Abstract
Ageing-induced skeletal muscle deterioration contributes to sarcopenia and frailty, adversely impacting the quality of life in the elderly. However, the molecular mechanisms behind primate skeletal muscle ageing remain largely unexplored. Here, we show that SIRT5 expression is reduced in aged primate skeletal muscles from both genders. SIRT5 deficiency in human myotubes hastens cellular senescence and intensifies inflammation. Mechanistically, we demonstrate that TBK1 is a natural substrate for SIRT5. SIRT5 desuccinylates TBK1 at lysine 137, which leads to TBK1 dephosphorylation and the suppression of the downstream inflammatory pathway. Using SIRT5 lentiviral vectors for skeletal muscle gene therapy in male mice enhances physical performance and alleviates age-related muscle dysfunction. This study sheds light on the molecular underpinnings of skeletal muscle ageing and presents the SIRT5–TBK1 pathway as a promising target for combating age-related skeletal muscle degeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data have been deposited in the Genome Sequence Archive in the National Genomics Data Center, Beijing Institute of Genomics (China National Center for Bioinformation) of the Chinese Academy of Sciences, under accession numbers HRA007459 and CRA017135 (refs. 96,97). The data based on LC–MS/MS have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) through the iProX partner repository under accession numbers PXD052992 and PXD052984 (refs. 98,99). Source data are provided with this paper.
References
Cai, Y. et al. The landscape of aging. Sci. China Life Sci. 65, 2354–2454 (2022).
Huang, H., Ren, J. & Liu, G. H. Insights and interventions in age-associated inflammation. Curr. Opin. Genet. Dev. 91, 102306 (2025).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Frontera, W. R. & Ochala, J. Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int. 96, 183–195 (2015).
Wolfe, R. R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 84, 475–482 (2006).
Larsson, L. et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 99, 427–511 (2019).
Aging Biomarker Consortium et al. A framework of biomarkers for skeletal aging: a consensus statement by the Aging Biomarker Consortium. Life Med. 2, https://doi.org/10.1093/lifemedi/lnad045 (2023).
Shur, N. F. et al. Age-related changes in muscle architecture and metabolism in humans: the likely contribution of physical inactivity to age-related functional decline. Ageing Res. Rev. 68, 101344 (2021).
Bao, H. et al. Biomarkers of aging. Sci. China Life Sci. 66, 893–1066 (2023).
Aging Biomarker Consortium et al. A framework of biomarkers for skeletal muscle aging: a consensus statement by the Aging Biomarker Consortium. Life Med. https://doi.org/10.1093/lifemedi/lnaf001 (2025).
Stokes, T., Hector, A. J., Morton, R. W., McGlory, C. & Phillips, S. M. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 10, 180 (2018).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646 (2019).
Tieland, M., Trouwborst, I. & Clark, B. C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 9, 3–19 (2018).
Brett, J. O. et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat. Metab. 2, 307–317 (2020).
Gheller, B. J., Riddle, E. S., Lem, M. R. & Thalacker-Mercer, A. E. Understanding age-related changes in skeletal muscle metabolism: differences between females and males. Annu. Rev. Nutr. 36, 129–156 (2016).
Pette, D. Metabolic heterogeneity of muscle fibres. J. Exp. Biol. 115, 179–189 (1985).
Al-Sofiani, M. E., Ganji, S. S. & Kalyani, R. R. Body composition changes in diabetes and aging. J. Diabetes Complications 33, 451–459 (2019).
Lai, Y. et al. Multimodal cell atlas of the ageing human skeletal muscle. Nature 629, 154–164 (2024).
Kedlian, V. R. et al. Human skeletal muscle aging atlas. Nat. Aging. 4, 727–744 (2024).
Distefano, G. & Goodpaster, B. H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 8, a029785 (2018).
He, Z. et al. Sugt1 loss in skeletal muscle stem cells impairs muscle regeneration and causes premature muscle aging. Life Med. 2, Inad039 (2023).
Amorim, J. A. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 18, 243–258 (2022).
Guo, S. et al. Muscle PARP1 inhibition extends lifespan through AMPKα PARylation and activation in Drosophila. Proc. Natl Acad. Sci. USA 120, e2213857120 (2023).
Zhou, Z. et al. Drp1 controls complex II assembly and skeletal muscle metabolism by Sdhaf2 action on mitochondria. Sci. Adv. 10, eadl0389 (2024).
Crescenzo, R. et al. Skeletal muscle mitochondrial energetic efficiency and aging. Int. J. Mol. Sci. 16, 10674–10685 (2015).
Baker, S. A. & Rutter, J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 24, 355–374 (2023).
Zhong, Q. et al. Post-translational regulation of muscle growth, muscle aging and sarcopenia. J. Cachexia Sarcopenia Muscle 14, 1212–1227 (2023).
Anderson, R. M. & Colman, R. J. Prospects and perspectives in primate aging research. Antioxid. Redox Signal. 14, 203–205 (2011).
Jones, R. A. et al. Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep. 21, 2348–2356 (2017).
Mercken, E. M. et al. Conserved and species-specific molecular denominators in mammalian skeletal muscle aging. NPJ Aging Mech. Dis. 3, 8 (2017).
Zhao, Q. et al. An efficient protocol for studying human pluripotent stem cell-derived myotube senescence. Biophys. Rep. 9, 232–240 (2023).
Wu, Z. et al. m6A epitranscriptomic regulation of tissue homeostasis during primate aging. Nat. Aging 3, 705–721 (2023).
Jing, Y. et al. SESN1 is a FOXO3 effector that counteracts human skeletal muscle ageing. Cell Prolif. 56, e13455 (2023).
Jing, Y. et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell 14, 497–512 (2023).
Zhang, B. et al. SenoIndex: S100A8/S100A9 as a novel aging biomarker. Life Med. 2, Inad022 (2023).
Satoh, A., Imai, S. I. & Guarente, L. The brain, sirtuins, and ageing. Nat. Rev. Neurosci. 18, 362–374 (2017).
Ji, Z., Liu, G. H. & Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genomics 49, 287–298 (2022).
Bi, S. et al. SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein Cell 11, 483–504 (2020).
Bi, S. et al. The sirtuin-associated human senescence program converges on the activation of placenta-specific gene PAPPA. Dev. Cell 59, 991–1009.e12 (2024).
Du, J. et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011).
Yang, X. et al. SHMT2 desuccinylation by SIRT5 drives cancer cell proliferation. Cancer Res. 78, 372–386 (2018).
Ma, X. et al. Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc. Natl Acad. Sci. USA 109, 9378–9383 (2012).
Ahmad, L., Zhang, S. Y., Casanova, J. L. & Sancho-Shimizu, V. Human TBK1: a gatekeeper of neuroinflammation. Trends Mol. Med. 22, 511–527 (2016).
Sun, S. C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2, 17023 (2017).
Wu, Z., Ren, J. & Liu, G.-H. Glutathione restoration: a sword to combat skeletal muscle stem cell aging. Life Med. 2, load012 (2023).
Zhou, B. et al. Cardioprotective role of SIRT5 in response to acute ischemia through a novel liver-cardiac crosstalk mechanism. Front. Cell Dev. Biol. 9, 687559 (2021).
Chang, L. et al. SIRT5 promotes cell proliferation and invasion in hepatocellular carcinoma by targeting E2F1. Mol. Med. Rep. 17, 342–349 (2018).
Lu, W., Zuo, Y., Feng, Y. & Zhang, M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol. 35, 10699–10705 (2014).
Xu, L. et al. SIRT5 as a biomarker for response to anthracycline-taxane-based neoadjuvant chemotherapy in triple-negative breast cancer. Oncol. Rep. 39, 2315–2323 (2018).
Abril, Y. L. N. et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene 40, 1644–1658 (2021).
Portanier, E. et al. Introduction history overrides social factors in explaining genetic structure of females in Mediterranean mouflon. Ecol. Evol. 7, 9580–9591 (2017).
Baek, J. et al. The deacylase sirtuin 5 reduces malonylation in nonmitochondrial metabolic pathways in diabetic kidney disease. J. Biol. Chem. 299, 102960 (2023).
Fiorentino, F., Castiello, C., Mai, A. & Rotili, D. Therapeutic potential and activity modulation of the protein lysine deacylase sirtuin 5. J. Med. Chem. 65, 9580–9606 (2022).
Cutler, A. A., Jackson, J. B., Corbett, A. H. & Pavlath, G. K. Non-equivalence of nuclear import among nuclei in multinucleated skeletal muscle cells. J. Cell Sci. 131, jcs207670 (2018).
Ye, Y. et al. SIRT2 counteracts primate cardiac aging via deacetylation of STAT3 that silences CDKN2B. Nat. Aging 3, 1269–1287 (2023).
Aging Biomarker Consortium et al. A biomarker framework for cardiac aging: the Aging Biomarker Consortium consensus statement. Life Med. 2, lnad035 (2023).
Zhang, Z. et al. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 7, 58–63 (2011).
Bringman-Rodenbarger, L. R., Guo, A. H., Lyssiotis, C. A. & Lombard, D. B. Emerging roles for SIRT5 in metabolism and cancer. Antioxid. Redox Signal. 28, 677–690 (2018).
Peng, C. et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteomics 10, M111.012658 (2011).
Nishida, Y. et al. SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol. Cell 59, 321–332 (2015).
Rardin, M. J. et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 18, 920–933 (2013).
Wang, F. et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 19, 2331–2344 (2017).
Rudrappa, S. S. et al. Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance—a qualitative review. Front. Physiol. 7, 361 (2016).
Song, Z. et al. An NAD+-dependent metabolic checkpoint regulates hematopoietic stem cell activation and aging. Nat. Aging 4, 1384–1393 (2024).
Ohkubo, R. et al. The hepatic integrated stress response suppresses the somatotroph axis to control liver damage in nonalcoholic fatty liver disease. Cell Rep. 41, 111803 (2022).
Xiang, S. et al. TANK-binding kinase 1 (TBK1): an emerging therapeutic target for drug discovery. Drug Discov. Today 26, 2445–2455 (2021).
Li, J. et al. A single-cell transcriptomic atlas of primate pancreatic islet aging. Natl Sci. Rev. 8, nwaa127 (2021).
Zhang, W. et al. A single-cell transcriptomic landscape of primate arterial aging. Nat. Commun. 11, 2202 (2020).
Zhang, H. et al. Nuclear lamina erosion-induced resurrection of endogenous retroviruses underlies neuronal aging. Cell Rep. 42, 112593 (2023).
Zhang, H. et al. Single-nucleus transcriptomic landscape of primate hippocampal aging. Protein Cell 12, 695–716 (2021).
Zhang, Y. et al. Single-nucleus transcriptomics reveals a gatekeeper role for FOXP1 in primate cardiac aging. Protein Cell 14, 279–293 (2023).
Sun, S. et al. CHIT1-positive microglia drive motor neuron ageing in the primate spinal cord. Nature 624, 611–620 (2023).
Deacon, R. M. J. Measuring the strength of mice. J. Vis. Exp. 2, e2610 (2013).
Shang, G. K. et al. Sarcopenia is attenuated by TRB3 knockout in aging mice via the alleviation of atrophy and fibrosis of skeletal muscles. J. Cachexia Sarcopenia Muscle 11, 1104–1120 (2020).
Zhao, Q. et al. Single-cell profiling reveals a potent role of quercetin in promoting hair regeneration. Protein Cell 14, 398–415 (2023).
Yang, Y. et al. Metformin decelerates aging clock in male monkeys. Cell 187, 6358–6378.e29 (2024).
Geng, L. et al. A comparative study of metformin and nicotinamide riboside in alleviating tissue aging in rats. Life Med. 2, lnac045 (2023).
Liu, X. et al. Migrasomes trigger innate immune activation and mediate transmission of senescence signals across human cells. Life Med. 2, lnad050 (2023).
Deng, L. et al. Stabilizing heterochromatin by DGCR8 alleviates senescence and osteoarthritis. Nat. Commun. 10, 3329 (2019).
He, Y. et al. 4E-BP1 counteracts human mesenchymal stem cell senescence via maintaining mitochondrial homeostasis. Protein Cell 14, 202–216 (2023).
Diao, Z. et al. SIRT3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Res. 49, 4203–4219 (2021).
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Ning, W. et al. HybridSucc: a hybrid-learning architecture for general and species-specific succinylation site prediction. Genomics Proteomics Bioinformatics 18, 194–207 (2020).
Hasan, M. M., Yang, S., Zhou, Y. & Mollah, M. N. SuccinSite: a computational tool for the prediction of protein succinylation sites by exploiting the amino acid patterns and properties. Mol. Biosyst. 12, 786–795 (2016).
Xu, Y. et al. iSuc-PseAAC: predicting lysine succinylation in proteins by incorporating peptide position-specific propensity. Sci. Rep. 5, 10184 (2015).
Lu, H. et al. Aging hallmarks of the primate ovary revealed by spatiotemporal transcriptomics. Protein Cell 15, pwad063 (2023).
Huang, D. et al. A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis. Protein Cell 14, 888–907 (2023).
Chen, T. et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics 19, 578–583 (2021).
CNCB-NGDC Members and Partners. Database resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 50, D27–D38 (2022).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2022).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Acknowledgements
We thank Z. Diao for helping with the generation and characterization of SIRT5+/+ and SIRT5−/− hES cells. We thank L. Bai, R. Bai, J. Lu, Y. Yang and X. Li for their administrative assistance, and J. Jia and C. Xie for their help with animal experiments. We also thank J. Jia (Institute of Biophysics, Chinese Academy of Sciences) for help with FACS experiments, and J. Wang (Institute of Biophysics, Chinese Academy of Sciences) for his help in LC–MS/MS. This work was supported by the National Natural Science Foundation of China (82488301 to G.-H.L. and J.Q.; 82122024 to S.W.; 82125011 to J.Q.; 82071588 to S.W.; 92468303 to S.W. and X.F.; 81921006 to G.-H.L. and J.Q.; 92149301 to G.-H.L. and S.W.; 92168201 to G.-H.L.; 82361148131 to W.Z.; 82172447 to X.Z.; 82472459 to X.Z.), the STI2030-Major Projects (2021ZD0202400 to S.W. and Q.Z.), the National Key Research and Development Program of China (2020YFA0804000 to G.-H.L. and S.W.; 2022YFA1103700 to W.Z., S.M. and J.Q.; 2023YFC3605400 to Q.Z.), Non-Communicable Chronic Diseases-National Science and Technology Major Project (2024ZD0530400 to J.Q.), the National Natural Science Foundation of China (82330044 to G.-H.L.; 32341001 to G.-H.L.; 32121001 to W.Z.; 82192863 to W.Z.; 82361148130 to G.-H.L. and J.Q.; 8231101626 to J.Q.; 32300980 to H.H.; 82322025 to S.M.; 82271600 to S.M.; 82471586 to S.W.; 32400968 to Y.J.; 82422031 to H.S.), CAS Project for Young Scientists in Basic Research (YSBR-076to G.-H.L. and J.Q.; YSBR-012 to W.Z.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDC0200000 to S.M.; XDA0460403 to W.Z.), the Program of the Beijing Natural Science Foundation (Z240018 to G.-H.L. and S.W.; JQ24044 to W.Z.; Z230011to J.Q.), the Informatization Plan of Chinese Academy of Sciences (CAS-WX2022SDC-XK14 to G.-H.L.), New Cornerstone Science Foundation through the XPLORER PRIZE (2021-1045 to G.-H.L.), Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes (JYY2023-13 to W.Z.), CAS Youth Interdisciplinary Team to W. Z., Key Laboratory of Alzheimer’s Disease of Zhejiang Province (ZJAD-2024001 to J.Q.), Shenzhen Medical Research Fund (C2406001 to G.-H.L.), Beijing Hospitals Authority Youth Programme (QML20230806 to Q.Z.), Excellent Young Talents Program of Capital Medical University (12300927 to S.W.), The Project for Technology Development of Beijing-affiliated Medical Research Institutes (11000023T000002036310 to S.W.), Excellent Young Talents Training Program for the Construction of Beijing Municipal University Teacher Team (BPHR202203105 to S.W.), Young Elite Scientists Sponsorship Program by CAST (2021QNRC001 to S.M.), Youth Innovation Promotion Association of CAS (2022083 to S.M.), Beijing Science and Technology Nova Cross Program (20220484180 to X.Z.) and Initiative Scientific Research Program, Institute of Zoology, Chinese Academy of Sciences (2023IOZ0202 to J.Q.; 2023IOZ0102 to S.M.; 2024IOZ0103 to J.Q.).
Author information
Authors and Affiliations
Contributions
G.-H.L., S.W. and J.Q. conceived the work and supervised the overall experiments. Q.Z. and Y.J. performed the experiments related to the phenotypic and mechanistic analyses. X.J. performed bioinformatic analyses. G.-H.L., S.W., Q.J., Q.Z., Y.J., X.J., X.Z., F.L., H.H., Z.Z., H.W., S.S., S.M., W.Z., Y.Y., X.F. and G.Z. performed manuscript writing, reviewing and editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Danica Chen, Vera Gorbunova and Young Jang for their contribution to the peer review of this work. Primary Handling Editors: Jean Nakhle and Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
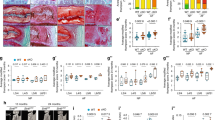
Extended Data Fig. 1 Information summary of cynomolgus monkeys and phenotypical analysis in young and old monkey skeletal muscles.
a. Information summary of the cynomolgus monkeys analyzed in this study. b.WGA staining in young and old monkey skeletal muscles. Left, representative images. Scale bars, 100 μm. Right, the percentages of central nuclei were quantified. Arrows indicate the central nuclei in skeletal muscles. c.The percentage of type IIX (MYH1-positive) fires in young and old monkey skeletal muscles was quantified. d, e. Immunofluorescence staining of LAP2 (d) and HP1γ (e) in young and old monkey skeletal muscles. Left, representative images. Scale bars, 100 μm and 25 μm (zoomed in). Right, the proportion of positively stained nuclei was quantified. Arrows indicate the positively stained nuclei in b, d and e. Two-tailed Student’s t-test was used for statistical analysis and data are shown as the mean ± s.e.m. n = 8 monkeys from both genders per group in b–e. P values are annotated in the figures.
Extended Data Fig. 2 Gender-based analysis of transcriptome and quantitative proteome of young and old monkey skeletal muscle.
a. Ring plots showing upregulated and downregulated DEGs in male old versus male young monkey skeletal muscles, and female old versus female young monkey skeletal muscles. b. Representative GO terms and pathways for upregulated and downregulated DEGs in male old versus male young monkey skeletal muscles, and female old versus female young monkey skeletal muscles. c. Ring plots showing upregulated and downregulated DEPs in male old versus male young monkey skeletal muscles, and female old versus female young monkey skeletal muscles. d. Representative GO terms and pathways for upregulated and downregulated DEPs in male old versus male young monkey skeletal muscles, and female old versus female young monkey skeletal muscles. e. Lollipop plots showing the shared upregulated and downregulated GO terms and pathways for upregulated and downregulated DEGs and DEPs in male old versus male young monkey skeletal muscles, and female old versus female young monkey skeletal muscles. Hypergeometric tests were performed in b, d and e. mkMuscle, monkey muscle.
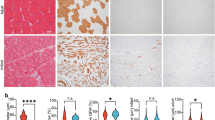
Extended Data Fig. 3 Generation and characterization of WT and SIRT5-deficient hES cells.
a. A quantitative PCR with reverse transcription (RT-qPCR) analysis of the relative transcript levels of SIRT1-7 genes in young and old monkey skeletal muscles. Two-tailed Student’s t-test was used for statistical analysis. n = 8 monkeys from both genders per group. b. Schematic representation of CRISPR-Cas9-mediated SIRT5 targeting strategy in hES cells. c. No off-target (OT) cleavage in top 10 predictive off-target sites against SIRT5 sgRNA in SIRT5−/− hES cells by genomic DNA PCR and DNA sequencing. d. Western blotting analysis of SIRT5 protein level in SIRT5+/+ and SIRT5−/− hES cells. β-tubulin was used as loading control. e. Immunostaining of SOX2, OCT4 and NANOG in SIRT5+/+ and SIRT5−/− hES cells. Scale bars, 50 μm. f. Karyotyping analysis of SIRT5−/− hES cells. g. Immunostaining of TUJ1, SMA and FOXA2 to evaluate the differentiation potentials of SIRT5+/+ and SIRT5−/− hES cells. Scale bars, 250 μm. h. Immunostaining of Ki67 in SIRT5+/+ and SIRT5−/− hES cells. Scale bars, 100 μm. n = 3 biological replicates. Two-tailed Student’s t-test was used for statistical analysis. Data are presented as the mean ± s.e.m. in a and h. The representative results from one of three independent experiments in d and e. P values are annotated in the figures.
Extended Data Fig. 4 Analysis of mitochondrial activity in SIRT5-deficient human myotubes.
a. Snapshot showing the transcript levels of SIRT1-7 gene in SIRT5+/+ and SIRT5−/− hMyotubes. b. Left, representative images of MyHC-positive myotubes in SIRT5+/+ and SIRT5−/− hMyotubes. Scale bars, 150 μm. Right, the differentiation efficiency of hMyotube was quantified. c. Western blotting analysis of the expression levels of oxidative respiratory chain complex proteins in SIRT5+/+ and SIRT5−/− hMyotubes. GAPDH was used as loading control. The representative results from one of three independent experiments. d. Left, analysis of mitochondria superoxide levels in SIRT5+/+ and SIRT5−/− hMyotubes by using MitoSOXTM Red probe. Scale bars, 100 μm. Right, positively stained area was quantified. e. Western blotting analysis of 4-HNE modified protein and SOD1 protein levels in SIRT5+/+ and SIRT5−/− hMyotubes. GAPDH was used as loading control. Two-tailed student’s t-test was used for statistical analysis and data are presented as the mean ± s.e.m. n = 3 biological replicates in b–e. P values are annotated in the figures.
Extended Data Fig. 5 SIRT5 interacts with TBK1 and protects skeletal muscle from aging.
a. TBK1 and SDHA were identified as SIRT5-interacting proteins by Co-IP and mass spectrometry. b. Immunofluorescence staining of TOM20, FLAG-TBK1 and HA-SIRT5 in hMyotubes. Scale bars, 160 μm and 20 μm (zoomed in). c. Western blotting analysis of TBK1 and p-TBK1 (S172) protein expression levels in hMyotubes transduced with lentivirus vectors expressing FLAG-Luc, FLAG-TBK1-WT or FLAG-TBK1-K137R. β-tubulin was used as loading control. d. RT-qPCR analysis of the transcript levels of IL6, IL8 and MCP1 in hMyotubes transduced with lentivirus vectors expressing FLAG-Luc, FLAG-TBK1-WT or FLAG-TBK1-K137R. e. Immunofluorescence staining of IL-6 in mouse skeletal muscle of Young-Luc, Old-Luc and Old-SIRT5 groups. Left, representative images. Scale bars, 100 μm and 25 μm (zoomed in). Right, IL6-positive cells were quantified. n = 6 male mice per group. f. Principal component (PC) analysis of RNA-seq data of mouse skeletal muscles in Young-Luc, Old-Luc and Old-SIRT5 groups. g. Volcano plot showing upregulated and downregulated DEGs of the skeletal muscles of Old-Luc versus Young-Luc and Old-SIRT5 versus Old-Luc-transduced mice. Benjamini-Hochberg P values were performed. Two-tailed student’s t-test was used for statistical analysis and data are presented as the mean ± s.e.m. in c–e. n = 3 biological replicates in c and d. P values are annotated in the figures. mMuscle, mouse muscle.
Supplementary information
Supplementary Tables 1–6
Supplementary Table 1: DEPs between old and young cynomolgus monkey skeletal muscles. Supplementary Table 2: DEGs identified by RNA-seq analysis between SIRT5+/+ and SIRT5−/− human myotubes. Supplementary Table 3: The candidate SIRT5-interacting proteins identified by LC–MS/MS. Supplementary Table 4: DEGs identified by RNA-seq analysis in mouse skeletal muscles. Supplementary Table 5: Antibodies used in this study. Supplementary Table 6: The sequences of siRNAs and primers used in this study.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Q., Jing, Y., Jiang, X. et al. SIRT5 safeguards against primate skeletal muscle ageing via desuccinylation of TBK1. Nat Metab 7, 556–573 (2025). https://doi.org/10.1038/s42255-025-01235-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01235-8
This article is cited by
-
SIRT5-mediated desuccinylation of MTHFD2 enhances chemoresistance in breast cancer cells by reducing therapy-induced senescence
Communications Biology (2025)
-
SIRT5 slows skeletal muscle ageing by alleviating inflammation
Nature Metabolism (2025)
-
Biomarkers of ageing of humans and non-human primates
Nature Reviews Molecular Cell Biology (2025)