Abstract
Animals select food based on hungers that reflect dynamic macronutrient needs, but the hormonal mechanisms underlying nutrient-specific appetite regulation remain poorly defined. Here, we identify tachykinin (Tk) as a protein-responsive gut hormone in Drosophila and female mice, regulated by conserved environmental and nutrient-sensing mechanisms. Protein intake activates Tk-expressing enteroendocrine cells (EECs), driving the release of gut Tk through mechanisms involving target of rapamycin (TOR) and transient receptor potential A1 (TrpA1). In flies, we delineate a pathway by which gut Tk controls selective appetite and sleep after protein ingestion, mediated by glucagon-like adipokinetic hormone (AKH) signalling to neurons and adipose tissue. This mechanism suppresses protein appetite, promotes sugar hunger and modulates wakefulness to align behaviour with nutritional needs. Inhibiting protein-responsive gut Tk prolongs lifespan through AKH, revealing a role for nutrient-dependent gut hormone signalling in longevity. Our results provide a framework for understanding EEC-derived nutrient-specific satiety signals and the role of gut hormones in regulating food choice, sleep and lifespan.
Similar content being viewed by others
Main
Animals’ survival depends on their ability to adapt their feeding behaviour to changing internal nutritional conditions. This homeostatic regulation of food intake is governed by neural and endocrine signals that control appetite or feeding drive, ensuring a balance between total energy intake and expenditure1,2,3,4. Furthermore, it has become clear that such mechanisms govern not only animals’ general appetite but also their preferences for foods containing specific macronutrients, as a way to satisfy physiological needs5,6,7,8 and to maintain proper balance between nutrients. Central to this balancing is the ability of animals to select food with the specific nutrients they require. Food selection is driven by ‘specific appetites’ for distinct nutrients, a phenomenon exhibited across animal species, including in humans1,5,9. Nonspecific appetite is understood to be governed by hormonal signalling, with many endocrine factors known to contribute to general hunger and satiety regulation. However, the endocrine signals that control nutrient-specific appetite and food choice, which are key aspects of homeostatic feeding behaviour, remain unclear. An essential component of these regulatory mechanisms is that the intake of a given macronutrient must be sensed in some way to drive nutrient-specific negative feedback that decreases future preference for the ingested nutrient. In line with this, we recently found that the hormone neuropeptide F (NPF) is released from endocrine cells of the mated female Drosophila gut in direct response to these cells’ detection of consumed sugar. This hormone targets neurosecretory cells, providing them with the feedback necessary to suppress sugar appetite10. Thus, sugar-induced gut NPF induces a sugar satiety mechanism, balancing sugar consumption.
Dietary protein intake influences growth, metabolism, fecundity and lifespan across species. However, the mechanisms that selectively regulate the ingestion of protein remain poorly understood. The protein-leverage hypothesis suggests that animals, including humans, prioritize protein over other macronutrients such as carbohydrates and fats when making dietary choices and thus consume available food until protein need is satisfied. In the context of modern diets, in which low-protein but high-calorie foods are prevalent, this often leads to overconsumption of accompanying sugars and fats along the path to protein satiety11,12. This behavioural response represents a form of homeostatic food selection by which the organism adjusts its food choices based on protein requirements. When dietary protein intake is insufficient, the resulting internal state can modulate feeding behaviours to favour protein consumption to restore balance and ensure optimal physiological function. In mice, protein restriction has been shown to shift preference toward protein-containing food. This response is regulated at least in part by FGF21, a liver-derived endocrine signal that acts on the brain to adapt feeding behaviour13. Similarly, the fruit fly Drosophila modulates its food choices based on its internal state5. In mammals, liver FGF21 is upregulated under low-protein feeding via the transcription factor ATF4, and a similar ATF4-dependent adaptive endocrine response has been identified in the fly equivalent of the mammalian liver and adipose tissue to counter protein restriction14.
In Drosophila, signals from peripheral organs play a large role in controlling appetite, with specialized endocrine cells of the gut (enteroendocrine cells, EECs) gaining attention for their functions in governing feeding behaviour and metabolism9,10,15,16,17,18, paralleling extensive human and rodent research19,20. These endocrine cells produce diverse hormones that signal to the brain to adjust feeding behaviour based on the nutritional status of the gut. Indeed, therapies centred around the human EEC-derived glucagon-like peptide 1 (GLP-1) have shown great potential in appetite reduction for weight loss21. In Drosophila, the gut–brain axis was recently found to detect deficiencies in essential amino acids and to induce a compensatory appetite for these nutrients16. However, in this case, it is the absorptive enterocytes, rather than the EECs, that sense the lack of diet- and microbiome-derived amino acids, releasing the peptide CNMamide (CNMa), which signals the brain to induce amino acid-specific hunger. Although both FGF21 and CNMa induce a preference for protein-rich food under protein deficiency conditions, the mechanisms activated by protein intake that induce protein-specific satiety remain poorly defined.
Here, we present our findings that EECs of the adult female Drosophila midgut produce the conserved gut hormone Tk22,23. Upon sensing protein-rich food intake, these cells release Tk, which acts on cells producing the glucagon-like factor AKH to suppress further intake of protein-rich food while promoting sugar consumption, forming a feedback loop that drives homeostatic nutrient consumption. Furthermore, we demonstrate that the same nutrient-sensing pathways that regulate the expression and secretion of EEC-derived Tk in flies also control mammalian gut Tk in mice. Inhibiting this protein-responsive Tk in Drosophila modulates sleep and extends lifespan in an AKH-dependent manner, highlighting conserved mechanisms of nutrient sensing and gut hormone function. These discoveries advance our understanding of how gut-mediated protein sensing regulates food choices for nutritional balance and how the gut influences lifespan.
Results
Gut-derived hormone Tk modulates protein and sugar appetite
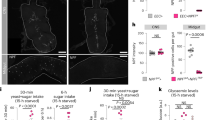
We recently found, in parallel with colleagues, that sugar consumption triggers the release of gut NPF, which in turn curbs appetite for sugar and adjusts systemic sugar metabolism10,18. Interested in whether animals also modulate their protein intake based on their internal state, we decided to examine their dietary choices in that respect. We allowed mated female flies to consume either a protein-rich diet (10% yeast) or a sugar diet (10% sucrose) for 15 h to induce protein or sugar satiety, respectively. We then measured the amount of 10% yeast or 10% sucrose these animals consumed during a 1-h dye food-consumption assay. Sugar-fed animals exhibited a decreased intake of the sugar diet compared to those that had consumed a yeast diet, which displayed an increased appetite for sugar (Fig. 1a). These observations are consistent with our findings that dietary sugar is sensed by NPF+ EECs, leading to the suppression of appetite for this nutrient10. Intriguingly, a similar effect was observed for protein consumption: yeast-fed animals consumed less when subsequently presented with protein-rich food than those that had previously consumed sugar (Fig. 1a). This difference suggests that animals adjust their consumption of protein and sugar based on their recent intake of both of these nutrients, aiming for a balanced diet.
a, Intake of sugar and yeast over a 1-h dye-feeding assay for flies previously fed a sugar or yeast diet for 15 h (two-tailed unpaired Student’s t-test). b–d, Dye-feeding intake of sugar (b, 1 h), yeast (c, 1 h) and coconut oil (d, 4 h) after 15 h of yeast feeding (Kruskal–Wallis with Dunnett’s test). Sh, short-hairpin RNAi collection; KK, long-hairpin phiC31 RNAi collection, both from the Vienna Drosophila Resource Center. e, Representative confocal images of UAS-mCD8::GFP (GFP) expression in the midgut and brain driven by Tk::2A::GAL4 either alone (Tk>) or in combination with the pan-neuronal GAL4 inhibitor R57C10-GAL80 (thus Tkgut>). The mean number of GFP+ cells ±s.e.m. in each region (R1 + R2. R4, R4 and R5) is noted (n = 7–8 guts). Scale bars, 200 µm (midgut) and 50 µm (brain). f, Tk transcripts in dissected midguts and the central nervous systems (CNS) of Tkgut> and Tkgut>Tk-RNAi flies (two-tailed unpaired Student’s t-test). g, Six-hour CAFÉ assay for sugar intake in Tkgut>TkRNAi animals (Kruskal–Wallis with Dunn’s test). h, Twenty-two-hour FLIC assay for sugar-feeding behaviour of Tkgut>TkRNAi flies (one-way ANOVA with Dunnett’s test). i–l, Total ‘sips’ from sugar (i), yeast (j), lipid-depleted yeast (k) and mixed amino acid (l) media measured by the 1-h flyPAD assay (two-tailed Mann–Whitney U-tests). m, Preference index for yeast over sugar in the two-choice flyPAD assay following 15 h of being fed a yeast diet (two-tailed Mann–Whitney U-test). n, Two-hour yeast intake in a dye assay with TrpA1 activation of Tk+ EECs, with and without simultaneous Tk-RNAi (one-way ANOVA with Dunnett’s test). o, Model summarizing gut-derived Tk regulation of sugar and protein intake. Sample sizes (n) and P values are indicated in each plot. In a–d,g–n, n represents animals per group. In f, n represents central nervous system or midgut samples. Behavioural experiments were performed following 15 h of yeast feeding unless otherwise stated. Box plots show the median, the bounds of the box (25th and 75th percentiles) and whiskers representing minimum and maximum values. Dot plots in f,h include mean ± s.e.m., whereas plots in i–m indicate the median and 95% confidence interval. NS, not significant (P > 0.05).
Our findings led us to ask whether this protein satiety effect was triggered by gut-mediated amino acid detection. We had found that the EEC-derived peptide Tk affects feeding behaviour and sleep through an in vivo RNA interference (RNAi) screen targeting EEC-secreted factors in adult Drosophila10. This peptide is reported to be expressed in a subset of midgut EECs that are responsive to amino acids22, and therefore we investigated whether Tk plays a role in the increased sugar intake and reduced protein appetite observed after protein consumption. To uncover the mechanism directing feeding responses upon detection of consumed protein, we fed mated females a 10% yeast diet for 15 h before feeding tests to induce protein satiety and hunger for dietary sugar in all subsequent experiments, unless specified otherwise. Animals with Tk knockdown in Tk-expressing (Tk+) EECs consumed less sugar (Fig. 1b), while they ingested high amounts of protein-rich yeast food despite their recent ad libitum intake of yeast food (Fig. 1c). We observed similar effects with two independent RNAi lines, ruling out off-target effects. Furthermore, we also examined male flies and found that the knockdown of gut Tk similarly affected sugar and protein consumption in these animals, suggesting a fundamental non-sex-specific role of this gut-derived hormone in regulating dietary preferences (Extended Data Fig. 1a). However, because feeding effects were more pronounced in female flies, which typically display more dynamic feeding responses, we focused our subsequent experiments on females. As yeast is not only rich in protein but also contains lipids, we tested whether EEC-derived Tk affected lipid consumption. We found no effect on lipid intake in animals with EEC-specific Tk knockdown (Fig. 1d), suggesting that the regulation of food preferences by gut Tk is specific to a preference for protein rather than for dietary lipids.
We further demonstrated that our approach specifically targets Tk in the gut, without affecting Tk expression in the nervous system, where it is also produced. This specificity for gut-derived Tk, as opposed to neuronal Tk, was demonstrated by the lack of detectable brain expression of UAS-GFP induced by our driver line (Tk::2A::GAL4 combined with pan-neuronal R57C10-GAL80 to inhibit neuronal activity of the transcription factor GAL4 (ref. 9), a combination hereafter referred to as Tkgut>) (Fig. 1e). Using this setup to drive RNAi against Tk strongly reduced Tk expression in the midgut without inducing detectable neuronal knockdown (Fig. 1f). We also excluded the possibility that the UAS transgene itself might have influenced the sugar-feeding phenotype by crossing the UAS-Tk-RNAi (Tk-RNAi) line with the w1118 control background. The results from this UAS-only genotype were consistent with those from the Tkgut> driver-only control (Extended Data Fig. 1b).
We next confirmed the decreased sugar appetite phenotype using a longer-term food-consumption assay (capillary feeder; CAFÉ24) and an automated behaviour-monitoring system, the Fly Liquid-Food Interaction Counter (FLIC), that tracks how often and for how long single animals interact with sugar-only liquid food25. As they did in the short-term food-consumption assay, yeast-satiated females expressing gut-specific Tk knockdown consumed less sugar over a 6-h period and exhibited reduced feeding time with sugar food than control animals (Fig. 1g,h), including UAS-only genotypes (Extended Data Fig. 1c,d). To further confirm the regulation of sugar and protein feeding by gut Tk, we used another automated system, the flyPAD apparatus, that allows the detection of individual sips of sugar- or protein (yeast)-containing solid food as well as the measurement of animals’ preference between these nutrients26. In line with our previous observations, yeast-fed females with gut-specific Tk knockdown exhibited a decrease in sugar feeding accompanied by elevated protein feeding (Fig. 1i,j). To ensure that the observed feeding behaviour was specifically due to protein or amino acids, rather than lipid content or an appetite for lipids in the yeast, we tested lipid-depleted yeast. Females with Tk knockdown in EECs exhibited an increased number of sips of lipid-depleted yeast, confirming that the increased yeast consumption is not driven by lipids (Fig. 1k). Furthermore, these animals similarly increased sipping of a synthetic mixture of dietary amino acids, supporting the idea that the loss of gut Tk specifically drives the increased intake of protein-rich food (Fig. 1l). As expected, females with EEC-specific Tk loss displayed an increased preference for protein-rich yeast food over sugar when presented with both options in a two-choice assay (Fig. 1m). Collectively, these findings imply that the observed feeding phenotypes arise from the EEC-specific loss of Tk. They further suggest that gut Tk serves as a satiety signal that is required after a protein-rich meal for curbing further protein intake and for promoting appetite for carbohydrate-rich food.
To assess whether gut-derived Tk inhibits protein consumption, we introduced the thermosensitive cation channel TrpA1 (ref. 27) into Tk+ EECs, permitting induction of Tk release. We conditioned mated females by providing them with sugar-based food for 15 h, thereby priming their appetite for a protein-rich diet. During the last 2 h of this period, the animals were incubated at 29 °C to trigger TrpA1-induced Tk release. This manipulation resulted in the complete inhibition of protein intake, despite the animals’ being fed only sugar for the preceding 15 h (15-h protein deprivation; Fig. 1n), a treatment that induces control animals to consume yeast (Fig. 1a). As Tk+ EECs express multiple peptides28,29 and TrpA1 activation presumably leads to the release of all of them, we tested whether the observed effect requires Tk. Concurrent Tk knockdown attenuated the protein-aversion phenotype of TrpA1 induction (Fig. 1n), indicating that Tk itself is indeed required. Together, these results demonstrate that EEC-derived Tk can suppress protein appetite, even under a condition in which this appetite is normally strongly upregulated (Fig. 1o).
Enteroendocrine hormones are also known for their role in modulating metabolism, and metabolic status has a strong influence on feeding behaviour, raising the possibility that the observed feeding changes arise in response to Tk-induced metabolic shifts rather than as a direct behavioural response brought about by Tk. We thus asked whether gut Tk loss might affect nutrient storage or metabolism. Animals with gut-specific Tk knockdown displayed no alteration in whole-body triacylglyceride (TAG) or glycogen levels under fed conditions (Extended Data Fig. 1e,f). However, there was a small decrease in lipid depletion during starvation, suggesting slower mobilisation, which was consistent with these animals’ slightly prolonged survival during starvation (Extended Data Fig. 1g). These findings support the idea that the observed effects of Tk on feeding arise directly through Tk-driven effects on behavioural centres, rather than being secondary effects of metabolic perturbations.
Dietary protein induces Tk release from EECs
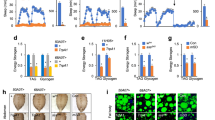
EECs can sense dietary nutrients, and EECs of the posterior midgut of the fly are activated by dietary amino acids22. Because EEC-specific knockdown of Tk led to phenotypes that are consistent with the hormone being released in response to protein intake, we investigated whether Tk+ EECs are indeed activated by protein-rich food. We employed the calcium-dependent nuclear import of LexA (CaLexA) reporter system, in which green fluorescent protein (GFP) expression, reflective of recent calcium-signalling activity history30, enables the measurement of EEC activity in freely moving animals (Fig. 2a). Although the release of Tk from midgut EECs has been linked with starvation23, we did not observe any activation of Tk+ EECs or any change in their intracellular Tk peptide immunofluorescence, used as a proxy for peptide levels, after a short-term (4-h) period of fasting (Extended Data Fig. 2a,b). We then asked whether short-term feeding with either sugar or yeast would influence Tk+ EEC activity and Tk peptide release. To observe short-term responses, we fasted animals for 25 h beforehand to stimulate feeding drive and then refed them with sugar or yeast medium for 3 h. We found that intracellular Tk peptide levels in yeast-refed animals were lower than those seen in sugar-fed animals (Extended Data Fig. 2c,d). This effect was not linked to any changes in Tk transcript levels, and no transcriptional Tk changes were observed even after 6 h of feeding on standard food containing both sugar and yeast (Extended Data Fig. 2e,f). Thus, the low Tk peptide levels observed after 3 h of yeast refeeding are likely caused by greater release of Tk under these dietary conditions, although this was not accompanied by any significant change in the cells’ calcium activity, perhaps due to the relatively short period. We therefore examined the effects of prolonged feeding and found that calcium activity in Tk+ EECs was significantly increased during longer-term yeast feeding compared to 3 h (Extended Data Fig. 2g), as determined using a modified CaLexA reporter system in which expression of luciferase rather than GFP reflected recent calcium-signalling activity9,31. We then analysed the effect of longer-term yeast feeding on gut Tk peptide levels. After 18 h of feeding on protein-rich yeast, Tk peptide levels within EECs were again low, but, with the extended feeding period, calcium activity in Tk+ EECs was elevated (Fig. 2b,c). This suggests that Tk+ EECs are more responsive to dietary protein than to sugar.
a, Representative images of Tk+ EECs expressing the activity reporter CaLexA from flies fed adult-optimized food. Top, Tk+ EECs (Tkgut>tdTomato, magenta) and active cells (Tkgut>CaLexA-induced GFP, green). Middle, active Tk+ EECs (GFP). Bottom, anti-Tk staining. Insets, regions R1–R5. Scale bars, 200 µm (whole midgut) and 50 µm (insets). Similar expression patterns were observed in five independent gut samples. b,c, Calcium-dependent activity (GFP/tdTomato ratio) in Tk+ EECs across the midgut (b) and anti-Tk staining intensity (c) in flies fed for 18 h with sugar, yeast or both (Kruskal–Wallis with Dunn’s test). d,e, Region-specific CaLexA analysis of Tk+ EECs (d) and Tk peptide-staining intensity (e) in midgut regions R1 + R2, R3 and R5 following 15 h of feeding on sugar or yeast (two-tailed Mann–Whitney U-tests). f,g, GCaMP6s activity in Tk+ EECs of R5 over 20 min after sugar or yeast ingestion. f, Representative GCaMP fluorescence images of Tk+ EECs. Scale bars, 10 µm. g, Left, heatmap of GCaMP signal (ΔF/F0) of single Tk+ EECs over time. g, Right, calcium indicator activity (ΔF/F0) in R5 Tk+ EECs after sugar or yeast feeding (two-tailed unpaired Student’s t-test). h, Tk+ EEC calcium activity and Tk peptide staining in R5 of flies fed for 15 h with sugar, yeast, lipid-depleted yeast or coconut oil (Kruskal–Wallis with Dunn’s test). i, Calcium activity in Tk+ EECs across the midgut after 15 h of sugar or peptone feeding (CaLexA luciferase reporter, two-tailed Mann–Whitney U-tests). j, Calcium activity (GFP/tdTomato) and anti-Tk staining in R5 Tk+ EECs after 15 h of sugar or peptone feeding (two-tailed Mann–Whitney U-tests). k, Anti-Tk staining in R5 EECs after 15 h of sugar, yeast or amino acid feeding (Kruskal–Wallis with Dunn’s test). l, A data-supported model. Sample sizes (n) and P values are indicated in each plot. In b–e,h,j,k, n represents EECs. In g, n represents midguts, and, in i, n represents midgut samples. Plots in b–e,h,j,k indicate the median and 95% confidence interval, and plots in g,i show mean ± s.e.m. NS, P > 0.05.
We then investigated whether yeast feeding might differentially activate Tk+ EECs of the anatomically and functionally distinct regions (named R1–R5) of the midgut. We observed that, after 15 h of yeast feeding, Tk+ EECs throughout the gut exhibited increased calcium activity compared to those in sugar-fed conditions. This was correlated with decreased Tk peptide levels across all regions (R1–R5), with a pronounced effect observed in R5 (Fig. 2d,e). These observations imply that protein-rich food induces the release of Tk from Tk+ EECs throughout the midgut, with strong effects in the posterior R5 region. We next established an ex vivo midgut setup for calcium imaging and employed Tk::2A::GAL4 to express GCaMP6s32, a genetically encoded calcium sensor, in Tk+ EECs. We observed that Tk+ EECs in the posterior-most region (R5) exhibited significantly increased calcium activity when animals had been fed with yeast before observation, compared to sugar feeding (Fig. 2f,g and Extended Data Fig. 2h). To verify that amino acids are the dietary component influencing the activity of Tk+ EECs and the levels of Tk peptide within these cells, we examined the effects of lipid-depleted yeast and a pure lipid diet. We found that, similar to complete yeast, lipid-depleted yeast increased the activity of Tk+ EECs in the posterior midgut (R5) while decreasing Tk peptide levels compared to a sugar-based diet (Fig. 2h). These findings indicate that dietary protein, rather than lipids, present in yeast drives the activation of Tk+ EECs and promotes Tk release.
To further confirm the sufficiency of dietary amino acids in driving these effects, we tested the effects of media containing only protein or a pure mixture of amino acids. Peptone feeding increased calcium activity in Tk+ EECs and reduced Tk peptide levels in R5 (Fig. 2i,j). Similarly, lipid-depleted casein, a pure protein source, reduced Tk peptide levels in the posterior gut, further supporting the sufficiency of dietary amino acids in driving these effects (Extended Data Fig. 2i). Importantly, we confirmed that these dietary treatments did not alter Tk transcript levels in the midgut, ruling out transcriptional regulation as the cause of reduced Tk peptide levels (Extended Data Fig. 2j,k). Furthermore, we found that feeding animals amino acids alone was sufficient to decrease Tk peptide levels in these EECs in the absence of other dietary components (Fig. 2k). These results provide strong evidence that amino acids are a key dietary component activating Tk+ EECs and driving Tk release in the posterior midgut (Fig. 2l).
Amino acid-sensing TOR and reactive oxygen species signalling regulate gut Tk
As these data indicate that Tk is released in response to protein feeding, we asked whether Tk+ EECs detect amino acids cell autonomously. Based on available single-cell sequencing data33, the amino acid transporter Slimfast (Slif), which plays a role in amino acid sensing in the fat body34, is expressed in a subset of Tk+ EECs33. Knocking down slif in Tk+ EECs resulted in intracellular accumulation of Tk peptide, primarily in the R3 and R5 regions, with the most significant effect observed in R5, whereas a small but significant decrease was indeed observed in the anterior R1 and R2 segments (Fig. 3a). The observed alterations in Tk peptide levels were not accompanied by changes in midgut Tk transcript levels (Extended Data Fig. 3a), indicating that the increase in intracellular Tk levels is likely due to peptide retention rather than altered transcription. We also examined the potential involvement of other amino acid transporters expressed in the gut33,35 by knocking them down in Tk+ EECs. Knockdown of these transporters did not lead to significant alterations in Tk peptide levels, indicating that they are not required for regulating Tk release in response to protein feeding (Extended Data Fig. 3b). To determine whether Tk+ EECs are an important site of slif expression in the midgut, we assessed the reduction in midgut slif transcript levels following EEC-specific knockdown. Targeting slif knockdown specifically in Tk+ EECs led to a measurable decrease in slif transcript levels in whole-midgut samples, confirming that slif is expressed in Tk+ EECs (Extended Data Fig. 3c). To further validate the regional expression of slif in Tk+ EECs, we performed fluorescent in situ hybridisation against slif, pros (prospero, a marker of EECs) and Tk in midguts. We found clear coexpression of slif with Tk and pros in EECs in R5, confirming the single-cell sequencing data, validating our ability to knock down slif and demonstrating that Slif is expressed in Tk+ EECs in the posterior midgut (Fig. 3b).
a, Tk peptide-staining intensity in midgut regions of Tk> controls and Tk>slif-RNAi animals fed for 18 h with yeast (two-tailed Mann–Whitney U-tests). b, Representative in situ hybridisation images localizing Tk, pros and slif transcripts in R5 EECs. Scale bar, 50 µm. Z projection of confocal substacks. Similar localisation patterns were observed in five independent gut samples from different animals. c,d, Expression of Tk in midguts (c) and anti-Tk staining in R5 (d) of controls and animals with reduced TOR signalling (Tor-RNAi or Tsc1 and Tsc2 overexpression) in Tk+ EECs fed for 15 h with yeast (c, one-way ANOVA with Dunnett’s test; d, Kruskal–Wallis with Dunn’s test). e, Representative images of Tk immunostaining and TrpA1::GFP (anti-GFP) coexpression in R5. Scale bars, 5 µm. Similar coexpression patterns were observed in five independent gut samples. In R5, 140 of 143 Tk+ EECs across three midguts coexpressed detectable TrpA1::GFP. f, Calcium activity (GFP/tdTomato ratio) and anti-Tk staining in Tk+ EECs throughout the midgut on a normal adult-optimized diet (ND) and medium supplemented with 5% H2O2 for 18 h, in controls and in animals with RNAi against the TrpA1 transcript in Tk+ EECs (two-tailed Mann–Whitney U-tests). g, Anti-Tk staining intensity in R5 in animals fed for 15 h on a yeast diet, with and without TrpA1 knockdown in Tk+ EECs (two-tailed Mann–Whitney U-test). h, One-hour dye-feeding assay for sugar consumption for controls and slif knockdown in Tk+ EECs, following 15 h of yeast feeding (Kruskal–Wallis with Dunn’s test). GD, long-hairpin RNAi P-element collection from Vienna Drosophila Resource Center. i, Sugar consumption over a 90-min dye-feeding assay of controls and animals with reduced TOR signalling Tk+ EECs, following 15 h of yeast feeding (Kruskal–Wallis, Dunn’s test). Sample sizes (n) and P values are indicated in each plot. In a,d,f,g, n represents EECs. In c, n represents midgut samples, and, in h,i, n represents animals per group. Plots in a,d,f,g show median and 95% confidence interval, and plots in c show mean ± s.e.m. Box plots show the median, the bounds of the box (25th and 75th percentiles) and whiskers representing minimum and maximum values. NS, P > 0.05.
To further investigate the route by which dietary amino acids promote Tk release, we examined the potential involvement of the nutrient-sensing TOR pathway and the amino acid-responsive general control nonderepressible 2 (GCN2) system. We found that knocking down Tor or inhibiting TOR through overexpression of tuberous sclerosis complex proteins 1 and 2 (Tsc1 and Tsc2, gigas) specifically in Tk+ EECs resulted in a strong reduction of Tk expression, while knocking down Gcn2 had no effect (Fig. 3c and Extended Data Fig. 3d), indicating that TOR activity in Tk+ EECs is required for Tk production. Consistent with a reduction in Tk production, we observed significantly lower Tk peptide levels in R5 when the TOR pathway was inhibited in Tk+ EECs (Fig. 3d). These findings support the involvement of the TOR pathway, rather than GCN2, in mediating amino acid sensing and Tk production in Tk+ EECs.
While the TOR pathway plays a critical role in amino acid sensing for Tk production, we next explored additional signalling mechanisms that might regulate Tk release in response to intestinal conditions and dietary intake. Specifically, we examined the expression of receptors in Tk+ EECs that might respond to environmental cues to regulate Tk release. Single-cell sequencing data33 revealed that Tk+ EECs express TrpA1, a sensor of the redox state that is activated by reactive oxygen species (ROS), the levels of which are tightly regulated by the cellular redox balance and linked to metabolism and nutrient-driven signalling36,37,38,39,40,41. To confirm the expression of TrpA1 in Tk+ EECs, we used a GFP knockin at the native TrpA1 locus, resulting in GFP-tagged TrpA1 expression. This confirmed that Tk+ EECs in the R5 region specifically express TrpA1, suggesting a role for this receptor in regulating Tk release (Fig. 3e). We thus next asked whether ROS signalling via TrpA1 might influence Tk peptide release. We found that ROS do indeed activate Tk+ EECs, as feeding animals with H2O2-laced (5%) food that induces intestinal ROS42 increased calcium activity in these cells (Extended Data Fig. 3e). Knockdown of TrpA1 in Tk+ EECs significantly decreased these cells’ activity and increased intracellular Tk peptide levels under conditions of intestinal ROS (Fig. 3f). This effect indicates that TrpA1 facilitates Tk release in response to ROS generated in the gut. Furthermore, in yeast-fed animals, but not sugar-fed ones, TrpA1 knockdown caused Tk accumulation in EECs of R5 (Fig. 3g and Extended Data Fig. 3f), reinforcing the idea that TrpA1-mediated signalling is critical for Tk release and that this process is also essential under conditions of protein feeding. Together, these findings support a model in which ROS signalling through TrpA1 complements amino acid sensing via the TOR pathway to regulate Tk release from EECs.
Collectively, our findings suggest that the consumption of protein-rich food activates Tk+ EECs, leading to the release of the Tk peptide. This release mechanism appears to involve both Slif- and TOR-dependent amino acid sensing, at least in the posterior portion of the midgut. Indeed, animals with inhibition of slif or TOR in Tk+ EECs displayed reduced sugar intake over a 1-h period following 15 h of yeast-only feeding (Fig. 3h,i and Extended Data Fig. 3g–i), mirroring the behaviour of animals with EEC-targeted Tk knockdown (Fig. 1b). However, these animals exhibited no accompanying increase in yeast feeding (Extended Data Fig. 3j–n), implying that the absence of Slif or TOR signalling in Tk+ EECs does not fully phenocopy Tk loss. Disrupting amino acid sensing through the knockdown of Slif or TOR may influence the release of other gut hormones in addition to Tk, as Tk+ EECs are also known to produce both NPF and diuretic hormone 31 (Dh31)29,43. In any case, our findings suggest that Slif and TOR are both required for the amino acid sensing involved in the regulation of Tk production and release from EECs.
Protein diet, TOR and TRPA1 regulate gut Tk in mice
Tks are peptide hormones encoded by an evolutionarily conserved gene family. Like the Drosophila gut, the mammalian gut contains Tk-producing endocrine cells44. To investigate whether the mechanisms regulating Tk release that we identified in Drosophila are conserved in mammals, we used intestinal organoids derived from female mice. These organoids are three-dimensional (3D) structures that replicate physiological and structural features of the in vivo intestinal epithelium and produce distinct enterochromaffin (EC) cells45. EC cells are specialized EECs that produce serotonin alongside the Tk family peptide substance P (SP), which is encoded by the conserved Tk gene Tac1. SP exerts its effects through a conserved Tk family receptor, which mediates diverse functions across species44. Recent studies suggest that this peptide hormone may play a role in regulating food intake and metabolism44,46,47. However, the anatomical sources of SP, which is primarily produced by neurons in the brain and endocrine cells in the gut, as well as the regulatory pathways operating in these cells, remain unclear.
To investigate the mechanisms that regulate SP in the mouse gut, we derived organoids from a transgenic mouse line in which EC cells are marked by expression of the GFP derivative cyan fluorescent protein (CFP) under the control of the promoter of Tph1, which encodes tryptophan hydroxylase 1, a key enzyme in serotonin biosynthesis45. We confirmed the coexpression of Tph1-driven CFP and SP using immunofluorescence, demonstrating that CFP-positive EC cells produce SP and, thus, express the Tac1 gene (Fig. 4a and Extended Data Fig. 4a,b). Although SP-positive EC cells are believed to respond to nutrients and microbial metabolites, the molecular mechanisms underlying chemical sensing and its coupling to SP release remain largely unexplored48. To explore whether the TOR pathway governs Tac1 and SP expression and release in EC cells in response to dietary and metabolic cues as it does in Drosophila EECs, we examined the effects of inhibiting TOR in cultured intestinal organoids using Torin1, a potent TOR inhibitor. Torin1 treatment significantly reduced intestinal organoid Tac1 expression and intracellular SP levels in EC cells (Fig. 4b,c), suggesting that TOR-mediated nutrient sensing is part of a conserved mechanism that promotes tachykinin expression in gut endocrine cells of both flies and mammals in response to nutritional conditions. Whether this nutrient-sensing pathway regulates the expression or synthesis and release of other known and unknown factors produced by EC cells, including serotonin, remains an interesting subject for future studies.
a, Representative confocal images of mouse intestinal organoids derived from Tph1-CFP transgenic mice. Top, overview images illustrating organoid morphology along with CFP-expressing ECs (teal), F-actin (orange) and nuclei (blue). Scale bars, 50 µm (left) and 20 µm (right). Bottom, colocalization of CFP (teal) and SP (orange); nuclei (blue). Scale bar, 20 µm. Similar results were observed in five independent organoid samples. DAPI, 4,6-diamidino-2-phenylindole. b, Tac1 expression in organoids treated with the mechanistic TOR (mTOR) inhibitor Torin1 (two-tailed unpaired Student’s t-test). c, Anti-SP staining intensity within organoid EC cells (two-tailed Mann–Whitney U-test). d, Tac1 expression in organoids treated with the TRPA1 antagonist A-967079 (A-079), the TRPA1 agonist cinnamaldehyde (CNA) or the mTOR inhibitor Torin1 (one-way ANOVA with Dunnett’s test). e, Anti-SP staining intensity in EC cells treated with A-967079, CNA or Torin1 (Kruskal–Wallis with Dunn’s test). f, Region-specific Tac1 expression in the small intestine of animals fed a low- or high-protein diet for 3 d (left) or 7 d (right) (two-tailed unpaired Student’s t-tests). g, Plasma SP levels of mice fed a low- or high-protein diet for 1 or 3 d, measured by enzyme-linked immunosorbent assay (two-tailed unpaired Student’s t-tests). h, Schematic of Tac1 and SP regulation in mouse EC cells. Plots in b,d,f,g include mean ± s.e.m., and plots in c,e indicate the median and 95% confidence interval. Sample sizes (n) and P values are indicated in each plot. In b,d, n represents organoid samples. In c,e, n represents EC cells. In f, n represents guts. In g, n represents plasma samples. NS, P > 0.05.
Because we found that TrpA1 is expressed by Drosophila EECs and regulates their release of Tk (Fig. 3e–g), we next investigated the role of this excitatory cation channel in mouse intestinal organoids. Although TrpA1 is known to be expressed in mammalian EC cells and to regulate their serotonin release49, its involvement in SP secretion remains unknown. To assess whether TrpA1-dependent SP regulatory mechanisms might operate in mammalian EC cells, intestinal organoids were treated with the TRPA1 agonist cinnamaldehyde (CNA) or the antagonist A-967079. CNA treatment significantly increased Tac1 expression, suggesting that TRPA1 activation might promote SP production (Fig. 4d). Consistent with this hypothesis, we observed elevated SP levels in agonist-treated EC cells (Fig. 4e). On the other hand, antagonist-mediated inhibition of TRPA1 resulted in SP accumulation within EC cells without altering Tac1 transcription (Fig. 4d,e), indicating that TrpA1 inhibition reduces SP release, leading to its retention in EC cells, similar to the effect of TrpA1 loss on Tk release by EECs of the Drosophila gut. These findings suggest that TRPA1 regulates both SP production and secretion, likely through TRPA1-mediated calcium-dependent regulatory mechanisms. In contrast to this inhibition of TRPA1, which did not affect expression of Tac1 or SP, inhibition of the TOR pathway through Torin1 treatment did reduce Tac1 expression and concomitantly decreased EC cell SP levels in intestinal organoids (Fig. 4d,e), reinforcing the key role of TOR signalling in regulating SP production. In sum, these findings demonstrate that TRPA1- and TOR-mediated signalling pathways jointly promote the expression of Tac1 and the secretion of SP in mouse EC cells.
To further investigate nutrient-dependent regulation of Tac1 in vivo, we examined its expression over time in the small intestine of female mice fed a low- or high-protein diet. Similar to our observations in Drosophila, which indicate that protein-rich food activates Tk+ EECs in the posterior part of the midgut, we found that a high-protein diet led to upregulated Tac1 expression in only the posterior region of the mouse small intestine (the ileum), with higher Tac1 expression levels in high-protein-fed animals observed after both 3 and 7 d of feeding (Fig. 4f). This increase in Tac1 expression correlated with an increase in the circulating plasma SP concentration, indicating that high-protein feeding promotes not only Tac1 transcription but also SP release (Fig. 4g). The stabilisation of Tac1 expression by day 7, with no further increase beyond day 3, suggests the involvement of regulatory mechanisms such as negative feedback that prevent excessive accumulation. These findings align with our results from cultured organoids and support the notion that dietary protein content promotes Tac1 expression and SP secretion through TOR and TRPA1, which enable EC cells to sense metabolic and environmental inputs (Fig. 4h). In sum, these results highlight the evolutionary conservation of mechanisms coupling dietary inputs to Tk hormone release.
Gut Tk regulates glucagon-like signalling
To pinpoint the target tissue(s) through which gut Tk influences behaviour, we examined the expression of its cognate receptor, TkR99D, in flies. We found that this receptor is broadly expressed in the central nervous system, with notable expression in the neuroendocrine cells that produce AKH (AKH-producing cells (APCs); Fig. 5a), a glucagon-like hormone, as supported by earlier RNA-seq data50. AKH acts on the fat body (an organ comparable to the mammalian liver and adipose tissue) to induce the breakdown and release of stored energy during periods of starvation9,51,52,53,54. We recently discovered that the release of AKH from APCs is enhanced by the EEC-derived peptide allatostatin C (orthologous with mammalian somatostatin), released when nutrients are scarce9. We and others have also recently shown that the consumption of dietary sugar triggers the release of NPF from EECs10,18. This gut-derived NPF inhibits AKH release and thus blocks AKH-induced mobilisation of stores, thereby allowing consumed sugar to be taken up by adipose tissue and stored. Furthermore, we observed that gut-derived NPF, through regulation of AKH, can also influence food preference, regulating the choice between sugar and protein-rich yeast food10. We thus explored whether gut-derived Tk might also modulate AKH release and, if so, whether this mechanism might mediate the observed Tk-driven behavioural food choice adaptations to protein intake.
a, Confocal-microscopy image of a single preparation containing the brain and the ventral nerve cord, the gut and ovaries, stained for a Tk receptor reporter (TkR99D>GFP, yellow), AKH peptide (red), filamentous actin (phalloidin (phal), magenta) and DAPI (marking nuclei, blue). APCs are indicated and enlarged in the inset. Scale bars, 100 µm (main image) and 20 µm (inset). Similar expression and localisation patterns were observed in five independent samples. b–e, Anti-AKH staining intensity within APCs and whole-body Akh transcript levels. Scale bars 20 μm. b,c, AKH staining intensity (b) and Akh expression (c) in controls and flies with RNAi against TkR99D in APCs using Akh-GAL4 (Akh>), following 15 h of feeding on sugar or yeast medium (Kruskal–Wallis with Dunn’s test). TRiP, RNAi collection from the Harvard Transgenic RNAi Project. d, AKH staining intensity in controls and flies with knockdown of Tk in EECs (Tkgut>Tk-RNAi) after 15 h of sugar or yeast feeding (one-way ANOVA with Tukey’s test). e, AKH staining in controls and animals with RNAi against slif in Tk+ EECs (Tkgut>slif-RNAi) after 15 h of yeast feeding (two-tailed Mann–Whitney U-test). f, AKH staining in controls and animals with attenuated TOR signalling in Tk+ EECs, following 15 h of yeast feeding (Kruskal–Wallis with Dunn’s test). Sample sizes (n) and P values are indicated in each plot. In b,d–f, n represents APCs. In c, n represents APC tissue samples. Indicated central tendencies in b,c,e,f are the median and 95% confidence interval and in d are mean ± s.e.m. NS, P > 0.05.
In mammals, glucagon is released during periods of fasting and exercise that trigger the breakdown of energy stores and may lead to reduced blood sugar concentration. This function is mirrored by the role of AKH in insect energy store mobilisation. Interestingly, protein-rich foods can also trigger glucagon release55. Based on this, we hypothesized that Tk, released from EECs in response to protein intake, might stimulate APCs to release AKH. Supporting this notion, we observed reduced intracellular AKH levels in the APCs of animals that had consumed yeast compared to those of animals on a sugar diet (Fig. 5b). This depletion was not associated with changes in Akh transcript levels (Fig. 5c), suggesting that it can be attributed to increased secretion of the AKH peptide in yeast-fed animals. However, this effect was completely abrogated when TkR99D was knocked down in APCs using Akh-GAL4 (Akh>): yeast feeding did not lead to AKH depletion from APCs in these animals (Fig. 5b,c). Together, our data imply that food high in protein and low in carbohydrates triggers AKH secretion and that TkR99D in APCs is essential for this effect.
We then investigated whether the release of Tk from the midgut is required for the induction of AKH release observed in yeast-fed animals. Phenocopying APC-specific TkR99D knockdown, EEC-specific Tk knockdown blocked the yeast-feeding-induced drop in intracellular AKH peptide levels (Fig. 5d). This effect suggests that gut-derived Tk promotes AKH secretion through a humoral mechanism in response to the intake of protein-rich food. Given our results indicating that amino acid sensing mediated by Slif in Tk+ EECs is important for the release of Tk, we investigated whether the loss of slif in these cells in the midgut, which leads to Tk retention, would also impair AKH release from APCs. As expected, slif knockdown in Tk+ EECs resulted in greater AKH peptide levels in APCs after yeast feeding (Fig. 5e). To further investigate whether the nutrient-sensing pathways that regulate gut Tk-mediated AKH secretion involve TOR, we tested the effect of TOR inhibition in Tk+ EECs on AKH levels in APCs. Similar to the loss of slif, Tor knockdown or Tsc1 and Tsc2 overexpression in Tk+ EECs led to increased intracellular AKH peptide levels in APCs compared to controls in yeast-fed animals (Fig. 5f). These results indicate that TOR signalling in Tk+ EECs plays a critical role in linking dietary protein intake to Tk release and subsequent AKH secretion from APCs. Together, our data suggest that food high in protein and low in carbohydrates triggers Tk release from EECs through amino acid-sensing mechanisms involving Slif and TOR signalling. Tk then acts on APCs through TkR99D to promote AKH secretion.
Tk regulates food choice via glucagon-like signalling
Given the role of gut-derived Tk in regulating the release of AKH, a factor that can influence food preferences10, we asked whether the observed Tk-dependent appetite effects are mediated by AKH. We first investigated whether Tk modulates feeding on sugar- versus protein-containing foods through TkR99D in APCs. Like animals with Tk knockdown in EECs, mated adult females with APC-specific TkR99D knockdown displayed diminished sugar consumption over a 1-h period following a 15-h period of yeast consumption (Fig. 6a). On the other hand, these protein-satiated animals exhibited an increased appetite for protein compared to controls, consuming a greater amount of yeast food (Fig. 6b). In both tests, two distinct RNAi lines showed consistent phenotypes (Fig. 6a,b and Extended Data Fig. 5a). We validated this elevated sugar consumption through a longer-duration feeding test and confirmed increased sugar-feeding behaviour in both medium-term and long-term consumption assays and the automated FLIC and flyPAD systems (Fig. 6c–e and Extended Data Fig. 5b–e). We examined whether TkR99D in APCs influences metabolism and found a minor increase in starvation resistance but no changes in TAG or glycogen levels in animals with APC-specific TkR99D knockdown under fed conditions (Extended Data Fig. 5f), mirroring the effects observed with EEC-specific Tk knockdown (Extended Data Fig. 1e–g). Together with the rapid effects on feeding behaviour induced by activation of Tk release from the gut, these results collectively suggest that behaviour is governed by EEC-derived Tk’s action on APCs via TkR99D.
a,b, Dye-consumption assays for sugar (a) and yeast (b) in controls and animals with RNAi against TkR99D in APCs (a, one-way ANOVA with Dunnett’s test; b, Kruskal–Wallis with Dunn’s test). c,d, Longer-term feeding behaviour of similar animals: 6-h CAFÉ assay for sugar (Kruskal–Wallis with Dunn’s test) (c) and sugar-feeding behavioural data recorded for 22 h using the FLIC system (one-way ANOVA with Dunnett’s test) (d). e, Sugar sips in a 1-h flyPAD assay for controls and animals with APC-specific TkR99D knockdown (two-tailed Mann–Whitney U-test). f,g, Short-term dye-consumption nutrient-intake assays for sugar (f) and yeast (g) in controls and animals expressing APC-specific Akh RNAi (two-tailed Mann–Whitney U-test (f), two-tailed unpaired Student’s t-test (g)). h,i, Six-hour sugar intake in the CAFÉ assay (h) and 22-h sugar-feeding (i) behaviour (FLIC) of controls and animals expressing Akh knockdown in APCs (two-tailed Mann–Whitney U-test). j, Dye-consumption assay for sugar in animals with EEC-specific Tk-RNAi, in an Akh-loss-of-function background (two-tailed Mann–Whitney U-test). k, Schematic for l–n showing 1-h dye assays for sugar and yeast intake. l,m, Pan-neuronal (l) or fat body (m) knockdown of AkhR (two-tailed Mann–Whitney U-tests). n, AkhR knockdown in subsets of neurons targeted by their respective GAL4 drivers: interoceptive sensory neurons (ISNs) (R34G02-GAL4); insulin-producing cells (IPCs; Ilp2>); major neurosecretory cells (dimm>); neurons of the central circadian clock (tim>); and octopaminergic (Tdc2>), dopaminergic (Tyrosine hydroxylase, TH>), glutamatergic (Vesicular glutamate transporter 1, VGlut1>) and cholinergic (ChAT>) neuronal populations (two-tailed unpaired Student’s t-test). o, Feeding behaviour toward sugar measured over 22 h in the FLIC apparatus in controls and animals with RNAi against AkhR in the cholinergic cell population (Kruskal–Wallis with Dunn’s test). p, Model that is consistent with these data. Sample sizes (n) and P values are indicated in each plot. n represents animals per group. Behavioural experiments were performed following 15 h of yeast feeding. Box plots show the median, the bounds of the box (25th and 75th percentiles) and whiskers representing minimum and maximum values. Plots in d,g indicate mean ± s.e.m., and plots in e,i,o indicate the median and 95% confidence intervals. NS, P > 0.05.
To ascertain whether AKH mediates the effects of Tk or TkR99D loss on feeding, we first assessed the impact of Akh knockdown on nutrient-specific appetite. Like gut-specific Tk knockdown or APC-targeted TkR99D knockdown, Akh knockdown in APCs led to reduced sugar consumption by yeast-satiated animals (Fig. 6f), and this was not attributable to the RNAi construct alone (Extended Data Fig. 5h). By contrast, these Akh-knockdown animals consumed more yeast-based food than controls (Fig. 6g). Knockdown of Akh also reduced yeast-fed animals’ sugar consumption over a longer observation period and decreased their sugar-feeding behaviour when compared to controls and animals with the RNAi transgene alone (Fig. 6h,i and Extended Data Fig. 5i,j). Thus, Akh loss has effects on feeding similar to those seen with gut Tk loss or knockdown of TkR99D in APCs. To discern whether AKH mediates the effects of Tk signalling on feeding patterns, we analysed the ability of gut Tk to alter sugar feeding in Akh-deficient animals following 15 h of feeding on a yeast diet. As gut-specific Tk knockdown leads to reduced sugar intake (Fig. 1b,g–i), we analysed food consumption over an extended 2-h window to detect any variations. In Akh mutants, EEC-specific Tk knockdown had no effect on sugar intake, suggesting that AKH is the primary factor mediating the influence of gut Tk on feeding behaviour (Fig. 6j). Collectively, our results suggest that gut-derived Tk, acting through TkR99D on APCs, stimulates AKH release following a protein-rich diet, and AKH inhibits further protein intake and enhances the appetite for sugar.
To determine specific tissues mediating food choice effects of AKH, we knocked down the AKH receptor (AkhR) in neurons using R57C10-GAL4 (R57C10>) and the fat body using cg-GAL4 (cg>), two key tissues known to express this receptor56. Although loss of AkhR in the fat body affected food intake, it was not sufficient alone to drive the food choice effects observed with AKH loss (Fig. 6k–m). By contrast, pan-neuronal AkhR knockdown significantly altered food choice, reducing sugar intake and increasing yeast consumption following a 15-h period of yeast feeding, thus mimicking the phenotype observed in animals lacking AKH. These findings suggest that neuronal AKH signalling plays a critical role in mediating food choice behaviours.
We next interrogated neuronal populations that might mediate these effects by using drivers targeting distinct neuronal subsets. Our results showed that knockdown of AkhR in glutamatergic neurons influenced yeast intake, but loss of AkhR in cholinergic neurons specifically recapitulated the food choice effects observed with pan-neuronal AkhR knockdown (Fig. 6n). Importantly, the phenotype associated with AkhR loss in cholinergic neurons was confirmed with a second RNAi construct targeting AkhR, which recapitulated decreased sugar-feeding behaviour (Fig. 6o) and decreased sugar consumption and increased yeast intake (Extended Data Fig. 5k–m). Consistent with these results, both single-cell PCR with reverse transcription54 and single-cell RNA-sequencing data33 (Extended Data Fig. 5n) indicate that AkhR is expressed in a subset of cholinergic neurons (choline acetyltransferase positive, ChAT+), further supporting the role of this neuronal population in mediating AkhR-regulated food choice behaviours. These findings indicate that AKH signalling in cholinergic neurons is important for driving the food choice adaptations mediated by gut Tk and APC TkR99D. Together, these results suggest that gut Tk, released in response to dietary protein, regulates AKH signalling via TkR99D in APCs. AKH mediates the observed food choice behaviours through neuronal AkhR signalling, specifically involving cholinergic neurons (Fig. 6p).
Protein-rich food impacts sleep via gut TK and AKH pathways
Feeding and sleep are interconnected behaviours, with diet exerting an important influence on sleep and activity patterns. In times of food scarcity, animals often suppress their sleep to prioritize foraging, which increases their activity. Under starvation conditions, the release of AKH suppresses sleep and leads to hyperactivity53, and our recent findings reveal that the gut hormone allatostatin C contributes to this adaptive starvation-induced sleep suppression by modulating AKH9. The macronutrient composition of the diet also seems to influence sleep depth and quality57,58. Although the underlying mechanisms remain largely elusive, recent findings suggest that dietary protein intake may influence arousability (the ease with which stimuli can induce a response during sleep) through peptides released from the gut59. Given that gut Tk promotes AKH release in response to protein-rich food intake, we rationalized that this would suppress sleep during protein feeding. We examined sleep during the midday period, when animals naturally exhibit a period of sleep after being active and eating in the morning, to enhance sensitivity in detecting diet-dependent effects. We also measured sleep during the night, when animals generally are inactive. Animals with EEC-specific Tk knockdown exhibited markedly different midday sleep patterns depending on their diet. Under yeast-feeding conditions, these animals displayed elevated midday sleep compared to controls (P < 0.0001, interaction between diet and genotype), while no significant differences were observed under the sugar-feeding condition commonly used in Drosophila sleep studies (Fig. 7a). In contrast to the diet-dependent effects of Tk on sleep during the midday period, animals with gut Tk knockdown exhibited increased sleep on both diets, indicating that gut Tk mainly exerts dietary influence on sleep during the midday period (Extended Data Fig. 6a). To validate these findings, we replicated the experiment using a second independent RNAi line, confirming the robustness of the observed effects (Extended Data Fig. 6b). This suggests that gut-derived Tk mediates the effect of dietary protein on midday sleep regulation, possibly by modulating AKH signalling to promote wakefulness under protein-rich dietary conditions, with minimal influence under sugar-fed conditions. We also examined sleep in males and found similar effects of gut Tk loss, indicating an essential and non-sex-specific role for this gut-derived hormone in sleep regulation around midday, particularly in response to protein intake (Extended Data Fig. 6c–e).
a, Left, sleep profile graphs showing the percentage of flies asleep over time for controls and gut-specific Tk-knockdown animals consuming sugar and yeast diets. The shaded area represents s.e.m. Right, quantification of sleep during the midday ‘siesta’ from Zeitgeber time (ZT) 4 to 8 h when flies normally exhibit high sleep, in animals with Tk knockdown in the gut on sugar and yeast diets (two-tailed Mann–Whitney U-tests). b,c, Left, sleep profiles of controls and animals with RNAi against TkR99D (b) or Akh (c) in APCs. Right, quantification of midday sleep in these animals (two-tailed Mann–Whitney U-tests). d,e, Left, sleep profiles of controls and animals with RNAi against AkhR in the fat body (d) or pan-neuronally (e). Right, quantification of midday sleep in these animals (two-tailed Mann–Whitney U-tests). f, A model of Tk function consistent with these data. Sample sizes (n) and P values are indicated in each plot. n represents animals per group. Dot plots indicate the median and 95% confidence interval. NS, P > 0.05.
We therefore explored whether Tk exerts this sleep modulatory effect through TkR99D in APCs, and we probed whether AKH also governs sleep responses to protein feeding. Mirroring the effects of Tk knockdown in the gut, TkR99D knockdown in APCs resulted in increased sleep during the midday period specifically under yeast-feeding conditions (P < 0.0001, interaction between diet and genotype; Fig. 7b and Extended Data Fig. 6f–i), consistent with the hypothesis that gut-derived Tk influences sleep patterns through its receptor in APCs. Similarly, animals with loss of AKH function displayed increased midday sleep only when fed yeast compared to controls (P < 0.0001, interaction between diet and genotype), indicating that AKH is required for the sleep-suppressing effects of dietary protein at this time of day (Fig. 7c and Extended Data Fig. 6j). Together, these findings indicate that, in environments characterized by diets high in protein and low in carbohydrates, the modulation of AKH release by gut-derived Tk orchestrates adaptive feeding and sleep behaviours. This likely ensures a balance in nutrient intake and prioritizes feeding over restorative behaviours, such as sleep.
Next, we investigated whether the sleep-regulating effects of AKH are mediated through neuronal or fat body AkhR signalling. Knockdown of AkhR in the fat body resulted in increased sleep during the midday period on a protein-rich diet (P < 0.0001, interaction between diet and genotype; Fig. 7d and Extended Data Fig. 6k–n). Similarly, knockdown of AkhR in neurons led to elevated midday sleep specifically under yeast-feeding conditions (P < 0.001, interaction between diet and genotype; Fig. 7e and Extended Data Fig. 6o–r), indicating that both neuronal and fat body AkhR signalling pathways are involved in mediating AKH-regulated sleep adaptations in response to dietary protein. Given our results indicating cholinergic mediation of AKH-induced food choice effects (Fig. 6n,o), we next tested whether this class of neurons is also involved in mediating the effects of AKH on sleep behaviour in response to yeast feeding. However, knockdown of AkhR in the cholinergic population had no significant effect on midday sleep or on the influence of diet on this behaviour (Extended Data Fig. 7a–c). We also tested octopaminergic neurons, which have been reported to play a role in modulating AKH-dependent activity patterns54,60, but knockdown of AkhR in these neurons using Tdc2-GAL4 did not significantly influence sleep or alter the effects of diet on this behaviour, suggesting that the sleep-regulating effects of AKH signalling are not mediated through these neuronal populations (Extended Data Fig. 7d–f). In sum, these findings suggest that AKH signalling through AkhR in both neurons and the fat body plays a cooperative role in balancing sleep and activity under protein-rich dietary conditions (Fig. 7f).
Gut Tk–AKH axis influences reproduction and longevity
Dietary modifications have substantial effects on lifespan and fecundity across taxa61,62. A balanced diet generally supports longevity, whereas fecundity in flies is mainly affected by dietary yeast content. Because gut Tk is responsive to protein-rich food consumption and modulates dietary choices to maintain nutritional balance, we sought to investigate its potential influence on fecundity and lifespan. We assessed fecundity by quantifying the viable offspring of genetically manipulated females allowed for 24 h to mate with w1118 control males. On a standard diet, which includes both sugar and yeast, reduction of Tk expression in the females’ EECs with either of two independent RNAi lines resulted in an increased number of offspring (Fig. 8a and Extended Data Fig. 8a). Conversely, females in which Tk release was induced through TrpA1 activation exhibited a marked decrease in offspring number compared to controls. This effect was abolished by concurrent Tk knockdown, indicating that the reduction in offspring requires gut-derived Tk (Fig. 8b and Extended Data Fig. 8b). Next, we investigated whether, as observed with feeding, this gut Tk-regulated process involves TkR99D and AKH in APCs. Consistent with the consequences of gut Tk knockdown, the downregulation of TkR99D or Akh in these cells resulted in a statistically significant, albeit modest, elevation in fecundity (Fig. 8c and Extended Data Fig. 8c), aligning with reports that diminished AKH signalling increases female fecundity63. As TkR99D is also expressed in the nervous system (Fig. 5a), we examined the effect of pan-neuronal TkR99D knockdown on fecundity. Knocking down TkR99D in all neurons did not affect the number of offspring produced (Extended Data Fig. 8d), which also suggests that the effects observed with APC knockdown are not due to the presence of the RNAi transgene itself. Our data indicate that gut-derived Tk influences the number of offspring a female produces after mating, at least in part by modulating AKH signalling, establishing links between the yeast-responsive gut factor Tk, AKH pathway activity and fecundity.
a, Cumulative number of offspring measured over a 22-d period for controls (Tkgut>) and females with EEC-specific RNAi against Tk (Tkgut > Tk-RNAi; two-way ANOVA for genotype × day, followed by Fisher’s least significant difference test; for clarity, only P ranges are indicated here, with numerical values given in Extended Data Fig. 8a). b, Cumulative offspring count over 8 d for control females and animals undergoing TrpA1-mediated exogenous activation of Tk+ EECs, with and without simultaneous knockdown of Tk (two-way ANOVA for genotype × day with Dunnett’s test; numerical P values are given in Extended Data Fig. 8b). c, Fecundity assessment of female flies with knockdown of TkR99D or Akh in APCs (two-way ANOVA for genotype × day with Fisher’s least significant difference test; numerical P values are given in Extended Data Fig. 8c). d–g, Survival curves for female flies under the given dietary conditions. d, Lifespan of controls, animals with EEC-specific Tk knockdown and animals with TrpA1 activation of Tk+ EECs (with and without simultaneous Tk knockdown) on 10% and 20% yeast diets. e,f, Lifespan of animals with APC-specific knockdown of TkR99D (e) or Akh (f) on different dietary yeast concentrations. g, Longevity comparison between controls and gut-specific Tk-knockdown animals, both in an Akh-null background, on 20% and 10% yeast diets. h, Lifespan of animals with AkhR knockdown in all neurons (left, driven by R57C10-GAL4) or in fat tissue (right, with cg>), compared with their respective controls. i, A comprehensive model for Tk–SP regulation and function in mice and flies. a–c indicate mean ± s.e.m. Pairwise P values in d–h were obtained using log-rank (Mantel–Cox) tests. Sample sizes (n) and P values are indicated in each plot. In a–c, n represents independent groups of animals. d–h, n represents animals per group. NS, P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Dietary yeast concentration influences the lifespan of Drosophila, with increasing levels reducing longevity64,65. Given that gut Tk is secreted in response to dietary yeast, we explored whether Tk might be involved in determining longevity. Silencing gut Tk via either one of two independent RNAi lines resulted in lifespan extension on diets containing 9% sugar supplemented with low (1%), normal (10%) or elevated (20%) concentrations of yeast (Fig. 8d and Extended Data Fig. 8e). Conversely, to mimic high protein consumption with respect to Tk signalling, in animals not consuming a high-protein diet, we induced Tk release via TrpA1 expression in Tk+ EECs, which led to decreased longevity on both diets, with a more pronounced effect observed on the 10% yeast diet (a 4-d reduction) than on the higher-yeast medium (a 2.1-d reduction). This difference may arise because Tk+ EECs are presumably already more active on the 20% yeast diet. Although the calcium activity of Tk+ EECs was not elevated on a 20% yeast diet compared to a 10% yeast diet, Tk peptide levels in EECs were reduced, indicating that there may be an increased release of Tk from the gut (Extended Data Fig. 8f). On the 10% yeast diet, the negative impact on lifespan of activating Tk+ EECs was partially abrogated by concurrent Tk knockdown, suggesting that the lifespan-shortening effect is partially driven by Tk release. Some Tk+ EECs also produce NPF, which responds to dietary sugar10,18, and this second factor may give rise to a confounding effect in animals fed the 10% yeast diet, in which sugar and yeast are relatively more balanced, potentially triggering greater NPF release. Consistent with this view, NPF+ EECs exhibited higher calcium activity on the 10% yeast diet than on richer medium (Extended Data Fig. 8g), suggesting that NPF release may indeed contribute to the phenotype observed on the 10% yeast diet, which is relatively richer in sugar than the 20% yeast diet. By contrast, on the 20% yeast diet, in which dietary yeast exceeds sugar content, the knockdown of gut Tk completely counteracted the reduced longevity observed with exogenous stimulation of Tk+ EECs. This may suggest that, on a high-yeast diet, Tk is the predominant hormone released by Tk+ EECs that influences lifespan.
We then assessed whether the influence of gut Tk on lifespan was mediated by TkR99D-regulated AKH release from APCs. APC-specific knockdown of TkR99D or Akh itself led to an extended lifespan on the diets with different yeast concentrations (Fig. 8e,f and Extended Data Fig. 8h), mirroring the lifespan extension observed with gut-specific Tk knockdown. All these manipulations mimic reduced protein intake with respect to this signalling axis, and these results suggest that gut-derived Tk, released in response to protein intake and acting via its receptor on APCs, governs AKH secretion, which, in turn, modulates lifespan. To further substantiate this notion, we posited that gut-specific Tk knockdown should not alter lifespan in AKH-deficient flies. Confirming this hypothesis, EEC-specific RNAi against Tk had no effect on longevity in Akh-null mutant flies (Fig. 8g). As these animals carry the Tk-RNAi construct, this experiment also rules out the possibility that the transgene itself causes effects independent of AKH. To further explore tissue-specific contributions of AkhR signalling, we tested neuronal and fat body-specific AkhR knockdown on diets containing 10% and 1% yeast. Both manipulations led to an extended lifespan on the 10% yeast diet, consistent with the idea that reduced AKH signalling improves survival under protein-rich conditions (Fig. 8h). However, on the 1% yeast diet, only fat body AkhR knockdown extended lifespan significantly (Extended Data Fig. 8i,j). Together, these findings indicate distinct yet complementary roles for neuronal and fat body AkhR signalling in integrating dietary protein cues to modulate lifespan. Collectively, our results demonstrate that gut-secreted Tk modulates behavioural adaptations in food selection and sleep following the consumption of protein-rich yeast food and also influences lifespan. These effects are mediated by the glucagon-like hormone AKH, which exerts distinct effects via signalling to neurons and the fat body (Fig. 8i).
Discussion
EECs of both flies and humans can sense nutrients including sugars, amino acids and fatty acids, triggering the release of hormones that modulate systemic metabolism and influence behaviours including feeding9,10,18,22,66. We find that protein intake activates Tk+ EECs through a mechanism requiring the conserved amino acid transporter Slif (orthologous with mammalian SLC7A family amino acid transporters) and the nutrient-sensing TOR pathway in flies. Furthermore, our findings suggest that the ROS sensor TrpA1 complements these mechanisms. This may enable Tk+ EECs to integrate redox signals, which are associated with nutrient-induced metabolic changes67, to regulate Tk release. Like Drosophila Tk, the mammalian Tk ortholog Tac1 and its encoded peptide SP are also expressed in a subset of EECs68,69, but the regulation of Tac1 and the function of SP derived from these EECs (EC cells) remain poorly defined. Our data demonstrate that gut Tac1 and SP are regulated by TOR- and TRPA1-dependent mechanisms in mouse EC cells, suggesting that Tac1 and SP are protein responsive in mammals. Our findings using intestinal organoids suggest that TOR promotes Tac1 expression, while TRPA1 regulates both synthesis and release of SP in EC cells. Mechanistically, TRPA1 activation increases intracellular calcium levels, triggering calcium-dependent signalling cascades that may regulate Tac1 expression and promote SP release via calcium-driven exocytosis of SP-containing vesicles. While intestinal organoids offer valuable insights into the regulation of Tac1 expression and SP release in EC cells, we acknowledge certain limitations of the model. Specifically, technical challenges in generating a sufficient number of organoids hindered reliable quantification of SP secretion from EC cells, a small subset of the intestinal epithelium, using established immunoassay methods. Additionally, regional differences in nutrient transporters, TRPA1 expression and downstream signalling pathways observed in vivo may be less distinct in organoid models, complicating direct comparisons of region-specific Tk regulatory mechanisms. However, we show that dietary protein regulates Tk specifically in the posterior gut of both flies and female mice, suggesting that its regulation is driven by amino acid sensing in this region of the gut and that Tk regulatory mechanisms may be shaped by region-specific signalling within the intestine. The biological basis of this regionalisation, potentially driven by differences in TrpA1 expression or nutritional sensors upstream of TOR, presents an interesting avenue for future research. These findings underscore the conservation of nutrient- and redox-sensing pathways in EECs that govern gut Tk release across species. SLC7A cationic amino acid transporters are also expressed in the mammalian intestine70. Future investigations should decipher the role of SLC7 family amino acid transporters in linking dietary amino acids to EEC-mediated secretion of hormones that govern mammalian feeding behaviours. EC cells produce both SP and serotonin, and it is likely that the regulatory pathways controlling their calcium-driven secretion overlap. However, the mechanisms controlling synthesis and pre-exocytic trafficking of these signalling molecules may diverge, as Tac1 and SP expression and serotonin biosynthesis follow distinct regulatory routes. Thus, whether TOR also governs serotonin synthesis remains an open question.
Nutrient-specific appetites drive food selection based on internal macronutrient requirements in animals, including humans71. Although many hormones are known to regulate appetite in general, the endocrine mechanisms underlying the regulation of nutrient-specific hungers are not well understood. Protein intake induces a strong satiety response compared to fat and carbohydrate, and gut-derived hormones are postulated to mediate this protein-induced satiety response72. We present evidence that the EEC-derived hormone Tk is indeed such a regulator of selective appetite that induces protein-specific satiety in flies, which may help explain the endocrine mechanisms underlying the satiating effects of protein. We previously found that gut-derived NPF, the Drosophila homologue of mammalian peptide YY (PYY), is secreted by EECs in response to carbohydrate consumption and acts as a sugar-specific satiety hormone10. Here, we propose that EEC-derived Tk and NPF function antagonistically to balance protein- and sugar-specific appetites, thereby balancing consumption of these nutrients with the needs of the animal. Gut-derived Tk stimulates AKH release following protein intake, which promotes protein satiety, whereas gut-derived NPF inhibits AKH secretion after sugar intake, thereby promoting sugar satiety10. Our results here indicate that AKH mediates these effects of gut-derived Tk through AkhR signalling in neurons, specifically cholinergic neurons, which are important for driving food choice in response to protein intake.
In addition to influencing feeding, dietary inputs also affect sleep and activity patterns, highlighting the interconnected nature of these behaviours73. Studies by others indicate that dietary protein also induces the EEC-mediated release of the hormone CCHamide-1, which influences arousal59. Our findings demonstrate that gut Tk modulates sleep in response to protein intake, promoting wakefulness through its regulation of AKH signalling in both neuronal and fat body tissues, underscoring the multitissue integration required for balancing sleep and activity under protein-rich dietary conditions. This highlights the importance of gut-derived hormones including Tk in coordinating feeding and sleep behaviours in response to dietary cues.
Dietary restriction, particularly the limitation of protein intake, is one of the main factors that extends lifespan across species74,75. Protein restriction induces hepatic FGF21, a protein-specific hunger hormone13,76, which has been shown to prolong lifespan in mice77. By contrast, we observed that EEC-derived Tk, stimulated by protein consumption, affects lifespan in the opposite direction: high levels lead to a reduction in lifespan, whereas its inhibition promotes longevity. Our findings indicate that gut Tk may mediate the link between high-protein intake and shortened lifespan. Further research is needed to decipher the molecular and cellular pathways through which gut Tk and AKH affect longevity, presenting an interesting direction for future investigation. Given the surge in popularity of metabolic disorder therapies based on gut hormones such as GLP-1, an understanding of how these hormones influence overall healthspan and longevity is becoming increasingly important.
In conclusion, our study contributes to the understanding of nutrient-specific satiety signals and the role of EEC-derived hormones in regulating food choices and lifespan. These insights have important implications for understanding the mechanisms of nutrient sensing and appetite regulation, which may potentially be targeted in preventive or therapeutic strategies for metabolic disorders and aging.
Methods
Fly stocks and maintenance
Animals were raised on a standard laboratory medium (82 g l−1 cornmeal, 60 g l−1 sucrose, 34 g l−1 yeast, 8 g l−1 agar, 4.8 ml l−1 propionic acid and 1.6 g l−1 methyl-4-hydroxybenzoate preservative) at 25 °C with 60% humidity and a 12-h light–dark cycle. Before experiments, adult males and females were transferred together to an adult-optimized diet lacking cornmeal (90 g l−1 sucrose, 80 g l−1 yeast, 10 g l−1 agar, 5 ml l−1 propionic acid and 15 ml l−1 of 10% methyl-4-hydroxybenzoate in ethanol)78 for 6–7 d. One day before experiments, flies were sorted by sex (15–20 flies per vial), and mainly mated females were used for experiments. Unless otherwise was stated, flies were treated (fed) for 15 h with a 10% yeast diet before further experiments. Several special-purpose diets were also used, all of which are based on 1% agar in water containing 0.15% methyl-4-hydroxybenzoate (0.1% for dye-feeding media). These diets included a sugar diet (10% sucrose), a yeast diet (10% dry yeast), lipid-depleted yeast extract (10%, Sigma, Y1625), peptone (10%, Gibco, 211677), coconut oil (10%, ‘organic’, obtained from a local grocery store), lipid-depleted casein (10%, Sigma, C5679) and a synthetic amino acid mixture79. Yeast extract and casein were depleted of lipids by six chloroform extractions: dry material was stirred with five volumes of chloroform at room temperature for at least 6 h in a fume hood, and insoluble material was retained by filtration (Whatman paper in a ceramic funnel). The material was completely air dried before use in medium. Animals were transferred to fresh vials of food every 2 d. Fly lines are listed in Supplementary Table 1. To minimize genetic background effects, GAL4 and GAL80 lines were backcrossed for several generations into the same non-isogenized but genetically well-regimented background population of w1118 before they were used to drive RNAi lines or crossed to this same w1118 as a control.
Feeding assays
Before feeding assays, mated females were protein or sugar sated during a 15-h period of feeding on yeast medium (10% yeast alone in 1% agar) or sugar medium (10% sucrose alone in 1% agar), as indicated in each figure panel. All food-intake experiments were performed during subjective breakfast time, beginning at ZT1 (1 h after lights-on). Short-term consumption was assessed using a dye-based feeding assay80, whereas 1-h feeding behaviour (‘sips’) was recorded using the flyPAD apparatus26. Longer-term consumption was measured using the CAFÉ assay24, and longer-term feeding behaviour was captured using the FLIC system25.
Dye feeding
Flies were transferred without anaesthesia to a vial containing the indicated medium dyed with 0.5% erioglaucine (Sigma-Aldrich, 861146) and allowed to feed for 60 min (90 min for Figs. 2h and 3i, 2 h for Figs. 1n and 6j and 4 h in Fig. 1d). In parallel, similar flies were transferred to undyed food for measurement of the baseline absorbance of fly lysates. Lysate samples were prepared by homogenizing single flies in 100 μl phosphate buffer, pH 7.5, using a TissueLyser LT (Qiagen) bead mill with 5-mm stainless steel beads (Qiagen, 69989). These lysates were clarified by a 5-min centrifugation step at 16,000g, after which 50 μl supernatant was loaded into a 384-well plate. Sample absorbance was measured at 629 nm on an EnSight multimode plate reader (PerkinElmer). A standard curve for erioglaucine was used to convert absorbance values to food-consumption amounts.
FlyPAD system
This electronic system was used to monitor sips of single diets as well as to assess preferences in two-choice behavioural assays. A 3-μl droplet of the indicated medium was loaded into the food pedestals of each flyPAD. Flies were briefly cold immobilized for an extremely short duration, just long enough to facilitate transfer to the flyPAD behavioural arenas. This procedure ensured minimal potential effects on behaviour, as flies quickly recovered upon transfer. Each fly’s behaviour was recorded for 1 h using the developer’s software within the Bonsai data stream-processing environment. Analysis was performed using the developer’s software (https://flypad.rocks/).
CAFÉ assay
A 5-μl capillary tube (Hirschmann, 9000105), filled with 10% sucrose in water containing preservatives (0.1% propionic acid and 0.1% methyl-4-hydroxybenzoate), was inserted through a hole in the lid of a 2-ml Eppendorf tube. Individual flies were briefly cold anaesthetized for transfer into these tubes, and similar tubes containing no flies were used as controls for evaporation. The prepared tubes were then kept inside a moist chamber within a standard fly incubator, and the movement of the liquid medium meniscus within the capillary was recorded over time.
Fly Liquid-Food Interaction Counter system
Drosophila feeding monitors (DFMs; Sable Systems) were maintained in an incubator (25 °C, 70% humidity, 12:12-h light–dark cycle). The food tank of each DFM was topped up at lights-on before experiments with a solution of 10% sucrose in water. Single animals were placed in each of the 12 DFM chambers, and feeding behaviours were recorded by the system for 6–22 h. Data were analysed with the published FLIC package25 (https://github.com/PletcherLab/FLIC_R_Code).
Immunohistochemistry and confocal imaging
To visualize EEC activity, we used the CaLexA system, in which calcium concentration is reflected in GFP expression30. Because of the relatively long perdurance of GFP, its abundance can be measured after the fact as a reporter of integrated recent calcium-signalling activity. Coexpressed calcium-independent tdTomato acts as a ratiometric control for normalizing GAL4 expression in each cell. Animals expressing UAS-CaLexA-GFP and UAS-tdTomato in Tk-producing EECs under the control of Tkgut> were fed for the indicated interval on the indicated media. After feeding, guts or APCs (the latter as part of a tissue ensemble that included the proventriculus, not as isolated single cells) were dissected in cold PBS and fixed in 4% formaldehyde in PBS for 1 h (guts) or 20 min (APC ensembles) at room temperature with agitation. Tissues were washed three times, each time for 20 min, with PBST (PBS + 0.1% Triton X-100) and blocked with 5% normal goat serum in PBST for 30 min, all at room temperature with agitation. Tissues were stained with mouse anti-GFP (Thermo Fisher, A11120, 1:500), rat anti-mCherry (for the mCherry derivative tdTomato; Thermo Fisher, M11217, 1:1,000) and rabbit anti-Tk81 or rabbit anti-AKH82 (both kind gifts from J. Veenstra, University of Bordeaux, 1:1,000) antibodies overnight at 4 °C with agitation.
Tissues were then washed again three times with PBST before being incubated overnight at 4 °C in secondary antibody solution (Alexa Fluor 488-conjugated goat anti-mouse (Thermo Fisher, A32723), Alexa Fluor 555-conjugated goat anti-rat (Thermo Fisher, A21434), Alexa Fluor 594-conjugated goat anti-rabbit (Thermo Fisher, A11012) or Alexa Fluor Plus 647-conjugated goat anti-rabbit (Thermo Fisher, A32733) antibodies, all diluted 1:500 in PBST). Tissues were washed three more times and mounted in ProLong Glass (Invitrogen, 36984, for stains including Alexa Fluor 647, which is quenched by Vectashield83), Fluoroshield with DAPI (Sigma, F6057) or Vectashield (Vector Laboratories, H-1200) antifade mountants, either on slides coated with poly-l-lysine (Sigma, P8920) and sealed using a 0.12-mm spacer (Grace Bio-Labs, 654006) or on 35-mm glass-bottom dishes (MatTek, P35G-0-10-C) sealed with a 0.1-mm glass coverslip. In cases where Alexa Fluor 647-conjugated phalloidin (Thermo Fisher, A30107) was used, it was included (1:1,000 in PBST) in the first wash step. Slides were imaged using a Zeiss LSM 900 confocal microscope and ZEN acquisition software. All samples that were quantitatively compared to one another were prepared simultaneously with the same staining preparations and imaged with the same hardware and software settings. Image analysis was performed using the open-source programme ImageJ and a MATLAB script10 to automate the process of identifying cells in confocal image stacks, quantifying the fluorescence signal in each channel and subtracting the local background intensity. CaLexA signal was normalized to the tdTomato intensity, and the reported data points represent the ratio of GFP to tdTomato within each cell to control for variation in GAL4 activity.
In situ hybridisation
To visualize target mRNA coexpression, we employed a hybridisation chain reaction (HCR) in situ protocol using a fluorescent probe-based detection system. The protocol was adapted from previously developed methods84 with minor modifications. Reagents and buffers were sourced from Molecular Instruments. Whole guts were dissected in cold PBS and fixed in 4% paraformaldehyde (PFA) for 1 h at room temperature with gentle agitation. After fixation, tissues were rinsed and washed three times, each for 10 min, with PBS containing 0.1% Tween-20 (Sigma, P1379) at room temperature. Tissue was permeabilized for 30 min at 37 °C in buffer containing 1% SDS, 0.5% Tween-20, 50 mM Tris-HCl, 1 mM EDTA and 150 mM NaCl, pH 7.5. Permeabilization buffer was removed, and warm (37 °C) hybridisation buffer (Molecular Instruments, ‘HCR buffers pack’) was then added, and tissues were incubated for 30 min at 37 °C. Warm hybridisation buffer containing probes synthesized by Molecular Instruments (slimfast probes with B3 initiator tag, B4-tagged tachykinin probes and B5-tagged prospero probes, each at a final concentration of 15 nM) was then added, and tissues were incubated overnight at 37 °C. Guts were washed four times, for 15 min each, with warmed probe wash buffer (Molecular Instruments, ‘HCR buffers pack’) at 37 °C. This was followed by two 5-min washes with 5× SSCT buffer (20× SSC buffer concentrate (Sigma, S6639) with 0.1% Tween-20 (Sigma, P1379)) at room temperature. Amplification buffer (Molecular Instruments, ‘HCR buffers pack’) was then applied, and tissues were incubated at room temperature for 40 min. Amplification buffer containing fluorophore-labelled hairpins (Molecular Instruments, HCR amplifiers Alexa Fluor 488-B3, Alexa Fluor 647-B4 and Alexa Fluor 546-B5, 120 nM each) was added, and tissues were incubated overnight at room temperature in the dark. Tissues were washed five times, for 10 min each time, with 5× SSCT buffer at room temperature. This was followed by six 15-min room temperature washes with PBS at room temperature. Guts were mounted on slides coated with poly-l-lysine (Sigma, P8920) in ProLong Glass Antifade Mountant (Invitrogen, 36984) and sealed with a 0.12-mm spacer and a 0.1-mm glass coverslip. Imaging was performed using a Zeiss LSM 900 confocal microscope with ZEN acquisition software.
Luciferase activity measurement
Tissue luciferase activity was measured as previously described10. Midguts from Tk>CaLexA, LexAop-luciferase females were excised and placed into lysis buffer (Promega, E266A/E2661). Four midguts per replicate were homogenized in 100 µl lysis buffer in 2-ml round-bottom Eppendorf tubes using a TissueLyser LT (Qiagen) and 5-mm stainless steel beads (Qiagen, 69989). The lysates were incubated for 10 min at room temperature for tissue lysis and equilibration of extracts to room temperature for optimal luciferase activity and then clarified by centrifugation at 17,000g for 5 min, after which supernatants were transferred into fresh tubes. A 20-µl aliquot of supernatant was dispensed into a 384-well plate, to which an equal volume of ‘Steady-Glo’ reagent (Promega, E2510) equilibrated to room temperature was added. The plate was then incubated at room temperature and protected from light for 10 min, after which luciferase activity was measured using the luminescence setting on an EnSight multimode plate reader (PerkinElmer).
Calcium imaging
An ex vivo midgut setup was prepared for transient calcium imaging using Tk::2A::GAL4 to drive the expression of GCaMP6s32 in Tk+ EECs. Midguts were dissected from the respective genotypes in a modified Schneider’s medium, specifically formulated for adult Drosophila tissues85. The dissected midguts were then carefully mounted on a small bed of low-melting-temperature agarose (Merck, A9414) pipetted onto a glass-bottom dish (MatTek). To immobilize the tissues, a droplet of 0.5% low-melting-temperature agarose was applied on top of each midgut. After the agar solidified, 100 µl of the modified Schneider’s medium was added to cover the tissue. Live imaging of these samples was performed for 1 h using a Zeiss LSM 900 microscope with a ×20, 0.8-NA objective lens. GCaMP was excited with a 488-nm laser line. The imaging aimed to compare calcium activity in particular midgut regions from animals fed yeast versus sugar for 2 h following 15-h starvation before imaging. Intensity over time was quantified for each EEC in the midgut using a custom ImageJ86 macro. Calcium responses were quantified as the maximum fold change in fluorescence intensity, defined by the formula ΔF/F0 = (F − F0)/F0, where F represents the peak fluorescence signal and F0 denotes the baseline fluorescence signal under the given condition. To account for variability in expression levels, we coexpressed GCaMP6s as the calcium indicator and tdTomato as a ratiometric calibrator. Fluorescence signals were normalized to tdTomato levels, and background fluorescence was subtracted before calculating ΔF/F0 values.
Transcript analysis using quantitative PCR
Total RNA was purified using the NucleoSpin RNA kit (Macherey-Nagel, 740955). For Drosophila experiments, five to six replicates for each genotype or condition, each replicate containing five central nervous systems or midguts, were homogenized in 2-ml Eppendorf tubes containing RA1 lysis buffer with 1% β-mercaptoethanol using a TissueLyser LT bead mill (Qiagen) and 5-mm stainless steel beads (Qiagen, 69989). For mouse small intestine samples, four replicates for each experimental condition and part of the intestine (anterior or duodenum; middle or jejunum; or posterior or ileum) were homogenized in RA1 lysis buffer, supplemented with 1% β-mercaptoethanol as described above for Drosophila samples, but only aliquots containing 9 mg tissue were loaded onto RNA extraction columns. For quantification of organoid gene expression, treatments were added to fresh ENR culture media, and organoids were incubated for 24 h at 37 °C in a humidified incubator with 5% CO2. Organoid–Matrigel domes were lifted using ice-cold Advanced DMEM/F12 medium. Organoids from wells underwent a gentle digestion process as described in detail below in ‘Mouse small intestinal organoid culture’ to dissociate the organoids from the Matrigel mixture. Following the final centrifugation step, lysis buffer from the RNA kit, supplemented with 1% β-mercaptoethanol, was added to the pellet. In all cases, complementary DNA was prepared using the High-Capacity cDNA Synthesis kit (Applied Biosystems, 4368814). RealQ Plus 2× SYBR Green Master Mix (Ampliqon, A324402) was used in qPCR reactions on a QuantStudio 5 (Applied Biosystems) machine. The housekeeping genes Ribosomal protein 49 (Rp49, Drosophila) and Rplp0 (mice) were used to normalize gene expression values. The oligonucleotides used are listed in Supplementary Table 2.
Metabolite measurements
Established protocols were used to measure whole-body levels of TAGs and glycogen78. Ten samples containing four flies each were homogenized in PBS containing 0.05% Tween-20 (Sigma, 1379) in a TissueLyser LT (Qiagen) bead mill with 5-mm stainless steel beads. TAGs were hydrolysed using Triglyceride Reagent (Sigma, T2449) to liberate the glycerol backbone, and the amount of this compound was determined using Free Glycerol Reagent (Sigma, F6428) in a colorimetric assay. Glycogen was measured in the same extracts by hydrolysing it into glucose using amyloglucosidase (Sigma, A7420), followed by a colorimetric glucose assay (Sigma, GAGO20). The absorbance of each reacted sample at 540 nm was measured in a 384-well plate using an EnSight multimode plate reader (PerkinElmer); standard curves were used to calculate TAG and glycogen concentrations. Protein levels were measured in the same extracts using a bicinchoninic acid assay (bicinchoninic acid solution, Sigma, B9643; copper sulphate solution, C2284; and BSA protein standards, P0914), with absorbance measured at 562 nm. Standard curves were used to calculate the protein concentration in each fly extract, and TAG and glycogen levels were normalized to these values to control for variability in the number and size of animals.
Starvation survival assay
Animals were transferred to vials containing 1% agar in water (starvation medium), 15 per vial. Vials were placed in an incubator at 25 °C, and the number of dead animals in each vial was recorded every 8 h until no flies remained alive. The 50% survival time was calculated by linearly interpolating between the measurements flanking 50% survival.
Longevity assay
Mated females were transferred to vials (20 flies per vial) containing either 10% or 20% yeast medium (consisting of 10% or 20% yeast, 9% sucrose and 1% agar in water). At least four vials per genotype with 20 flies per vial were used. The number of surviving flies was recorded every second day at the same time, continuing until no flies remained alive. To ensure optimal survival conditions, flies were transferred to fresh medium every 96 h.
Fecundity assay
Ten female flies of the genotype of interest were allowed to mate with five w1118 males for 24 h at 25 °C in vials containing adult-optimized medium. The mated females were then anaesthetized using CO2 and transferred to fresh vials for 1 d of acclimation. The following day, and subsequently every 24 h until the end of the experiment, at the same time of day, flies were transferred to fresh experimental vials containing adult food. For experiments with TrpA1 (Fig. 8b), flies were placed at 29 °C to induce TrpA1 activation, whereas embryos and developing larvae were moved to 25 °C once flipped to avoid developmental effects. The number of pupae present in each vial was recorded after a few days.
Sleep and activity assays
Sleep and activity patterns were monitored using the Drosophila Activity Monitoring System (TriKinetics). Six-to-seven-day-old flies were transferred under CO2 anaesthesia to glass tubes stoppered at one end with a foam plug and sealed at the other end with a removable 250-μl PCR tube containing 90 μl of either 5% sucrose or 5% yeast in 1% agarose in water, supplemented with 0.5% propionic acid and 0.15% methyl-4-hydroxybenzoate, and locomotor activity was recorded beginning at lights-on. After 24 h, the food tubes were replaced with fresh food. Intervals of at least 5 min without detected activity were classified as ‘sleep’. Data were analysed using a custom MATLAB script (MathWorks).
Mouse small intestinal organoid culture
Small intestinal organoids were generated following established protocols with minor modifications87,88,89 as described45. Organoids were derived from 15–32-week-old adult female Tph1-CFP mice, obtained from Jackson Laboratory (stock 028366)90. Mice were housed at 22 °C with a relative humidity of 55% and maintained on the SAFE D30 diet (SAFE-Diets, U8004G10R), a complete breeding diet formulated for mice. All animal procedures were conducted in accordance with institutional and national ethical guidelines and were approved by the Danish Animal Experiments Inspectorate (permit number 2023-15-0202-00197). This study also complies with the European Convention on the protection of animals used for scientific purposes. Adult female Tph1-CFP mice were killed by cervical dislocation, and small intestinal organoid cultures were prepared following published methods88,89,91. The proximal small intestine (duodenum, approximately 3–5 cm distal to the pyloric sphincter) was harvested, the tissue was chopped into pieces of approximately 2 × 2 mm using a surgical blade, and the pieces were transferred to a 50-ml Falcon tube containing 30 ml ice-cold PBS (without Ca2+ and Mg2+). The tissue was washed extensively (10–20 times) with PBS until the wash solution ran clear. After the final wash, PBS was removed, and the extracellular matrix was loosened by a 30-min incubation in 2 mM EDTA with gentle rotation at 4 °C. This solution was then removed, and the tissue was rinsed with 10 ml cold PBS. Crypt-enriched fractions were collected through sequential fractionation and filtered through a 70-µm cell strainer to remove villi and debris. The filtrate was centrifuged at 200g for 5 min, and the pellet was resuspended in basal medium. A subsequent 5-min centrifugation step at 150g separated unwanted single cells (supernatant) from the desired crypts (pellet). Crypts were resuspended in ~20 µl of prewarmed organoid basal culture medium (prepared below). To generate 3D organoids, crypt suspensions were mixed with 200 µl ice-cold Matrigel extracellular matrix formulation (Corning, 354230), and ~17 µl of the crypt–Matrigel mixture was plated as hemispherical domes in the centre of prewarmed wells of a 48-well plate. Plates were incubated at 37 °C with 5% CO2 for 15 min to polymerize the Matrigel. To each well was added 250 µl ENR medium, prepared below. Organoids were maintained at 37 °C with 5% CO2, and the medium was replaced every 2–3 d. Organoids grown in ENR medium developed crypt–villus structures, while supplementation with WNT3A (WENR medium) maintained a cystic organoid state. For all experiments, organoids were cultured in ENR medium for a minimum of 5 d to develop mature crypts.
Preparation of basal and supplemented media
Basal culture medium was prepared by supplementing Advanced DMEM/F12 medium with the components listed in Supplementary Table 3, followed by filter sterilisation. ENR medium was prepared by adding further components listed in that table to the basal medium and the addition of 20% R-spondin-conditioned medium (preparation described below). For WENR medium, 15 µl WNT3A-conditioned medium per 15 ml ENR medium was added alongside ROCK inhibitor before use. To generate WNT3A- and R-spondin1-conditioned media for 3D organoid culture, we followed established protocols87,92. Cultrex HA-R-Spondin1-Tc 293T cells (Trevigen) were cultured in DMEM medium (high glucose, GlutaMAX, Gibco, 31966021) supplemented with 10% FBS, 1% penicillin–streptomycin and 300 µg ml−1 zeocin. Once the cells reached ~75–80% confluency, the medium was replaced with organoid basal medium, and R-spondin1-conditioned medium was collected after 1 week of incubation. L-Wnt3a cells93 were cultured in DMEM supplemented with 10% FBS, 1% penicillin–streptomycin and 125 µg ml−1 zeocin. WNT3A-conditioned medium was collected after 1 week of incubation in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin.
Maintenance and splitting of organoids
Organoids were passaged between days 6 and 10, depending on experimental requirements and density. Cultures were split when darkened lumens, indicative of dead cell buildup, were observed. Organoids were removed from Matrigel by aspirating the culture medium and adding ice-cold Advanced DMEM/F12 medium. Matrigel domes were gently scraped to release the organoids, which were pooled in a 15-ml Falcon tube. Additional gentle pipetting released residual organoids from the Matrigel. The mixture was centrifuged at 150g for 5 min at room temperature, and the pellet was resuspended in 0.5 ml warm (37 °C) TrypLE Express (Invitrogen, 12605010) digestion medium. After 2–4 min of incubation at 37 °C, digestion was halted with 1 ml neutralisation medium (90% Advanced DMEM/F12, 10% FBS, 1:1,000 ROCK inhibitor). Organoids were further dissociated by pipetting 10–20 times and centrifuged at 150g for 5 min. The resulting crypt pellet was resuspended in Matrigel, and hemispherical domes were plated as described above. For clearing dead cells before experiments, gentler digestion was applied, involving resuspension in ice-cold TrypLE Express for 30 s to 1 min at room temperature, followed by standard processing.
Plating 3D organoids as two-dimensional epithelial monolayers
To generate two-dimensional (2D) epithelial monolayers for experiments such as immunocytochemistry, healthy 3D budding organoids with crypt–villus structures grown in ENR medium were dissociated into cell clusters and plated onto glass coverslips, following an adapted protocol91. Glass coverslips (15 mm) were sterilized under ultraviolet light for 30 min in a 12-well plate. After sterilisation, the coverslips were coated with a 2% Matrigel solution and incubated at 37 °C for 1 h to ensure uniform coating. Organoids were dissociated with warmed TrypLE Express enzyme. Organoids were collected in Advanced DMEM/F12, centrifuged at 200g for 5 min, and resuspended in 0.5 ml warmed TrypLE Express. The mixture was incubated at room temperature for 1–3 min, after which digestion was halted with 1 ml of neutralisation medium (90% Advanced DMEM/F12, 10% FBS and 10 µM ROCK inhibitor). Organoids were gently triturated approximately 12 times to produce cell clusters while avoiding the formation of single cells. The resulting suspension was centrifuged at 200g for 5 min, and the pellet was resuspended in 2-D medium. The 2-D medium consisted of DMEM (Sigma-Aldrich, D6546) supplemented with 10% FBS, 2 mM GlutaMAX, 0.1 U ml−1 penicillin and 0.1 mg ml−1 streptomycin, with 10 µM ROCK inhibitor added immediately before use. An 80-µl suspension of cell clusters was pipetted onto the centre of each Matrigel-coated coverslip. Plates were incubated at 37 °C for 1 h to allow cell attachment. Subsequently, wells were flooded with 800 µl warm 2-D medium containing ROCK inhibitor. Cultures were maintained at 37 °C in a humidified incubator under 5% CO2 and were ready for use in experiments 24 h after plating. Monolayer cultures were used within 24–72 h after plating to ensure optimal cell viability and consistency.
Drug treatments of organoids and two-dimensional monolayer cultures
Concentrations of the TRPA1 agonist CNA94,95 and antagonist A-967079 (ref. 49) and the mTORC1 and mTORC2 inhibitor Torin1 (refs. 96,97) for use with 3D organoids and 2D monolayer cultures were determined based on literature review and sensitivity testing for both culture types (data not shown). All compounds were reconstituted and diluted in sterile DMSO so that equal volumes could be added to the warmed culture media. Treatments were conducted for 24 h at 37 °C in a humidified incubator with 5% CO2. The concentration of DMSO in the culture media did not exceed 1.7% in single treatments. Appropriate DMSO-only controls were included to account for any vehicle effects. Torin1 (Sigma-Aldrich, 475991) was diluted to a stock concentration of 15 µM and used at 250 nM. Compound A-967079 (Sigma-Aldrich, A-967079) was dissolved at a stock concentration of 0.6 mM and used at 10 µM. CNA (Sigma-Aldrich, W228613) was diluted to a stock concentration of 6 mM and used at 100 µM for 3D cultures and at 50 µM for 2D cultures.
Immunostaining enterochromaffin cells in two-dimensional monolayer cultures
Two-dimensional epithelial monolayers were fixed within 24–48 h of plating using freshly prepared 4% PFA. Coverslips carrying cell cultures were washed three times with PBS to remove residual medium, followed by the addition of 1 ml of 4% PFA to each well. Plates were incubated on a shaker at 12 rpm for 30 min at room temperature. After fixation, coverslips were washed three times with PBS. Fixed samples were either processed immediately for immunostaining or stored at 4 °C for up to 48 h. Samples were blocked using a solution of PBS containing 10% serum (matched to the secondary antibody species) and 0.3% Triton X-100. Coverslips were incubated in 800 µl blocking solution per well on a plate rocker at 12 rpm for 45 min at room temperature. Primary antibody incubation was performed in an antibody buffer containing PBS, 5% appropriate serum and 0.1% Triton X-100. Coverslips were incubated for 2 h at room temperature or overnight at 4 °C. After incubation, coverslips were washed four times with PBS. Secondary antibodies were diluted in antibody buffer, and coverslips were incubated with the solution for 1 h at room temperature, followed by four additional PBS washes. The antibodies used are anti-GFP (Abcam, Ab13970, chicken IgY, 1:2,000), anti-SP (Abcam, Ab14184, mouse IgG1, validated for use in mice, 1:1,500; specificity validated in Extended Data Fig. 4b), goat anti-chicken IgY, Alexa Fluor 488 conjugate (Abcam, Ab150169, 1:500) and goat anti-mouse IgG1 cross-absorbed secondary, Alexa Fluor 647 conjugate (Thermo Fisher, A21240, 1:2,000). Alexa Fluor 647-conjugated phalloidin (Thermo Fisher, A30107) and DAPI (Sigma, D9542) were used as counterstains. Coverslips were mounted onto glass slides using ProLong Glass Antifade mounting medium (Invitrogen, P36984), ensuring minimal bubble formation. After curing at room temperature for 1 h, slides were sealed with clear nail polish and stored at 4 °C. Cells were imaged using a Zeiss LSM 900 confocal microscope with the ZEN 3.1 (blue edition) software package. Quantification of SP staining within the cells was performed using ImageJ with local background subtraction.
Immunostaining of three-dimensional organoids
To remove dead cell buildup within the organoids, a gentle digestion was performed using ice-cold TrypLE 1 d before fixation. Organoids were lifted and partially digested as described above, ensuring the retention of intact organoid structures. To maintain 3D architecture while reducing Matrigel thickness, an adjusted plating protocol was used. Approximately 17 µl of the organoid–Matrigel mixture was spread into a circle 5–10 mm in diameter at the centre of sterile UV-treated coverslips kept at room temperature. The room temperature coverslips allowed the Matrigel to spread evenly, forming a thinner layer, approximately 1–2 mm thick. The coverslips, in 12-well plates, were incubated at 37 °C for 20 min to solidify the Matrigel. Afterward, 800 µl ENR medium containing ROCK inhibitor was added to each well. Organoids were allowed to recover for 24 h before fixation. To preserve the integrity of the Matrigel matrix, which can degrade at ice-cold temperatures, fixation was performed at room temperature. Culture medium was carefully removed, and organoids were washed three times with room temperature PBS. Fixation was carried out using 1 ml of freshly prepared 4% PFA per well, and samples were incubated for 30 min at room temperature with gentle shaking. Organoids were then washed three additional times with PBS and stored at 4 °C until immunostaining. Fixed organoids were permeabilized and blocked in PBS containing 10% goat serum and 0.3% Triton X-100 for 2 h at room temperature with gentle shaking. Primary antibodies were diluted in an antibody buffer consisting of PBS containing 5% goat serum and 0.1% Triton X-100, and organoids were incubated with this solution at 4 °C for 48 h with gentle shaking. Following primary antibody staining, organoids were washed three times with PBS for 1 h per wash at room temperature with gentle shaking. Secondary antibodies were prepared in antibody buffer and applied to organoids, which were then incubated overnight at 4 °C with gentle shaking. Following secondary antibody staining, organoids were washed once with PBS for 1 h at room temperature. Organoids were then stained for filamentous actin using Alexa Fluor 647-conjugated phalloidin (Thermo Fisher, A30107, 1:100) in PBS for 1.5 h at room temperature. If no actin staining was used, organoids were instead washed for 1.5 h using PBS. Following the actin stain, the organoids were washed for an additional hour with PBS, after which nuclei were stained with DAPI for 2 h. Counterstained organoids were washed three times for 30 min each using PBS. To preserve their 3D structure, stained organoids were mounted in ProLong Glass Antifade Mountant (Life Technologies, P36984) on coverslips with a double stack of SecureSeal adhesive imaging spacers (Grace Bio-Labs, 620001, total thickness of 0.24 mm). Imaging was performed using a Zeiss LSM 900 confocal microscope, followed by post-processing in ZEN 3.1 and Fiji.
Mouse dietary interventions and sample collection for gut hormone analysis
The mouse study was conducted at the University of Copenhagen, Denmark. Female C57BL/6J mice, aged 10 weeks, were obtained from a commercial breeder (Taconic). The animals were housed under controlled conditions at 22 °C with a 12:12-h light–dark cycle and a relative humidity of 55%. They had ad libitum access to water and a low-protein diet (LPD: protein contributes 5.2% of food mass and 4% of calories; D11092301, Research Diets) or a high-protein diet (protein contributes 20.3% of the diet’s mass and 18% of its calories; D11051801, Research Diets). To ensure environmental enrichment, all mice were provided with standardized nesting materials, hiding structures and toys for play and manipulation. The mice were housed in groups of six per cage. The experiment was conducted in compliance with the European Convention for the Protection of Vertebrate Animals Used for Scientific Purposes and was approved by the Danish Animal Experimentation Inspectorate (license number 2020-15-0201-00628).
Female C57BL/6J mice were block randomized into two groups, receiving LPD or high-protein diet. Additionally, the LPD and high-protein diet groups were further block randomized into three subgroups based on the duration of dietary intervention: 1 d, 3 d or 7 d. Throughout the experiment, the mice had ad libitum access to water and their assigned diets, which were monitored and measured daily. Body weight was also recorded daily during the study. On the morning of days 1, 3 or 7 of dietary intervention, the corresponding subgroup was euthanized by decapitation. Thoracic blood was collected in heparinized tubes and centrifuged in the cold, and plasma was then frozen. The gut was harvested, rinsed in saline and segmented into the duodenum, jejunum and ileum, with each segment snap-frozen in liquid nitrogen. Plasma and tissue samples were stored at −70 °C until further analysis.
Quantification of plasma substance P
To measure SP levels in plasma from mice fed either low- or high-protein diets, we used the SP Competitive ELISA Kit (Thermo Fisher Scientific, EEL013) following the manufacturer’s instructions. Plasma samples (50 μl each) were prepared using EDTA as an anticoagulant and centrifuged at 1,000g for 15 min at 4 °C. The assay was performed in a microplate precoated with SP antigen, and samples were added alongside SP standards (0–5,000 pg ml−1) to generate a standard curve. Samples and standards were incubated with biotinylated detection antibodies and HRP conjugates, followed by substrate addition and signal detection at 450 nm using a microplate reader. SP concentrations in plasma were calculated based on the standard curve and normalized to sample dilution.
Statistics
All statistics were computed using the Prism analysis package (version 9, GraphPad). Pairwise differences between survival curves were analysed using Mantel–Cox log-rank tests. Preference indices for option A over option B were calculated as (amount of A − amount of B)(amount of A + amount of B)−1. Numeric data were checked for normality and tested for equal variance before determinations of significance were made. Pairwise comparisons between normal data were made using two-tailed unpaired Student’s t-tests, and multiple normally distributed samples were compared using one-way ANOVA with Tukey’s or Dunnett’s multiple comparisons. Data not normally distributed were compared using two-tailed unpaired Mann–Whitney U-tests or one-way Kruskal–Wallis ANOVA followed by Dunn’s multiple comparisons. In some cases, two-way ANOVA was performed to examine interactions between variables. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications9,10,18,98. Animals and samples were randomly assigned to experimental groups when applicable, but no additional randomisation procedures were applied. Data collection and analysis were not performed blind to experimental conditions. No data points or animals were excluded from analyses. Bar and dot plots of normal data indicate mean ± s.e.m., and, for non-normal data, the median and 95% confidence interval are indicated. Statistical tests and central tendency indication are noted in each legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting this study’s findings are included in the article and/or its Supplementary Information. Additional inquiries for resources and reagents should be directed to the corresponding author, K.R., and will be fulfilled upon request. Source data are provided with this paper.
Code availability
The scripts10 used for analysing and quantifying EEC fluorescence can be accessed publicly at https://doi.org/10.5281/zenodo.6641933 (ref. 99). The script for analysing sleep is available publicly at https://zenodo.org/records/5772445 (ref. 100).
References
Carreiro, A. L. et al. The macronutrients, appetite, and energy intake. Annu. Rev. Nutr. 36, 73–103 (2016).
Small, C. J. & Bloom, S. R. Gut hormones and the control of appetite. Trends Endocrinol. Metab. 15, 259–263 (2004).
Woods, S. C. & D’Alessio, D. A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 93, S37–S50 (2008).
Murphy, K. G. & Bloom, S. R. Gut hormones in the control of appetite. Exp. Physiol. 89, 507–516 (2004).
Munch, D., Ezra-Nevo, G., Francisco, A. P., Tastekin, I. & Ribeiro, C. Nutrient homeostasis — translating internal states to behavior. Curr. Opin. Neurobiol. 60, 67–75 (2020).
Sorensen, A., Mayntz, D., Raubenheimer, D. & Simpson, S. J. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity 16, 566–571 (2008).
Hewson-Hughes, A. K. et al. Geometric analysis of macronutrient selection in the adult domestic cat, Felis catus. J. Exp. Biol. 214, 1039–1051 (2011).
Raubenheimer, D. & Simpson, S. J. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10, 151–179 (1997).
Kubrak, O. et al. The gut hormone allatostatin C/somatostatin regulates food intake and metabolic homeostasis under nutrient stress. Nat. Commun. 13, 692 (2022).
Malita, A. et al. A gut-derived hormone suppresses sugar appetite and regulates food choice in Drosophila. Nat. Metab. 4, 1532–1550 (2022).
Khan, M. S. et al. Protein appetite at the interface between nutrient sensing and physiological homeostasis. Nutrients 13, 4103 (2021).
Simpson, S. J. & Raubenheimer, D. Obesity: the protein leverage hypothesis. Obes. Rev. 6, 133–142 (2005).
Hill, C. M. et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 27, 2934–2947 (2019).
Kosakamoto, H. et al. Sensing of the non-essential amino acid tyrosine governs the response to protein restriction in Drosophila. Nat. Metab. 4, 944–959 (2022).
Cai, X. T. et al. Gut cytokines modulate olfaction through metabolic reprogramming of glia. Nature 596, 97–102 (2021).
Kim, B. et al. Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit. Nature 593, 570–574 (2021).
Lin, H. H. et al. A nutrient-specific gut hormone arbitrates between courtship and feeding. Nature 602, 632–638 (2022).
Yoshinari, Y. et al. The sugar-responsive enteroendocrine neuropeptide F regulates lipid metabolism through glucagon-like and insulin-like hormones in Drosophila melanogaster. Nat. Commun. 12, 4818 (2021).
Wachsmuth, H. R., Weninger, S. N. & Duca, F. A. Role of the gut–brain axis in energy and glucose metabolism. Exp. Mol. Med. 54, 377–392 (2022).
Holst, J. J. GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat. Metab. 6, 1866–1885 (2024).
Finer, N. Future directions in obesity pharmacotherapy. Eur. J. Intern. Med. 93, 13–20 (2021).
Park, J. H. et al. A subset of enteroendocrine cells is activated by amino acids in the Drosophila midgut. FEBS Lett. 590, 493–500 (2016).
Song, W., Veenstra, J. A. & Perrimon, N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 9, 40–47 (2014).
Ja, W. W. et al. Prandiology of Drosophila and the CAFE assay. Proc. Natl Acad. Sci. USA 104, 8253–8256 (2007).
Ro, J., Harvanek, Z. M. & Pletcher, S. D. FLIC: high-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS ONE 9, e101107 (2014).
Itskov, P. M. et al. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 5, 4560 (2014).
Hamada, F. N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008).
Chen, J., Kim, S. M. & Kwon, J. Y. A systematic analysis of Drosophila regulatory peptide expression in enteroendocrine cells. Mol. Cells 39, 358–366 (2016).
Guo, X. et al. The cellular diversity and transcription factor code of Drosophila enteroendocrine cells. Cell Rep. 29, 4172–4185 (2019).
Masuyama, K., Zhang, Y., Rao, Y. & Wang, J. W. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenet. 26, 89–102 (2012).
Guo, F., Chen, X. & Rosbash, M. Temporal calcium profiling of specific circadian neurons in freely moving flies. Proc. Natl Acad. Sci. USA 114, E8780–E8787 (2017).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Li, H. et al. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432 (2022).
Colombani, J. et al. A nutrient sensor mechanism controls Drosophila growth. Cell 114, 739–749 (2003).
Wright, Y. & Armstrong, A. R. Differential amino acid transporter expression in adult Drosophila melanogaster tissues. MicroPubl. Biol. https://doi.org/10.17912/micropub.biology.001121 (2024).
Jones, R. M., Mercante, J. W. & Neish, A. S. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr. Med. Chem. 19, 1519–1529 (2012).
Arenas, O. M. et al. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci. 20, 1686–1693 (2017).
Faris, P. et al. Transient receptor potential ankyrin 1 (TRPA1) mediates reactive oxygen species-induced Ca2+ entry, mitochondrial dysfunction, and caspase-3/7 activation in primary cultures of metastatic colorectal carcinoma cells. Cell Death Discov. 9, 213 (2023).
Gu, C., Shi, Y. & Le, G. Effect of dietary protein level and origin on the redox status in the digestive tract of mice. Int. J. Mol. Sci. 9, 464–475 (2008).
Tan, B. L., Norhaizan, M. E. & Liew, W. P. Nutrients and oxidative stress: friend or foe? Oxid. Med. Cell. Longev. 2018, 9719584 (2018).
Sinenko, S. A., Starkova, T. Y., Kuzmin, A. A. & Tomilin, A. N. Physiological signaling functions of reactive oxygen species in stem cells: from flies to man. Front. Cell Dev. Biol. 9, 714370 (2021).
Malita, A. et al. Glia-mediated gut–brain cytokine signaling couples sleep to intestinal inflammation. eLife 13, RP99999 (2024).
Hung, R. J. et al. A cell atlas of the adult Drosophila midgut. Proc. Natl Acad. Sci. USA 117, 1514–1523 (2020).
Campo, A., Dufour, S. & Rousseau, K. Tachykinins, new players in the control of reproduction and food intake: a comparative review in mammals and teleosts. Front. Endocrinol. 13, 1056939 (2022).
Shaaban, A. et al. Dissecting functional, structural, and molecular requirements for serotonin release from mouse enterochromaffin cells. Preprint at bioRxiv https://doi.org/10.1101/2021.05.28.446100 (2021).
Karagiannides, I. & Pothoulakis, C. Substance P, obesity, and gut inflammation. Curr. Opin. Endocrinol. Diabetes Obes. 16, 47–52 (2009).
Karagiannides, I. et al. Role of substance P in the regulation of glucose metabolism via insulin signaling-associated pathways. Endocrinology 152, 4571–4580 (2011).
Lund, M. L. et al. Enterochromaffin 5-HT cells — a major target for GLP-1 and gut microbial metabolites. Mol. Metab. 11, 70–83 (2018).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198 (2017).
Braco, J. T., Nelson, J. M., Saunders, C. J. & Johnson, E. C. Modulation of metabolic hormone signaling via a circadian hormone and biogenic amine in Drosophila melanogaster. Int. J. Mol. Sci. 23, 4266 (2022).
Inagaki, H. K., Panse, K. M. & Anderson, D. J. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron 84, 806–820 (2014).
Koyama, T. et al. A nutrient-responsive hormonal circuit mediates an inter-tissue program regulating metabolic homeostasis in adult Drosophila. Nat. Commun. 12, 5178 (2021).
Lee, G. & Park, J. H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323 (2004).
Yu, Y. et al. Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. eLife 5, e15693 (2016).
Ang, T., Bruce, C. R. & Kowalski, G. M. Postprandial aminogenic insulin and glucagon secretion can stimulate glucose flux in humans. Diabetes 68, 939–946 (2019).
Koyama, T., Texada, M. J., Halberg, K. A. & Rewitz, K. Metabolism and growth adaptation to environmental conditions in Drosophila. Cell. Mol. Life Sci. 77, 4523–4551 (2020).
Peuhkuri, K., Sihvola, N. & Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 32, 309–319 (2012).
Murphy, K. R. et al. Postprandial sleep mechanics in Drosophila. eLife 5, e19334 (2016).
Titos, I. et al. A gut-secreted peptide suppresses arousability from sleep. Cell 186, 2273–2274 (2023).
Pauls, D. et al. Endocrine signals fine-tune daily activity patterns in Drosophila. Curr. Biol. 31, 4076–4087 (2021).
Piper, M. D. W. et al. Dietary restriction and lifespan: adaptive reallocation or somatic sacrifice? FEBS J. 290, 1725–1734 (2023).
Skorupa, D. A., Dervisefendic, A., Zwiener, J. & Pletcher, S. D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 7, 478–490 (2008).
Wat, L. W., Chowdhury, Z. S., Millington, J. W., Biswas, P. & Rideout, E. J. Sex determination gene transformer regulates the male–female difference in Drosophila fat storage via the adipokinetic hormone pathway. eLife 10, e72350 (2021).
Mair, W., Piper, M. D. & Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3, e223 (2005).
Bass, T. M. et al. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 62, 1071–1081 (2007).
Koliaki, C., Liatis, S., Dalamaga, M. & Kokkinos, A. The implication of gut hormones in the regulation of energy homeostasis and their role in the pathophysiology of obesity. Curr. Obes. Rep. 9, 255–271 (2020).
Lennicke, C. & Cocheme, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021).
Aiken, K. D. & Roth, K. A. Temporal differentiation and migration of substance P, serotonin, and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Dev. Dyn. 194, 303–310 (1992).
Bai, L. et al. Enteroendocrine cell types that drive food reward and aversion. eLife 11, e74964 (2022).
Liu, J., Yu, K. & Zhu, W. Amino acid sensing in the gut and its mediation in gut–brain signal transduction. Anim. Nutr. 2, 69–73 (2016).
Hopkins, M. & Blundell, J. E. in Appetite and Food Intake: Central Control (ed. Harris, R. B. S.) 259–276 (2017).
Morrison, C. D. & Laeger, T. Protein-dependent regulation of feeding and metabolism. Trends Endocrinol. Metab. 26, 256–262 (2015).
Catterson, J. H. et al. Dietary modulation of sleep–wake behaviour. PLoS ONE 5, e12062 (2010).
Lu, J. et al. Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nat. Aging 1, 60–72 (2021).
Richardson, N. E. et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat. Aging 1, 73–86 (2021).
Laeger, T. et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 124, 3913–3922 (2014).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Tennessen, J. M., Barry, W. E., Cox, J. & Thummel, C. S. Methods for studying metabolism in Drosophila. Methods 68, 105–115 (2014).
Piper, M. D. et al. A holidic medium for Drosophila melanogaster. Nat. Methods 11, 100–105 (2014).
Wong, R., Piper, M. D., Wertheim, B. & Partridge, L. Quantification of food intake in Drosophila. PLoS ONE 4, e6063 (2009).
Veenstra, J. A., Agricola, H. J. & Sellami, A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334, 499–516 (2008).
Isabel, G., Martin, J. R., Chidami, S., Veenstra, J. A. & Rosay, P. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R531–R538 (2005).
Arsic, A., Stajkovic, N., Spiegel, R. & Nikic-Spiegel, I. Effect of Vectashield-induced fluorescence quenching on conventional and super-resolution microscopy. Sci. Rep. 10, 6441 (2020).
Bruce, S. H. et al. Hybridization chain reaction (HCR) in situ protocol. protocols.io https://doi.org/10.17504/protocols.io.bunznvf6 (2021).
Marchetti, M., Zhang, C. & Edgar, B. A. An improved organ explant culture method reveals stem cell lineage dynamics in the adult Drosophila intestine. eLife 11, e76010 (2022).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato, M., Shames, D. S. & Hasegawa, Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology 17, 1048–1059 (2012).
Li, H. J. et al. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology 146, 754–764 (2014).
Goldspink, D. A. et al. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol. Metab. 7, 90–101 (2018).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Farin, H. F., Van Es, J. H. & Clevers, H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143, 1518–1529 (2012).
Camacho, S. et al. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 5, 7919 (2015).
Macpherson, L. J. et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 (2007).
Koh, V. et al. Hedgehog transcriptional effector GLI mediates mTOR-induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 518, 59–71 (2021).
Atanasova, V. S. et al. Selective eradication of colon cancer cells harboring PI3K and/or MAPK pathway mutations in 3D culture by combined PI3K/AKT/mTOR pathway and MEK inhibition. Int. J. Mol. Sci. 24, 1668 (2023).
Lin, H. H. et al. Spint1 disruption in mouse pancreas leads to glucose intolerance and impaired insulin production involving HEPSIN/MAFA. Nat. Commun. 15, 10537 (2024).
Nagy, S. Cell fluorescence analysis for midgut enteroendocrine cells. Zenodo https://doi.org/10.5281/zenodo.6641933 (2022).
Nagy, S. Custom DrosophilaActivityMonitor data analysis. Zenodo https://doi.org/10.5281/zenodo.5772445 (2021).
Acknowledgements
We thank J. Veenstra (University of Bordeaux) for generously providing the anti-Tk and anti-AKH antibodies. We thank J.R. Quale (University of Copenhagen) for providing guidance and support on organoid culture techniques. This work was supported by the Novo Nordisk Foundation (grant NNF19OC0054632) and the Lundbeck Foundation (grant R313-2019-772) to K.R. T.K. and K.V.H. were supported by funding from the Danish Independent Research Council—Natural Sciences (9064-00009B) to K.V.H. C.I. was supported by funding by the Lundbeck Foundation (R307-2018-2973). E.A.R. was supported by the Novo Nordisk Foundation (grant NNF23OC0084246). The Zeiss LSM 900 confocal microscope and the PerkinElmer EnSight plate reader were purchased with generous grants from the Carlsberg Foundation (CF19-0353 and CF17-0615, respectively) to K.R.
Author information
Authors and Affiliations
Contributions
N.A. and K.R. conceived and designed the study. N.A., O.K., M.L., A.M., T.K., A.S.F., C.M.S., A.J., P.E.H.M., K.V.H., S.N., C.I., E.A.R., M.J.T. and K.R. designed, performed and analysed experiments. M.J.T. and K.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Hans-Rudolf Berthoud, Joep Beumer, Fumiaki Obata and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effects of gut-specific Tk knockdown on feeding behavior and metabolic homeostasis in males and mated female flies.
Comparing controls (Tkgut>) and gut-specific Tk knockdowns (Tkgut > Tk-RNAi). a, One-hour dye-feeding intake of sugar (left) and yeast (right) by male flies fed on a yeast diet for 15 h (two-tailed unpaired Student’s t test). b, One-hour dye-feeding sugar intake for UAS-Tk-RNAish alone (two-tailed Mann-Whitney U). c, Six-hour CAFÉ assay for sugar intake for UAS-Tk-RNAiKK alone (two-tailed Mann-Whitney U test). d, FLIC observation of sugar-directed feeding behavior over 22 h for UAS-Tk-RNAish alone (two-tailed unpaired Student’s t test). e and f, Whole-body triacylglyceride (e) and glycogen (f) levels measured under fed and 24-hour-starved conditions for controls and animals expressing EEC-specific RNAi against Tk (one-way ANOVA/Dunnett’s). g, Starvation-survival curves for controls and animals expressing EEC-specific RNAi targeting Tk (log-rank Mantel-Cox tests). Sample sizes (N) and p values are indicated in each plot. In a–d and g, N = animals per group. In e and b, N = whole-body samples. Box plots show the median, the bounds of the box (25th and 75th percentiles), and whiskers representing the minimum and maximum values. Panels d, e, and f indicate the mean ± SEM. ns, non-significant (p > 0.05).
Extended Data Fig. 2 Midgut Tk+ EEC activity, Tk peptide levels, and transcripts under different nutritional conditions in mated female flies.
a-b, Assessment of calcium-signaling activity measured by CaLexA in Tk+ EECs (a; GFP normalized to tdTomato) and Tk peptide intensity (b) in the whole midgut of mated females fed standard adult-optimized diet (9% sugar and 8% yeast) or starved on 1% agarose for four hours (two-tailed Mann-Whitney U tests). c-d, Effects on calcium signaling (c) and anti-Tk staining intensity (d) of a 3-hour period of refeeding with 10% sugar alone, 10% yeast alone, or both, following 25-hour starvation (Kruskal-Wallis/Dunn’s). e-f, Midgut Tk transcript levels in mated females: (e) animals were starved for 25 h and then refed for 3 h with 10% sugar or 10% yeast, and (f) animals were starved for 22 h and then refed with sugar+yeast medium for a range of time periods before dissection [two-tailed unpaired Student’s t test (e) and one-way ANOVA with Dunnett’s test (f)]. g, Tk+ EEC activity in dissected midguts, reflected in calcium-LexA-driven Luciferase, measured over a range of yeast-feeding durations following normal feeding on adult diet (one-way ANOVA/Dunnett’s). h, Quantification of GCaMP6s signal (ΔF/F₀) averaged across the R1-R3 Tk+ EECs in sugar- and yeast-fed conditions (two-tailed Mann-Whitney U). i, Anti-Tk staining intensity in R5 EECs of animals fed for 15 h on media containing 10% of either (naturally lipid-free) sugar or lipid-depleted casein (two-tailed Mann-Whitney U). j, Tk transcript levels in dissected midguts of animals fed for 15 h on either sugar, yeast, or the peptide mixture peptone (one-way ANOVA/Dunnett’s). k, Tk transcript levels in midguts of animals fed for 15 h on media containing either sugar, yeast, a mixture of amino acids, lipid-depleted yeast, or lipid-depleted casein (one-way ANOVA/Dunnett’s). Sample sizes (N) and p values are indicated in each plot. In a–b and i, N = EECs. In e–h, j, and k, N = midgut samples. Panels a, b, c, d, h, and i indicate medians and 95%-confidence intervals, and panels e, f, g, j, and k indicate means ± SEM. ns, non-significant (p > 0.05).
Extended Data Fig. 3 Nutrient-intake regulation, metabolic consequences, and sleep effects of TkR99D deficiency in the APCs and loss of AKH signaling in mated female flies.
a-d, Analysis of midgut Tk peptide and transcript (a, b, d), and slif (c) transcript levels in flies expressing Tk+-EEC-specific RNAi against slif (a, c), a range of other amino-acid transporters (b), or the central regulatory gene Gcn2 (d) in animals fed 15 h with yeast. (two-tailed unpaired Student’s t tests in a, c, d; one-way ANOVA/Dunnett’s in b.) e, Calcium activity (CaLexA>GFP normalized to tdTomato) in all Tk+ midgut EECs of animals fed for 3 h on normal adult optimized diet (ND) or on medium containing hydrogen peroxide (two-tailed Mann-Whitney U). f, anti-Tk staining intensity of R5 EECs in 15 h sugar-fed controls and animals expressing RNAi against TrpA1 in Tk+ EECs (two-tailed Mann-Whitney U). g, 1-hour FlyPAD assay of sugar-directed feeding behavior for animals expressing RNAi against slif in the Tk+ EECs (two-tailed Mann-Whitney U). h-i, 1-hour dye-feeding assay for sugar consumption for UAS-slif-RNAi alone (two-tailed Mann-Whitney U tests). j-k, FlyPAD feeding behavior (j) and dye-feeding consumption (k) of yeast in controls and animals expressing RNAi against slif in the Tk+ EECs [two-tailed Mann-Whitney U (j) and Kruskal-Wallis/Dunn’s (k)]. l, Dye-feeding consumption of yeast in animals exhibiting attenuated TOR signaling in the Tk+ EECs (Kruskal-Wallis/Dunn’s). m-n, One-hour dye-feeding assay for yeast intake for UAS-slif-RNAi alone (two-tailed Mann-Whitney U tests). Behavioral experiments were performed following 15 h of yeast feeding. Sample sizes (N) and p values are indicated in each plot. In a, c, and d, N = midgut samples. In b, e, and f, N = EECs. In g–n, N = animals per group. Box plots show the median, the bounds of the box (25th and 75th percentiles), and whiskers representing the minimum and maximum values. Dot plots in a-d include mean ± SEM, and in e, f, g, and j indicate the median and 95%-confidence interval. ns, non-significant (p > 0.05).
Extended Data Fig. 4 Controls for organoid stains.
a, bright-field (left) and confocal (right) images of a single entire mouse-intestinal organoid. Serotonin/Substance-P-expressing enterochromaffin cells are marked with CFP expressed under the Tph1 promoter (cyan); rich actin staining with phalloidin to stain F-actin marks the luminal surface of the organoid (orange), and nuclei are marked with DAPI (blue). Scale bars span 50 µm. Similar results were observed in five independent organoid samples. b, Antibody negative control for SP staining. Top row: tissue stained with anti-SP primary (left, orange), also visualized with Tph1-CFP and DAPI for context; bottom row: tissues processed similarly except for the omission of anti-SP. Scale bar indicates 20 µm. Similar results were observed in five independent organoid samples.
Extended Data Fig. 5 Tk-mediated regulation of the AKH system regulates aspects of physiology and behavior in mated female flies.
a-c, One-hour dye-feeding assay (a) or 6-hour CAFÉ assays (b and c) for sugar in animals expressing RNAi against TkR99D in the APCs, compared to UAS-TkR99D-RNAi alone (two-tailed Mann-Whitney U tests). d and e, one-hour FlyPAD assay (d) and 22-hour FLIC assay (e) for sugar-feeding behavior comparing APC-specific TkR99D knockdowns to UAS-TkR99D-RNAi alone (two-tailed Mann-Whitney U tests). f, Starvation-survival curves for controls and animals expressing RNAi against TkR99D in the APCs [log-rank (Mantel-Cox) test]. g, Whole-body triacylglyceride (left) and glycogen (right) levels in controls and animals expressing RNAi against TkR99D in the APCs (two-tailed unpaired Student’s t tests). h and i, One-hour dye-consumption assay (h) and six-hour CAFE assay (i) for sugar intake in animals carrying UAS-Akh-RNAi alone (two-tailed Mann-Whitney U tests). j, Six-hour FLIC assay for sugar-directed feeding behavior in animals carrying UAS-Akh-RNAi alone (two-tailed Mann-Whitney U). k, a schematic of the experimental setup underlying panels l-m: controls and animals expressing RNAi against AkhR in the indicated pattern were fed yeast medium for 15 h prior to a one-hour dye assay with sugar or yeast. l and m, intake testing for AkhR-knockdown effects in the glutamatergic (VGlut1>) and cholinergic (ChAT>) neuronal populations. (two-tailed Mann-Whitney U tests, except for yeast feeding in cholinergic neurons, where two-tailed unpaired Student’s t test was used). n, Single-cell RNAseq data from fly heads indicates co-expression of AkhR and ChAT. Data from FlyCellAtlas34 was visualized using the SCope viewer105. Behavioral experiments were performed following 15 h of yeast feeding. Sample sizes (N) and p values are indicated in each plot. In a–e, f, and h–m, N = animals per group. In g, N = whole-body samples. Box plots show the median, the bounds of the box (25th and 75th percentiles), and whiskers representing the minimum and maximum values. Panels d, e, and j indicate median with 95%-confidence intervals. Panel g indicates means with SEM. ns, not significant (p > 0.05).
Extended Data Fig. 6 Dietary effects on sleep are mediated by signaling via gut-derived Tk and AKH signaling in males and mated female flies.
Sleep profiles and summed minutes of sleep during the mid-day ‘siesta’ (ZT 4-8) are shown, along with total nighttime (ZT 12-24) sleep. Animals had access to sugar-only food on day 1 and yeast-only food on day 2. a, Nighttime sleep in female controls and flies expressing RNAi against Tk in the Tk+ EECs (two-tailed Mann-Whitney U tests). b, Sleep profile (left) and sleep during the midday ‘siesta’ for female controls and animals expressing RNAi against Tk in the Tk+ EECs (two-tailed Mann-Whitney U tests). c-e, Sleep profile (c) midday sleep (d), and nighttime sleep (e) for males expressing RNAi targeting Tk in the midgut EECs (Kruskal-Wallis/Dunn’s). f, Nighttime sleep for female controls and animals expressing RNAi against TkR99D in the APCs (two-tailed Mann-Whitney U tests). g-i, Sleep profile (g), midday sleep (h), and nighttime sleep (i) for female controls and animals expressing RNAi against TkR99D in the APCs (two-tailed Mann-Whitney U tests). j, Nighttime sleep in female controls and animals expressing RNAi against Akh in the APCs (two-tailed Mann-Whitney U tests). k, Nighttime sleep for female controls and animals expressing RNAi against AkhR in the fat body (two-tailed Mann-Whitney U tests). l-n, Sleep profile (l), midday sleep (m), and nighttime sleep (n) for female controls and animals expressing RNAi against AkhR in the fat body (two-tailed Mann-Whitney U tests). o, Nighttime sleep for female controls and animals expressing RNAi against AkhR in all neurons (two-tailed Mann-Whitney U tests). p-r, Sleep profile (p), midday sleep (q), and nighttime sleep (r) for female controls and animals expressing RNAi against AkhR in all neurons (two-tailed Mann-Whitney U tests). Shaded areas in sleep profiles indicate SEM. Sample sizes (N) and p values are indicated in each plot. In all panels, N = animals per group. Data in dot plots are presented with medians and 95%-confidence intervals. Two-way ANOVA was used to determine interaction between genotype and diet. ns, non-significant (p > 0.05).
Extended Data Fig. 7 AKH signaling in cholinergic and octopaminergic neurons does not mediate dietary effects on sleep in mated female flies.
Sleep profiles and summed minutes of sleep during the mid-day ‘siesta’ (ZT 4-8) and total nighttime sleep are shown. During the first day of the assay, animals had access to sugar-only medium within the sleep-monitoring apparatus, and on the second day, the animals had access to yeast-only food. a-c, Sleep profile (a), midday sleep (b), and nighttime sleep (c) for female controls and animals expressing RNAi targeting AkhR in the cholinergic fraction of the nervous system using ChAT > (Kruskal-Wallis/Dunn’s). d-f, Sleep profile (d), midday sleep (e), and nighttime sleep (f) for female controls and animals expressing RNAi against AkhR in the octopaminergic neuronal population using Tdc2 > (Kruskal-Wallis/Dunn’s). Shaded areas in sleep profiles indicate SEM. Sample sizes (N) and p values are indicated in each plot. In all panels, N = animals per group. Data in dot plots are presented with medians and 95%-confidence intervals. Two-way ANOVA was used to determine interaction between genotype and diet. ns, non-significant (p > 0.05).
Extended Data Fig. 8 Offspring number and lifespan in mated females flies with pan-neuronal TkR99D knockdown and significance p values related to Fig. 8a-c.
a-c, p values (a) p values for Fig. 8a, (b) p values for Fig. 8b, and (c) p values for Fig. 8c (one-way ANOVA/Dunnett’s). d, Analysis of fecundity in mated females expressing pan-neuronal TkR99D knockdown under the control of R57C10 > [two-way ANOVA (genotype × days) with Dunnett’s multiple-comparisons test]. e, Lifespan of animals with and without EEC-specific Tk knockdown, on media containing 10% or 1% yeast. Pairwise p-values derived by log-rank Mantel-Cox tests. f, Quantification of calcium-dependent activity (Tk>CaLexA), as indicated by the GFP:tdTomato ratio, in Tk+ EECs of R5, along with the intensity of anti-Tk staining, in flies fed on diets containing 10% sugar and either 10% or 20% yeast for 15 h (two-tailed Mann-Whitney U tests). g, Quantification of calcium-dependent activity, as indicated by the GFP:tdTomato ratio (driven by NPF-GAL4), in the middle midgut (R3) of flies assayed as in panel f (two-tailed Mann-Whitney U). h-j, Lifespan of female flies kept on medium containing either 10% or 1% yeast (with 10% sugar in both cases). h, controls and animals expressing RNAi targeting TkR99D in the APCs; i, flies expressing RNAi against AkhR pan-neuronally with R57C10> vs. controls; and j, animals with or without knockdown of AkhR in the fat body. Pairwise p-values in h-j determined by log-rank Mantel-Cox survival tests. Sample sizes (N) and p values are indicated. In d, N = independent groups of animals. In e, i, and j, N = animals per group. In f, N = EECs. Panel d indicates means ± SEM, whereas panels f and g indicate median and 95%-confidence intervals. ns, non-significant (p > 0.05).
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Source data
Source Data Fig. 1
Numerical values for charts in Fig. 1.
Source Data Fig. 2
Numerical values for charts in Fig. 2.
Source Data Fig. 3
Numerical values for charts in Fig. 3.
Source Data Fig. 4
Numerical values for charts in Fig. 4.
Source Data Fig. 5
Numerical values for charts in Fig. 5.
Source Data Fig. 6
Numerical values for charts in Fig. 6.
Source Data Fig. 7
Numerical values for charts in Fig. 7.
Source Data Fig. 8
Numerical values for charts in Fig. 8.
Source Data Extended Data Fig. 1
Numerical values for charts in Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Numerical values for charts in Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Numerical values for charts in Extended Data Fig. 3.
Source Data Extended Data Fig. 5
Numerical values for charts in Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Numerical values for charts in Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Numerical values for charts in Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Numerical values for charts in Extended Data Fig. 8.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahrentløv, N., Kubrak, O., Lassen, M. et al. Protein-responsive gut hormone tachykinin directs food choice and impacts lifespan. Nat Metab 7, 1223–1245 (2025). https://doi.org/10.1038/s42255-025-01267-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01267-0
This article is cited by
-
Insect neuropeptides as agents for pest control: potential and challenges
Biological Research (2025)
-
A gut hormone governing protein appetite and longevity
Nature Metabolism (2025)
-
What goes down may come up: regurgitative behaviors and their underlying gut–brain axes—insights from genetic models and related pathologies
Journal of Comparative Physiology A (2025)