Abstract
Advanced colorectal cancer (CRC) consensus molecular subtype 4 (CMS4) or CRC with a low immunoscore is associated with shorter survival times. Non-metastatic CRC with microsatellite instability (MSI) is associated with a lower risk of recurrence. We evaluated outcome (lymph node metastases [LNM] or cancer recurrence) in these tumor subtypes in patients with surgically-removed non-pedunculated T1 CRC by performing a multicenter case-cohort study. We included all patients in 13 hospitals in the Netherlands from 2000–2014 (n = 651). We randomly selected a subgroup of patients (n = 223) and all patients with LNM or recurrence (n = 63), and median follow-up of 44 months. We centrally reviewed tumor-slides, and constructed and immunostained tissue microarrays determining MSI, CMS (MSI/CMS1, CMS2/3, or CMS4), and immunoscore (I-low/I-high). We used weighted Cox proportional hazard models to evaluate the association of MSI, CMS, and immunoscore with LNM or recurrence, adjusting for conventional histologic risk factors. In the randomly selected subgroup of patients, 7.1% of tumors were MSI/CMS1, 91.0% CMS2/3, 1.8% CMS4, and 25% I-low. In the case-cohort, patients with CMS4 tumors had an increased risk for LNM or recurrence compared with patients with tumors of other CMSs (adjusted hazard ratio [HR], 3.97; 95% CI, 1.12–14.06; P = 0.03). Albeit not significant, tumors with MSI had a lower risk for LNM or recurrence than other tumor subtypes (adjusted HR, 0.52; 95% CI, 0.12–2.30; P = 0.39), whereas tumors with a low immunoscore had an increased risk for LNM or recurrence (adjusted HR, 1.30; 95% CI, 0.68–2.48; P = 0.43). In conclusion, in a case-cohort study of patients with non-pedunculated T1 CRC, MSI, and immunoscore were not significantly associated with adverse outcome after surgery. CMS4 substantially increased the risk of adverse outcome. However, CMS4 is rare in T1 CRCs, limiting its value for determining the risk in patients.
Similar content being viewed by others
Introduction
The prognosis and therapeutic decision making in patients with colorectal cancer (CRC) rely on the TNM classification, concerning tumor invasion and metastasis [1]. Recently, two new promising prognostic molecular-based classification systems for CRC have been developed [2]. First, an international consortium has evaluated genome-wide gene-expression data to classify patients into four consensus molecular subtypes (CMS) [3]. CMS1, 13% of CRCs, represents immune-active tumors with high-mutational load, Microsatellite instability (MSI) or BRAF-mutations, and has been recognized as a favorable prognostic subtype in early-stage CRC [4]. CMS2, the canonical type and comprising 37% of CRCs, is characterized by activated WNT- and MYC-pathways, high somatic copy-number alterations, and increased EGFR expression with a mutated TP53 gene. CMS3, the metabolic type and comprising 13% of CRCs, is characterized by low somatic copy-number alterations, KRAS mutations, and elevated metabolic signatures. CMS4, representing 23% of CRC, is characterized by tumors with epithelial-mesenchymal transition, TGF-beta pathway, and stromal activation. CMS4 is identified as a subtype with a significantly worse relapse-free and overall survival of 62 and 60%, respectively [3]. Furthermore, stage III tumors with CMS4 have no benefit from adjuvant therapy such as FOLFOX and they also seem resistant to anti-EGFR therapy [5, 6]. A residual group of 13% represents unclassified patients or patients with a mixed subtype [3, 7].
Second, the immunoscore, focusing on infiltrating lymphocytes rather than the tumor cells was developed [8]. A low density of CD3 and CD8 T-cells infiltrating the tumor’s center (TC) and invasive margin (IM), i.e., a low immunoscore, has been associated with a worse prognosis in patients with CRC [9, 10]. However, these promising risk stratification scores have been developed and validated in patients with mostly advanced tumor stages (T2–T4) or lymph node or distant metastasis (stage III or IV), whereas their prevalence and relevance in T1 CRC are unknown [11]. Risk stratification in T1 CRC (both pedunculated and non-pedunculated) is currently based on conventional histologic markers, such as poor differentiation, lymphovascular invasion, deep submucosal invasion, and tumor budding. The therapeutic decision to perform additional oncologic surgery is based on these markers, but due to low specificity, this leads to unnecessary surgery for patients classified as high-risk but without actual lymph node metastasis (LNM) being present (false positives) [12,13,14]. If the newer classification systems, CMS and immunoscore, are proven useful in patients with T1 CRC, they could enable organ preservation in selected patients.
In this large multicenter cohort study of patients with non-pedunculated T1 CRC, we aimed to evaluate the frequency distribution of Microsatellite (MS)-status, CMS, and immunoscore. Furthermore, we assessed the association with currently used histologic markers for T1 CRC. Finally, we evaluated whether these molecular- and immune-based scores could be of clinical value in the selection of high-risk patients, based on their prediction of an adverse outcome (LNM or recurrence).
Materials and methods
Study design and source population
A total of 13 Dutch hospitals (1 academic, 12 non-academic) participated in this multicenter T1 CRC registration cohort study of the Dutch T1 CRC Working Group. This cohort consists of all patients diagnosed with T1 CRC between 2000 and 2014, as identified by the Netherlands Cancer Registry. All medical records were reviewed, and exclusion criteria as defined previously were applied (i.e., hereditary predisposition for CRC, inflammatory bowel disease, synchronous CRC, non-CRC related death within 1 year, non-adenocarcinoma, neo-adjuvant radiotherapy, and missing reports). The definition for morphology was based on the endoscopist’s judgment prior to resection, as presumed morphology can be altered by resection techniques or fixation procedures. Tumors were considered pedunculated when the presence of a stalk was reported or when a tumor was classified as Paris 0-1p. T1 CRCs with pedunculated or unknown morphology were excluded, as they harbor lower risks for LNM and recurrence compared with non-pedunculated T1 CRCs [15]. The remaining group of T1 CRCs was classified as non-pedunculated and consisted of sessile or flat tumors. We selected all patients with known lymph node status after surgical resection, resulting in a cohort of 651 patients with non-pedunculated T1 CRC. Patients were either treated with an oncologic surgical resection without a prior attempt of a curative endoscopic resection (primary surgical resection, n = 471) or surgery after an endoscopic resection had been performed (secondary surgical resection, n = 180). All included cases had negative resection margins (R0).
This study was approved by the Medical Ethics Review Committee of the University Medical Center Utrecht (approval for data-collection, reference number: 15-487; approval for histologic assessment, reference number 15-716), and performed in accordance with the Helsinki Declaration.
Case-cohort approach
We adopted a case-cohort approach in which we selected all patients with an endpoint of interest (LNM and/or recurrence) during follow-up and a random 50% subgroup from the full cohort at baseline (Fig. 1). This approach has all the benefits of a regular cohort approach for prognostic factor analysis but is more efficient as fewer samples need assessment for tumor markers. Due to the low number of events for each individual outcome, we chose a composite endpoint ‘adverse outcome’, defined as the presence of LNM in the resection specimen and/or recurrent cancer during follow-up. Both LNM and recurrence are relevant outcomes to identify those patients who will benefit from a surgical resection. Distant recurrence was defined as metastasis to extracolonic organs confirmed with imaging or histology. Local recurrence was defined as malignant tissue at the site of the anastomosis. In the subgroup, we assessed the distribution of MS-status, CMS, and immunoscore, and their association with conventional histologic risk factors. The case-cohort set was used to evaluate the prognostic value of these molecular and immune-based classifications. The follow-up period started at the time of diagnosis and finished at 5 years, date of recurrence, death, or last date of follow-up. Adverse outcomes (LNM and/or recurrent cancer) were treated as events with LNM at baseline being analyzed as an event at 0.01 months.
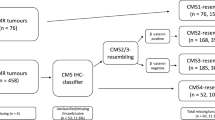
The subgroup consisted of a random subsample (50%) from the full cohort. The case-cohort set consisted of the random subgroup and any additional cases from the full cohort that met the composite endpoint (lymph node metastasis or recurrent cancer). Five patients with LNM also developed recurrent cancer. CRC colorectal carcinoma, IM invasive margin, TC tumor center, LNM lymph node metastasis.
Pathology review
The hematoxylin-eosin (H&E) slides and formalin-fixed paraffin-embedded (FFPE) tumor tissue blocks of the case-cohort set were collected. Two expert pathologists (ML & GJO) both blinded for the clinical characteristics, patient outcome, and the original histologic report reviewed the H&E slides to confirm the diagnosis of T1 CRC. Patients were excluded if T1 CRC (defined as invasion through the muscularis mucosae and into, but not beyond, the submucosa) could not be confirmed [1]. Next, the H&E slides were reviewed for the conventional histologic risk factors (i.e., differentiation grade, lymphovascular invasion, invasion depth, and tumor budding) by one pathologist (ML). The grade of tumor differentiation was assessed according to the World Health Organization classification of tumors [16]. Deep invasion was defined as invasion depth ≥1000 µm or Kikuchi level SM2-3. If the muscularis mucosae could be identified, submucosal depth was measured with the muscularis mucosae used as baseline. When the muscularis mucosae could not be identified, submucosal depth was measured from the surface of the tumor at the site of invasion, as described previously [17]. Lymphovascular invasion was defined as the presence of cancer cells within endothelial-lined channels [16]. Tumor budding was measured, defined and scored using the standardized, evidence-based method for tumor budding assessment as described previously: a cancer cell nest consisting of 1 or <5 cells that infiltrates the interstitium at the invasive margin of the tumor; after selecting one field where budding is the most intensive, the number of buds is counted in a field measuring 0.785 mm². Depending on the number of buds, the grade of budding is defined as grade 1 (0–4 buds), grade 2 (5–9 buds), or grade 3 (>10 buds). Grade 1 is considered low-grade tumor budding, whereas grades 2 and 3 are considered high-grade tumor budding [18].
Tissue microarray (TMA)
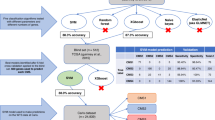
For each tumor specimen, H&E slides containing tumor center (TC) and invasive margin (IM) regions were selected (Fig. 2a). For each region, three cores (diameter: 0.6 mm) were punched out and set into a recipient paraffin block using an automated tissue microarrayer (TMA Grand Master 3D Histech, Sysmex) (Fig. 2b). In order to minimize batch effects, patients with and without events were mixed on the TMA-blocks.
a For each tumor specimen, H&E stained slides containing tumor center (TC) and invasive margin (IM) regions were selected by two expert pathologists. b For each region, three cores (diameter, 0.6mm) were punched out and were set into a recipient paraffin block using an automated tissue microarrayer (TMA Grand Master 3D Histech, Sysmex). c TMA core stained with antibodies against CD3 (tumor in blue; CD3 in brown). d The density (number of positive cells/surface area analyzed) of lymphocytes within the tumor center and invasive margin was quantified with QuPath image analysis. e The immunoscore was based on the enumeration of two lymphocyte populations (CD3 and CD8). The consensus-molecular-subtype was based on a panel of five IHC stains (CDX2, FRMD6, HTR2B, ZEB1, KER) together with microsatellite status. #Formula for immunoscore in non-pedunculated T1 CRCs: (((log(CD3 IM+6.35)−6.5185)/0.9117)+((log(CD3 TC+19.325)−6.4082)/0.8030) + ((log(CD8 IM+0.3333)−4.4885)/1.4498) + ((log(CD8 TC+0.5)−4.1358)/1.4636))/4. If the result is≤-0.64175 patient is I-Low. If the result is >-0.64175 patient is I-High. CD3 IM = CD3 density at invasive margin; CD3 TC = CD3 density at tumor center; CD8 IM = CD8 density at invasive margin; CD8 TC = CD8 density at tumor center.
Immunohistochemistry (IHC)
Tumors were stratified in different consensus-molecular subtypes with the use of an IHC-based classifier as developed and validated by Trinh et al. [19]. TMA-blocks were cut into 4-µm-thick sections. CMS1 patients were first grouped using MS-status, identified with antibodies against MLH1 (1:10; Pharmingen, G168-15), MSH2 (RTU; Cellmarque, G219-1129), MSH6 (1:200; Abcam; ERP3945) and PMS2 (1:25; Dako, EP51). To further distinguish CMS2/CMS3 from CMS4 patients, a panel of five IHC stains was used with antibodies against: CDX2 (1:200; Immunologic; EPR2764Y), FRMD6 (1:500; Sigma; HPA001297), HTR2B (1:75; Sigma; HPA012867), ZEB1 (1:500; Sigma; HPA027524), and Pancytokeratin (1:500; Thermo Scientific; AE1/AE3). Tumors were stratified in different immune-subtypes by IHC with antibodies against CD3 (1:100; Dako) and CD8 (1:100, Dako; CD8/144B) as proposed by Galon et al. [8]. MLH1, MSH2, MSH6, PMS2, CDX2, Pancytokeratin, CD3, and CD8 were stained with an automated slide staining system (Ventana Bench Mark Ultra, Roche). FRMD6, HTR2B, and ZEB1 were stained manually as described previously [19].
Determining MSI, CMS, and Immunoscore by immunohistochemistry
Immunostained TMA slides were scanned at ×40 magnifications (NanoZoomer-XR, Hamamatsu), (Fig. 2c–e). CMS1 patients were first grouped using MS-status, as determined by two pathologists (LB and GJO) as presence or loss of expression of (one of) the mismatch repair proteins MLH1, MSH2, MSH6 and PMS2. The other five CMS-stainings were assessed by digital image analysis. We distinguished CMS2/3 from CMS4 tumors using an automated image analysis approach, whereby digital images of TMA cores were segmented and quantitated as previously described [19]. A random forest probability score was calculated for each core and cores with a score >60% were classified as mesenchymal. At least half the cores were required to be mesenchymal to consider a tumor CMS4 (a detailed description can be found in the Supplementary Methods). We distinguished immune-subtypes by determining the density (number of positive cells/surface area analyzed) of CD3- and CD8-expressing lymphocytes within each of the tissue compartments (TC and IM). This was done with QuPath image analysis (Fig. 2d) [20]. To define the immunoscore per patient we followed the steps as described in the paper by Pagès et al. [9], but, because no cut-off values are provided in this paper we used our own subgroup of non-pedunculated T1 CRCs to provide a reproducible method for establishing the immunoscore in non-pedunculated T1 CRCs. Using the random subgroup, we first established the mean density per tumor region for each individual patient, yielding four separate values per patient (mean density of CD3 IM, CD3 TC, CD8 IM, and CD8 TC). To allow meaningful normalization in a future step, the mean CD3 and CD8 densities of each region were first natural log-transformed to account for the right-skewedness of the data across patients (after adding half of the lowest value for each mean density to allow for zero values). These natural log mean densities were then transformed to a standard normal distribution (mean 0, standard deviation 1). As a final step, the four values of each patient were averaged, and this single score was used to identify the cut-off values for the 25th and 70th percentile, which were used to categorize patients into immunoscore groups. Lastly, we applied the data normalization steps as learned in the subgroup to categorize cases outside the subgroup into two groups (≤25th percentile: Low; >25th percentile: High). The provided formula (Fig. 2) may be used for classifying non-pedunculated T1 CRCs, and the aforementioned method can be used to establish cut-off values in different cohorts. In our Supplementary materials, we also divided patients into three groups (≤25th percentile: Low; 25th–70th percentile: Intermediate; ≥70th percentile: High). In addition, in our Supplementary materials, we used the classification as described by Galon et al., in which the immunoscore was determined to range from I0 when low densities of both cell types were found in both the TC and IM, to I4 when high densities were found in both regions [10]. With this method patients were then further categorized as immunoscore Low (I0-I1) or High (I2-I4) (Table S1) [8].
Statistical analysis
Baseline characteristics were analyzed using standard descriptive statistics. We used the subgroup to evaluate the distribution of MS-status and CMS in patients with T1 CRC. We evaluated the distribution of CMS per tumor location (left colon vs. right colon vs. rectum) and per immuno-subtype (I-low vs. I-high). Next, we evaluated the association between these molecular- and immune-based scores and the conventional histologic factors (i.e., differentiation grade, lymphovascular invasion, invasion depth, and tumor budding) with Fisher’s exact test. Per histologic factor, patients with unknown status for this factor were excluded from the analysis.
We used a Cox proportional hazard approach weighted by Prentice’s method to account for the case-cohort sampling to evaluate whether MS-status (MSI vs. MSS), CMS (CMS4 vs. CMS1/2/3) and immuno-subtypes (I-low vs. I-high) were associated with adverse outcome (LNM and recurrent cancer) [21]. Multivariable analysis was performed with adjustment for conventional histologic risk factors. Results are reported as hazard ratios (HRs) and 95%-CIs. We also calculated HRs for the immunoscore as a continuous variable (the averaged and normalized natural log-transformed mean densities of each patient), assuming linearity as the number of events was too limited to meaningfully evaluate non-linearity (Supplementary materials).
GraphPad Prism version 8.01 (GraphPad Software Inc., San Diego, CA, USA) was used to draw figures. IBM SPSS Statistics version 25 (SPSS Inc., Chicago, IL, USA) and R version 3.5.1 were used for the selection of the 50% subgroup and statistical analysis respectively. The random subgroup was drawn using a random number generator, without oversampling or matching based on any clinicopathological variables. It can, therefore, be considered as an accurate representation of the patient mix of the full cohort. A two-sided p value < 0.05 was considered significant for all tests.
Results
Study population
We identified 651 patients with non-pedunculated T1 CRC treated with surgery within the participating hospitals, with a median follow-up time of 44 months (Interquartile range (IQR) 17–78). The baseline characteristics of the full cohort and the random subgroup were comparable (Table 1). After the exclusion of ineligible patients or tumor material, a final subgroup of 223 patients and a final case-cohort of 261 patients remained eligible for analysis (Fig. 1).
We used the case-cohort to evaluate whether MS-status, CMS, and immunoscore were associated with adverse outcome. The case-cohort consisted of 198 non-cases (without an event) and 63 cases, 46 patients with LNM (10.0% of the full cohort), and 22 patients with recurrent cancer (4.8% of the full cohort). Five of these patients with LNM also developed recurrent cancer. Of 22 patients with recurrent cancer, nine patients had local, eight patients had distant and five patients had both local and distant recurrence.
Distribution of molecular subtypes and Immunoscore
We evaluated the distribution of MS-status, CMS, and immunoscore in the subgroup. Among 223 patients, 7.2% were identified as CMS1 according to their MSI status, 91.0% of patients as CMS2/3, and 1.8% as CMS4 (Table 2). CMS1 tumors were significantly more often located in the right-sided colon (81.3% right-sided vs. 18.8% left-sided, p < 0.001).
Based on our subgroup we identified cut-off values for the immunoscore in non-pedunculated T1 CRC classifying 53 patients as I-low (25%) and 159 patients as I-high (75%) (Table 2). No significant association was observed between CMS and immunoscore, although all four patients in the CMS4 group were classified as I-High.
Association with conventional histologic risk factors
The associations between MS-status, the CMS, and the immunoscore and conventional histologic risk factors (i.e., poor differentiation, lymphovascular invasion, deep submucosal invasion, and high-grade tumor budding) are shown in Table 2. Tumors with high-grade tumor budding were classified as I-low more often than tumors with low-grade tumor budding (37.7% vs. 20.1%, p = 0.02). CMS4 tumors did not show high-grade tumor budding more often than other CMS groups (p = 0.82). No correlation was found between conventional histologic risk factors and tumors with CMS4 at the invasive margin (Supplementary Table S2).
Prognostic value of MS-status, CMS classification and the Immunoscore
At 5 years, 86.4% and 94.1% of MSS (CMS2/3/4) and MSI (CMS1) patients were event-free. There was a reduced (non-significant) risk for an adverse outcome in MSI vs. MSS tumors (HR 0.42, 95%CI 0.10–1.81, p = 0.25) (Fig. 3a). Adjusted for conventional histologic risk factors (i.e., differentiation grade, invasion depth, lymphovascular invasion, and tumor budding) these results did not change substantially (adjusted HR 0.52, 95%CI 0.12–2.30, p = 0.39).
5-year event-free proportion curves for adverse outcome based on the case-cohort dataset, reweighted to full cohort proportion by taking the random subgroup sampling fraction into account for (a) MSI (CMS1) vs. MSS (CMS2/3/4) tumors (b) CMS4 vs CMS1/2/3 tumors (c) I-low (≤25th percentile) vs. I-high (>25th percentile). Multivariable HR adjusted for conventional histologic risk factors (differentiation grade, lymphovascular invasion, submucosal invasion depth, and tumor budding). HR Hazard Ratio as calculated with Prentice’s method; MSI microsatellite instable, MSS microsatellite stable, CMS Consensus Molecular Subtype, I-low Immunoscore Low, I-high Immunoscore High.
The 5-year event-free percentage was 87.5% and 66.7% for CMS1/2/3 and CMS4 respectively. Compared with CMS1/2/3, CMS4 was associated with an increased risk for an adverse outcome (HR 3.56, 95%CI 1.02–12.4, p = 0.046) (Fig. 3b). Adjusted for conventional risk factors, CMS4 remained associated with an increased risk for an adverse outcome (adjusted HR 3.97, 95%CI 1.12–14.06, p = 0.03).
The 5-year event-free percentage was 81.5% and 88.5% for I-Low and I-High, respectively. A low immunoscore suggests a (non-significant) increased risk for an adverse outcome (HR 1.58, 95%CI 0.88-2.83, p = 0.13) (Fig. 3c). This risk was attenuated by differences in conventional risk factors between patients with a high and low immunoscore (adjusted HR 1.30, 95%CI 0.68–2.48, p = 0.43).
Alternative methods for determining the immunoscore did not result in an improved risk stratification compared with the method described in our “Methods” section, although immunoscore as a continuous variable did show an increased risk for lower scores (HR 0.67, 95%CI 0.63–0.96, with coefficient: −0.40, p = 0.03; adjusted HR 0.78 (0.53–1.13), with coefficient −0.26, p = 0.18) (Supplementary Table S3 and Fig. S1A and B).
All conventional risk factors (poor differentiation grade, lymphovascular invasion, deep submucosal invasion, and high-grade tumor budding), showed an increased risk for adverse outcome, but it reached statistical significance only for high-grade tumor budding (HR 2.51, 95%CI 1.45–4.36, p = 0.001) and lymphovascular invasion (HR 1.72, 95%CI 1.01–2.93, p = 0.046). Adjusted for the other conventional risk factors and new features (MSI, CMS, and immunoscore), high-grade tumor budding remained associated with a statistically significant increased risk (adjusted HR 1.98, 95%CI 1.05–3.76, p = 0.04), while for lymphovascular invasion the risk became less (adjusted HR 1.66, 95%CI 0.90–3.05, p = 0.10) (Table S4).
Because local recurrence may be the result of the surgical procedure itself and might not reflect aggressive behavior of the tumor, we calculated the adjusted HRs excluding patients with local recurrence only (n = 9) and these HRs were comparable or even stronger than the original ones: MSI vs. MSS 0.63 (95%CI 0.14–2.80); CMS4 vs. CMS1/2/3 4.36 (95%CI 1.21–15.6); I-low vs. I-high 1.42 (95%CI 0.72–2.79).
Discussion
In this large multicenter study, we are the first to evaluate the frequency distribution and the prognostic value of the MS-status, CMS-classification, and the immunoscore in patients with non-pedunculated T1 CRC.
In our study, MSI showed a decreased risk and a low immunoscore an increased risk for adverse outcome, although both risks did not reach statistical significance. CMS4 is uncommon in T1 CRC, but if present, it substantially increases the risk for LNM and recurrent cancer.
Contrary to previous studies evaluating CMS in advanced CRC, the proportion of CMS4 tumors was much smaller in our study (23% vs. 1.8%) [3]. This percentage is more in line with the one seen in adenomas (0–2%) [22, 23]. We can only speculate about the biology underlying this observation. It might be that between the adenoma stage and advanced carcinoma stage there is some point in time at which the CMS of the invasive cells changes from CMS1/2/3 to CMS4. Another possibility is that tumors classified as CMS4 grow faster and thus it is less likely to find them at an early stage because there might be only a short interval during which a CMS4 tumor resides in T-stage 1. In that case the tumor stage would reflect tumor biology rather than the occurrence of conversion of a subtype. A third explanation could be that a reduced amount of desmoplastic cancer stroma surrounding early invasive cancer cells compared with advanced tumors accounts for fewer T1 CRCs classified as CMS4, because, besides the tumor cells, the stroma also attributes considerably to the classification of this subtype [24, 25]. These hypotheses are all in line with the CMS landmark paper that concluded that CMS4 tended to be present more often in advanced stages of diagnosis [3]. In any case, the rarity of CMS4 in T1 CRC should be taken into account when CMS is applied because this low incidence makes its value less in clinical decision-making.
Although a low immunoscore was not statistically significantly associated with adverse outcome in our cohort, the hazard ratio pointed towards an increased risk. This concurs with the results of a recent meta-analysis in which I-low was associated with poor prognosis in advanced tumors [11]. Importantly, we used cut-off values based on just T1 tumors because lymphocyte counts can vary between tumor stages [26]. Therefore, the method we used provides a reproducible and precise manner of determining immunoscore in non-pedunculated T1 CRCs. Because I-low was associated with high-grade tumor budding, a strong predictor of LNM in T1 CRC, the risk for I-low patients became less in our multivariable analysis [27].
An important finding of this study is that the mesenchymal group (CMS4) had a significant risk for adverse outcome compared with non-CMS4 patients. This finding is in line with CMS4 patients in studies with advanced CRC, in which this subgroup also had a worse prognosis compared with non-CMS4 patients [3]. Our IHC-classifier for CMS used five IHC-markers to distinguish CMS4 from CMS2/3 and several studies have also related mesenchymal-like markers with LNM and poor survival. Sugai et al. demonstrated that the expression of mesenchymal-like markers was correlated with LNM in T1 CRC. ZEB1, used in their study, was also used in our CMS-classifier, but other biomarkers such as Twist1 and α-SMA were not [28]. Also, by assessing five separate mesenchymal markers in stage II/III CRC patients, Roseweir et al. showed that patients at risk for micrometastases and poor survival could be adequately identified [29]. As such, mesenchymal markers may contribute to risk stratification in CRC, however, due to the low prevalence of CMS4 in non-pedunculated T1 CRCs, it seems unlikely that this prognostic marker will contribute considerably to clinical decision-making in these patients.
This study has some limitations. First, for efficiency reasons we used a case-cohort design instead of evaluating the full-cohort, limiting the number of patients within subgroups for evaluation of the frequency distribution of the different classifiers. Thus, the number of patients classified as CMS1 or CMS4 was low. Nevertheless, we believe that our cohort is representative for T1 CRC in a Western population, including all T1 CRC patients from a tertiary and multiple secondary hospitals between 2000 and 2014. Only 11 patients in our cohort were detected by the nation wide screening program for CRC initiated in The Netherlands in 2014. CRCs found through screening might have different tumor biology and behavior [30]. Because of the small proportion of screen-detected patients in our cohort (4.2%), we could not evaluate whether the detection method may influence the distribution of MSI, CMS or immunoscore, nor whether the same association with poor outcome as we observed in an almost exclusively non-screen detected cohort is present in screen-detected T1 CRCs. Second, we used TMAs rather than whole tumor slides, risking sampling error. Nonetheless, earlier studies have shown that TMA analysis is reliable for determining MS-status, CD8 counts, and our used CMS-classifier [19, 31,32,33]. Furthermore, by analyzing cores from both the TC and IM, we tried to reduce sampling bias in heterogeneous tumors, a phenomenon observed by Ubink et al. when comparing multi-region endoscopic biopsies from CRC resection specimens [34]. Spatial variation in stromal composition throughout the tumor could influence subtype stratification and multi-region testing for CMS4 is needed, which could be problematic in T1 CRC when tissue may be scarce. Third, although we were able to distinguish CMS1 (MSI) and CMS4 (mesenchymal)-type tumors, the classifier does not discriminate between CMS2 and CMS3. On the other hand, CMS1 and CMS4, are the two groups that discriminate best regarding prognosis and are therefore the most important subtypes to distinguish [3, 19]. The use of this validated IHC-based classifier, makes it more readily available in the pathology department and clinical practice as transcription-based methods are yet to be widely implemented. Other methods for CMS-subtyping, such as RNA-analysis are expensive, time-consuming, and not routinely performed in clinical practice compared with IHC-analysis [4, 19, 35]. Furthermore, not all of these analyses can be easily performed on FFPE, still the golden standard for processing tissue in pathology departments, especially when tumor material is scarce as in T1 CRC tumors [36]. Lastly, even though our cohort is one of the largest T1 CRC series, and despite using a large sampling fraction of 50% of the total cohort, this revised cohort still has limited power to detect weak to intermediate prognostic factors such as I-low or MSI; for this much larger cohorts would be needed. Combining large T1 CRC cohorts in future studies might clarify whether such effects exists in T1 CRC.
In conclusion, this is the first study performed in a large cohort of patients with non-pedunculated T1 CRC focusing on promising prognostic markers, namely MS-status, CMS, and immunoscore based on immunohistochemical analysis. Albeit non-significant, our data showed that MSI is associated with a decreased and I-low with an increased risk for adverse outcome. CMS4 is uncommon in non-pedunculated T1 CRC, but if present, it increased the risk for LNM and recurrence of cancer significantly in our study. These results should be taken into account when molecular- and immune-based scores are being implemented in clinical practice for CRC.
Notes
Author names in bold designate shared co-first authorship
References
Author names in bold designate shared co-first authorship
Sobin LH, Gospodarowicz MWC, Wittekind Ch (eds.). TNM classification of malignant tumors. UICC International Union Against Cancer. Hoboken, NJ: Wiley-Blackwell, 2009.
Müller M, Ibrahim A, Arends M. New molecular classification of colorectal cancer. Virchows Arch. 2016;469:125–34.
Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6.
Battaglin F, Naseem M, Lenz H-J, Salem ME. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol. 2018;16:735–47.
Roepman P, Schlicker A, Tabernero J, Majewski I, Tian S, Moreno V, et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int J Cancer. 2014;134:552–62.
De Sousa E Melo F, Wang X, Jansen M, Fessler E, Trinh A, De Rooij LPMH, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–8.
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92.
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumors. J Pathol. 2014;232:199–209.
Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–39.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–5.
Sun G, Dong X, Tang X, Qu H, Zhang H, Zhao E. The prognostic value of immunoscore in patients with colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2019;8:182–9.
Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: A pooled-data analysis. Dis Colon Rectum. 2005;48:1588–96.
Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–9.
Bosch SL, Teerenstra S, de Wilt JHW, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827–34.
Kessels K, Backes Y, Elias SG, van den Blink A, Offerhaus GJA, van Bergeijk JD, et al. Pedunculated morphology of T1 colorectal tumors associates with reduced risk of adverse outcome. Clin Gastroenterol Hepatol. 2019;17:1112–20.
Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. college of american pathologists consensus statement 1999. Arch Pathol Lab Med. 2000;124:979–94.
Kitajima K, Fujimori T, Fuji S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–43.
Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299–311.
Trinh A, Trumpi K, De Sousa E, Melo F, Wang X, De Jong JH, Fessler E, et al. Practical and robust identification of molecular subtypes in colorectal cancer by immunohistochemistry. Clin Cancer Res. 2017;23:387–98.
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:1–7.
Onland-Moret NC, van der A DL, van der Schouw YT, Buschers W, Elias SG, van Gils CH. et al. Analysis of case-cohort data: a comparison of different methods. J Clin Epidemiol. 2007;60:350–5.
Komor MA, Bosch LJW, Bounova G, Bolijn AS, Delis-van Diemen PM, Rausch C, et al. Consensus molecular subtype classification of colorectal adenomas. J Pathol. 2018;246:266–76.
Chang K, Willis JA, Reumers J, Taggart MW, San Lucas FA, Thirumurthi S, et al. Colorectal premalignancy is associated with consensus molecular subtypes 1 and 2. Ann Oncol. 2018;29:2061–7.
Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47:312–9.
Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320–9.
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95.
Cappellesso R, Luchini C, Veronese N, Lo Mele M, Rosa-Rizzotto E, Guido E, et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol. 2017;65:62–70.
Sugai T, Uesugi N, Kitada Y, Yamada N, Osakabe M, Eizuka M, et al. Analysis of the expression of cancer-associated fibroblast- and EMT-related proteins in submucosal invasive colorectal cancer. J Cancer. 2018;9:2702–12.
Roseweir AK, Kong CY, Park JH, Bennett L, Powell AGMT, Quinn J, et al. A novel tumor-based epithelial-to-mesenchymal transition score that associates with prognosis and metastasis in patients with Stage II/III colorectal cancer. Int J Cancer. 2019;144:150–9.
Mengual-Ballester M, Pellicer-Franco E, Valero-Navarro G, Soria-Aledo V, Garcia-Marin JA, Aguayo-Albasini JL. Population-based screening improves histopathological prognostic factors in colorectal cancer. Int J Colorectal Dis. 2018;33:23–28.
Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51.
Ziai J, Gilbert HN, Foreman O, Eastham-Anderson J, Chu F, Huseni M, et al. CD8+ T cell infiltration in breast and colon cancer: a histologic and statistical analysis. PLOS ONE. 2018;13:e0190158.
Hendriks Y, Franken P, Dierssen JW, De Leeuw W, Wijnen J, Dreef E, et al. Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol. 2003;162:469–77.
Ubink I, Elias SG, Moelans CB, Laclé MM, Van Grevenstein WMU, Van Diest PJ, et al. A novel diagnostic tool for selecting patients with mesenchymal-type colon cancer reveals intratumor subtype heterogeneity. J Natl Cancer I. 2017;109:1–8.
Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in Phenotyping Colorectal Tumors. J Clin Oncol. 2002;20:1043–8.
Farragher SM, Tanney A, Kennedy RD, Paul Harkin D. RNA expression analysis from formalin fixed paraffin embedded tissues. Histochem Cell Biol. 2008;130:435–45.
Acknowledgements
The authors would like to thank the pathology departments of the participating hospitals for their cooperation in providing the H&E stained slides and tumor blocks, and D. Castigliego and N. Stathanikos of the Department of Pathology for the excellent technical assistance. The authors would also like to thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice.
Funding
This investigator-initiated study was supported by a grant from the Dutch Digestive Diseases Foundation (reference MG/2015-040). This study was performed and written independently of the funder.
Author information
Authors and Affiliations
Consortia
Contributions
Study concept and design: YB, KH, SE, GJO, ML, LM; acquisition of data: all authors; analysis and interpretation of data: all authors; drafting of the manuscript: KH, YB; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: KH, YB, SE, ML, LM; histologic review: GJA, ML; study supervision: KH, YB, LM, SE, ML.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Haasnoot, K.J.C., Backes, Y., Moons, L.M.G. et al. Associations of non-pedunculated T1 colorectal adenocarcinoma outcome with consensus molecular subtypes, immunoscore, and microsatellite status: a multicenter case-cohort study. Mod Pathol 33, 2626–2636 (2020). https://doi.org/10.1038/s41379-020-0598-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41379-020-0598-9
This article is cited by
-
Development of a prognostic risk model for colorectal cancer based on microsatellite stability-associated genes
BMC Cancer (2025)
-
Survival machine learning model of T1 colorectal postoperative recurrence after endoscopic resection and surgical operation: a retrospective cohort study
BMC Cancer (2025)
-
The prognostic value of immunoscore in the early-onset colorectal cancer
BMC Gastroenterology (2025)
-
Risk assessment of lymph node metastasis and comparison of treatment modalities for low-risk T1b colorectal cancer: a meta-analysis
Surgical Endoscopy (2025)
-
Utility of quantitative pathologic analysis of pT1 colorectal carcinomas to improve prediction of lymph node metastasis
Virchows Archiv (2025)