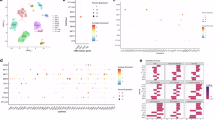
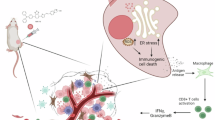
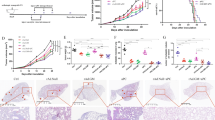
Abstract
Bacteria-based therapies hold great promise for cancer treatment due to their selective tumor colonization and proliferation. However, clinical application is hindered by the need for safe, precise control systems to regulate local therapeutic payload expression and release. Here we developed a near-infrared (NIR) light-mediated PadC-based photoswitch (NETMAP) system based on a chimeric phytochrome-activated diguanylyl cyclase (PadC) and a cyclic diguanylate monophosphate-dependent transcriptional activator (MrkH). The NETMAP-engineered bacteria exhibited antitumor performance in mouse tumor models with different levels of immunogenicity. Specifically, in immunogenic lymphoma tumors, NIR-induced PD-L1 and CTLA-4 nanobodies enhanced the activation of adaptive immunity. In low-immunogenic tumors—including mouse-derived colon cancer models, an orthotopic human breast cancer cell line-derived xenograft model and a colorectal cancer patient-derived xenograft model—NIR-induced azurin and cytolysin A predominantly led to tumor inhibition. Our study identifies an NIR light-mediated therapeutic platform for engineered bacteria-based therapies with customizable outputs and precise dosage control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data associated with this study are available within the Article, Supplementary Information, Source Data file and from the corresponding authors upon request. Source data are provided with this paper.
References
Wang, H. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
Arranja, A. G. et al. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 115, 87–95 (2017).
Zhou, S. et al. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018).
Yi, X. et al. Bacteria-triggered tumor-specific thrombosis to enable potent photothermal immunotherapy of cancer. Sci. Adv. 6, eaba3546 (2020).
Chen, Y. et al. Genetically engineered oncolytic bacteria as drug delivery systems for targeted cancer theranostics. Acta Biomater. 124, 72–87 (2021).
Kim, S. H. et al. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 69, 5860–5866 (2009).
Pan, H. et al. Engineered NIR light-responsive bacteria as anti-tumor agent for targeted and precise cancer therapy. Chem. Eng. J. 426, 130842 (2021).
Zheng, J. H. et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 9, eaak9537 (2017).
Chen, Y. et al. Spatiotemporal control of engineered bacteria to express interferon-γ by focused ultrasound for tumor immunotherapy. Nat. Commun. 13, 4468 (2022).
Chait, R. et al. Pervasive selection for and against antibiotic resistance in inhomogeneous multistress environments. Nat. Commun. 7, 10333 (2016).
Gao, T. et al. Sonogenetics-controlled synthetic designer cells for cancer therapy in tumor mouse models. Cell Rep. Med. 5, 101513 (2024).
Lim, J. et al. ASIC1a is required for neuronal activation via low-intensity ultrasound stimulation in mouse brain. eLife 10, e61660 (2021).
Yang, F. et al. Principles and applications of sono-optogenetics. Adv. Drug Deliv. Rev. 194, 114711 (2023).
Chen, X. et al. An extraordinary stringent and sensitive light-switchable gene expression system for bacterial cells. Cell Res. 26, 854–857 (2016).
Ribeiro, I. M. A. et al. Spatial and temporal control of expression with light-gated LOV-LexA. G3 12, jkac178 (2022).
Wang, Z. et al. A single-component blue light-induced system based on EL222 in Yarrowia lipolytica. Int. J. Mol. Sci. 23, 6344 (2022).
Tan, P. et al. Optophysiology: illuminating cell physiology with optogenetics. Physiol. Rev. 102, 1263–1325 (2022).
Weissleder, R. A clearer vision for in vivo imaging: progress continues in the development of smaller, more penetrable probes for biological imaging. Nat. Biotechnol. 19, 316–317 (2001).
Schmidl, S. R. et al. Refactoring and optimization of light-switchable Escherichia coli two-component systems. ACS Synth. Biol. 3, 820–831 (2014).
Kaberniuk, A. A. et al. Single-component near-infrared optogenetic systems for gene transcription regulation. Nat. Commun. 12, 3859 (2021).
Multamäki, E. et al. Optogenetic control of bacterial expression by red light. ACS Synth. Biol. 11, 3354–3367 (2022).
Ong, N. T. et al. Engineering an E. coli near-infrared light sensor. ACS Synth. Biol. 7, 240–248 (2018).
Gnach, A. et al. Upconverting nanoparticles: assessing the toxicity. Chem. Soc. Rev. 44, 1561–1584 (2015).
Gourinchas, G. et al. Influence of the N-terminal segment and the PHY-tongue element on light-regulation in bacteriophytochromes. J. Biol. Chem. 294, 4498–4510 (2019).
Wegele, R. et al. The heme oxygenase(s)-phytochrome system of Pseudomonas aeruginosa. J. Biol. Chem. 279, 45791–45802 (2004).
Wilksch, J. J. et al. MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog. 7, e1002204 (2011).
Ryu, M. H. & Gomelsky, M. Near-infrared light responsive synthetic c-di-GMP module for optogenetic applications. ACS Synth. Biol. 3, 802–810 (2014).
Solano, C. et al. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43, 793–808 (2002).
Solano, C. et al. Genetic reductionist approach for dissecting individual roles of GGDEF proteins within the c-di-GMP signaling network in Salmonella. Proc. Natl Acad. Sci. USA 106, 7997–8002 (2009).
Lovelace, A. H. et al. RpoS contributes in a host-dependent manner to Salmonella colonization of the leaf apoplast during plant disease. Front. Microbiol. 13, 999183 (2022).
Kim, J. K. et al. Purine biosynthesis-deficient Burkholderia mutants are incapable of symbiotic accommodation in the stinkbug. ISME J. 8, 552–563 (2014).
Howe, K. et al. Development of stable reporter system cloning luxCDABE genes into chromosome of Salmonella enterica serotypes using Tn7 transposon. BMC Microbiol. 10, 197 (2010).
Danino, T. et al. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 7, 289ra284 (2015).
McKenzie, G. J. & Craig, N. L. Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6, 39 (2006).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 12, 1 (2020).
Sandage, B. W. et al. Abstract 2619: combination of ECP1014 and anti-PD-L1 reduces tumor growth in the CT26 murine colon carcinoma model of a cold tumor. Cancer Res. 77, 2619–2619 (2017).
Zhang, Y. et al. Escherichia coli Nissle 1917 targets and restrains mouse B16 melanoma and 4T1 breast tumors through expression of azurin protein. Appl. Environ. Microbiol. 78, 7603–7610 (2012).
Jia, X. et al. Delayed oseltamivir plus sirolimus treatment attenuates H1N1 virus-induced severe lung injury correlated with repressed NLRP3 inflammasome activation and inflammatory cell infiltration. PLoS Pathog. 14, e1007428 (2018).
Wu, Y. et al. Secreting-lux/pT-ClyA engineered bacteria suppresses tumor growth via interleukin-1beta in two pathways. AMB Express 9, 189 (2019).
Ohlendorf, R. & Möglich, A. Light-regulated gene expression in bacteria: fundamentals, advances, and perspectives. Front. Bioeng. Biotechnol. 10, 1029403 (2022).
Baumschlager, A. et al. Dynamic blue light-inducible T7 RNA polymerases (Opto-T7RNAPs) for precise spatiotemporal gene expression control. ACS Synth. Biol. 6, 2157–2167 (2017).
Richter, F. et al. Engineering of temperature- and light-switchable Cas9 variants. Nucleic Acids Res. 44, 10003–10014 (2016).
Castillo-Hair, S. M. et al. Optogenetic control of Bacillus subtilis gene expression. Nat. Commun. 10, 3099 (2019).
Zhou, Y. et al. A small and highly sensitive red/far-red optogenetic switch for applications in mammals. Nat. Biotechnol. 40, 262–272 (2022).
Azzouzi, A.-R. et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 18, 181–191 (2017).
Banerjee, S. M. et al. Photodynamic therapy in primary breast cancer. J. Clin. Med. 9, 483 (2020).
Li, X. et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Qiao, L. et al. A sensitive red/far-red photoswitch for controllable gene therapy in mouse models of metabolic diseases. Nat. Commun. 15, 10310 (2024).
Zhou, J. et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 13, 3432 (2022).
Wang, X. et al. The EspF N-terminal of enterohemorrhagic Escherichia coli O157: H7 EDL933w imparts stronger toxicity effects on HT-29 cells than the C-Terminal. Front. Cell Infect. Microbiol. 7, 410 (2017).
Jiang, Y. et al. Multigene editing in the Escherichia coli genome via the CRISPR–Cas9 system. Appl. Environ. Microbiol. 81, 2506–2514 (2015).
Acknowledgements
We thank the Iñigo Lasa Laboratory (Universidad Pública de Navarra) for the generous donation of S. enteritidis 3934 and S. enteritidis 3934 ΔXII and the Andreas Winkler Laboratory (Graz University of Technology) for generously providing the PadC plasmids. In addition, we thank the Sheng Yang Laboratory (Institute of Plant Physiology and Ecology, Chinese Academy of Sciences) for generously providing the pCas/pTargetF system. H.Y. is a Shanghai Academy of Natural Sciences Exploration Scholar. This work was financially supported by grants from the National Natural Science Foundation of China (NSFC; number 32250010, number 32261160373, number 32430064), the Noncommunicable Chronic Diseases-National Science and Technology Major Project (number 2023ZD0501300), the Science and Technology Commission of Shanghai Municipality (numbers 23HC1410100 and 22N31900300), and the Fundamental Research Funds for the Central Universities to H.Y. This work was also partially supported by the NSFC (number 31901023) to N.G. and the Young Scientists Fund of the NSFC (number 32300458), the Science and Technology Commission of Shanghai Municipality (number 23YF1410700), and China Postdoctoral Science Foundation (number 2022M721163 and number BX20230128) to Y.Z. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thank the ECNU Multifunctional Platform for Innovation (011) for supporting the mice experiments and the Instruments Sharing Platform of the School of Life Sciences, ECNU.
Author information
Authors and Affiliations
Contributions
H.Y. conceived the project. H.Y., L.Q., N.G., and L.N. designed the experiments, analyzed the results, and wrote the paper. L.Q., L.N., Z.W., D.D., Z.D., X.M., Y.Z., D.K., Q.W., J.Y., L.J., J.S., B.F., W.L., and N.G. performed the experimental work. L.Q., L.N., Z.W., D.D., F.C., N.G., and H.Y. analyzed and interpreted the experiments. All authors edited and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
H.Y., L.N., L.Q., Z.W., and N.G. are listed as inventors on a Chinese patent application (number 202210607219.2: “A prokaryotic far-red light-regulated transcriptional activation system: construction method and application in tumor therapy”) submitted by ECNU, which covers the optogenetic NETMAP system. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Intracellular c-di-GMP concentrations across Salmonella strains.
a, Schematic depiction of the experimental procedure and schedule for evaluating the intracellular c-di-GMP concentrations throughout different Salmonella strains. b, Intracellular c-di-GMP concentrations in WT (S. enteritidis 3934), ∆XII (S. enteritidis 3934 ∆XII), and ∆XIV (S. enteritidis 3934 ∆XIV). c, NETMAP-engineered ∆XIV (∆XIV-NETMAP) cells were exposed to NIR light (710 nm, 1 mW cm-2) for two hours. After growth for nine hours, cells from different groups were harvested and ultrasonically crushed for ELISA assay. Data (b, c) are presented as mean ± s.d.; n = 3 independent experiments. Statistical analyses in b were performed using one-way ANOVA followed by Tukey’s test with multiple comparisons. Statistical analyses in c were performed using a two-tailed unpaired t-test.
Extended Data Fig. 2 Characterization of S. enteritidis 3934 ΔXIV (ΔXIV) for A20 lymphoma therapy.
a, Bacterial abundance and metabolic activity of ΔXIV within A20 tumors at the indicated time points. A20 tumor-bearing mice received an intratumoral injection of 5 × 106 c.f.u. of ΔXIV-lux (n = 5 mice per group). Bacterial abundance was measured by colony counting (c.f.u., left axis), and metabolic activity was evaluated by RLU (right axis) at the indicated time points. b, Representative photographs of LB agar plates spread with tissue homogenate of tumors and major organs. For example, 1/10 means that the tissue homogenate supernatant is diluted 10 times with sterile PBS, and then 100 μL supernatant of tissue homogenate with this dilution ratio is plated on the agar. c, Bacterial counts in tumor tissues and five major organs (n = 5 mice per group). Tumors and major organs, including heart, liver, spleen, lung, and kidney, were collected from A20 tumor-bearing mice at the indicated time points after intratumoral injection of 5 × 106 c.f.u. of ΔXIV, homogenization and plating on LB solid medium for colony counting. d, e, IL-6 levels in tumor tissues and serum. A20 tumor-bearing mice received an intratumoral injection of 5 × 106 c.f.u. of ΔXIV (n = 5 mice per group). IL-6 levels in tumor homogenates (d) and serum (e) of mice were quantified at the indicated time points. Data are expressed as means ± s.e.m.; Statistical analyses in d, e were performed using one-way ANOVA followed by Tukey’s test with multiple comparisons.
Extended Data Fig. 3 Complete blood count and blood biochemistry examination in mice.
CT26 tumor-bearing mice were intratumorally injected with 5 × 106 c.f.u. of ΔXIV. Blood samples were collected on the indicated days post-bacterial injection for a complete blood count and blood biochemistry investigation. (a) Alkaline phosphatase (ALP), (b) aspartate aminotransferase (AST), (c) alanine aminotransferase (ALT), (d) white blood cells, (e) red blood cells, (f) hematocrit, (g) time-course albumin/globin ratios, (h) mean corpuscular hemoglobin, (i) mean corpuscular volume, (j) creatinine (CRE), and (k) blood urea nitrogen (BUN) from healthy mice (control) and bacteria-treated mice were detected. Data are presented as means ± s.e.m.; n = 4 mice per group. Statistical analyses were performed using a one-way ANOVA followed by Tukey’s test with multiple comparisons.
Extended Data Fig. 4 Schematic depicting the genetic arrangement of the plasmid.
a, Plasmid layouts of the NETMAP system inducing the expression of PD-L1nb and CTLA-4nb, respectively. b, Schematic depicting the genetic arrangement of the plasmid for ΔXIVPadC4 construction employing the Tn7 transposon system. The coding sequences of PadC4 and BphO, along with their constitutive promoter Ptac, were placed into the genome of ΔXIV using the Tn7 transposon, forming ΔXIVPadC4. c, Schematic diagram of the experimental procedure for developing a stable ΔXIVPadC4 strain.
Extended Data Fig. 5 Evaluation of the anti-tumor effects of ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb in an A20 lymphoma model.
a, Schematic of the experimental procedure and schedule for evaluating the anti-tumor performance of ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb with a single intratumoral injection against A20 lymphoma in mice. A20 tumor-bearing mice were divided into four groups when the tumor volume reached ~100 mm3, and were intratumorally injected with PBS (G1), ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb Dark (5 × 106 c.f.u., G2), ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb 710 nm (5 × 106 c.f.u., G3), or recombinant PD-L1nb (450 ng) and CTLA-4nb (450 ng) proteins (G4) on Day 0. The G3 mice received 2 h of illumination (710 nm, 10 mW cm−2) every day from Day 0 to Day 9. b, Tumor growth curves of female mice with the indicated treatments. c, d, Images of isolated tumors (c) and tumor weights (d) for the indicated female mouse groups (c, n = 4 mice per group). e, Tumor growth curves of male mice with the indicated treatments. f, g, Images of isolated tumors (f) and tumor weights (g) for the indicated male mouse groups. h, Tumor inhibition rates in male and female mice on Day 9 across the indicated treatment groups. Data (b, d, e, g, h) are presented as means ± s.e.m.; n = 5 mice per group. Statistical analyses in (b, e) were performed using two-way ANOVA followed by Sidak’s test with multiple comparisons. Statistical analyses in (d, g) were performed using one-way ANOVA with a Turkey’s test. Statistical analyses in h were performed using a two-tailed unpaired t-test.
Extended Data Fig. 6 Flow cytometric quantification of immune cells for A20 lymphoma immunotherapy.
Immune cells in tumors, spleen, and lymph nodes were isolated from A20-bearing mice on the 7th day of various treatments and analyzed by flow cytometry. a, Flow cytometry quantification of NK cells (CD49b+CD69+) in the tumor. b, Flow cytometry quantification of NK cells (CD49b+CD69+) in the spleen. c, Flow cytometry quantification of NK cells (CD49b+CD69+) in lymph nodes. d, Flow cytometry quantification of M1 macrophage cells (F4/80+CD80+) in tumor. e, Flow cytometry quantification of M1 macrophage cells (F4/80+CD80+) in spleen. f, Schematic diagram of the immune responses caused by ΔXIVPadC4-NETMAP in A20-bearing mice. PD-L1nb and CTLA-4nb produced by ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb inhibit A20 tumor growth through activation of immune responses, including inducing the proliferation and activation of CD8+ T cells and NK cells, reducing Treg cells, and promoting macrophage polarization to M1 phenotype. Data (a-e) are presented as means ± s.e.m.; n = 5 mice per group. Statistical analyses were conducted using one-way ANOVA followed by a Tukey’s test with multiple comparisons. The specific FACS sorting strategies for panels a-c were provided in Supplementary Fig. 4a, and for panels d, e were provided in Supplementary Fig. 5.
Extended Data Fig. 7 Evaluation of ΔXIV biodistribution and detection of checkpoint blockade nanobodies expression in a bilateral tumor model.
a, Schematic of the experimental procedure and schedule for evaluating the biodistribution of ΔXIV in a bilateral A20 lymphoma mouse model. Mice received an intratumoral injection of ΔXIV-lux (5 × 106 c.f.u.) in the left flank tumors when the tumor volume reached ~100 mm3. b, The bioluminescence signal intensity was quantified with an in vivo imaging system on Days 0, 1, 3, 6, 9, and 12 after bacteria injection to track the colonization of ΔXIV in tumors (n = 4 mice per group). c, Bioluminescence quantification of the IVIS imaging in b. d, Schematic illustrating the experimental procedure and schedule for evaluating immune checkpoint blockade nanobodies expression via ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb in a bilateral A20 lymphoma mouse tumor model. A bilateral A20 lymphoma mouse model was established. ΔXIVPadC4-NETMAPPD-L1nb+CTLA-4nb (5 × 106 c.f.u.) were injected into the left hind flank tumor and exposed to 710 nm light for 2 h daily. Four days after illumination, tumors on both sides were collected and stained for immunofluorescence. e, Immunofluorescence staining of tumor slices. PD-L1nb-Flag tag (red) and CTLA-4nb-HA tag (green) secreted by ΔXIV-NETMAPPD-L1nb+CTLA-4nb were stained with anti-Flag and anti-HA antibodies. Nuclei were stained with DAPI (blue). Three times the experiment was repeated with similar results. Images are representative of 3 mice per group. Scale bar, 25 μm. Data (c) are presented as means ± s.e.m.; n = 4 mice per group. Statistical analyses in c were performed using a two-tailed unpaired t-test.
Extended Data Fig. 8 Evaluation of the anti-tumor effects of ΔXIVPadC4-NETMAPAzurin+ClyA in a CT26 colon tumor model.
a, Schematic of the experimental procedure and schedule for evaluating the anti-tumor performance of ΔXIVPadC4-NETMAPAzurin+ClyA against CT26 colon tumors in mice. CT26 tumor-bearing mice were divided into four groups when the tumor volume reached ~100 mm3, and were intratumorally injected with PBS (G1), ΔXIVPadC4-NETMAPAzurin+ClyA Dark (5 × 106 c.f.u., G2), ΔXIVPadC4-NETMAPAzurin+ClyA 710 nm (5 × 106 c.f.u., G3), or recombinant Azurin (650 ng) and ClyA (750 ng) proteins (G4) on Day 0. The G3 mice received 2 h of illumination (710 nm, 10 mW cm−2) every day from Day 0 to Day 9. b, Tumor growth curves of female mice with the indicated treatments. c, d, Images of isolated tumors (c) and tumor weights (d) for the indicated female mouse groups (c, n = 4 mice per group). e, Tumor growth curves of male mice with the indicated treatments. f, g, Images of isolated tumors (f) and tumor weights (g) for the indicated male mouse groups. h, Tumor inhibition rates in male and female mice on Day 12 across the indicated treatment groups. Data (b, d, e, g, h) are presented as means ± s.e.m.; n = 5 mice per group. Statistical analyses in (b, e) were performed using two-way ANOVA followed by Sidak’s test with multiple comparisons. Statistical analyses in (d, g) were performed using one-way ANOVA with a Turkey’s test. Statistical analyses in h were performed using a two-tailed unpaired t-test.
Extended Data Fig. 9 Evaluation of NETMAP-mediated anti-tumor effects in an orthotopic CT26 colon tumor mouse model.
a, Schematic illustrating the experimental procedure and schedule for assessing the colonization performance of ΔXIV-lux in the orthotopic CT26 colon tumor mouse model. Wild-type (WT) BALB/c mice and BALB/c mice bearing orthotopic CT26 tumors were intraperitoneally injected with ΔXIV-lux (100 μL, 5 × 106 c.f.u.) on Day 0. b, The bioluminescence signal intensity from ΔXIV-lux was quantified with an in vivo imaging system on Days 0, 2, 4, and 7 after bacteria injection (n = 5 mice per group). c, Bioluminescence signal quantification from ΔXIV-lux in b. d, Schematic illustrating the experimental procedure and schedule for evaluating the anti-tumor performance of ΔXIVPadC4-NETMAPAzurin+ClyA against the orthotopic CT26 colon tumor mouse model. e, Bioluminescence imaging of mice bearing orthotopic CT26 colon tumors expressing luciferase (n = 5 mice per group). f, Bioluminescence quantification of mice in panel e. g, Kaplan-Meier curves for mouse survival in the indicated mouse groups for 20 days. Data (c, f, g) are presented as means ± s.e.m., n = 5 mice per group. Statistical analyses in c, f were performed using two-way ANOVA followed by Sidak’s test with multiple comparisons. Statistical analyses in g were performed using the Log-rank (Mantel-Cox) test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10.
Supplementary Tables
Supplementary Table 1, the ribosome binding sites. Supplementary Table 2, sequence analysis of the gene deletion. Supplementary Table 3, list of strains used in this study. Supplementary Table 4, list of reagents, antibodies and cells. Supplementary Table 5, plasmids designed and used in this study. Supplementary Table 6, amino acid sequence information of NETMAP.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, L., Niu, L., Wang, Z. et al. Engineered bacteria for near-infrared light-inducible expression of cancer therapeutics. Nat Cancer 6, 612–628 (2025). https://doi.org/10.1038/s43018-025-00932-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00932-3
This article is cited by
-
The application of AI-driven and engineered intratumoral microbes in cancer therapy
Journal of Translational Medicine (2025)
-
Engineering bacteria for enhanced tumor therapy: from surface modification to synthetic genetic circuits
Journal of Hematology & Oncology (2025)
-
Probiotic-based oral vaccine mucosal delivery system enabling genetically encoded dual-antigen arrays
Nature Communications (2025)
-
Micro-nano microbial fuel cell-driven bioelectrochemical tumor therapy
Nature Communications (2025)