Abstract
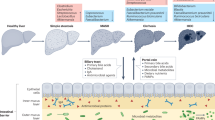
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a common condition linked to obesity and the metabolic syndrome, yet its transkingdom connections have been under-investigated. We performed high-resolution multi-omic profiling—including stool metagenomes, metatranscriptomes and metabolomes—in 211 MASLD cases and 502 controls from a cohort of female nurses. Here we show that MASLD is associated with shifts in 66 gut bacterial species, including widespread enrichment of oral-typical microbes, and transkingdom dysbiosis involving not only bacterial but also viral taxa. Streptococcus spp. are more abundant in non-lean versus lean MASLD, the latter being a paradoxical subtype of a disease typically associated with increased adiposity. These microbial changes correspond with shifts in transcripts and metabolites, including increases in polyamines and acylcarnitines and reductions in secondary bile acids. We highlight gut viral perturbations in MASLD, showing that expansions of bacteriophage targeting oral-typical bacteria correspond to expansions of their bacterial hosts in the gut. We provide a comprehensive resource for understanding MASLD and highlight transkingdom multi-omic microbial shifts as potential contributors to its aetiopathogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw metagenomic and paired metatranscriptomic sequences are available in the Sequence Read Archive under accession no. PRJNA1246224. Metagenomic sequencing data (under the data category ‘raw reads’), metabolomic profiles (under data category ‘mbx’) and clinical phenotype data have also been deposited at the BIOM-Mass under project ‘MLSC_BtB’ (https://biom-mass.org/projects). This work is also scheduled for inclusion in the next major release of the widely used curatedMetagenomicData package99. Further details on the NHS II parent cohort are available at http://www.nurseshealthstudy.org. Source data are provided with this paper.
Code availability
Analysis programs have been deposited on GitHub at https://github.com/biobakery/MASLD.
References
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Yu, L.-X. & Schwabe, R. F. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 14, 527–539 (2017).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153 (2017).
Simon, T. G., Roelstraete, B., Khalili, H., Hagström, H. & Ludvigsson, J. F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 70, 1375–1382 (2021).
Cleveland, E., Bandy, A. & VanWagner, L. B. Diagnostic challenges of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin. Liver Dis. 11, 98–104 (2018).
VanEvery, H., Franzosa, E. A., Nguyen, L. H. & Huttenhower, C. et al. Microbiome epidemiology and association studies in human health. Nat. Rev. Genet. 24, 109–124 (2023).
Shen, F. et al. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobil. Pancreat. Dis. Int. 16, 375–381 (2017).
Mouzaki, M. et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 58, 120–127 (2013).
Leung, J. C.-F. et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology 65, 54–64 (2017).
Ye, Q. et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 5, 739–752 (2020).
Eslam, M., Fan, J.-G. & Mendez-Sanchez, N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol. Hepatol. 5, 713–715 (2020).
Everett, C. et al. Overview of the Microbiome Among Nurses study (Micro-N) as an example of prospective characterization of the microbiome within cohort studies. Nat. Protoc. 16, 2724–2731 (2021).
Bao, Y. et al. Origin, methods, and evolution of the Three Nurses’ Health Studies. Am. J. Public Health 106, 1573–1581 (2016).
Blanco-Míguez, A. et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 41, 1633–1644 (2023).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Maioli, T. U. et al. Possible benefits of Faecalibacterium prausnitzii for obesity-associated gut disorders. Front. Pharmacol. 12, 740636 (2021).
Oh, T. G. et al. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab. 32, 878–888.e876 (2020).
Li, F., Ye, J., Shao, C. & Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: a systematic review and Meta-analysis. Lipids Health Dis. 20, 22 (2021).
Lloyd-Price, J. et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 550, 61–66 (2017).
Bruno, G. et al. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J. Gastroenterol. 25, 2706–2719 (2019).
Kim, H., Nzabarushimana, E., Huttenhower, C., Chan, A. T. & Nguyen, L. H. Altered microbial transcription in long-term proton pump inhibitor use: findings from a United States cohort study. Gastroenterology 167, 405–408.e3 (2024).
Tsukasaki, M. et al. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 9, 701 (2018).
Chen, F. et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology 71, 1213–1227 (2020).
Thompson, K. N. et al. Alterations in the gut microbiome implicate key taxa and metabolic pathways across inflammatory arthritis phenotypes. Sci. Transl. Med. 15, eabn4722 (2023).
Voloshin, I., Hahn-Obercyger, M., Anavi, S. & Tirosh, O. L-arginine conjugates of bile acids-a possible treatment for non-alcoholic fatty liver disease. Lipids Health Dis. 13, 69 (2014).
Canbay, A. & Sowa, J.-P. L-Ornithine L-aspartate (LOLA) as a novel approach for therapy of non-alcoholic fatty liver disease. Drugs 79, 39–44 (2019).
Maeda, T. et al. Role of polyamines derived from arginine in differentiation and proliferation of human blood cells. Biol. Pharm. Bull. 29, 234–239 (2006).
Rieck, J., Skatchkov, S. N., Derst, C., Eaton, M. J. & Veh, R. W. Unique chemistry, intake, and metabolism of polyamines in the central nervous system (CNS) and its body. Biomolecules 12, 501 (2022).
Zhang, Y., Thompson, K. N., Huttenhower, C. & Franzosa, E. A. Statistical approaches for differential expression analysis in metatranscriptomics. Bioinformatics 37, i34–i41 (2021).
Testerman, T., Li, Z., Galuppo, B., Graf, J. & Santoro, N. Insights from shotgun metagenomics into bacterial species and metabolic pathways associated with NAFLD in obese youth. Hepatol. Commun. 6, 1962–1974 (2022).
Xiong, Y. et al. Dynamic alterations of the gut microbial pyrimidine and purine metabolism in the development of liver cirrhosis. Front. Mol. Biosci. 8, 811399 (2021).
Franzosa, E. A. et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol 4, 293–305 (2019).
Li, W. et al. A bacterial bile acid metabolite modulates Treg activity through the nuclear hormone receptor NR4A1. Cell Host Microbe 29, 1366–1377.e1369 (2021).
Sato, Y. et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599, 458–464 (2021).
Mouzaki, M. et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS ONE 11, e0151829 (2016).
Codoñer-Franch, P., Tavárez-Alonso, S., Murria-Estal, R., Herrera-Martín, G. & Alonso-Iglesias, E. Polyamines are increased in obese children and are related to markers of oxidative/nitrosative stress and angiogenesis. J. Clin. Endocrinol. Metab. 96, 2821–2825 (2011).
Enooku, K. et al. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 9, 10663 (2019).
Chang, Y. et al. A targeted metabolomic profiling of plasma acylcarnitines in nonalcoholic fatty liver disease. Eur. Rev. Med. Pharm. Sci. 24, 7433–7441 (2020).
Rutkowsky, J. M. et al. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 306, E1378–E1387 (2014).
Fujiwara, N. et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut 67, 1493–1504 (2018).
Ghazi, A. R. et al. High-sensitivity pattern discovery in large, paired multiomic datasets. Bioinformatics 38, i378–i385 (2022).
Pugin, B. et al. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis. 28, 1353881 (2017).
Nanduri, B. & Swiatlo, E. The expansive effects of polyamines on the metabolism and virulence of Streptococcus pneumoniae. Pneumonia 13, 4 (2021).
Hussain, T. et al. Polyamines: therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids 49, 1457–1468 (2017).
Fernandez-Garcia, J. C. et al. Type 2 diabetes is associated with a different pattern of serum polyamines: a case-control study from the PREDIMED-plus trial. J. Clin. Med. Res. 8, 71 (2019).
Oellerich, M. & Armstrong, V. W. The MEGX test: a tool for the real-time assessment of hepatic function. Ther. Drug Monit. 23, 81–92 (2001).
Lang, S. et al. Intestinal virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology 159, 1839–1852 (2020).
Cao, Z. et al. The gut ileal mucosal virome is disturbed in patients with Crohn’s disease and exacerbates intestinal inflammation in mice. Nat. Commun. 15, 1638 (2024).
Nakatsu, G. et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e525 (2018).
Colson, P. et al. Pepper mild mottle virus, a plant virus associated with specific immune responses, fever, abdominal pains, and pruritus in humans. PLoS ONE 5, e10041 (2010).
Dutilh, B. E. et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat. Commun. 5, 4498 (2014).
Kieft, K., Zhou, Z. & Anantharaman, K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome 8, 90 (2020).
Moxley, R. A., Jarrett, H. W. & Mitra, S. Methods for transcription factor separation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 797, 269–288 (2003).
Roux, S. et al. iPHoP: an integrated machine learning framework to maximize host prediction for metagenome-derived viruses of archaea and bacteria. PLoS Biol. 21, e3002083 (2023).
Hernández Medina, R. et al. Machine learning and deep learning applications in microbiome research. ISME Commun. 2, 98 (2022).
Statnikov, A. et al. A comprehensive evaluation of multicategory classification methods for microbiomic data. Microbiome 1, 11 (2013).
Pasolli, E., Truong, D. T., Malik, F., Waldron, L. & Segata, N. Machine learning meta-analysis of large metagenomic datasets: tools and biological insights. PLoS Comput. Biol. 12, e1004977 (2016).
FitzGerald, J. D. et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 72, 744–760 (2020).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Zhang, Y. et al. Discovery of bioactive microbial gene products in inflammatory bowel disease. Nature 606, 754–760 (2022).
Bhosle, A. et al. Integrated annotation prioritizes metabolites with bioactivity in inflammatory bowel disease. Mol. Syst. Biol. 20, 338–361 (2024).
Koh, A. et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 175, 947–961.e917 (2018).
Haghikia, A. et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 43, 518–533 (2022).
Meijnikman, A. S. et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 28, 2100–2106 (2022).
Begley, M., Gahan, C. G. M. & Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005).
VanHook, A. M. Bile acids for immune tolerance. Sci. Signal. 14, eabm3135 (2021).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Świderska, M. et al. The interplay between Th17 and T-regulatory responses as well as adipokines in the progression of non-alcoholic fatty liver disease. Clin. Exp. Hepatol. 3, 127–134 (2017).
Sarparanta, J., García-Macia, M. & Singh, R. Autophagy and mitochondria in obesity and type 2 diabetes. Curr. Diabetes Rev. 13, 352–369 (2017).
Koves, T. R. et al. Peroxisome proliferator-activated receptor-γ co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 280, 33588–33598 (2005).
Koves, T. R. et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56 (2008).
Mihalik, S. J. et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 18, 1695–1700 (2010).
Yang, M. et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 14, 228 (2023).
Park, S.-Y., Noureddin, M., Boushey, C., Wilkens, L. R. & Setiawan, V. W. Diet quality association with nonalcoholic fatty liver disease by cirrhosis status: the multiethnic cohort. Curr. Dev. Nutr. 4, nzaa024 (2020).
Lemons, J. M. S. et al. Enterobacteriaceae growth promotion by intestinal acylcarnitines, a biomarker of dysbiosis in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 17, 131–148 (2024).
Tanes, C. et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 29, 394–407.e395 (2021).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Rajakovich, L. J., Fu, B., Bollenbach, M. & Balskus, E. P. Elucidation of an anaerobic pathway for metabolism of l-carnitine-derived γ-butyrobetaine to trimethylamine in human gut bacteria. Proc. Natl Acad. Sci. USA 118, e2101498118 (2021).
Joyce, S. A. & Clarke, D. J. Microbial metabolites as modulators of host physiology. Adv. Micro. Physiol. 84, 83–133 (2024).
Meadows, J. A. & Wargo, M. J. Carnitine in bacterial physiology and metabolism. Microbiology 161, 1161–1174 (2015).
Hsu, C. L., Duan, Y., Fouts, D. E. & Schnabl, B. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J. Hepatol. 75, 1465–1475 (2021).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015).
Vehik, K. et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 25, 1865–1872 (2019).
Bajaj, J. S. et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 70, 1162–1173 (2021).
Aron-Wisnewsky, J. et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 17, 279–297 (2020).
Loomba, R. et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 25, 1054–1062.e1055 (2017).
Kim, M. N. et al. Weight gain during early adulthood, trajectory of body shape and the risk of nonalcoholic fatty liver disease: a prospective cohort study among women. Metabolism 113, 154398 (2020).
Franzosa, E. A. et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl Acad. Sci. USA 111, E2329–E2338 (2014).
Quince, C., Walker, A. W., Simpson, J. T., Loman, N. J. & Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 35, 833–844 (2017).
Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 10, e65088 (2021).
Caspi, R. et al. The MetaCyc database of metabolic pathways and enzymes - a 2019 update. Nucleic Acids Res. 48, D445–D453 (2020).
Shishkin, A. A. et al. Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods 12, 323–325 (2015).
Bhattacharyya, R. P. et al. Simultaneous detection of genotype and phenotype enables rapid and accurate antibiotic susceptibility determination. Nat. Med. 25, 1858–1864 (2019).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Oksanen, J. et al. The vegan package: community ecology package (2007).
Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17, e1009442 (2021).
Wirbel, J. et al. Microbiome meta-analysis and cross-disease comparison enabled by the SIAMCAT machine learning toolbox. Genome Biol. 22, 93 (2021).
Pasolli, E. et al. Accessible, curated metagenomic data through ExperimentHub. Nat. Methods 14, 1023–1024 (2017).
Acknowledgements
This work was supported by the National Institutes of Health grants U01CA176726, T32CA009001 (to H.K.), R35CA253185 (to A.T.C.), R24DK110499 (to C.H.) and K23DK125838 (to L.H.N.), the American Gastroenterological Association Research Foundation’s Research Scholars Award (to L.H.N.) and Crohn’s and Colitis Foundation Career Development Award (to L.H.N.). A.T.C. is supported by the American Cancer Society Research Professorship. L.H.N. is the Massachusetts General Hospital/Chen Institute Transformative Scholar in Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. Empress Therapeutics supported molecular data generation. This work was also supported by the Massachusetts Life Sciences Center. Computational work was conducted on the Harvard FASRC Cannon cluster supported by the FAS Division of Science Research Computing Group at Harvard University. The authors also acknowledge their colleagues in the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Author information
Authors and Affiliations
Contributions
C.H. and L.H.N. conceived of the study. H.K., P.N., E.N., J.S. and N.L. performed analyses. J.J., A.B., B.B., G.F., J.L., L.M. and E.A.F. provided technical support. C.L., C.E., F.B.H., T.G.S., A.T.C., B.H. and K.N.T. provided datasets and resources. H.K. wrote the initial draft of the paper. C.H. and L.H.N. co-supervised this work. All authors contributed to writing and revising the paper.
Corresponding authors
Ethics declarations
Competing interests
P.N. and B.H. were employees of Empress Therapeutics. C.H. is on the Scientific Advisory Board of Empress Therapeutics, Seres Therapeutics and ZOE Nutrition. All others declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Tae Gyu Oh, Sonja Lang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bacterial alpha- and beta-diversity differ by MASLD case status and MASLD subtype.
a. There was a small but statistically significant difference in beta-diversity based on case/control status, largely attributable to characteristic and previously documented trade-offs between Bacteroidetes and Firmicutes. The plot shows a principal coordinates analysis (PCoA) based on Bray–Curtis dissimilarity. Multivariable PERMANOVA adjusting for age, body mass index, physical activity, diabetes mellitus, and diet quality was performed among prevalent species (after 10% prevalence filter) with two-sided p-value reported. b. In 211 individuals with MASLD (compared to 502 non-MASLD controls), alpha-diversity was reduced, which is a broad measure of overall community structure and indicates lower species richness and evenness. Boxplots are presented as median with the lower and upper hinges corresponding to the interquartile range. The lower and upper whiskers show the smallest and largest value within the 1.5 × interquartile range. Statistical comparisons were performed using the Wilcoxon rank-sum test (p-value = 2.1e-6). ***: Two-sided p-value ≤ 0.001 c. Alpha-diversity was similarly lower among 37 participants with lean MASLD and 174 participants with non-lean MASLD. Boxplots are presented as median with the lower and upper hinges corresponding to the 25% and 75%, respectively. The lower and upper whiskers show the smallest and largest value within the 1.5 × interquartile range. Statistical comparisons were performed using the Wilcoxon rank-sum test (p-value = 9.2e-5 comparing non-lean MASLD vs. controls; p-value = 4.1e-4 comparing lean MASLD vs. controls; p-value = 0.12 comparing non-lean MASLD vs. lean MASLD). ***: Two-sided p-value ≤ 0.001.
Extended Data Fig. 2 Differences in overall metabolomic profiles in MASLD and correlations between bacteria and acylcarnitines by chain length in controls.
a. The community-level metabolomic abundance in MASLD and controls is depicted by principal coordinates analysis (PCoA) using Bray–Curtis dissimilarity. Multivariable PERMANOVA results (with two-sided p-value) demonstrate distinct metabolomic landscapes between the groups. b. Similar to samples from MASLD, among controls, there were clear clustering patterns between bacteria and acylcarnitines based on chain length and dietary intake. Alternative Healthy Eating Index (AHEI) and fibre represent long-term dietary intake using the cumulative average prior to stool collection (Methods). Cells are coloured by Spearman correlation coefficient. *: PFDR < 0.20, adjusted for multiple comparisons between metabolites.
Extended Data Fig. 3 Distinct correlation patterns between oral-typical bacteria and MASLD-associated metabolites in non-lean vs. lean MASLD.
a. The correlations between oral-typical bacteria and metabolites vary between non-lean and lean MASLD cases. The analysis includes all oral-typical bacteria and MASLD-associated metabolites. Dot size represents the magnitude of the absolute difference in correlations between non-lean and lean MASLD cases (that is, |⍴(bacteria and metabolites) for non-lean - ⍴(bacteria and metabolites) for lean|). Dot colour reflects the directionality of the correlations in each group: for example, green dots signify correlations that are negative for non-lean and positive for lean MASLD, whereas blue dots indicate correlations that are positive for non-lean and negative for lean MASLD, respectively. This visualization highlights the nuanced interplay between oral-typical microbes and metabolites across MASLD phenotypes. b. Selected microbe-metabolite pairs show different interaction patterns between non-lean vs. lean MASLD (Supplementary Table 9). Spearman correlation test was used to fit the line with the corresponding two-sided p-value shown. Bacteria are arcsine square root transformed and metabolites are log2 transformed.
Extended Data Fig. 4 Distinct correlation patterns between bacterial taxa and long-chain acylcarnitines in non-lean vs. lean MASLD.
The analysis includes bacterial taxa with at least four instances of absolute correlation differences greater than 0.3 and acylcarnitines. Dot size represents the magnitude of the absolute difference in correlations between non-lean and lean MASLD cases (that is, |⍴(bacteria and acylcarnitines) for non-lean - ⍴(bacteria and acylcarnitines) for lean|). Dot colour reflects the directionality of the correlations in each group. This visualization highlights the interplay between microbes and acylcarnitines (based on chain length) across MASLD phenotypes.
Extended Data Fig. 5 Alpha-diversity and gut viral taxa.
a. Compared to non-MASLD controls (502 individuals), both non-lean MASLD (174 individuals) and lean MASLD (37 individuals) had reduced viral alpha-diversity. Boxplots are presented as median with the lower and upper hinges corresponding to the 25% and 75%, respectively. The lower and upper whiskers show the smallest and largest value within the 1.5 × interquartile range. Statistical comparisons were performed using the Wilcoxon rank-sum test (p-value = 9.1e-4 comparing non-lean MASLD vs. controls; p-value = 7.5e-3 comparing lean MASLD vs. controls; p-value = 0.24 comparing non-lean MASLD vs. lean MASLD). ***: Two-sided p-value ≤ 0.001; **: p-value ≤ 0.01 b. Similar to metagenomic analysis, metatranscriptomic analysis also demonstrated that lean MASLD had a different proportion of classified/unclassified viruses compared to non-lean MASLD. ꞵ coefficients of non-lean MASLD (vs. controls) are plotted against ꞵ coefficients of lean MASLD (vs. controls) from multivariable linear models. Black dots indicate classified or known RNA viral species, while grey dots indicate unclassified RNA viral species. c. As with bacteria/archaea, in MASLD, there were distinct clustering patterns between MASLD-associated viruses (largely unclassified) and acylcarnitines based on chain length. *: PFDR < 0.20, adjusted for multiple comparisons between metabolites. The heatmap includes viral taxa with at least ten significant correlations (PFDR < 0.20) with acylcarnitines.
Extended Data Fig. 6 Co-occurrence and co-exclusion of oral-typical bacteria and MASLD-associated viruses in lean MASLD.
In lean MASLD, hierarchical all-against-all association testing demonstrated broad co-occurrence and co-exclusion of oral-typical bacteria and MASLD-associated viruses. *: differentially abundant bacteria in MASLD.
Extended Data Fig. 7 Co-occurrence and co-exclusion of oral-typical bacteria and MASLD-associated viruses in non-lean MASLD.
In non-lean MASLD, hierarchical all-against-all association testing demonstrated broad co-occurrence and co-exclusion of oral-typical bacteria and MASLD-associated viruses. *: differentially abundant bacteria in MASLD.
Extended Data Fig. 8 Comparison of machine learning models.
Random forest, kernel support vector machine, linear support vector machine, elastic net, LASSO, and ridge regression models using bacterial/archaeal, metabolomic, viral, and metatranscriptomic features along with clinical metadata, with random forest model showing a comparatively high area under the receiver operating curve (AUC = 0.691) and the highest area under the precision-recall curve (0.599).
Extended Data Fig. 9 Top multi-omic features distinguishing non-lean vs. lean MASLD and comparative classification performance across MASLD subtypes.
a. Feature importance for the comprehensive random forest model (that is, with all multi-omic data types with clinical metadata) differentiating non-lean MASLD cases vs. lean MASLD cases. Z-scores for the top selected features with high median Gini importance (Supplementary Table 12) are displayed as a heatmap. b. The classifications of non-lean vs. lean MASLD cases, non-lean MASLD cases vs. controls, lean MASLD cases vs. controls, non-lean MASLD cases vs. non-lean cases, and lean MASLD cases vs. lean controls were performed using bacteria/archaea, metabolites (MBX), unstratified metatranscriptomic (MTX) pathways, and viral features.
Extended Data Fig. 10 Strong correlation between cases with and without defined cardiometabolic diagnostic criteria.
a. Confirmed MASLD cases and potential non-MASLD cases demonstrated reasonable correlation given the small sample size of steatotic liver disease without a confirmed cardiometabolic comorbidity (N = 11). ꞵ coefficients for bacteria/archaea among potential non-MASLD cases (vs. controls) are plotted against ꞵ coefficients of confirmed MASLD cases (vs. controls) from multivariable linear models adjusted for age, body mass index, physical activity, diabetes mellitus, and diet quality. Spearman correlation test was used to fit the line, with the corresponding two-sided p-value shown. b. Confirmed MASLD cases and all potential MASLD cases demonstrated high correlation. ꞵ coefficients for bacteria/archaea among all potential MASLD cases (vs. controls) are plotted against ꞵ coefficients of confirmed MASLD cases (vs. controls) from multivariable linear models. Spearman correlation test was used to fit the line, with the corresponding two-sided p-value shown. c. Effect estimates in microbial differences from multivariable linear modelling between MASLD vs. controls with and without MASLD-defining comorbidities were generally concordant. Comparing the effect estimates for cases vs. controls without comorbid conditions (body mass index ≥ 25 kg/m2, have type 2 diabetes, high blood pressure, high cholesterol, or reported using medications for hypertension, diabetes, or high cholesterol) demonstrated high correlation with those for cases vs. all controls. Multivariable ꞵ coefficients for confirmed MASLD cases vs. controls without cardiometabolic comorbidities are on the y-axis vs. ꞵ coefficients for confirmed MASLD cases vs. all controls are on the x-axis. Spearman correlation test was used to fit the line with the corresponding two-sided p-value shown.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table 1
Supplementary Tables 1–14.
Source Data for Supplementary Fig. 1
Source Data for Supplementary Fig. 1.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Nelson, P., Nzabarushimana, E. et al. Multi-omic analysis reveals transkingdom gut dysbiosis in metabolic dysfunction-associated steatotic liver disease. Nat Metab 7, 1476–1492 (2025). https://doi.org/10.1038/s42255-025-01318-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01318-6
This article is cited by
-
Functional roles and regulatory mechanisms of paeonol in the treatment of liver disease
Natural Products and Bioprospecting (2026)
-
Viewing MASLD through an integrative gut microbiome lens
Nature Metabolism (2025)