Abstract
Gliomas are a major cause of cancer-related deaths in adolescents and young adults (AYAs; ages 15–39 years). Different molecular alterations drive gliomas in children and adults, leading to distinct biology and clinical consequences, but the implications of pediatric- versus adult-type alterations in AYAs are unknown. Our population-based analysis of 1,456 clinically and molecularly characterized gliomas in patients aged 0–39 years addresses this gap. Pediatric-type alterations were found in 31% of AYA gliomas and conferred superior outcomes compared to adult-type alterations. AYA low-grade gliomas with specific RAS–MAPK alterations exhibited senescence, tended to arise in different locations and were associated with superior outcomes compared to gliomas in children, suggesting different cellular origins. Hemispheric IDH-mutant, BRAF p.V600E and FGFR-altered gliomas were associated with the risk of malignant transformation, having worse outcomes with increased age. These insights into gliomagenesis may provide a rationale for earlier intervention for certain tumors to disrupt the typical behavior, leading to improved outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The clinical and molecular (immunohistochemistry, ddPCR) source dataset for the AYA population is available as Supplementary Data. Survival data are not publicly available per CCO policy. Newly generated panel sequencing data and copy number analysis of AYA gliomas are available at the European Genome–phenome Archive (EGA) under study ID EGAD50000000560. Previously generated panel sequencing data of pediatric HGGs are available at the EGA under study ID EGAS50000000221 and dataset ID EGAD50000000326. The data are available under controlled access to comply with data protection regulations and can be accessed by application to the data access committee via C.H. (cynthia.hawkins@sickkids.ca). Previously published RNA and targeted DNA sequencing data for pediatric LGG are available at the EGA (EGAD00001005987) and can be accessed by application to the data access committee via C.H. (cynthia.hawkins@sickkids.ca). Additional clinical data and molecular characterization for the pediatric LGG cohort (using immunohistochemistry, fluorescence in situ hybridization, NanoString gene fusion panels, single-nucleotide polymorphism array) are available as source data in the manuscript website at https://doi.org/10.1016/j.ccell.2020.03.011 (ref. 17). Methylation data discussed in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO)79 and are accessible through GEO Series accession number GSE290136.
The publicly available PBTA raw data are available through KidsFirstPortal (https://portal.kidsfirstdrc.org/login) accession codes PBTA-CBTN and PBTA-PNOC and Cavatica (https://cavatica.sbgenomics.com/u/cavatica/openpbta) upon request to the Children’s Brain Tumor Network (CBTN), and processed summary files are accessible via GitHub at https://github.com/AlexsLemonade/OpenPBTA-analysis.
Source data are provided with this paper. All other data supporting the findings of this study are available upon request from the corresponding author.
References
Ng, S. et al. An epidemiology report for primary central nervous system tumors in adolescents and young adults: a nationwide population-based study in France, 2008–2013. Neuro Oncol. 22, 851–863 (2020).
Ostrom, Q. T. et al. American Brain Tumor Association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 18, i1–i50 (2016).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 24, v1–v95 (2022).
Mupparapu, N., Brewster, L., Ostrom, K. F. & Elshahawi, S. I. Late-stage chemoenzymatic installation of hydroxy-bearing allyl moiety on the indole ring of tryptophan-containing peptides. Chemistry 28, e202104614 (2022).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2016–2020. Neuro Oncol. 25, iv1–iv99 (2023).
Price, M. et al. CBTRUS statistical report: American Brain Tumor Association & NCI Neuro-Oncology Branch adolescent and young adult primary brain and other central nervous system tumors diagnosed in the United States in 2016–2020. Neuro Oncol. 26, iii1–iii53 (2024).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Nobre, L. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis. Oncol. 4, 561–571 (2020).
Kaley, T. et al. BRAF inhibition in BRAFV600-mutant gliomas: results from the VE-BASKET study. J. Clin. Oncol. 36, 3477–3484 (2018).
Hargrave, D. R. et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin. Cancer Res. 25, 7303–7311 (2019).
Fangusaro, J. et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 20, 1011–1022 (2019).
Mellinghoff, I. K. et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N. Engl. J. Med. 389, 589–601 (2023).
Bouffet, E. et al. Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N. Engl. J. Med. 389, 1108–1120 (2023).
Eckel-Passow, J. E. et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N. Engl. J. Med. 372, 2499–2508 (2015).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Yeo, K. K. et al. Multi-institutional study of the frequency, genomic landscape, and outcome of IDH-mutant glioma in pediatrics. Neuro Oncol. 25, 199–210 (2023).
Ryall, S. et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37, 569–583 (2020).
Yan, Y. et al. Landscape of genomic alterations in IDH wild-type glioblastoma identifies PI3K as a favorable prognostic factor. JCO Precis. Oncol. 4, 575–584 (2020).
Labussière, M. et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 83, 1200–1206 (2014).
Mohile, N. A. et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: ASCO–SNO guideline. J. Clin. Oncol. 40, 403–426 (2022).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Tesileanu, C. M. S. et al. Temozolomide and radiotherapy versus radiotherapy alone in patients with glioblastoma, IDH-wildtype: post hoc analysis of the EORTC randomized phase III CATNON trial. Clin. Cancer Res. 28, 2527–2535 (2022).
Zhang, J. et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 45, 602–612 (2013).
Jones, D. T. W. et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 68, 8673–8677 (2008).
Pfister, S. et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J. Clin. Invest. 118, 1739–1749 (2008).
Lassaletta, A. et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J. Clin. Oncol. 35, 2934–2941 (2017).
Krishnatry, R. et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer 122, 1261–1269 (2016).
Mackay, A. et al. Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY phase II randomized trial. Cancer Cell 33, 829–842 (2018).
Mistry, M. et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J. Clin. Oncol. 33, 1015–1022 (2015).
Jakacki, R. I. et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol. 18, 1442–1450 (2016).
Grill, J. et al. Phase II, open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J. Clin. Oncol. 36, 951–958 (2018).
Coccia, P. F. et al. Adolescent and young adult oncology, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 16, 66–97 (2018).
Close, A. G., Dreyzin, A., Miller, K. D., Seynnaeve, B. K. N. & Rapkin, L. B. Adolescent and young adult oncology—past, present, and future. CA Cancer J. Clin. 69, 485–496 (2019).
Roux, A. et al. High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro Oncol. 22, 1190–1202 (2020).
Buckner, J. C. et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N. Engl. J. Med. 374, 1344–1355 (2016).
van den Bent, M. J. et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366, 985–990 (2005).
Wick, W. et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 18, 1529–1537 (2016).
van den Bent, M. J. et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet 390, 1645–1653 (2017).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC–NCIC trial. Lancet Oncol. 10, 459–466 (2009).
van den Bent, M. J. et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 22, 813–823 (2021).
Wen, P. Y. et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 22, 1073–1113 (2020).
Wen, P. Y. et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 23, 53–64 (2022).
Bouffet, E. et al. Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600-mutant low-grade glioma. J. Clin. Oncol. 41, 664–674 (2023).
Listernick, R., Charrow, J., Greenwald, M. & Mets, M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J. Pediatr. 125, 63–66 (1994).
Shapiro, J. A. et al. OpenPBTA: the open Pediatric Brain Tumor Atlas. Cell Genom. 3, 100340 (2023).
Fridman, A. L. & Tainsky, M. A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 27, 5975–5987 (2008).
Buhl, J. L. et al. The senescence-associated secretory phenotype mediates oncogene-induced senescence in pediatric pilocytic astrocytoma. Clin. Cancer Res. 25, 1851–1866 (2019).
Saul, D. et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 13, 4827 (2022).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7 (2013).
Lee, D. Y., Gianino, S. M. & Gutmann, D. H. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell 22, 131–138 (2012).
Brossier, N. M., Thondapu, S., Cobb, O. M., Dahiya, S. & Gutmann, D. H. Temporal, spatial, and genetic constraints contribute to the patterning and penetrance of murine neurofibromatosis-1 optic glioma. Neuro Oncol. 23, 625–637 (2021).
Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56 (2009).
Persson, A. I. et al. Non-stem cell origin for oligodendroglioma. Cancer Cell 18, 669–682 (2010).
Capper, D. et al. DNA methylation-based classification of central nervous system tumours. Nature 555, 469–474 (2018).
Fukuoka, K. et al. Clinical impact of combined epigenetic and molecular analysis of pediatric low-grade gliomas. Neuro Oncol. 22, 1474–1483 (2020).
Lambert, S. R. et al. Differential expression and methylation of brain developmental genes define location-specific subsets of pilocytic astrocytoma. Acta Neuropathol. 126, 291–301 (2013).
Meurer, L., Ferdman, L., Belcher, B. & Camarata, T. The SIX family of transcription factors: common themes integrating developmental and cancer biology. Front. Cell Dev. Biol. 9, 707854 (2021).
Bishop, K. M., Rubenstein, J. L. R. & O’Leary, D. D. M. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J. Neurosci. 22, 7627–7638 (2002).
Huang, T.-N. & Hsueh, Y.-P. Brain-specific transcriptional regulator T-brain-1 controls brain wiring and neuronal activity in autism spectrum disorders. Front. Neurosci. 9, 406 (2015).
Sharma, M. K. et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 67, 890–900 (2007).
Das, A. et al. Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nat. Med. 28, 125–135 (2022).
Merchant, T. E., Conklin, H. M., Wu, S., Lustig, R. H. & Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 27, 3691–3697 (2009).
Villani, A. et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li–Fraumeni syndrome: a prospective observational study. Lancet Oncol. 12, 559–567 (2011).
Durno, C. et al. Survival benefit for individuals with constitutional mismatch repair deficiency undergoing surveillance. J. Clin. Oncol. 39, 2779–2790 (2021).
Miller, A. M. et al. Next-generation sequencing of cerebrospinal fluid for clinical molecular diagnostics in pediatric, adolescent and young adult brain tumor patients. Neuro Oncol. 24, 1763–1772 (2022).
Reyes-Botero, G. et al. Molecular analysis of diffuse intrinsic brainstem gliomas in adults. J. Neurooncol. 116, 405–411 (2014).
Zhao, S., Agafonov, O., Azab, A., Stokowy, T. & Hovig, E. Accuracy and efficiency of germline variant calling pipelines for human genome data. Sci. Rep. 10, 20222 (2020).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26, 589–595 (2010).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Uhrig, S. et al. Accurate and efficient detection of gene fusions from RNA sequencing data. Genome Res. 31, 448–460 (2021).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Layer, R. M., Chiang, C., Quinlan, A. R. & Hall, I. M. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 15, R84 (2014).
Kronenberg, Z. N. et al. Wham: identifying structural variants of biological consequence. PLoS Comput. Biol. 11, e1004572 (2015).
Hovestadt, V. & Zapatka, M. Conumee: enhanced copy-number variation analysis using Illumina DNA methylation arrays. R Package Version 1.9.0 bioconductor.org/packages/conumee/ (2017).
Aryee, M. J. et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369 (2014).
Wilkerson, M. D. & Hayes, D. N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics 26, 1572–1573 (2010).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Cheng, D. T. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Acknowledgements
We would like to thank the patients and their families. J.B. was supported by a Garron Family Cancer Centre Research Fellowship and the Aflac Archie Bleyer Young Investigator Award in Adolescent and Young Adult Oncology from the Children’s Oncology Group. A.L.L. was supported by the Brain Tumor Foundation of Canada Richard Motyka Research Fellowship, the SickKids Clinician Scientist Training Program and the Canadian Institutes of Health Research (CIHR) Fellowship. Funding for this project was provided by the CIHR (grant nos. 159805 (C.H.) and 480606 (C.H.)), b.r.a.i.n.child and the Canadian Cancer Society/Brain Canada Sparks grant (SPARK-21, #707089, U.T.). This study was supported by Cancer Care Ontario (CCO), and these data have the following restrictions: parts of this material are based on data and information provided by Ontario Health (CCO) and include data received by Ontario Health (CCO) from the Canadian Institute for Health Information (CIHI). The opinions, reviews, views and conclusions reported in this publication are those of the authors and do not necessarily reflect those of Ontario Health (CCO) and/or the CIHI. No endorsement by Ontario Health (CCO) and/or the CIHI is intended or should be inferred. Ontario Health is prohibited from making the data used in this research publicly accessible if they include potentially identifiable personal health information and/or personal information as defined in Ontario law, specifically the Personal Health Information Protection Act (PHIPA) and the Freedom of Information and Protection of Privacy Act (FIPPA). Due to these legal and ethical restrictions, data will not be made publicly available. However, upon request, data deidentified to a level suitable for public release may be provided. This research was supported in part by the National Cancer Institute Cancer Center Support Grant P30CA008748 to the MSKCC, the Molecular Diagnostics Service in the Department of Pathology and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
This project was conceptualized by J.B., U.T. and C.H. Clinical data were collected by J.B. Resources including patient samples were provided by C.H., D.G.M., J.K., N.L., L. Nguyen and A.G. Investigations were carried out by J.B., J.S., K.F., L. Nobre, L. Negm, J.C., M.K., M.J., M.R., R.S., A.B.L., N.M.N. and S.R. Additional data were provided by S.F.S., M.A.K., T.A.B., A.M., A.G.-L., B.K.L., A.L.L., E.B., M.D.C., S.D., J.D., P.D., P.K., M.J.L.-F., W.P.M., J.R.P., A.S., D.S.T., N.L. and G.Z. Funding acquisition was done by U.T. and C.H. Analysis, visualization and manuscript draft preparation was done by J.B., A.B.L. and S.K. All authors participated in manuscript review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Adam Green, Quinn Ostrom and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Approach to molecular testing and CONSORT diagram.
(A) The tiered approach that was taken to molecular testing of the AYA cohort. (B) A CONSORT diagram outlining the population of study.
Extended Data Fig. 2 Characteristics of Toronto 0-40 cohort.
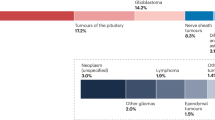
(A) Age at diagnosis divided by sex in 0-40 Toronto cohort (n = 1456 patients). P-value calculated using unpaired t-test (two-tailed). (B) Tumor location in the pediatric ( < 15 years) Toronto cohort (n = 583 patients). (C) Tumor location of IDH-WT tumors in 0-40 Toronto cohort (n = 738 patients). P-value calculated using Tukey’s multiple comparison test. (D) PFS and OS of PLGG in treated vs untreated AYAs (n = 202 patients). P-value calculated using Log-rank test. (E) 2021 WHO diagnosis of tumors in the Toronto pediatric cohort (n = 530 tumors).
Extended Data Fig. 3 Additional findings of IDH-mutant glioma in ages 0-40.
(A) Age of diagnosis in IDH-mutant astrocytoma (n = 241 patients). P-value calculated using Tukey’s multiple comparison test. (B) Age of diagnosis in IDH-mutant ODG (n = 101 patients). P-value calculated using unpaired t-test (two-tailed). (C) Age of diagnosis of IDH1 p.R132H vs non-canonical IDH mutant tumors in the Toronto cohort (n = 368 patients). P-value calculated using unpaired t-test (two tailed). (D) OS of IDH-mutant astrocytoma based on age of diagnosis in the Toronto cohort (n = 230 patients). P-value calculated using Log-rank test. (E) OS of IDH-mutant astrocytoma with based on ATRX and TP53 status (including MSKCC cohort for those with incomplete phenotype, n = 75 patients). P-value calculated using Log-rank test. (F) OS of IDH-mutant ODG based on age of diagnosis in the Toronto cohort (n = 101 patients). P-value calculated using Log-rank test. (G) OS of IDH-mutant ODG with and without co-occuring TERT promoter mutation (including MSKCC cohort for those without TERT promoter mutation, n = 80 patients). P-value calculated using Log-rank test. (H) Non-canonical IDH mutation and tumor type within the Toronto cohort (n = 48 tumors). (I) OS of IDH-mutant astrocytomas stratified by mutation type and grade (MSKCC cohort included for non-canonical IDH-mutant tumors, n = 253 patients). P-value calculated using Log-rank test. (J) OS of ODGs stratified by mutation type and grade (MSKCC cohort included for non-canonical IDH-mutant tumors, n = 111 patients). P-value calculated using Log-rank test. (K) Multivariate analysis of factors affecting OS in IDH-mutant astrocytoma. P-value calculated using Cox regression, error bars show 95% confidence intervals. (L) Multivariate analysis of factors affecting OS in ODG. P-value calculated using Cox regression, error bars show 95% confidence intervals.
Extended Data Fig. 4 Additional findings in FGFR-altered glioma in ages 0-40.
(A) Location of FGFR mutant tumors by age of diagnosis (n = 44 patients). P-value calculated using unpaired t-test (two-tailed). (B) Tumor grade (LGG/HGG) of FGFR mutant tumors by age of diagnosis (n = 45 patients). P-value calculated using ANOVA. (C) Location of FGFR fused tumors by age of diagnosis (n = 39 patients). P-value calculated using unpaired t-test (two-tailed). (D) Tumor grade (LGG/HGG) of FGFR fused tumors by age of diagnosis (n = 42 patients). P-value calculated using unpaired t-test (two-tailed).
Extended Data Fig. 5 Additional findings in BRAF-mutant glioma in ages 0-40.
(A) OS of HGG with different driver mutations (n = 97 patients). P-value calculated using Log-rank test. (B) PFS and OS of BRAF-mutant tumors in AYA Toronto cohort stratified by histology (n = 71 patients). P-value calculated using Log-rank test. (C) PFS of BRAF mutant LGG in AYA based on extent of resection (n = 57 patients). P-value calculated using Log-rank test. (D) PFS of BRAF mutant LGG based on location (n = 57 patients). P-value calculated using Log-rank test. (E) Multivariate analysis of factors influencing PFS in BRAF p.V600E mutant LGG. P-value calculated using Cox regression, error bars show 95% confidence intervals. (F) Multivariate analysis of factors influencing OS in BRAF p.V600E mutant HGG. P-value calculated using Cox regression, error bars show 95% confidence intervals.
Extended Data Fig. 6 Additional findings in BRAF-fused glioma in ages 0-40.
(A) Tumor location of BRAF fused glioma based on age of diagnosis in Toronto cohort (n = 207 patients). P-value calculated using Tukey’s multiple comparison test. (B) Age of diagnosis for KIAA1549-BRAF fusion vs non-canonical BRAF fusion partners in the Toronto cohort (n = 176 patients). P-value calculated using unpaired t-test (two-tailed). (C) Gene constructs of non-canonical BRAF fusions from AYA cohort. (D) BRAF fusion breakpoint/binding partner based on age of diagnosis in the Toronto cohort (n = 167 tumors). P-value calculated using one-way ANOVA, using Tukey’s multiple comparison test. (E) PFS and OS of BRAF fused glioma in the AYA cohort (including MSKCC cohort, n = 33 patients). P-value calculated using Log-rank test. (F) Multivariate analysis of factors influencing PFS in BRAF fused LGG. P-value calculated using Cox regression, error bars show 95% confidence intervals.
Extended Data Fig. 7 BRAF/FGFR Mutant Tumors in AYA.
(A) Bar graph showing proportion of LGG vs HGG in children vs AYA (n = 156 tumors). (B) Regression analysis showing probability of diagnosis of HGG if patient presents with BRAF or FGFR mutant tumor based on age (n = 362 tumors). (C) PFS of LGG harboring BRAF/FGFR SNV in children and AYA (n = 156 patients). P-value calculated using Log-rank test. (D) Regression analysis showing probability of progression of LGG based on age of diagnosis (n = 362 tumors).
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Supplementary Data
Clinical data for the pediatric and AYA cohorts from Toronto and the MSKCC.
Source data
Source Data Figs. 1, 3, 4 and 6 and Extended Data Figs. 2, 3 and 5
Data for OS curves, methylation data and analysis using the PBTA dataset.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bennett, J., Levine, A.B., Nobre, L. et al. A population-based analysis of the molecular landscape of glioma in adolescents and young adults reveals insights into gliomagenesis. Nat Cancer 6, 1102–1119 (2025). https://doi.org/10.1038/s43018-025-00962-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00962-x
This article is cited by
-
Central Nervous System Tumors in Adolescents and Young Adults
Current Neurology and Neuroscience Reports (2025)