Abstract
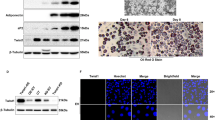
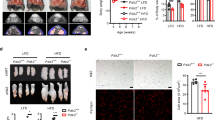
The preadipocyte-to-adipocyte differentiation (adipogenesis) is a key process in fat mass increase and thus it is regarded as a compelling target for preventing or treating obesity. Of adipogenic hormone receptors, peroxisome proliferator-activated receptor gamma (PPARγ) has crucial roles in adipogenesis and lipid accumulation within adipocytes. Here we demonstrate that the NEDD8 (neuronal precursor cell expressed, developmentally downregulated 8)-based post-translation modification (neddylation) of PPARγ is essential for adipogenesis. During adipogenesis, NEDD8 is robustly induced in preadipocytes and conjugates with PPARγ, leading to PPARγ stabilization. When the neddylation process was blocked by NEDD8-targeting siRNAs (or viral vectors) or an inhibitor MLN4924, adipocyte differentiation and fat tissue development were substantially impaired. We also demonstrate that MLN4924 effectively prevents the high-fat diet-induced obesity and glucose intolerance in mice. This study provides a better understanding of how the PPARγ signaling pathway starts and lasts during adipogenesis and a potential anti-obesity strategy that targets the neddylation of PPARγ.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- APPBP1:
-
amyloid beta precursor protein-binding protein 1
- CD36:
-
cluster of differentiation 36
- C/EBP:
-
CCAAT/enhancer-binding protein
- ChIP:
-
chromatin immunoprecipitation
- CT:
-
computed tomography
- DAPI:
-
4′,6-diamidino-2-phenylindole
- FABP4:
-
fatty acid-binding protein 4
- FASN:
-
fatty acid synthase
- H-ADSCs:
-
human adipose tissue-derived mesenchymal stem cells
- HFD:
-
high-fat diet
- LDL:
-
low-density lipoproteins
- MDM2:
-
murine double minute 2
- NCD:
-
normal control diet
- NEDD8:
-
neuronal precursor cell expressed developmentally downregulated 8
- Neddylation:
-
NEDD8-based post-translation modification
- PPARγ:
-
peroxisome proliferator-activated receptor gamma
- PPREs:
-
peroxisome proliferator response elements
- SENP8:
-
sentrin-specific protease 8
- TG:
-
triglycerides
References
Kopelman PG . Obesity as a medical problem. Nature 2000; 404: 635–643.
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O et al. Dynamics of fat cell turnover in humans. Nature 2008; 453: 783–787.
Camp HS, Ren D, Leff T . Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med 2002; 9: 442–447.
Cao Z, Umek RM, McKnight SL . Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 1991; 5: 1538–1552.
Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J et al. Propagation of adipogenic signals though an epigenomic transition state. Genes Dev 2010; 24: 1035–1044.
Yeh WC, Cao Z, Classon M, McKnight SL . Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 1991; 9: 168–181.
Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM . mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 1994; 8: 1224–1234.
Tontonoz P, Hu E, Spiegelman BM . Stimulation of adipogenesis in fibroblasts by PPARγ, a lipid-activated transcription factor. Cell 1994; 79: 1147–1156.
Osaka F, Kawasaki H, Aida N, Saeki M, Chiba T, Kawashima S et al. A new NEDD8-ligating system for cullin-4A. Genes Dev 1998; 12: 2263–2268.
Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD . The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci USA 2000; 97: 11371–11376.
Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 1999; 3: 151–158.
Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM . Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem 2000; 24: 18527–18533.
Berberich SJ, Litteral V, Mayo LD, Tabesh D, Morris D . Mdm-2 gene amplification in 3T3-L1 preadipocytes. Differentiation 1999; 64: 205–212.
Hallenborg P, Feddersen S, Francoz S, Murano I, Sundekilde U, Petersen RK et al. Mdm2 controls CREB-dependent transactivation and initiation of adipocyte differentiation. Cell Death Differ 2012; 19: 1381–1389.
Wade M, Li YC, Wahl GM . MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer 2013; 13: 83–96.
Tang QQ, Otto TC, Lane MD . Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 2003; 100: 44–49.
Rabut G, Peter M . Function and regulation of protein neddylation. 'Protein modifications: beyond the usual suspects' review series. EMBO Rep 2008; 9: 969–976.
Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009; 458: 732–736.
Knittle JL, Timmers K, Ginsberg-Fellner E, Brown RE, Katz DP . The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell-number and size. J Clin Invest 1979; 63: 239–246.
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012; 150: 620–632.
Camp HS, Tafuri SR . Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem 1997; 272: 10811–10816.
Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 2010; 466: 451–456.
Ohshima T, Koga H, Shimotohno K . Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem 2004; 279: 29551–29557.
Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005; 437: 759–763.
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 2013; 19: 557–566.
Tontonoz P, Hu E, Spiegelman BM . Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev 1995; 5: 571–576.
Tontonoz P, Hu E, Spiegelman BM . Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994; 79: 1147–1156.
Tontonoz P, Spiegelman BM . Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 2008; 77: 289–312.
Kilroy GE, Zhang X, Floyd ZE . PPAR-gamma AF-2 domain functions as a component of an ubiquitin-dependent degradation signal. Obesity 2009; 17: 665–673.
Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature 2008; 456: 350–356.
Ryu JH, Li SH, Park HS, Park JW, Lee B, Chun YS . Hypoxia-inducible factor α subunit stabilization by NEDD8 conjugation is reactive oxygen species-dependent. J Biol Chem 2011; 28: 6963–6970.
Noh EH, Hwang HS, Hwang HS, Min B, Im E, Chung KC . Covalent NEDD8 conjugation increases RCAN1 protein stability and potentiates its inhibitory action on calcineurin. PLoS One 2012; 7: e48315.
Embade N, Fernández-Ramos D, Varela-Rey M, Beraza N, Sini M, Gutiérrez de Juan V et al. Murine double minute 2 regulates Hu antigen R stability in human liver and colon cancer through NEDDylation. Hepatology 2012; 55: 1237–1248.
Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R et al. A synthetic antagonist for the peroxisome proliferator-activated receptor-g inhibits adipocyte differentiation. J Biol Chem 2000; 3: 1873–1877.
Rieusset J, Touri F, Michalik L, Escher P, Desvergne B, Niesor E et al. A new selective peroxisome proliferator-activated receptor-g antagonist with antiobesity and antidiabetic acitivity. Mol Endocrinol 2002; 11: 2628–2644.
Smith MA, Maris JM, Gorlick R, Kolb EA, Lock R, Carol H et al. Initial testing of the investigational NEDD8-activating enzyme inhibitor MLN4924 by the pediatric preclinical testing program. Pediatr Blood Cancer 2012; 2: 246–253.
Swords RT, Erba HP, DeAngelo DJ, Bixby DL, Altman JK, Maris M et al. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol 2015; 169: 534–543.
Hainer V, Hainerová IA . Do we need anti-obesity drugs? Diabetes Metab Res Rev 2012; 28: 8–20.
Lee HW, Nam SK, Choi WJ, Kim HO, Jeong LS . Stereoselective synthesis of MLN4924, an inhibitor of NEDD8-activating enzyme. J Org Chem 2011; 76: 3557–3561.
Chung SS, Ahn BY, Kim M, Kho JH, Jung HS, Park KS . SUMO modification selectively regulates transcriptional activity of peroxisome-proliferator-activated receptor γ in C2C12 myotubes. Biochem J 2011; 433: 155–161.
Ryu JH, Li SH, Park HS, Park JW, Lee B, Chun YS . Hypoxia-inducible factor α subunit stabilization by NEDD8 conjugation is reactive oxygen species-dependent. J Biol Chem 2011; 286: 6963–6970.
Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD . Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci USA 1997; 94: 4300–4305.
Jaffray EG, Hay RT . Detection of modification by ubiquitin-like proteins. Methods 2006; 38: 35–38.
Dixon AK . Abdominal fat assessed by computed tomography: sex difference in distribution. Clin Radiol 1983; 34: 189–191.
Acknowledgements
This work was supported by a Korean Health Technology R&D grant (A121106, A120476, HI15C2695), National Research Foundation grants of Korea government (2012R1A5A2A44671346, 2009-0090188). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
YSC designed experiments. HSP, UIJ, JYS, DHS and SWK performed experiments and interpreted results. JWP, JYC, LSJ, SYK, JY, HWL and YSC provided critical materials. HSP, JWP, JBK, KSP and YSC interpreted results and analyzed statistics. HSP, UIJ, JWP and YSC wrote the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Bazan
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Park, HS., Ju, UI., Park, JW. et al. PPARγ neddylation essential for adipogenesis is a potential target for treating obesity. Cell Death Differ 23, 1296–1311 (2016). https://doi.org/10.1038/cdd.2016.6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.6
This article is cited by
-
Neddylation of insulin receptor substrate acts as a bona fide regulator of insulin signaling and its implications for cancer cell migration
Cancer Gene Therapy (2024)
-
Latexin deficiency attenuates adipocyte differentiation and protects mice against obesity and metabolic disorders induced by high-fat diet
Cell Death & Disease (2022)
-
IP6-assisted CSN-COP1 competition regulates a CRL4-ETV5 proteolytic checkpoint to safeguard glucose-induced insulin secretion
Nature Communications (2021)
-
Neddylation of sterol regulatory element-binding protein 1c is a potential therapeutic target for nonalcoholic fatty liver treatment
Cell Death & Disease (2020)
-
The E3 ligase C-CBL inhibits cancer cell migration by neddylating the proto-oncogene c-Src
Oncogene (2018)