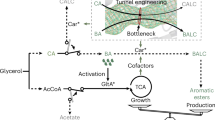
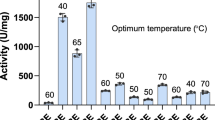
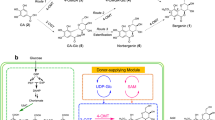
Abstract
Benzyl acetate is a valuable aromatic ester compound with diverse applications in the flavor and fragrance industries. However, its current synthesis primarily relies on inefficient plant extraction methods or chemical/enzymatic processes that depend on non-renewable substrates. Here we report a sustainable approach to benzyl acetate production from d-glucose using metabolically engineered Escherichia coli strains. We explored both benzoic acid-dependent and -independent synthetic pathways by either dividing the pathway between upstream and downstream strain pairs or by introducing the complete pathway into single, integrated strains. In an optimized two-phase extractive fermentation process, a delayed co-culture of an upstream strain that converts d-glucose to benzoic acid and a downstream strain that transforms benzoic acid into benzyl acetate yielded 2,238.3 ± 171.9 mg l−1 of benzyl acetate from d-glucose in 108 h (or 2,204.0 ± 192.2 mg l−1 in 96 h). The economic competitiveness of the microbial process for sustainable benzyl acetate production was also assessed by techno-economic analysis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All source data for figures have been deposited at figshare (https://doi.org/10.6084/m9.figshare.24026634).
Code availability
All codes used for calculating the theoretical maximum flux of benzyl acetate biosynthesis and techno-economic analysis of the microbial-based benzyl acetate production are available at https://github.com/kaistsystemsbiology/BenzylAcetateFluxAnalysis and https://github.com/kaistsystemsbiology/BenzylAcetateTEA.
References
Majumder, A. B., Singh, B., Dutta, D., Sadhukhan, S. & Gupta, M. N. Lipase catalyzed synthesis of benzyl acetate in solvent-free medium using vinyl acetate as acyl donor. Bioorg. Med. Chem. Lett. 16, 4041–4044 (2006).
Radnik, J. et al. Deactivation of Pd acetoxylation catalysts: direct observations by XPS investigations. Angew. Chem. Int. Ed. 44, 6771–6774 (2005).
Ahmed, N., Hanani, Y. A., Ansari, S. Y. & Anwar, S. in Essential Oils in Food Preservation, Flavor and Safety (ed Preedy, V. R.) 487–494 (Elsevier, 2016).
Mallavarapu, G., Gurudutt, K. & Syamasundar, K. in Essential Oils in Food Preservation, Flavor and Safety (ed Preedy, V. R.) 865–873 (Elsevier, 2016).
Knudsen, J. T., Tollsten, L. & Bergström, L. G. Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33, 253–280 (1993).
Garlapati, V. K., Kumari, A., Mahapatra, P. & Banerjee, R. Modeling, simulation, and kinetic studies of solvent-free biosynthesis of benzyl acetate. J. Chem. 2013, 451652 (2013).
Benhmid, A., Narayana, K. V., Martin, A. & Lucke, B. One-step synthesis of benzyl acetate by gas phase acetoxylation of toluene over highly active and selective Pd-Sb-TiO2 catalysts. Chem. Commun. https://doi.org/10.1039/b406396a (2004).
Komatsu, T., Inaba, K., Uezono, T., Onda, A. & Yashima, T. Nano-size particles of palladium intermetallic compounds as catalysts for oxidative acetoxylation. Appl. Catal. A 251, 315–326 (2003).
Nandiwale, K. Y., Galande, N. D., Raut, S. A. & Bokade, V. V. Benzylation of acetic acid to benzyl acetate over highly active and reusable micro/meso-HZSM-5. Chem. Eng. Res. Des. 93, 584–590 (2015).
Park, Y. C., Shaffer, C. E. & Bennett, G. N. Microbial formation of esters. Appl. Microbiol. Biotechnol. 85, 13–25 (2009).
Luo, Z. W., Cho, J. S. & Lee, S. Y. Microbial production of methyl anthranilate, a grape flavor compound. Proc. Natl Acad. Sci. USA 116, 10749–10756 (2019).
Perdomo, I. C. et al. Efficient enzymatic preparation of flavor esters in water. J. Agric. Food Chem. 67, 6517–6522 (2019).
Melo, A. D. Q. et al. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules https://doi.org/10.3390/molecules22122165 (2017).
Gómez, J. et al. Biosynthesis of benzyl acetate: optimization of experimental conditions, kinetic modelling and application of alternative methods for parameters determination. Bioresour. Technol. Rep. 11, 100519 (2020).
Lee, J. W. & Trinh, C. T. Microbial biosynthesis of lactate esters. Biotechnol. Biofuels 12, 226 (2019).
Pugh, S., McKenna, R., Halloum, I. & Nielsen, D. R. Engineering Escherichia coli for renewable benzyl alcohol production. Metab. Eng. Commun. 2, 39–45 (2015).
Luo, Z. W. & Lee, S. Y. Metabolic engineering of Escherichia coli for the production of benzoic acid from glucose. Metab. Eng. 62, 298–311 (2020).
Noda, S., Kitazono, E., Tanaka, T., Ogino, C. & Kondo, A. Benzoic acid fermentation from starch and cellulose via a plant-like beta-oxidation pathway in Streptomyces maritimus. Microb. Cell Fact. 11, 49 (2012).
Otto, M., Wynands, B., Marienhagen, J., Blank, L. M. & Wierckx, N. Benzoate synthesis from glucose or glycerol uising engineered Pseudomonas taiwanensis. Biotechnol. J. 15, e2000211 (2020).
Venkitasubramanian, P., Daniels, L., Das, S., Lamm, A. S. & Rosazza, J. P. Aldehyde oxidoreductase as a biocatalyst: reductions of vanillic acid. Enzyme Microb. Technol. 42, 130–137 (2008).
Akhtar, M. K., Turner, N. J. & Jones, P. R. Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities. Proc. Natl Acad. Sci. USA 110, 87–92 (2013).
Dudareva, N., D’Auria, J. C., Nam, K. H., Raguso, R. A. & Pichersky, E. Acetyl-CoA:benzylalcohol acetyltransferase—an enzyme involved in floral scent production in Clarkia breweri. Plant J 14, 297–304 (1998).
Venkitasubramanian, P., Daniels, L. & Rosazza, J. P. Reduction of carboxylic acids by Nocardia aldehyde oxidoreductase requires a phosphopantetheinylated enzyme. J. Biol. Chem. 282, 478–485 (2007).
Quadri, L. E. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37, 1585–1595 (1998).
Choi, K. R., Yu, H. E., Lee, H. & Lee, S. Y. Improved production of heme using metabolically engineered Escherichia coli. Biotechnol. Bioeng. 119, 3178–3193 (2022).
Choi, K. R., Yu, H. E. & Lee, S. Y. Production of zinc protoporphyrin IX by metabolically engineered Escherichia coli. Biotechnol. Bioeng. 119, 3319–3325 (2022).
Bhatia, S. P. et al. Fragrance material review on cinnamyl acetate. Food Chem. Toxicol. 45, S53–S57 (2007).
Cortes-Pena, Y., Kumar, D., Singh, V. & Guest, J. S. BioSTEAM: a fast and flexible platform for the design, simulation, and techno-economic analysis of biorefineries under uncertainty. ACS Sustain Chem. Eng. 8, 3302–3310 (2020).
Tribe, D. E. Novel microorganism and method. US patent 4,681,852 (1987).
Lee, Y. & Lee, S. Y. Enhanced production of poly (3-hydroxybutyrate) by filamentation-suppressed recombinant Escherichia coli in a defined medium. J. Environ. Polym. Degrad. 4, 131–134 (1996).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory, 2001).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Zhao, X. R., Choi, K. R. & Lee, S. Y. Metabolic engineering of Escherichia coli for secretory production of free haem. Nat. Catal. 1, 720–728 (2018).
Monk, J. M. et al. iML1515, a knowledgebase that computes Escherichia coli traits. Nat. Biotechnol. 35, 904–908 (2017).
Acknowledgements
This work was supported by the ‘Bio & Medical Technology Development Program (2021M3A9I4022740)’ of the National Research Foundation and funded by the Ministry of Science and ICT, Republic of Korea (K.R.C., Z.W.L., G.B.K., H.X. and S.Y.L.). This work was also supported by the ‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. RS-2021-RD009210)’ from the Rural Development Administration, Republic of Korea (K.R.C. and Z.W.L.).
Author information
Authors and Affiliations
Contributions
K.R.C. and S.Y.L. conceptualized the project. K.R.C., Z.W.L. and G.B.K. developed the methodology. K.R.C., Z.W.L., G.B.K. and H.X. conducted experiments. K.R.C., Z.W.L., G.B.K. and H.X. analyzed the data. K.R.C., Z.W.L. and G.B.K. performed visualization. K.R.C., Z.W.L. and G.B.K. wrote the original draft. K.R.C. and S.Y.L. reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Kristina Haslinger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Two-phase extractive fermentation to overcome benzyl acetate toxicity.
a, The growth curve of E. coli W3110 strain in shake-flask culture supplemented with 0-1.0 g L-1 of benzyl acetate. Solid symbols and error bars represent mean values and standard deviations of biological triplicates (n = 3). Smaller open symbols represent raw data points. b, Scheme of aqueous/organic two-phase extractive culture. Toxic products with high hydrophobicity and partition coefficient, such as benzyl acetate, can be effectively sequestered to droplets of organic solvents. As a result, the concentration of the toxic compounds in the aqueous phase and the cytoplasm decreases, leading to reduction of the toxic effects on microorganisms in the aqueous phase.
Extended Data Fig. 2 Non-averaged profiles of two-phase extractive fermentations.
a and b, Non-averaged profiles of the two-phase extractive fermentation for production of benzyl acetate by the co-culture of the Bn1 and Bn-BnAc3 strains. The averaged profile of these duplicated rounds of fermentations is presented in Fig. 2e. c and d, Non-averaged profiles of the two-phase extractive fermentation for production of benzyl acetate by the co-culture of the BnOH7 and BnOH-BnAc2 strains. The averaged profile of these duplicated rounds of fermentation is presented in Fig. 3d. e and f, Non-averaged profiles of the two-phase extractive fermentation for production of benzyl acetate by the BnAc30 strain. The averaged profile of these duplicated rounds of fermentations are presented in Fig. 4e.
Extended Data Fig. 3 Optimization of medium components to improve the cell density and benzyl acetate titre of the BnAc30 strain.
a, Perturbation of component levels in shake-flask culture of the BnAc30 strain supplemented with increased amount of d-glucose (20 g L-1), thiamine (Thi), l-tyrosine (Tyr), l-phenylalanine (Phe), l-aspartic acid (Asp) and yeast extract (YE) than the standard conditions. Bars and solid symbols represent mean values and error bars represent standard deviations of biological triplicates (n = 3). Smaller open symbols represent raw data points. b, Perturbation of component levels in shake-flask culture of the BnAc30 strain supplemented with 20 g L-1 of d-glucose, as well as replacement of yeast extract with l-leucine (Lue) and l-isoleucine (Ile). Bars and solid symbols represent mean values and error bars represent standard deviations of biological triplicates (n = 3). Smaller open symbols represent raw data points. c, Two-phase extractive fermentation of the BnAc30 strain with supplementation of 6 g L-1 of yeast extract and 0.1 g L-1 of l-tyrosine. Symbols represent raw data points (n = 1). d, Two-phase extractive fermentation of the BnAc30 strain with supplementation of 6 g L-1 of yeast extract and 0.2 g L-1 of l-tyrosine. Symbols represent raw data points (n = 1).
Extended Data Fig. 4 Co-culture of the auxotrophic Bn-BnAc5 strain with the auxotrophic Bn1 strain.
a, Population changes in co-culture of an auxotrophic upstream strain (strain 1) and a prototrophic downstream strain (strain 2). b, Population changes in co-culture of an auxotrophic upstream strain (strain 1) and an auxotrophic downstream strain (strain 2). c, Two-phase extractive fermentation profile for benzyl acetate production by co-culture of the auxotrophic Bn1 strain and the auxotrophic Bn-BnAc5 strain. Symbols represent raw data points (n = 1). d, Scheme of promiscuous conversion of trans-cinnamic acid into cinnamyl acetate in co-culture of an upstream strain (strain 1) and a downstream strain (strain 2) harbouring the benzoic acid-dependent pathway for benzyl acetate production.
Extended Data Fig. 5 Optimization of inoculation delay in delayed co-culture of the Bn1 and Bn-BnAc3 strains (part 1).
a, Two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (200 mL; OD600 = 8.5) was added after 36 h of fermentation. b, Apparent average biosynthesis rates of l-phenylalanine (Phe), trans-cinnamic acid (Cin), cinnamyl acetate (CinAc), benzoic acid (Bn), benzaldehyde (BnAl), benzyl alcohol (BnOH) and benzyl acetate (BnAc), calculated based on the changes in titres (panel a) and the molar masses of the compounds. c, Two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (200 mL; OD600 = 11.6) was added after 48 h of fermentation. d, Apparent average biosynthesis rates of benzyl acetate, cinnamyl acetate and intermediates between sampling points were calculated based on the changes in the titres (panel c) and the molar masses of the compounds. Symbols represent raw data points and bars represent apparent average biosynthesis rates.
Extended Data Fig. 6 Optimization of inoculation delay in delayed co-culture of the Bn1 and Bn-BnAc3 strains (part 2).
a, Two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (200 mL; OD600 = 7.8) was added after 72 h of fermentation. b, Apparent average biosynthesis rates of l-phenylalanine (Phe), trans-cinnamic acid (Cin), cinnamyl acetate (CinAc), benzoic acid (Bn), benzaldehyde (BnAl), benzyl alcohol (BnOH) and benzyl acetate (BnAc), calculated based on the changes in titres (panel a) and the molar masses of the compounds. c, Two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (200 mL; OD600 = 9.1) was added after 96 h of fermentation. d, Apparent average biosynthesis rates of benzyl acetate, cinnamyl acetate and intermediates between sampling points were calculated based on the changes in the titres (panel c) and the molar masses of the compounds. Symbols represent raw data points and bars represent apparent average biosynthesis rates.
Extended Data Fig. 7 Non-averaged profiles and average biosynthesis rates for the delayed co-culture of the Bn1 and Bn-BnAc3 strains shown in Fig. 5d.
a, Non-averaged two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (400 mL; OD600 = 13.1) was added after 48 h of fermentation. The profiles of d-glucose and organic acids are summarized in Extended Data Fig. 10a. b, Apparent average biosynthesis rates of l-phenylalanine (Phe), trans-cinnamic acid (Cin), cinnamyl acetate (CinAc), benzoic acid (Bn), benzaldehyde (BnAl), benzyl alcohol (BnOH) and benzyl acetate (BnAc), calculated based on the changes in titres (panel a) and the molar masses of the compounds. c, Non-averaged two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (400 mL; OD600 = 14.4) was added after 48 h of fermentation. The profiles of d-glucose and organic acids are summarized in Extended Data Fig. 10b. d, Apparent average biosynthesis rates of benzyl acetate, cinnamyl acetate and intermediates between sampling points were calculated based on the changes in the titres (panel c) and the molar masses of the compounds. Symbols represent raw data points and bars represent apparent average biosynthesis rates.
Extended Data Fig. 8 Two-phase extractive fermentation profile and average biosynthesis rates for the delayed co-culture of the Bn1 and Bn-BnAc3 strains with the delayed inoculum volume of 400 mL and inoculation delay of 36 h.
a, Two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (400 mL; OD600 = 13.8) was added after 36 h of fermentation. b, Apparent average biosynthesis rates of l-phenylalanine (Phe), trans-cinnamic acid (Cin), cinnamyl acetate (CinAc), benzoic acid (Bn), benzaldehyde (BnAl), benzyl alcohol (BnOH) and benzyl acetate (BnAc), calculated based on the changes in titres (panel a) and the molar masses of the compounds. Symbols represent raw data points and bars represent apparent average biosynthesis rates.
Extended Data Fig. 9 Non-averaged profiles and average biosynthesis rates for the delayed co-culture of the Bn1 and Bn-BnAc3 strains shown in Fig. 5e.
a, Non-averaged two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (400 mL; OD600 = 7.6) was added after 48 h of fermentation. The profiles of d-glucose and organic acids are summarized in Extended Data Fig. 10c. b, Apparent average biosynthesis rates of l-phenylalanine (Phe), trans-cinnamic acid (Cin), cinnamyl acetate (CinAc), benzoic acid (Bn), benzaldehyde (BnAl), benzyl alcohol (BnOH) and benzyl acetate (BnAc), calculated based on the changes in titres (panel a) and the molar masses of the compounds. c, Non-averaged two-phase extractive fermentation profile of delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1). The Bn-BnAc3 strain inoculum (400 mL; OD600 = 8.0) was added after 48 h of fermentation. The profiles of d-glucose and organic acids are summarized in Extended Data Fig. 10d. d, Apparent average biosynthesis rates of benzyl acetate, cinnamyl acetate and intermediates between sampling points were calculated based on the changes in the titres (panel c) and the molar masses of the compounds. Symbols represent raw data points and bars represent apparent average biosynthesis rates.
Extended Data Fig. 10 Non-averaged d-glucose and organic acid profiles for the delayed co-culture of the Bn1 and Bn-BnAc3 strains shown in Extended Data Figs. 7 and 9.
a, Non-averaged d-glucose and organic acid profiles during the delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1) shown in Extended Data Fig. 7a. The Bn-BnAc3 strain inoculum (400 mL; OD600 = 13.1) was added after 48 h of fermentation. b, Non-averaged d-glucose and organic acid profiles for the delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1) shown in Extended Data Fig. 7c. The Bn-BnAc3 strain inoculum (400 mL; OD600 = 14.4) was added after 48 h of fermentation. c, Non-averaged d-glucose and organic acid profiles for the delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1) shown in Extended Data Fig. 9a. The Bn-BnAc3 strain inoculum (400 mL; OD600 = 7.6) was added after 48 h of fermentation. d, Non-averaged d-glucose and organic acid profiles for the delayed co-culture of the Bn1 and Bn-BnAc3 strains (n = 1) shown in Extended Data Fig. 9c. The Bn-BnAc3 strain inoculum (400 mL; OD600 = 8.0) was added after 48 h of fermentation.
Supplementary information
Supplementary Information
Supplementary Discussions 1–13, Tables 1–4 and 11–14, and Supplementary Figs. 1 and 2.
Supplementary Tables
Supplementary Tables 5–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, K.R., Luo, Z.W., Kim, G.B. et al. A microbial process for the production of benzyl acetate. Nat Chem Eng 1, 216–228 (2024). https://doi.org/10.1038/s44286-023-00022-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-023-00022-0
This article is cited by
-
A combined pseudouridine biomanufacturing platform enabled by a streamlined designer pathway
Nature Communications (2025)
-
Comprehensive evaluation of the capacities of microbial cell factories
Nature Communications (2025)
-
Amorphous/crystalline HTiNbO5-X membranes for efficient confined flow synthesis of acetate ester flavours
Nature Communications (2025)
-
Metabolic engineering of Escherichia coli for high-level production of benzyl acetate from glucose
Microbial Cell Factories (2024)
-
A bacterial platform for producing aromatic esters from glycerol
Nature Chemical Engineering (2024)