Abstract
Lipid droplets (LDs), which are important storage structures for neutral lipids and organelles of diverse functions, participate in various cellular activities. In this study, BALB/c mice, fed a regular or a high-fat diet, were exposed to the synthetic perfluorinated compound, perfluorooctanoic acid (PFOA). PFOA-exposed mice had altered serum lipid and lipoprotein levels and hydropic degeneration or ballooning degeneration of hepatocytes. Moreover, we report for the first time that LDs accumulate in hepatic nuclei after PFOA exposure. As PFOA resembles fatty acids (FA) in its structure, this chemical may interfere with the transportation and metabolism of FA as well as LDs in the cell. This abnormal localization of LDs in the nucleus may be related to the cause of PFOA toxicity.
Similar content being viewed by others
Introduction
Lipids are essential components of biological membranes and important players in the storage of energy-rich compounds. Therefore, they participate in the intercellular communication and balance of energy1,2. Lipid droplets (LDs), which are ubiquitous storage structures for neutral lipids in various eukaryotic organisms, usually formulate in the endoplasmic reticulum and exit to the cytoplasm3. In addition to their role in metabolism and lipid storage, LDs are also involved in signal transduction, intracellular lipid trafficking and protein degradation4,5,6.
Perfluorooctanoic acid (PFOA) is a synthetic perfluorinated eight-carbon organic chemical. Industrial and regular uses of PFOA, which is considered to be a general pollutant, cause its accumulation in the environment7,8,9,10. PFOA can cause various toxicities, including hepatocyte developmental toxicity, carcinogenicity and immunotoxicity11,12. The similarity of PFOA to fatty acids (FA) suggests its effects on cellular and intercellular lipid metabolism. Recent studies on rodents and humans have confirmed that PFOA decreases serum cholesterol (CHOL) and triglyceride (TG) levels, while it increases total lipids in the liver13,14. However, the molecular mechanism of this action is still unclear. In this study, male BALB/c mice were exposed to increasing doses of PFOA for 14 days, while being fed a regular diet (RD) or a high-fat diet (HFD), serum lipid and lipoprotein levels were measured. Surprisingly, histological and ultrastructure examination of PFOA-treated livers showed that LDs accumulate in hepatocyte nuclei. Our results provide a novel insight into the biological mechanism of PFOA toxicity.
Results
Organ indexes and serum lipid levels after PFOA treatment
In a HFD there are more lipids available for lipid transportation and metabolism. Our results showed that the extra fat in the diet increased the liver index and elevated the serum CHOL and glucose, as well as the synthesis of high-density and low-density lipoprotein cholesterol (HDL-ch and LDL-ch, Table 1). However, the serum TG levels in the control groups were similar in RD and HFD groups (Table 1).
Decreased body weight was detected upon PFOA exposure compared with control mice, which was in a dose-dependent manner (Table 1). Livers and ventral fat were harvested from PFOA-exposed mice. In the HFD-fed mice the livers were significantly more swollen and the ventral fat was more atrophied than in the RD-fed mice (Shown as liver index and fat index in Table 1). PFOA had no effect on serum albumin levels, which participates in transportation of FA. Compared with the control group, RD mice exposed to PFOA had reduced levels of serum TG, CHOL and HDL-ch, which were the lowest in the 20 mg/kg/day PFOA group (P < 0.01); LDL-ch levels were only lower compared with the control when exposed to 20 mg/kg/day PFOA (P < 0.05). Serum glucose levels were reduced after PFOA exposure in both RD and HFD mice. In the control groups, hepatic glycogen in the HFD-fed mice was lower than in the RD-fed mice. Nevertheless, less glycogen content was detected in the livers of all PFOA-exposed mice, especially in the 10 and 20 mg/kg/day PFOA groups (P < 0.01).
Histological changes in livers after PFOA exposure
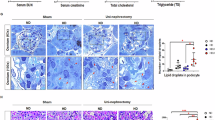
Pathological liver sections indicated that PFOA exposure caused hypertrophy of hepatic cells (Fig. 1). In RD-fed mice, 5 mg/kg/day PFOA caused no obvious change in the hepatic cells compared with the control mice (Fig. 1a, b). Hydropic degeneration with visible vacuoles in the hepatocytes was induced by 10 mg/kg/day PFOA (Fig. 1c) and ballooning degeneration occurred in 20 mg/kg/day PFOA-treated mice (Fig. 1d). Though the control HFD-fed mice showed enlarged intercellular space (Fig. 1e), undefined cell edges were observed in the 5 mg/kg/day PFOA group (Fig. 1f). The 10 and 20 mg/kg/day PFOA-treated mice displayed similar pathological changes to the RD-fed mice (Fig. 1g, h).
H & E stained liver sections from control and PFOA-exposed mice.
Liver sections were viewed under a light microscope. (a) Control mice fed a regular diet (RD); (b) RD-fed mice treated with 5 mg/kg/day PFOA; (c) RD-fed mice treated with 10 mg/kg/day PFOA; (d) RD-fed mice treated with 20 mg/kg/day PFOA; (e) Control mice fed a high-fat diet (HFD); (f) HFD-fed mice treated with 5 mg/kg/day PFOA; (g) HFD-fed mice treated with 10 mg/kg/day PFOA; (h) HFD-fed mice treated with 20 mg/kg/day PFOA. Hydropic degeneration with visible vacuoles in hepatocytes was observed in c and g; ballooning degeneration occurred in d and h. Scale bar in panel a, 200 μM.
PFOA causes LDs accumulation in the nucleus of hepatic cells
As PFOA caused changes in the serum lipid levels and in the histology of hepatocytes, we examined the pathologic ultrastructural changes in these cells (Figs. 2 and 3).
Ultrastructural changes in liver nuclei after 14 days of PFOA exposure.
(a) Control mice fed a regular diet (RD); (b) RD-fed mice treated with 5 mg/kg/day PFOA; (c) RD-fed mice treated with 10 mg/kg/day PFOA; (d) RD-fed mice treated with 20 mg/kg/day PFOA; (e) Control mice fed a high-fat diet (HFD); (f) HFD-fed mice treated with 5 mg/kg/day PFOA; (g) HFD-fed mice treated with 10 mg/kg/day PFOA; (h) HFD-fed mice treated with 20 mg/kg/day PFOA. L = lipid droplets. Scale bar in panel a, 2 μM.
Ultrastructures of liver organelles after 14 days of PFOA exposure.
(a) Control mice fed a regular diet (RD); (b) RD-fed mice treated with 5 mg/kg/day PFOA; (c) RD-fed mice treated with 10 mg/kg/day PFOA; (d) RD-fed mice treated with 20 mg/kg/day PFOA; (e) Control mice fed a high-fat diet (HFD); (f) HFD-fed mice treated with 5 mg/kg/day PFOA; (g) HFD-fed mice treated with 10 mg/kg/day PFOA; (h) HFD-fed mice treated with 20 mg/kg/day PFOA. L = lipid droplets. V = void formed by dilatation of endoplasmic reticulum; M = swollen mitochondria; C = condensed chromatin. Scale bar in panel a, 2 μM.
LDs were present in the cytoplasm of control animals (Fig. 2a, e). However, we observed that PFOA-exposed mice had LDs transferred from the cytoplasm to the nucleus, forming intranuclear inclusion in the hepatic cell. Specifically, PFOA increased the nuclear accumulation of LDs in a dose-dependent manner (Fig. 2b, c, d, f, g and h). The ratios in 0, 5, 10 and 20 mg/kg/day PFOA-treated mice were 0, 42, 67 and 78%, respectively, in RD-fed mice; while they were 5, 56, 62 and 82 in HFD-fed mice. The excessive entry into nuclei was not due to impairment of nuclear membranes in that they remained unaffected in the hepatocytes of the PFOA-treated mice (Fig. 2b, c, d, f, g and h).
RD-fed control and 5 mg/kg/day PFOA-treated mice had integral organelles in hepatic cells (Fig. 3a, b); while voids formed by dilatation of endoplasmic reticulum occurred in hepatocytes of 10 and 20 mg/kg/day PFOA-treated mice (Fig. 3c, d). In HFD-fed mice, although there were more LDs in the cytoplasm, normal endoplasm, mitochondria and uniform karyoplasm were observed in control and 5 mg/kg/day PFOA exposed hepatocytes (Fig. 3e, f). In contrast to the RD-fed mice, PFOA exposure led to mitochondrial swelling and irregular nucleus in HFD-fed mice (Fig. 3g, h). These cells also showed condensed chromatin in the nucleus, suggesting on-going apoptosis (Fig. 3g, h).
Expression of genes involved in lipid metabolism were altered in PFOA-treated mice
Peroxisome proliferator-activated receptor α (PPARα) controls the transcriptional expression of key enzymes that are involved in FA uptake and β-oxidation. While peroxisome proliferator-activated receptor γ (PPARγ) is involved in activating genes required for lipid synthesis and storage within adipocytes. Though a HFD caused fluctuating levels of PPARα in control mice, expression of this gene was not induced by PFOA (Fig. 4). No alternation could be observed in PFOA-exposed RD mice, but expression of PPARγ was stimulated in HFD individuals, which showed to be significant in 20 mg/kg/day PFOA group (Fig. 4).
Expression of PPARα and PPARγ after 14 day of PFOA exposure.
*p<0.05, **p<0.01 versus their respective controls or between the two groups indicated, using one-way ANOVA. Doses of PFOA are showed in the legends. RD = regular diet; HFD = high-fat diet. All values are represented as means ± SE (standard error); N = 4.
Discussion
LDs, the major storage structures of neutral lipids, have been recognized for their diverse components and functions4,5,15. The abundance of lipid metabolism-related enzymes in LDs indicates the important roles of LDs in storage and metabolism of TG and CHOL, as well as degradation of arachidonic acid6,16,17,18. Abnormal state of LDs may lead to disturbance in lipid metabolism and a series of effects on cellular activities.
In this study, in spite of the decreased body weight, lipid accumulation was observed in PFOA-exposed hepatic cells. A similar result has been reported in other PFOA-related studies19,20. The compelling point occurred as abnormal translocation of LDs to the hepatocyte nucleus in PFOA-treated mice. We also examined ultrastructures in thymus and spleen cells, but did not observe a similar phenomenon. As an important location for DNA storage, replication and transcription21, Nuclei invaded by LDs may lead to devastating consequences. As expected, we observed liver-associated abnormalties. Therefore, we reason that PFOA induces a specific accumulation of LDs in the nucleus of hepatic cells, which may be responsible for the PFOA-associated toxicity.
The activation of the transcription factor PPARα has been considered as a possible mechanism for PFOA-induced toxicities13,22. Here expression of PPARγ can be stimulated by 20 mg/kg/day PFOA, but PPARα expression in liver cells was not upregulated. Hence, it is unlikely that PPARα activation is responsible for the PFOA liver toxicity.
TG and CHOL in hepatocytes can be assembled into very low-density lipoproteins (VLDLs) and secreted into the circulation, or stored in the hepatic cytosol as LDs3,23. VLDLs turn into low-density lipoproteins (LDL) in the blood stream, which carries CHOL to extra-hepatic tissues; High-density lipoproteins (HDL) extracts lipids from VLDLs remnants and can carry CHOL stored in extra-hepatic tissues back to the liver21. In this study, the unaltered albumin levels in PFOA-exposed mice indicated that the transportation of exogenous lipids was normal. However, PFOA may block the export of lipids from the liver, resulting in less VLDLs secretion, leading to reduced levels of serum HDL and LDL. Adding extra fat to the diet may have balanced the serum lipids, but reduction in LDL was also detected. Though lipids were released from adipose tissue to supplement the decreasing serum TG and CHOL, LDs continued to accumulate in the hepatocytes. Therefore, the PFOA-exposed mice had intumescent livers and shrunken adipose tissues.
As PFOA resembles FA in structure, we reason that it would interfere with the transportation and metabolism of FA24,25,26. This interference may have blocked the lipids exportation from the liver and probably caused altered transportation of lipids to the nucleus. As a result, LDs accumulation in hepatocytes, especially in the nucleus, occurred in PFOA-treated mice. However, the mechanism for translocation LDs into the nucleus requires further investigation.
Methods
Reagents and animals
The experimental procedures were approved by the ethics committee of Jianghan University (Wuhan, China). Four-week-old male BALB/c mice were purchased from Hubei Research Center of Laboratory Animals (Hubei, China). The animals were fed either an RD or a HFD and were randomly divided for PFOA exposure (15 mice per group). In comparison with the RD, HFD contained 10% more lard and 3% more cholesterol. The food was purchased from Beijing HFK Bio-Technology co., LTD.
PFOA (CAS number, 335-67-1; purity, 96%, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.5% Tween 20 (Sigma-Aldrich) and was given to mice orally via gavage for 14 days at doses of 5, 10 or 20 mg/kg/day. Control mice were given 0.5% Tween 20 without PFOA.
Animal treatment
All animals were fasted overnight prior to execution. Blood samples of those animals were drawn from the orbital sinus. Serum was separated and analyzed for various biochemical parameters, such as albumin, glucose, CHOL, TG, HDL-ch and LDL-ch, by an Abbott Aeroset automated instrument analyzer (Abbott Diagnostics, Abbott Park, IL, USA). Next, the mice were sacrificed by cervical luxation. Livers and ventral fat were quickly removed and weighed. Cubical samples of liver were removed and immediately immersed in a 4% paraformaldehyde solution for histopathological observation, or in 2.5% glutaraldehyde for ultrastructure observation. The remaining liver samples were stored at −80°C for glycogen or gene analysis.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Procedures of qRT-PCR were performed as previously reported27. The forward (F) and reverse (R) primers were as follows: PPARα, F-TGAACAAAGACGGGATGC and R-GCCACAAACAGGGAAATG; PPARγ, F-TCAAGGGTGCCAGTTTCG and R-GAGGCCAGCATCGTGTAG.
Statistical analysis
Data were analyzed with SPSS 16.0 software (SPSS, Chicago, IL, USA). Results are presented as the mean ± standard error (SE) of at least four independent experiments. One-way ANOVA was used to determine the differences between the control and PFOA-treated groups. P values < 0.05 were considered statistically significant.
References
Fromm, H. J. & Hargrove, M. Essentials of biochemistry (Springer, 2012).
Vance, J. E. & Vance, D. E. Biochemistry of lipids, lipoproteins and membranes. Vol. 42 (Elsevier Science, 2008).
Guo, Y., Cordes, K. R., Farese Jr, R. V. & Walther, T. C. Lipid droplets at a glance. Journal of cell science 122, 749–752 (2009).
Bartz, R., Zehmer, J. K. & Liu, P. The new face of lipid droplets. Progress in Biochemistry and Biophysics 32, 387 (2005).
Goodman, J. M. The gregarious lipid droplet. Journal of Biological Chemistry 283, 28005–28009 (2008).
Farese, R. V. & Walther, T. C. Lipid droplets finally get a little RESPECT. Cell 139, 855–860 (2009).
Butenhoff, J. L., Olsen, G. W. & Pfahles-Hutchens, A. The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum. Environmental health perspectives 114, 1776 (2006).
Karstadt, M. L. Serum PFOA levels in residents of communities near a teflon-production facility. Environmental Health Perspectives 115, A486 (2007).
Olsen, G. W. & Zobel, L. R. Assessment of lipid, hepatic and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. International archives of occupational and environmental health 81, 231–246 (2007).
Shin, H. M., Vieira, V. M., Ryan, P. B., Steenland, K. & Bartell, S. M. Retrospective exposure estimation and predicted versus observed serum perfluorooctanoic acid concentrations for participants in the C8 Health Project. Environmental health perspectives 119, 1760 (2011).
Kudo, N. & Kawashima, Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. The Journal of Toxicological Sciences 28, 49–57 (2003).
Lau, C. et al. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicological Sciences 99, 366–394 (2007).
Kennedy, G. L. et al. The toxicology of perfluorooctanoate. Critical reviews in toxicology 34, 351–384 (2004).
Kudo, N. et al. Effects of Perfluorinated Fatty Acids with Different Carbon Chain Length on Fatty Acid Profiles of Hepatic Lipids in Mice. Biological and Pharmaceutical Bulletin 34, 856–864 (2011).
Liu, P. et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. Journal of Biological Chemistry 279, 3787–3792 (2004).
Athenstaedt, K., Zweytick, D., Jandrositz, A., Kohlwein, S. D. & Daum, G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. Journal of bacteriology 181, 6441–6448 (1999).
Zimmerman, A. & Veerkamp, J. New insights into the structure and function of fatty acid-binding proteins. Cellular and Molecular Life Sciences 59, 1096–1116 (2002).
Bartz, R. et al. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. Journal of proteome research 6, 3256–3265 (2007).
Okochi, E., Nishimaki-Mogami, T., Suzuki, K. & Takahashi, A. Perfluorooctanoic acid, a peroxisome-proliferating hypolipidemic agent, dissociates apolipoprotein B48 from lipoprotein particles and decreases secretion of very low density lipoproteins by cultured rat hepatocytes. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1437, 393–401 (1999).
Wolf, D. C. et al. Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-α knockout and wild-type mice. Toxicologic pathology 36, 632–639 (2008).
Lehninger, A. Principles of biochemistry. North, New York 361–393 (1985).
Abbott, B. D. et al. Perfluorooctanoic acid (PFOA)-induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR {alpha}). Toxicological sciences (2007).
Murphy, D. J. & Vance, J. Mechanisms of lipid-body formation. Trends in biochemical sciences 24, 109–115 (1999).
Luebker, D. J., Hansen, K. J., Bass, N. M., Butenhoff, J. L. & Seacat, A. M. Interactions of flurochemicals with rat liver fatty acid-binding protein. Toxicology 176, 175–185 (2002).
Wu, L. L., Gao, H. W., Gao, N. Y., Chen, F. F. & Chen, L. Interaction of perfluorooctanoic acid with human serum albumin. BMC structural biology 9, 31 (2009).
Han, X., Snow, T. A., Kemper, R. A. & Jepson, G. W. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chemical research in toxicology 16, 775–781 (2003).
Wang, Y. et al. Modulation of dietary fat on the toxicological effects in thymus and spleen in BALB/c mice exposed to perfluorooctane sulfonate. Toxicology letters 204, 174–182 (2011).
Acknowledgements
The authors wish to thank the Major State Basic Research Development Program of China (973 Program; 2009CB421605), the National Natural Science Foundation of China (grant numbers 21277062, 21107128) and the Program for New Century Excellent Talents in University (NCET-11-0964) for providing funding for this study.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the idea and designed the research with L.W. L.W., Y.W., Y.L. and J.L. carried out the experiment. L.W. analyzed the data and wrote the manuscript. Y.C.L. and J.Z. supplied theoretical background for the explanation. All the authors contributed to discussion of the results. J.J.F. and A.Q.Z. revised the manuscript. G.B.J. supervised the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, L., Wang, Y., Liang, Y. et al. Specific Accumulation of Lipid Droplets in Hepatocyte Nuclei of PFOA-exposed BALB/c Mice. Sci Rep 3, 2174 (2013). https://doi.org/10.1038/srep02174
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02174
This article is cited by
-
Association between perfluoroalkyl substances exposure and the prevalence of nonalcoholic fatty liver disease in the different sexes: a study from the National Health and Nutrition Examination Survey 2005–2018
Environmental Science and Pollution Research (2023)
-
Mechanisms of Environmental Contributions to Fatty Liver Disease
Current Environmental Health Reports (2019)
-
Bacterial lipid droplets bind to DNA via an intermediary protein that enhances survival under stress
Nature Communications (2017)
-
The lipid droplet: A conserved cellular organelle
Protein & Cell (2017)
-
Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach
Scientific Reports (2016)