The heatmap of 26 DRK-containing proteins in multiple cancer-associated metabolic processes, including glycolysis, TCA cycle, and fatty acid metabolism.
Extended Data Fig. 3 Performance analysis of pFunK.
a The UMAP analysis of lysine modification sites and other sites separated by pFunK-P. b The UMAP analysis of Kbhb sites and non-Kbhb lysine residues separated by pFunK-T. c ROC curves and AUC values demonstrating the performance of pFunK-T and other methods in distinguishing general lysine modification sites from other sites. d ROC curves and AUC values demonstrating the performance of pFunK and other methods in distinguishing general Kbhb sites from non-Kbhb sites. e ROC curves and corresponding AUC values illustrating the predictive performance of lysine modification sites for various lengths of peptide sequences. f ROC curves and corresponding AUC values illustrating the predictive performance of functional Kbhb sites with various ratios of positive and negative data.
Extended Data Fig. 4 Interpretation analysis of pFunK.
a The Deep SHAP analysis of positive KSP(30, 30) items for training pFunK-P. b The statistical analysis of 20 types of amino acid frequencies at flanking regions of 145,657 non-redundant lysine modifications sites against non-modified lysine residues, using the binomial distribution model. c The sequence logos of flanking sequences around lysine modification sites, using WebLogo 3.029. d Visualization of normalized attention weights for the relationships between different positions. e The 3D surface plot illustrating the contextual feature information between different positions of sequences around lysine modification sites. f, g Deep SHAP analyses of pFunK-T (f) and pFunK (g).
Extended Data Fig. 5 mRNA and protein expression levels of the 27 genes/proteins corresponding to the 33 sites predicted by pFunK.
a Transcript expression level of the 27 genes in Ctrl and KD mice. b Protein expression level of the 27 proteins in Ctrl and KD mice. c The GSEA analysis of the 5,304 Kbhb sites ranked by pFunK scores. Enriched pathways were indicated (p < 0.05, q < 0.25). d The protein expression levels of Aldob, Aldoa, and Aldoc in livers of KD and Ctrl mice, indicated by normalized intensity values. e The mRNA expression levels of ALDOB, ALDOA, and ALDOC in livers of KD and Ctrl mice, indicated by normalized expression values. f The word cloud analysis of GO biological process terms for the 27 proteins harboring at least one predicted functional Kbhb site. g, h The LC/MS-MS analysis of BDH1-derived peptides containing β-hydroxybutyrylated K91 (g) and K97 (h).
Extended Data Fig. 6 KD and Na-β-OHB treatment inhibits the mTOR signaling pathway.
a, b Immunoblotting shows liver Aldob protein levels in male mice fed Ctrl or KD for 5 weeks (n = 4 per group). The graph showed ratios of Aldob to GAPDH quantified by densitometry using Image J software. c, d Representative immunoblotting of post-translational modifications (PTMs) from SK-Hep1 cells treated with 10 mM Na-β-OHB for 24 h (n = 3 biological replicates). Ctrl Kbhb vs KD Kbhb: p value: 7.2e − 7; e Immunoblotting shows the time course of Kbhb and Kac of Aldob in GFP-Aldob HCC-LM3 cells. HCC-LM3 cells transiently transfected with GFP-Aldob were treated with 10 mM Na-β-OHB for multiple time points, and Aldob was immunoprecipitated by its GFP tag. Total Kbhb or Kac levels were also tested. f, g HCC-LM3 (f) or SK-Hep1 cells (g) were treated with 10 mM Na-β-OHB for 24 h, and whole cell lysates were probed by immunoblotting with indicated antibodies. h HCC-LM3 cells were transiently transfected with GFP-Aldob, treated with 10 mM Na-β-OHB for multiple time points, and Aldob was immunoprecipitated by its GFP tag. Samples were first quantified by immunoblotting. The enzymatic activity of Aldob was measured by monitoring the consumption of NADH. i, j Quantification of p-S6K (i) and p-4EBP1 (j) levels in the livers of male mouse fed Ctrl or KD for 5 weeks (n = 6 per group). k Immunoblotting shows p-AMPKα and total AMPKα levels in the livers of male mice fed Ctrl or KD for 5 weeks (n = 6 per group). l, m Quantification of p-AMPKα levels from liver whole-cell lysates (n = 6 per group). n, o Immunoblotting shows the levels of p-S6K and total S6K in HCC-LM3 cells (n) and SK-Hep1 cells (o) treated with Na-β-OHB at a variety of concentrations (1, 5, 10, 20 mM). Similar results were obtained in three independent experiments (c, e-h, n, o). Each dot represents an independent experiment with three technical replicates (d, h). Statistical data are presented as mean ± SEM (b-m), P values were determined by one-way ANOVA, followed by Dunnett’s test (h), or by unpaired two-tailed t test (b, d, i, j, l, m). ns (not significant).
Extended Data Fig. 7 KD and Na-β-OHB treatment increase the levels of K108bhb and K147bhb.
a Conservation of Aldob kbhb sites in multiple organisms. b Specificity of Aldob K108bhb antibody determined by a dot blot assay. Synthetic peptides with K108bhb, K108Ac or unmodified K108 were blotted with the Aldob K108bhb antibody. c Specificity of Aldob K147bhb antibody determined by a dot blot assay. Synthetic peptides with K147bhb, K147Ac or unmodified K147 were blotted with the Aldob K147bhb antibody. d, e Immunoblotting shows the Aldob K108bhb level in male mice livers (n = 3 per group). The mice were fed with a control diet or KD for 5 weeks. The graph showed ratios of K108bhb to Tubulin quantified by densitometry using Image J software. p value: 1.3e − 5. f, g Immunoblotting shows the Aldob K147bhb level in male mice livers (n = 3 per group). The mice were fed with a control diet or KD for 5 weeks. The graph showed ratios of K147bhb to Tubulin quantified by densitometry using Image J software. h, i HCC-LM3 cells were treated with 10 mM Na-β-OHB for 24 h, and whole cell lysates were probed by immunoblotting with anti-K108bhb or anti-Tubulin antibodies. The graph showed ratios of K108bhb to Tubulin quantified by densitometry using Image J software. j, k HCC-LM3 cells were treated with 10 mM Na-β-OHB for 24 h, and whole cell lysates were probed by immunoblotting with anti-K147bhb or anti-Tubulin antibodies. The graph showed ratios of K147bhb to Tubulin quantified by densitometry using Image J software. Experiments in (h, j) were performed in triplicate, each dot represents an independent experiment with three technical replicates (i, k). Statistical data are presented as mean ± SEM (e, g, i, k), P values were determined by unpaired two-tailed t test (e, g, i, k).
Extended Data Fig. 8 Aldob K108bhb attenuates the mTOR signaling.
a, b HCC-LM3 cells were transfected with Aldob WT, K108Q, or K147Q, respectively. After 24 h, cell lysates were immunoprecipitated with GFP beads and probed with antibodies against Aldob and LKB1 (a). The ratios of LKB1 to GFP-Aldob derived from a densitometric analysis of the blot were shown (b). c Cells were stably expressed with Aldob WT, K108Q, K230A, D34S, or K147Q, respectively, and starved for glucose (GS) for 2 h or not starved. The indicated protein levels were detected by immunoblotting. d Representative confocal images showing the colocalization between LAMP1 (purple) and mTORC1 (red) in cells stably expressed with Aldob WT, K108Q, K230A, D34S, or K147Q, respectively. Cells were starved for glucose (GS) for 2 h or not starved, GFP indicates Aldob (green). e Colocalization analysis of LAMP1 and mTORC1 in cells in (d). Each dot represents Mander’s overlap coefficients from one cell. At least 30 cells from each group were used for analysis. MOCK (-GS) vs MOCK ( + GS): p value: 3.2e − 6; WT (-GS) vs WT ( + GS): p value: 2.8e − 5; f Cells stably expressing RNAi-resistant Aldob WT or K108Q were transfected with control or a mix of siRNA targeting Aldoa, Aldob, and Aldoc. The p-S6K and total S6K protein levels were detected by immunoblotting. g Cells stably expressed Aldob WT, K108R, or K147R were treated with 10 mM Na-β-OHB for 24 h. The p-S6K, total S6K and total Aldob protein levels were detected by immunoblotting. h Representative confocal images showing the colocalization between LAMP1 (purple) and mTORC1 (red) in cells stably expressed with Aldob WT, K108R, or K147R. The cells were treated with 10 mM Na-β-OHB for 24 h. GFP indicates Aldob (green). Scale bar: 2 μm. i Colocalization analysis of LAMP1 and mTORC1 in cells in (h). Each dot represents Mander’s overlap coefficients from one cell. At least 30 cells from each group were used for analysis. Similar results were obtained in three independent experiments (a-i). Statistical data are presented as mean ± SEM (a-i), P values were determined by one-way ANOVA, followed by Dunnett’s test (b, i), or by two-way ANOVA, followed by Tukey’s test (e). ns (not significant).
Extended Data Fig. 9 Genetic incorporation of Kbhb at Aldob K108.
a Immunoblotting and coomassie brilliant blue staining analysis of purified Aldob WT and K108bhb. b Intact mass measurement by MALDI-TOF verified the successful introduction of protein β-hydroxybutyrylation at K108 of Aldob. Calculated mass: 43205. c MS/MS analysis confirmed the successful incorporation of Kbhb at Aldob K108. d Enzymatic activities of purified Aldob WT, Aldob mutants, Aldob-K108bhb and Aldob-K108ac. The enzymatic activity of Aldob was measured by monitoring the consumption of NADH. e Relative enzyme activity determined at 14 minutes in (d). Similar results were obtained in three independent experiments (a, d). Statistical data are presented as mean ± SEM (e), P values were determined by one-way ANOVA, followed by Dunnett’s test (e).
Extended Data Fig. 10 Stoichiometry of K108bhb in mice livers or in cells.
a, b Stoichiometry of K108bhb in mouse livers. The liver samples contained same amount of Aldob as purified Aldob-K108bhb protein. Aldob-K108bhb protein was diluted at different fold, loaded to a gel together with the liver samples, and probed using Aldob K108bhb-specific antibody. The stoichiometry of K108bhb was determined by comparing densitometry of the liver samples with that of purified protein. c Lysosome fractions were isolated and probed by immunoblotting with different marker antibodies. d, e The lysosomal samples contained same amount of Aldob as purified Aldob-K108bhb protein. Aldob-K108bhb protein was diluted at different fold, loaded to a gel together with the lysosome samples, and probed using Aldob K108bhb-specific antibody. The stoichiometry of K108bhb was determined by comparing densitometry of the lysosomal samples with that of purified protein. p value: 5.2e − 5; f, g Stoichiometry of K108bhb in HCC-LM3 cells expressing Flag-Aldob-K147R. Aldob-K147R was immunoprecipitated by the Flag tag, and same amount of Aldob-K147R was used as purified Aldob-K108bhb protein. Aldob-K108bhb protein was diluted at different fold, loaded to a gel together with Aldob-K147R, and probed using Aldob K108bhb-specific antibody as described above. h Immunoblotting and coomassie brilliant blue staining analysis of purified Aldob WT and K108ac. i Intact mass measurement by MALDI-TOF verified the successful introduction of protein acetylation at K108 of Aldob. Calculated mass: 43162. j, k Stoichiometry of Kac in HCC-LM3 cells expressing Flag-Aldob-K147R. Aldob-K147R was immunoprecipitated by the Flag tag, and same amount of Aldob-K147R was used as purified Aldob-K108ac protein. Aldob-K108ac protein was diluted at different fold, loaded to a gel together with Aldob-K147R, and probed using Kac antibody. Similar results were obtained in three independent experiments (a-h, j-k). Statistical data are presented as mean ± SEM (b, e, g, k), P values were determined by unpaired two-tailed t test (b, e, g, k).
Supplementary information
Supplementary Information
Supplementary Figs. 1–4
Supplementary Data 1
Statistical source data.
Supplementary Data 2
Statistical source data.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed blots or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed blots or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed blots or gels.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig./Table 1
Statistical source data.
Source Data Extended Data Fig./Table 6
Statistical source data.
Source Data Extended Data Fig./Table 6
Unprocessed blots or gels.
Source Data Extended Data Fig./Table 7
Statistical source data.
Source Data Extended Data Fig./Table 7
Unprocessed blots or gels.
Source Data Extended Data Fig./Table 8
Statistical source data.
Source Data Extended Data Fig./Table 8
Unprocessed blots or gels.
Source Data Extended Data Fig./Table 9
Statistical source data.
Source Data Extended Data Fig./Table 9
Unprocessed blots or gels.
Source Data Extended Data Fig./Table 10
Statistical source data.
Source Data Extended Data Fig./Table 10
Unprocessed blots or gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, J., Huang, X., Gou, S. et al. Ketogenic diet reshapes cancer metabolism through lysine β-hydroxybutyrylation. Nat Metab 6, 1505–1528 (2024). https://doi.org/10.1038/s42255-024-01093-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-024-01093-w
This article is cited by
-
TAF1 acetyltransferase promotes colorectal carcinoma metastasis by catalyzing β-hydroxybutyrylation of KCTD9
Oncogene (2026)
-
Targeting metabolic-epigenetic-immune axis in cancer: molecular mechanisms and therapeutic implications
Signal Transduction and Targeted Therapy (2026)
-
Regulatory Landscapes of Protein Acylations in Neuroinflammation: From Molecular Mechanisms to Therapeutic Targets
Neurochemical Research (2026)
-
Machine learning-based identification of kbhb-affected tumor cell subsets as prognostic and therapeutic targets in breast cancer
Journal of Translational Medicine (2025)
-
A metabolic switch for myelination
Cell Regeneration (2025)