Abstract
Infants undergo distinct ketogenesis during the preweaning period, yet its physiological implications remain unclear. Here, we show that preweaning ketosis promotes beige fat biogenesis and improves health outcomes in adulthood. Loss of ketogenesis in neonatal mice by early weaning or ablation of Hmgcs2 hinders beige adipogenesis, subsequently exacerbating metabolic dysregulation in high-fat diet-induced obesity. Enhanced ketogenesis during lactation through exogenous ketone supplements enhances energy expenditure, beige fat formation, and mitochondrial biogenesis and respiration. Using single-cell RNA sequencing, we identified a subset of β-hydroxybutyrate-responsive adipocyte progenitor cells (APCs) expressing Cd81 that showed high beige adipogenic potential. Enhanced ketogenesis promotes the recruitment of beige APCs and their differentiation into beige adipocytes. Mechanistically, ketogenesis-derived βHB induces a switch in the histone acetylome and β-hydroxybutyrylome for transcriptional activation of beige fat biogenesis genes. Notably, enhanced ketogenesis during lactation alleviates adverse metabolic effects predisposed by parental obesity. Our study highlights that targeting preweaning ketosis to drive beige adipogenesis may offer a therapeutic approach to combat obesity and metabolic diseases in adulthood.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The next-generation sequencing data generated in this study have been deposited in the Gene Expression Omnibus (GEO) under the following accession numbers: GSE267314 (bulk RNA-seq of iWAT and SVF), GSE267320 (ChIP–seq), GSE268811 (scRNA-seq) and GSE267285 (bulk RNA-seq of SVF from the PO model). Previously published ChIP–seq datasets (GSE175654 and GSE193463) were used to identify H3K27ac-enriched enhancer regions in iWAT and iWAT SVF. Source data are provided with this paper.
Code availability
The code used in this study is available on GitHub at https://github.com/FJLinLab/2025_ketone_biege.
References
Ebbeling, C. B., Pawlak, D. B. & Ludwig, D. S. Childhood obesity: public-health crisis, common sense cure. Lancet 360, 473–482 (2002).
World Health Organization. Obesity and Overweight (2021).
Kartiosuo, N. et al. Predicting overweight and obesity in young adulthood from childhood body-mass index: comparison of cutoffs derived from longitudinal and cross-sectional data. Lancet Child Adolesc. Health 3, 795–802 (2019).
Agras, W. S., Hammer, L. D., McNicholas, F. & Kraemer, H. C. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J. Pediatr. 145, 20–25 (2004).
Eriksson, J., Forsen, T., Osmond, C. & Barker, D. Obesity from cradle to grave. Int. J. Obes. Relat. Metab. Disord. 27, 722–727 (2003).
Whitaker, R. C., Wright, J. A., Pepe, M. S., Seidel, K. D. & Dietz, W. H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337, 869–873 (1997).
Geserick, M. et al. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 379, 1303–1312 (2018).
Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242–258 (2019).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Wang, W. et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 30, 174–189.e5 (2019).
Hasegawa, Y. et al. Repression of adipose tissue fibrosis through a PRDM16–GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 27, 180–194.e6 (2018).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
VanItallie, T. B. & Nufert, T. H. Ketones: metabolism’s ugly duckling. Nutr. Rev. 61, 327–341 (2003).
Reichard, G. A. Jr., Owen, O. E., Haff, A. C., Paul, P. & Bortz, W. M. Ketone-body production and oxidation in fasting obese humans. J. Clin. Invest. 53, 508–515 (1974).
Patikorn, C. et al. Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials. BMC Med. 21, 196 (2023).
Dowis, K. & Banga, S. The potential health benefits of the ketogenic diet: a narrative review. Nutrients 13, 1654 (2021).
Laffel, L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res Rev. 15, 412–426 (1999).
Lucas, A., Boyes, S., Bloom, S. R. & Aynsley-Green, A. Metabolic and endocrine responses to a milk feed in six-day-old term infants: differences between breast and cow’s milk formula feeding. Acta Paediatr. Scand. 70, 195–200 (1981).
Liu, F. et al. Breastfeeding and overweight/obesity among children and adolescents: a cross-sectional study. BMC Pediatr. 22, 347 (2022).
Harder, T., Bergmann, R., Kallischnigg, G. & Plagemann, A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am. J. Epidemiol. 162, 397–403 (2005).
Arenz, S., Ruckerl, R., Koletzko, B. & von Kries, R. Breast-feeding and childhood obesity—a systematic review. Int. J. Obes. Relat. Metab. Disord. 28, 1247–1256 (2004).
Chowdhury, G. M., Jiang, L., Rothman, D. L. & Behar, K. L. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J. Cereb. Blood Flow. Metab. 34, 1233–1242 (2014).
Seaton, T. B., Welle, S. L., Warenko, M. K. & Campbell, R. G. Thermic effect of medium-chain and long-chain triglycerides in man. Am. J. Clin. Nutr. 44, 630–634 (1986).
Bach, A., Schirardin, H., Weryha, A. & Bauer, M. Ketogenic response to medium-chain triglyceride load in the rat. J. Nutr. 107, 1863–1870 (1977).
Gors, S., Kucia, M., Langhammer, M., Junghans, P. & Metges, C. C. Technical note: milk composition in mice—methodological aspects and effects of mouse strain and lactation day. J. Dairy Sci. 92, 632–637 (2009).
Chong, D. et al. Neonatal ketone body elevation regulates postnatal heart development by promoting cardiomyocyte mitochondrial maturation and metabolic reprogramming. Cell Discov. 8, 106 (2022).
Asif, S. et al. Hmgcs2-mediated ketogenesis modulates high-fat diet-induced hepatosteatosis. Mol. Metab. 61, 101494 (2022).
Arima, Y. et al. Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation. Nat. Metab. 3, 196–210 (2021).
Jiang, C. L., Chen, Y. F. & Lin, F. J. Apolipoprotein E deficiency activates thermogenesis of white adipose tissues in mice through enhancing β-hydroxybutyrate production from precursor cells. FASEB J. 35, e21760 (2021).
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994).
Chawla, A., Schwarz, E. J., Dimaculangan, D. D. & Lazar, M. A. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135, 798–800 (1994).
Alimirah, F. et al. Crosstalk between the peroxisome proliferator-activated receptor gamma (PPARγ) and the vitamin D receptor (VDR) in human breast cancer cells: PPARγ binds to VDR and inhibits 1ɑ,25-dihydroxyvitamin D3 mediated transactivation. Exp. Cell. Res. 318, 2490–2497 (2012).
Pei, H., Yao, Y., Yang, Y., Liao, K. & Wu, J. R. Krüppel-like factor KLF9 regulates PPARγ transactivation at the middle stage of adipogenesis. Cell Death Differ. 18, 315–327 (2011).
Meirhaeghe, A. et al. Characterization of the human, mouse and rat PGC1 beta (peroxisome-proliferator-activated receptor-gamma co-activator 1 beta) gene in vitro and in vivo. Biochem. J. 373, 155–165 (2003).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Oguri, Y. et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 182, 563–577.e20 (2020).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Rondini, E. A., Ramseyer, V. D., Burl, R. B., Pique-Regi, R. & Granneman, J. G. Single cell functional genomics reveals plasticity of subcutaneous white adipose tissue (WAT) during early postnatal development. Mol. Metab. 53, 101307 (2021).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Terranova, C. J. et al. Reprogramming of H3K9bhb at regulatory elements is a key feature of fasting in the small intestine. Cell Rep. 37, 110044 (2021).
Karmodiya, K., Krebs, A. R., Oulad-Abdelghani, M., Kimura, H. & Tora, L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics 13, 424 (2012).
Nanduri, R. et al. Epigenetic regulation of white adipose tissue plasticity and energy metabolism by nucleosome binding HMGN proteins. Nat. Commun. 13, 7303 (2022).
Shao, M. et al. ZFP423 controls EBF2 coactivator recruitment and PPARγ occupancy to determine the thermogenic plasticity of adipocytes. Genes Dev. 35, 1461–1474 (2021).
Bostrom, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Lane, M., Zander-Fox, D. L., Robker, R. L. & McPherson, N. O. Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends Endocrinol. Metab. 26, 84–90 (2015).
Lu, J. F. et al. GDF15 is a major determinant of ketogenic diet-induced weight loss. Cell Metab. 35, 2165–2182.e7 (2023).
Ludwig, D. S. The ketogenic diet: evidence for optimism but high-quality research needed. J. Nutr. 150, 1354–1359 (2020).
Deemer, S. E. et al. Exogenous dietary ketone ester decreases body weight and adiposity in mice housed at thermoneutrality. Obesity (Silver Spring) 28, 1447–1455 (2020).
Joshi, S., Ostfeld, R. J. & McMacken, M. The ketogenic diet for obesity and diabetes—enthusiasm outpaces evidence. JAMA Intern. Med. 179, 1163–1164 (2019).
Da Eira, D., Jani, S., Stefanovic, M. & Ceddia, R. B. The ketogenic diet promotes triacylglycerol recycling in white adipose tissue and uncoupled fat oxidation in brown adipose tissue, but does not reduce adiposity in rats. J. Nutr. Biochem. 120, 109412 (2023).
Douris, N. et al. Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Mol. Metab. 6, 854–862 (2017).
Asrih, M., Altirriba, J., Rohner-Jeanrenaud, F. & Jornayvaz, F. R. Ketogenic diet impairs FGF21 signaling and promotes differential inflammatory responses in the liver and white adipose tissue. PLoS ONE 10, e0126364 (2015).
Jokinen, R. et al. Adipose tissue mitochondrial capacity associates with long-term weight loss success. Int. J. Obes. (Lond.) 42, 817–825 (2018).
Heinonen, S. et al. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia 60, 169–181 (2017).
Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 9, 2973 (2018).
Huypens, P. et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 48, 497–499 (2016).
Carriere, A. et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, 3253–3265 (2014).
Roh, H. C. et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab. 27, 1121–1137.e5 (2018).
Inoue, S. I. et al. Short-term cold exposure induces persistent epigenomic memory in brown fat. Cell Metab. 36, 1764–1778.e9 (2024).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Huang, H. et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci. Adv. 7, eabe2771 (2021).
Gao, M. et al. Metabolically controlled histone H4K5 acylation/acetylation ratio drives BRD4 genomic distribution. Cell Rep. 36, 109460 (2021).
Goudarzi, A. et al. Dynamic competing histone H4 K5K8 acetylation and butyrylation are hallmarks of highly active gene promoters. Mol. Cell 62, 169–180 (2016).
Tsusaka, T. et al. Non-specific recognition of histone modifications by H3K9bhb antibody. iScience 26, 107235 (2023).
Tiraby, C. et al. Acquirement of brown fat cell features by human white adipocytes. J. Biol. Chem. 278, 33370–33376 (2003).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Fan, H. et al. Cold-inducible Klf9 regulates thermogenesis of brown and beige fat. Diabetes 69, 2603–2618 (2020).
Norman, A. W. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 88, 491S–499S (2008).
Pramono, A., Jocken, J. W. E. & Blaak, E. E. Vitamin D deficiency in the aetiology of obesity-related insulin resistance. Diabetes Metab. Res. Rev. 35, e3146 (2019).
Wong, K. E. et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 286, 33804–33810 (2011).
Wong, K. E. et al. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am. J. Physiol. Endocrinol. Metab. 296, E820–E828 (2009).
Hoang, A. C. et al. Mitochondrial RNA stimulates beige adipocyte development in young mice. Nat. Metab. 4, 1684–1696 (2022).
O’Neill, B. & Raggi, P. The ketogenic diet: pros and cons. Atherosclerosis 292, 119–126 (2020).
Stubbs, B. J. et al. A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring) 26, 269–273 (2018).
Cox, P. J. et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 24, 256–268 (2016).
Acknowledgements
This work was supported by the National Science and Technology Council, Taiwan (NSTC 111-2320-B-002-037-MY3 and 114-2320-B-002-015 to F.-J.L.), NTU (113L894803 and 114L893003 to F.-J.L.) and Ministry of Education Higher Education Sprout Project (NTU 112L7131 to F.-J.L.). We thank the Technology Commons, College of Life Science, NTU, for providing access to the cell analyzer and Seahorse analyzer services. We thank W.-S. Yao at the Technology Commons in the College of Life Science, and the Instrumentation Center sponsored by the National Science and Technology Council, NTU, for technical support with the FACSAria III Cell Sorter. We also thank the Animal Resource Center and the Consortium of Integrative Biomedical Science Key Technology at NTU for their technical assistance with the single-cell experiment. We are grateful to the Taiwan Mouse Clinic, Academia Sinica and Taiwan Animal Consortium for support with body composition measurements and indirect calorimetry. We acknowledge the National Center for Biomodels (NCB), National Institute of Applied Research (NIAR), Taiwan, for providing the C57BL/6-Tg(Adipoq-FusRed,-cre/ERT2)13Narl/Narl mice (RMRC13297) and for technical support in assisted reproductive technology, contract breeding and testing services. We gratefully acknowledge Y.-C. Lee, C.-T. Chen and H.-M. Su for providing mouse cell lines, including 3T3-L1 fibroblasts, CT26.WT colon carcinoma cells and C2C12 myoblasts. We thank S.-H. Wang for the rederivation of Albumin-cre mice and H.-C. Tsai and Y.-J. Huang for assistance with the ChIP experiments. We are also grateful to C.-H. Lee and L.-J. Juan for their valuable discussions and critical reading of the manuscript. Finally, we thank all members of our laboratory for expert mouse husbandry and technical support throughout the study.
Author information
Authors and Affiliations
Contributions
C.-L.J. and F.-J.L. conceived the project, designed and performed the experiments, interpreted the data, prepared the figures and wrote the manuscript. P.-H.L. conducted experiments, performed the bioinformatics analysis of the scRNA-seq data and prepared figures. P.-C.Y. carried out library construction for ChIP–seq experiments, analysed ChIP–seq data, and prepared figures. C.-J.L. analysed the scRNA-seq data. H.-P.C.C. supervised the ChIP–seq experiments and contributed to data interpretation. J.-D.L. interpreted the scRNA-seq results. S.-J.L. and I.-S.Y. provided reagents. F.-J.L. supervised the project, designed experiments, interpreted data and generated figures with assistance from C.-L.J., P.-H.L. and P.-C.Y. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Yuichiro Arima, Da Jia, Yu-Hua Tseng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Revati Dewal, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Loss of endogenous ketogenesis in Hmgcs2-deficient mice impairs cold-induced beige fat formation and mitochondrial biogenesis.
a. Hepatic Hmgcs2 mRNA in control (CTL) and Hmgcs2 knockout (KO, Hmgcs2−/−) male mice at P0, P7, P14, and P21. b. Serum βHB levels in CTL and KO mice at P0, P4, P7, P14, and P21. c. Survival rate of Hmgcs2 heterozygous (HET) and KO mice (n = 42, 16). d. Ucp1 mRNA in iWAT of wild-type mice after 1, 2, 5, or 7 days at 4 °C (n = 3/group). e. Hmgcs2 mRNA in iWAT of wild-type mice after 7 days at 4 °C (n = 5/group). f. iWAT weights of CTL and KO mice housed at room temperature (RT) or 4 °C (Cold) for 7 days. g. Quantification of white and beige adipocytes from iWAT sections in Fig. 2c (n = 3/group). h–j. Transcriptomic analysis of iWAT SVF: pie charts (top) and volcano plots (bottom) of differentially expressed genes for (h) CTL Cold vs. RT; (i) KO Cold vs. RT, and (j) KO vs. CTL under Cold conditions. k. Ucp1 and Cidea mRNA in iWAT of mice from (f). l. iBAT weights in mice from (f). m. Ucp1 mRNA in iBAT from mice in (f). n. Fatty acid oxidation (FAO) stress test in SVF-derived adipocytes from CTL and KO mice using palmitic acid (PA)-BSA as substrate, followed by etomoxir (Eto) treatment. Real-time oxygen consumption rate (OCR) shown (n = 3 for CTL; 4 for KO). o. Quantification of basal and maximal FAO responses following Eto treatment (n = 3 for CTL; 4 for KO). p. Mitochondrial DNA (mtDNA) copy number in iWAT, expressed as the ratio of Nd1 to Lpl gene expression (n = 6/group). q. mRNA levels of mitochondrial biogenesis genes in iWAT. Data are expressed as mean ± SD. n = 4/group in a–b, f, k–m, q. Statistical analyses: two-sided Student’s t-test (a, b, d, e, o–q) and two-way ANOVA with Tukey’s post hoc test for multiple comparisons (f, k–m) and. P values in (d) are versus day 0.
Extended Data Fig. 2 Global and liver-specific deletion of Hmgcs2 impairs cold-induced beige fat formation in male mice.
a. Hmgcs2 mRNA in liver, iWAT, eWAT, and iBAT of P14 control mice (n = 3/group). b. Intracellular βHB concentrations in liver, iWAT, eWAT, and iBAT of P14 control mice (n = 4/group). c. Expression of Hmgcs2 (left) and Fabp4 (right) during adipogenic differentiation of SVF (n = 3/group). d. Schematic of global and conditional Hmgcs2 knockout models and experimental design. Eight-week-old male control (CTL), global knockout (KO, mediated by Sox2-Cre), liver-specific knockout (LiKO, mediated by Albumin-Cre), and adipocyte-specific knockout (AdKO, mediated by Adipoq-CreERT2) mice were maintained on chow and housed at room temperature (RT) or 4 °C (Cold) for 7 days. Rectal temperature was measured after 2 days; iWAT was collected after 7 days. e. Representative western blot of HMGCS2 protein in liver and iWAT. β-actin as loading control. f. Serum βHB levels at P14 in male mice (n = 6 for CTL; 5 for KO; 4 for LiKO; 4 for AdKO). g. Intracellular βHB in iWAT at P14 (n = 3/group). h. Serum βHB levels at P14 in female mice (n = 8 for CTL; 7 for KO; 6 for LiKO; 6 for AdKO). i. Changes in rectal temperature after 2 days at 4 °C in male mice. Delta rectal temperature = temperature at indicated time – baseline temperature (n = 8 for CTL RT; 8 for CTL Cold; 6 for KO; 6 for LiKO; 5 for AdKO). j. Gross morphology and representative H&E-stained iWAT sections. Grid:1 cm×1 cm. Scale bar, 100 µm. k. Quantification of white and beige adipocyte in (j) (n = 4/group). l. mRNA levels of thermogenic genes in iWAT (n = 6 for CTL RT; 6 for CTL Cold; 4 for KO; 4 for LiKO; 4 for AdKO). Data are expressed as means ± SD. Statistical analyses: one-way ANOVA with Tukey’s post hoc test for multiple comparisons (a–b, f–i, l) and two-sided Student’s t-test (c). P values in (c) are versus day 0 (c). Illustrations in d created in BioRender.com, https://biorender.com/hjq5y60.
Extended Data Fig. 3 Global deletion of Hmgcs2 impairs cold-induced beige fat formation in female mice.
a. Serum βHB levels in P14 male and female mice (n = 4 for males; 6 for females). b. Schematic of the Hmgcs2 KO mouse model and experimental design. Eight-week-old female control (CTL-F) and knockout (KO-F) mice were maintained on chow and housed at room temperature (RT) or 4 °C (Cold) for 7 days. Rectal temperature was measured after 2 days of cold exposure; iWAT was collected after 7 days of cold exposure. c. Gross morphology and representative H&E-stained iWAT sections. Grid:1 cm×1 cm. Scale bar, 100 µm. d. Quantification of white and beige adipocyte numbers in iWAT sections (n = 3/group). e. mRNA expression of thermogenic and white adipocyte-selective genes in iWAT (n = 4/group). f. Changes in rectal temperature after 2 days of cold exposure. Delta rectal temperature = temperature at indicated time – baseline temperature (n = 4/group). g. Representative H&E-stained iBAT sections. Scale bar, 100 µm. h. mRNA expression of Hmgcs2 and Ucp1 in iBAT (n = 4/group). Data are expressed as means ± SD. Statistical analyses: one-way ANOVA with Tukey’s post hoc test for multiple comparisons (e, f, h) and two-sided Student’s t-test (a). Illustrations in b created in BioRender.com, https://biorender.com/oql9znl.
Extended Data Fig. 4 Enhanced ketogenesis during lactation promotes mitochondrial biogenesis and Ucp1 expression in iWAT.
a. Serum βHB concentrations in 3-week-old C57BL/6 J male mice orally administrated water or 1,3-butanediol (1,3BD; 5 g/kg BW) (n = 3/group). b. Serum βHB levels in P14 mice orally administrated water or 1,3BD starting at P2 (n = 4, 6). c. Intracellular βHB concentrations in P14 mice described in (b) (n = 4, 6). d. Body weight of mice from Fig. 3a (n = 4/group). e. iWAT weight of mice from Fig. 3a (n = 4/group). f. Dio2 and Elovl3 mRNA expression in iWAT of mice from Fig. 3a (n = 4/group). g. Adrb1, Adrb2 and Adrb3 mRNA expression in iWAT of mice from Fig. 3a (n = 4/group). h. Gross morphology of iBAT from mice in Fig. 3a. i. iBAT weight of mice from Fig. 3a (n = 4/group). j. Ucp1 mRNA expression in iWAT and iBAT of mice from Fig. 3a (n = 5, 4, 3, 3). k. Dio2 and Elovl3 mRNA expression in iBAT of control, Hmgcs2 KO, and 1,3BD-treated mice (n = 4, 4, 3). l. Temporal changes and quantification of oxygen consumption rate (VO2) in mice from Fig. 3m, housed at thermoneutrality (30 °C) and cold exposure (4 °C) (n = 5/group). m. Temporal changes and quantification of carbon dioxide production rate (VCO2) in mice from Fig. 3m, housed at 30 °C and 4 °C (n = 5/group). Data are expressed as means ± SD. Statistical analyses: two-sided Student’s t-test (a–c, g, j, l–m), one-way ANOVA with Tukey’s post hoc test (k) and two-way ANOVA with Tukey’s post hoc test for multiple comparisons (d–f, i).
Extended Data Fig. 5 Enhanced ketogenesis during lactation induces transcriptomic and cellular changes in iWAT SVF.
a. Pie chart of differentially expressed genes in 1,3BD SVF vs. Water SVF. Water SVF and 1,3B -SVF were isolated from iWAT of P21 male mice orally administrated water or 1,3BD from P2–P21 (n = 3/group; each sample pools 3-5 animals). b. Pie chart of differentially expressed transcription factor genes in SVF samples in (a). c. Immunofluorescence staining of UCP1 in SVF samples from (a). d. Western blot analysis of PGC1α, PPARγ1/2, CD81, CD137, and β-actin in SVF from (a). β-actin as loading control. e. Densitometry analysis of (d) (n = 3/group). f. Proportion of CD34−:CD81+ cells in SVF (see Fig. 4i) (n = 3/group). g. Gating strategy for isolating CD81low and CD81high cells from SVF of iBAT in P21 CTL, Hmgcs2 KO and 1,3BD-treated mice. h. Quantification of CD81+ cell populations from (g) (n = 7 for CTL, 6 for KO, 7 for 1,3BD). i. Mean fluorescence intensity (MFI) of CD81 in cells from (g) (n = 7 for CTL, 6 for KO, 7 for 1,3BD). j. Proportions of CD81high and CD81low cells within the CD81⁺ population from (g) (n = 7 for CTL, 6 for KO, 7 for 1,3BD). k. Histogram of BrdU incorporation (left) and quantification of BrdU+ cells in CD81low, CD81high, CD81low cells treated with 2 mM βHB for 2 days (CD81low + βHB cells), and CD81high cells treated with 2 mM βHB for 2 days (CD81high + βHB cells) (n = 3/group). l. Oil Red O staining of differentiated CD81low, CD81high, CD81low + βHB, and CD81high + βHB cells. Cells were isolated from CTL iWAT and cultured under adipogenic conditions for 6 days. Data are expressed as means ± SD. Statistical analyses: two-sided Student’s t-test (e), one-way ANOVA with Tukey’s post hoc test for multiple comparisons (f, h–j) and two-way ANOVA with Tukey’s post hoc test for multiple comparisons (k).
Extended Data Fig. 6 Marker gene expression patterns across cell clusters from iWAT SVF.
a. Heatmap showing representative gene expression across identified cell clusters from iWAT SVF from P21 male mice (n = 1/group; each sample pools 3–5 animals). b. Violin plots depicting mRNA expression levels of indicated genes across cell clusters. c. UMAP plots and corresponding violin plots illustrating Pdgfra and Ly6a expression in each cluster. d. UMAP visualization of Pparg, Rreb1, Cd81, Tfam, Klf9, and Vdr expression in each cluster of adults (P56) condition. e. Venn diagram showing upregulated differentially expressed genes (DEGs) in ASC1 populations under KO vs. CTL and 1,3BD vs. CTL conditions. Data were obtained from scRNA-seq analysis and are presented as log-normalized gene expression values (a–d).
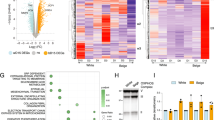
Extended Data Fig. 7 ChIP-seq analysis of H3K9ac, H3K14ac, and H3K9bhb in iWAT SVF.
a. Representative immunoblot of total lysine acetylation (Kac) in SVF from iWAT of P21 control (CTL), Hmgcs2 knockout (KO) and 1,3BD-treated mice. β-actin as loading control. b. Densitometry analysis of Fig. 6a (n = 4/group). c. Heatmap of Pearson’s correlation coefficients for genome-wide occupancy of H3K9ac, H3K14ac, and H3K9bhb between biological replicates of Water SVF and 1,3BD SVF. d. Co-localization of consensus enrichment peaks for H3K9ac, H3K14ac, and H3K9bhb in Water SVF and 1,3BD SVF. e. Metagene profiles of H3K9ac, H3K14ac and, H3K9bhb coverage around the transcription starts sites (TSS) ( ± 3 kb) of gene involved in cell differentiation, cell fate commitment, cold-induced thermogenesis, and fat cell differentiation in Water SVF and 1,3BD SVF. f–h. ChIP-qPCR validation of H3K9ac, H3K14ac, and H3K9bhb occupancy at the promoters of Ppargc1a (f), Klf9 (g), and Vdr (h) in Water SVF and 1,3BD SVF (n = 3/group). i. Genome browser view of H3K9ac, H3K14ac, and H3K9bhb occupancy at regulatory regions of indicated genes in Water SVF, KO SVF, and 1,3BD SVF. j. ChIP-qPCR validation of H3K9ac occupancy at Cd81 (left) and Ppargc1a (right) promoters in Water SVF and 1,3BD SVF (n = 3/group). k. H3K27ac ChIP-seq profiles from whole iWAT and SVF (GSE175654 and GSE193463) upstream regions of Cd81 (top) and Ppargc1a (bottom) TSS. Red boxes indicate regions targeted by ChIP-qPCR primers. H3K27ac signals are shown in dark blue peaks (GSE175654) and light blue boxes (GSE193463). l–m. ChIP-qPCR validation of H3K27ac occupancy at enhancers of Cd81 (l) and Ppargc1a (m) in Water SVF and 1,3BD SVF (n = 3/group). Data are expressed as means ± SD. n = 3/group in (f–h, l–m). Statistical analyses: one-way ANOVA with Tukey’s post hoc test for multiple comparisons (b) and two-sided Student’s t-test (f–h, j, l–m).
Extended Data Fig. 8 Exogenous ketone body supplementation induces enrichments of H3K9/14ac and H3K9bhb and gene expression in MEFs.
a. Schematic of the experimental design. b. Representative immunoblot of global H3K9ac, H3K14ac, H3K9bhb, and total histone H3 in wild-type mouse embryonic fibroblasts (MEFs) treated with or without βHB for 2 days. Histone H3 as loading control. c. Densitometry analysis of (b). d. ChIP-qPCR validation of H3K9ac, H3K14ac, and H3K9bhb occupancy at the promoter region of Ppargc1a in control (CTL) and βHB-treated MEFs. e. ChIP-qPCR validation of H3K9ac, H3K14ac, and H3K9bhb occupancy at the promoter region of Klf9 in CTL and βHB-treated MEFs. f. ChIP-qPCR validation of H3K9ac, H3K14ac, and H3K9bhb occupancy at the promoter region of Vdr in CTL and βHB-treated MEFs. g. mRNA expression of Ppargc1a, Klf9 and Vdr in CTL and βHB-treated MEFs. h. mRNA expression of beige progenitor markers Cd81 and Tmem26 in CTL and βHB-treated MEFs. Data are expressed as means ± SD. Sample sizes in b–h were n = 3 per group. Statistical analyses: one-way ANOVA with Tukey’s post hoc test for multiple comparisons (c) and two-sided Student’s t-test (d–h). Illustrations in a created in BioRender.com, https://biorender.com/nmszrfi.
Extended Data Fig. 9 Ppargc1a is required for βHB-induced beige adipogenesis.
a. Schematic of experimental design. Ppargc1a control (Ppargc1+/+) and knockout (Ppargc1a−/−) mice were orally administered water or 1,3-butanediol (1,3BD) during the preweaning period (P2-P21). SVF were isolated from iWAT of P21 mice and subjected to adipogenic induction. b. Ppargc1a mRNA expression in Ppargc1+/+ and Ppargc1a−/− SVF samples (n = 3/group; 3 animals/group). c. Representative Oil Red O (ORO) staining of differentiated SVF-derived adipocytes from (a). d. Ucp1 mRNA levels in isoproterenol-treated or untreated SVF-derived adipocytes from (a) (n = 3/group; 3 animals/group). e. Oxygen consumption rate (OCR) profiles from mitochondrial stress tests in SVF-derived adipocytes from (a). f. Quantification of basal respiration, maximal respiration, and spare respiratory capacity from data in (e) (n = 3/group; 3 animals/group). g. Mitochondrial DNA (mtDNA) content in SVF-derived adipocytes from (a) (n = 3/group). h. mRNA levels of mitochondrial genes in SVF-derived adipocytes from (a) (n = 3/group). Data are expressed as means ± SD. Statistical analyses: two-sided Student’s t-test (b) and two-way ANOVA with Tukey’s post hoc test for multiple comparisons (d, f–h). Illustrations in a created in BioRender.com, https://biorender.com/czow54b.
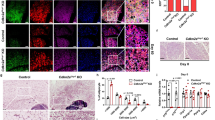
Extended Data Fig. 10 Enhanced ketogenesis during lactation protects against parental obesity-linked metabolic dysfunction.
a. Blood glucose levels in male offspring from lean (Ln-F1) and obese (Ob-F1) parents at P0, P7, P14, and P21 under ad libitum feeding (Ln-F1, n = 3, 3, 5, 4; Ob-F1, n = 4, 4, 4, 5). b. Serum βHB levels in the same mice as (a) (Ln-F1, n = 3, 5, 3, 4; Ob-F1, n = 4, 4, 4, 5). c. Hepatic Hmgcs2 mRNA expression in mice from (a) (Ln-F1, n = 3, 4, 5, 3; Ob-F1, n = 3, 5, 4, 4). d. Pie chart (left) and volcano plot (right) showing differentially expressed genes in SVF from P21 Ob-F1 vs. Ln-F1 mice (n = 1/group). e. Expression of ketone utilization genes in SVF from (d) (n = 3/group). f. Gross morphology (top) and weights (bottom) of eWAT and iBAT in 20-week-old mice (n = 12, 12, 6, 5). g. Serum glucose and systolic/diastolic blood pressure in mice from (f) (glucose: n = 12, 12, 6, 5; blood pressure: n = 5, 5, 3, 3 for blood pressure). h. Oral glucose tolerance test (OGTT) at 16 weeks (left) and AUC (right) in mice from (f) (n = 7, 6, 6, 6). i. Intraperitoneal insulin tolerance test (ipITT) at 18 weeks (left) and AUC (right) in mice from (f) (n = 7, 6, 6, 6). j. Representative western blot of total AKT (t-AKT) and phosphorylated AKT (p-AKT at S473) in iWAT of mice from (f). β-actin as loading control. W, Water; 1,3, 1,3BD. k. Densitometry analysis of (j) (n = 4 for untreated; n = 5 for insulin-treated). l. Gross morphology of iWAT from mice in Fig. 7o; Grid:1 cm×1 cm. m. Cidea and Cox8b mRNA expression in iWAT from Fig. 7o (n = 4/group). n. Representative western blot of UCP1 and β-actin in iWAT from Fig. 7o, with quantification (n = 4/group). β-actin as loading control. Data are means ± SD. Statistical analysis: two-sided Student’s t-test (a–c, e); two-way ANOVA with Tukey’s post hoc test for multiple comparisons (f–i, k, m–n). PO, parental obesity.
Supplementary information
Supplementary Information
Supplementary Figures 1–4, Supplementary Tables 1–2 and Supplementary Figure source data unprocessed Immunoblots
Supplementary Data 1
Supplementary figure source data
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 1
Unprocessed Immunoblots
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 3
Unprocessed Immunoblots
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 6
Unprocessed Immunoblots
Source Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 2
Unprocessed Immunoblots
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 5
Unprocessed Immunoblots
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 7
Unprocessed Immunoblots
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 8
Unprocessed Immunoblots
Source Data Extended Data Fig. 9
Statistical Source Data
Source Data Extended Data Fig. 10
Statistical Source Data
Source Data Extended Data Fig. 10
Unprocessed Immunoblots
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, CL., Lai, PH., Yang, PC. et al. Early-life ketone body signalling promotes beige fat biogenesis through changes in histone acetylome and β-hydroxybutyrylome. Nat Metab 7, 2045–2066 (2025). https://doi.org/10.1038/s42255-025-01378-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01378-8
This article is cited by
-
Infant ketones set the thermogenic tone
Nature Metabolism (2025)