Abstract
Current first-line antidepressants, such as selective serotonin reuptake inhibitors (SSRI), often present a delayed onset of action and fail to effectively treat a large proportion of patients, leaving a gap in the treatment of mood disorders. Psychedelics have recently emerged as promising alternatives due to their ability to produce fast-acting antidepressant effects through neuroplastic adaptations, but their hallucinogenic properties remain a major obstacle to their widespread therapeutic use. In this study, we characterized a novel class of halogenated DMT derivatives—5-F-DMT, 5-Cl-DMT, and 5-Br-DMT—for their pharmacological activity, behavioral effects, and therapeutic potential. Using a combination of in vitro assays, in silico modeling, and in vivo behavioral and gene expression studies, we found that halogen substitution at the 5-position modulates receptor affinity and selectivity across key serotonin (5-HT) receptors (5-HT1A/2 A/2B/2CR) and transporter (SERT). Notably, 5-Br-DMT was found to activate 5-HT2AR but did not induce the head twitch response (HTR) in mice, suggesting non-hallucinogenic activity. Furthermore, 5-Br-DMT upregulated immediate early genes (IEGs) associated with neuroplasticity in the mouse prefrontal cortex and hippocampus (Arc, Egr-1, -2 and -3) and promoted dendritic growth in cortical neurons. In a mouse model of stress-induced depression, a single administration (10 mg/kg, i.p.) of 5-Br-DMT resulted in a significant reduction in depressive-like behavior, reflecting rapid antidepressant effects. Collectively, our results highlight 5-Br-DMT as a non-hallucinogenic psychoplastogen with antidepressant properties, supporting its potential as a prototypical candidate for further study. Moreover, the evaluation and biological characterization of the halogenated DMT derivatives offers valuable information on structure-activity relationships that may guide the design of future therapeutic compounds.
Similar content being viewed by others
Introduction
First-line antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), may fail to provide relief to a substantial proportion of patients, and despite numerous clinical trials, their effectiveness is not well established [1]. Moreover, these medications present a delayed onset of action—often requiring several weeks before therapeutic effects are visible [2]—which prolongs the patient’s suffering, increasing the risk of suicide and affecting treatment adherence [3]. In this context, psychedelics have emerged as potential alternatives due to their fast-acting properties as psychoplastogens—substances that promote neuronal plasticity [4]. Tryptamines, a class of psychedelics characterized by an indole ring core, an ethyl side chain and a terminal amino group [5], act primarily at the serotonin (5-HT) 1 A, 2 A and 2 C receptors (5-HT1A/2 A/2CR) and transporter (SERT), with these interactions being intricately related to their hallucinogenic effects [6, 7]. While historically used in traditional-ritual indigenous practices due to their profound impact on consciousness [8], contemporary interest in tryptamines has grown due to their potential therapeutic applications [9].
Even though the role of the 5-HT receptors in the hallucinogenic response is incompletely understood, it is largely known that 5-HT2AR activation induces the head twitch response (HTR) in mice [6, 7, 10], which has been associated with the subjective psychedelic experience in humans [11]. Yet, the underlying mechanism driving these responses is still unclear [12, 13]. One proposed mechanism points to the βarr2 and Gq pathways within the 5-HT2AR signaling cascade, in which the preference for one pathway over the other (biased agonism) could potentially explain the diverse hallucinogenic effects exerted by psychedelics [14, 15]. Furthermore, recent studies demonstrated various interactions with other 5-HT receptors that also modulate the hallucinogenic response. In fact, 5-HT1AR plays an opposite role in the 5-HT2AR-induced HTR, blocking the hallucinogenic effects [16, 17], and might also be involved in structural plasticity in the brain [18, 19]. 5-HT2CR may also have an inhibitory effect in the HTR [20], although some discrepancies exist depending on the conditions of the experiments [7, 21, 22]. On the other hand, 5-HT2BR is primarily expressed in peripheral tissues, such as the heart and blood vessels, and its activation has been associated with adverse effects on cardiac function [23].
Although the binding to and activation of the 5-HT2AR is thought to be involved in the antidepressant effects of psychedelics, the specific ways in which these compounds initiate distinct downstream signaling pathways and subsequently induce changes at the neuronal and brain levels remain ambiguous [24]. While the influence of 5-HT2AR-mediated hallucinogenic effects on therapeutic outcomes remains an open question [25, 26], the significance of structural plasticity has long been recognized as a key indicator for the ensuing antidepressant effects [27, 28]. In fact, serotonergic psychedelics have been shown to promote expression of immediate early genes (IEGs) (e.g. Arc, Egr-1, Egr-2 and Egr-3) and brain-derived neurotrophic factor (Bdnf), which are widely used as molecular markers for neuroplastic adaptations [29, 30].
Studies have shown that dimethyltryptamine (DMT)—a naturally occurring psychedelic compound found in plants such as Psychotria viridis [31]—and some analogs can induce rapid and sustained antidepressant effects after a single administration. For instance, DMT was reported to induce structural and functional plasticity (i.e., dendritic growth) in cortical neurons after 24 h [4], as well as fast antidepressant and anxiolytic effects in rodent models [32]. Further, 5-MeO-DMT has been recently studied in clinical trials using a vaporized formulation for patients with treatment-resistant depression, providing favorable tolerability and potent and rapid antidepressant effects [33, 34]. In contrast to other DMT derivatives, the halogenated counterparts have been poorly investigated [35]. Incorporation of a halogen group to an active molecule is a strategy often used in rational drug design in order to increase membrane permeability, decrease metabolic degradation and fill space in the binding pocket of target receptors, thereby improving potency and target selectivity [35, 36]. Notably, 5-F-DMT, 5-Cl-DMT and 5-Br-DMT are novel molecules [35, 37] that might offer new avenues for research into the pharmacology and therapeutic potential of psychedelics. Moreover, although several tryptamine compounds have shown promising therapeutic potential, there are still safety concerns with their medical use due to the perceptual disruptions (e.g. hallucinations, sensory distortions…) they may induce. Thus, the discovery of non-hallucinogenic derivatives capable of inducing neuroplasticity could lead to a new approach in treating mental health conditions. In this sense, 5-Br-DMT has been reported to induce no HTR in mice [38], which renders it a promising candidate for further research.
Therefore, the aim of this study was to investigate this novel class of halogenated-DMT derivatives by (i) characterizing the pharmacological profile at 5-HT1A/2 A/2B/2CR and SERT, using a combination of in vitro assays and computational approaches; (ii) evaluating the HTR response in mice, as well as the thermoregulatory and locomotor effects; (iv) studying the changes in gene expression of plasticity-related IEGs in vivo and dendritic growth in vitro, and finally (v) exploring the antidepressant potential of the most promising DMT analog with reduced hallucinogenic-like effects.
Materials and methods
Drugs and materials
DMT, 5-F-DMT, 5-Cl-DMT and 5-Br-DMT were synthesized in the form of hydrochloride salts (Supplementary Material, Scheme S1), identified and characterized as described in the Supplementary Material. Reagents used for the assays, buffers and solutions are detailed in the Supplementary Material.
Subjects
Male and/or female Swiss CD-1 mice (Janvier, Le Genest, France) weighing 30–35 g (6–8 weeks old) were randomly assigned (block randomization) to an experimental group. Researchers were blinded to the group allocation, the outcome assessment and the data analysis, following the ARRIVE guidelines [39]. Housing conditions are detailed in Supplementary Material. All experimental procedures were approved by the Animal Ethics Committee of the University of Barcelona according to the guidelines of the European Community Council (2010/63/EU). The sample size was conducted using GPower software. The minimal significance was set at 0.05 and statistical power at 0.8.
HEK293 and CHO/K1 cell culture and membrane preparation
The generation and maintenance of Human Embryonic Kidney (HEK) 293 cells stably expressing the yellow-fluorescent protein (YFP)-tagged version of the human isoform of SERT (hSERT) (Perkin Elmer, Waltham, MA, USA) was carried out as previously described [40]. Cell culture for Chinese Hamster Ovarian (CHO) K1 cells expressing the human isoforms of 5-HT1A/2 A/2B/2 C receptors (h5-HT1A/2 A/2B/2CR) was carried out following the manufacturer’s protocol (Perkin Elmer, Waltham, MA, USA). Routine DAPI-Staining assays tested negative in mycoplasma contamination. Membrane preparation procedures were performed as detailed in Supplementary Material.
Mouse primary mixed cortical cell culture
Primary cortical cultures of astrocytes and neurons were prepared from cortical tissue of C57BL/6 mouse embryos at embryonic day 17 (E17). Cells were plated at a density of 250.000 cells/mL on poly-D-lysine (PDL)-coated 24 well plates. The details of the method are described in Supplementary Material.
Radioligand binding assays
Radioligand binding assays using membrane suspensions from CHO/K1 or HEK293 transfected cells were performed as previously described [41, 42], with details shown in Supplementary Material. All determinations were conducted in duplicate for a minimum of three independent experiments.
[3H]5-HT uptake inhibition assays
The [3H]5-HT uptake inhibition assay with HEK293 cells overexpressing hSERT was performed according to previous experiments [43]. The details of the procedure are shown in Supplementary Material. Each assay was performed per triplicate for a minimum of three independent experiments.
Calcium mobilization assays
5-HT2AR functional assays were conducted with CHO/K1 cells stably expressing h5-HT2AR. The Invitrogen™ Fluo-4 NW Calcium Assay Kit (Thermo Fisher, Waltham, MA, USA) was used for carrying out the experiments, as described in the manufacturer’s protocol. The details of the method are described in Supplementary Material. Each determination was conducted in triplicate for a minimum of three independent experiments.
cAMP accumulation inhibition assays
LANCE Ultra cAMP assay was conducted with CHO/K1 cells stably expressing h5-HT1AR, following the manufacturer’s guidelines (Perkin Elmer, Waltham, MA, USA), with details shown in Supplementary Material. Assays were conducted in triplicate for at least three independent experiments.
NanoBiT® recruitment assay by means of transient transfection
Two functional complementary assays, measuring β-arrestin 2 (βarr2) and miniGαq recruitment, were carried out using the NanoBiT® system, as previously described [44, 45]. HEK293T cells were transfected with the receptor construct (5-HT2AR fused to the LgBiT component of the NanoBiT® system) and either βarr2 or miniGαq (both fused to SmBiT), as described previously [44, 46, 47]. The details of the assay are shown in Supplementary Material. Assays were performed in duplicate for at least three independent experiments.
Computational studies
Molecular docking was performed using AutoDock Bias [48] with 5-HT1AR (PDB: 8FY8), 5-HT2AR (PDB: 7WC5), and 5-HT2CR (PDB: 6BQH), guided by key conserved interactions (Supplementary Material, Figures S1 and S2). The top poses were refined using Amber (Sander, GAFF force field) [49], and non-covalent interactions were analyzed with IGMPlot [50]. Visualizations were generated using VMD and PoseView [51]. The detailed methods are described in Supplementary Material.
In vivo gene expression
Swiss CD-1 male mice (N = 6/group) received an i.p. injection of saline solution or 10 mg/kg of DMT, 5-F-DMT, 5-Cl-DMT or 5-Br-DMT. Thirty min or 120 min after injection, mice were euthanized by cervical dislocation and the prefrontal cortex (PFC), hippocampus (HIP) or medial prefrontal cortex (mPFC) were dissected out as described [52] and stored at −80 °C until use. Further procedures for assessment of the changes in gene expression are detailed in Supplementary Material. Each sample was tested in duplicate. N = 6 samples/group.
Immunocytochemistry
Mouse primary mixed cortical cells of astrocytes and neurons were treated with 5-Br-DMT for 24 h at day 4 in vitro (DIV 4). Afterwards, an immunocytochemistry against MAP-2 was performed to measure the resulting morphological changes in the dendritic arbor at DIV7 using Sholl analysis. Details of the method are described in Supplementary Material. All imaging and analyses were conducted by a blinded experimenter to treatment conditions. N = 3 independent experiment (40 neurons per cell culture).
Behavioral experiments
Head twitch response
HTR experiments were performed as previously described [53]. Swiss CD-1 male mice (N = 10/group) were administered with saline or different doses of DMT, 5-F-DMT, 5-Cl-DMT or 5-Br-DMT (0.3, 1, 3, 10, 30, 60 mg/kg, i.p.). In a different batch of animals (N = 7/group), mice were pretreated with 5-Br-DMT (10 mg/kg, i.p.) and one minute after injection, they were also administered 5-F-DMT (1 mg/kg i.p.). Then, mice were placed into an observation arena (25x25x40 cm) and recorded for the next 10 min, corresponding to the time period HTR typically occurs, based on pilot data. Video recordings were analyzed by two blinded observers who assessed the number of head twitch events, defined as a quick rotational jerk of the head, which is not contiguous with any grooming or scratching behavior [54].
Core body temperature measurements
Rectal temperature was measured in Swiss CD-1 male mice (N = 10/group) 60 min after i.p. administration of saline solution or test compound at the same doses used in the HTR experiments – this time point corresponded to the peak hypothermic response, based on pilot experiments. A thermocouple probe (YS451, Panlab, Barcelona, Spain) connected to a digital thermometer (TMP812RS, Panlab, Barcelona, Spain) was inserted 2 cm into the rectum and steady temperature readout was obtained after 10 s of insertion, as described in a previous study [53].
Horizontal locomotor activity (HLA)
The same animals used for the HTR experiments (N = 10/group) were administered with saline or different doses of test compound (0.3, 1, 3, 10, 30, 60 mg/kg, i.p.), placed into an observation arena (25x25x40 cm) and video-recorded for 30 min, as previously described [53] – a time window selected since animals typically become sedentary after that period. Video recordings were analyzed using a tracking software (Smart 3.0 Panlab, Barcelona, Spain) to measure the total travelled distance.
Tail suspension test (TST)
Swiss CD-1 male (N = 10/group) and female (N = 10/group) mice were suspended by their tails using adhesive tape for 6 min per day over 4 consecutive days to induce depression-like behavior through repeated exposure to stress (adapted from Ruan and colleagues [55]). Twenty-four hours before the test, mice received an i.p. injection of saline solution or 5-Br-DMT (10 mg/kg). Sessions were recorded, and two blinded observers quantified the immobility time (last 4 min of each session) on the first day (baseline) and test day. Decreased immobility time was used as a measure of reduced behavioral despair, reflecting an antidepressant-like effect [56].
Data analysis
Data were expressed as means ± SEM. All data were analyzed using GraphPad Prism 10 (San Diego, CA, USA), with the detailed methods shown in Supplementary Material. For behavioral studies, data were fitted by non-linear regression to calculate ED50 and Emax values with the corresponding 95% CI. One-way ANOVA with Dunnett’s post-hoc test was used to compare all conditions to saline in dose-response and in vivo gene expression experiments when data were normally distributed. Otherwise, a non-parametric test (Kruskal-Wallis with Dunn’s post-hoc test) was used. Student’s t-tests (two-sided) and mixed-effects analysis with Bonferroni’s post-hoc test were used in the TST experiments. Student’s t-tests (two-sided) were used for Sholl analysis. Alpha was set at 0.05. Outliers were excluded following ROUT’s method (Q = 0.1%). Bias factors (βi) were calculated using the “intrinsic relative activity” (RAi) approach [45, 57, 58], in which βi equals to 0 for the reference agonist; >0 if the compound shows more relative tendency towards βarr2 recruitment; or <0 if it tends towards miniGαq recruitment relative to the reference.
Results
Halogen substituents in DMT derivatives modify 5-HT2AR binding selectivity without affecting 5-HT2AR biased agonism
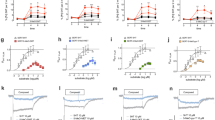
The activity at the serotonergic receptors involved in the hallucinogenic response (h5-HT1A/2A/2 CR), hSERT, and h5-HT2B was studied by radioligand binding and functional assays. The binding affinities for h5-HT1A/2A/2C/2BR and hSERT, and EC50 and Emax for h5-HT1A/2AR functional assays are summarized in Table 1 and depicted in Fig. 1.
A [3H]8-OH-DPAT binding to h5-HT1AR. B [3H]ketanserin binding to h5-HT2AR. C [3H]mesulergine binding to h5-HT2BR. D [3H]mesulergine binding to h5-HT2CR. E [3H]imipramine binding to hSERT. Assays were conducted in duplicate. F Correlation between affinity (Ki) at h5-HT1AR and molecular volume (p < 0.01; R2 = 0.9858). G h5-HT1AR-mediated cAMP accumulation inhibition assay. H h5-HT2AR-mediated calcium mobilization assay. Assays were conducted in triplicate. %E was normalized as 5-HT maximum response. I 5-HT uptake inhibition at hSERT. Assays were conducted in duplicate. Data are expressed as percentage of control uptake (absence of compound). J Biased agonism: schematic representation of the two pathways (βarr2 and/or miniGαq recruitment) triggered upon 5-HT2AR activation by 5-halo-DMT derivatives. Image source: adapted from Servier Medical Art, CC BY 3.0, via https://smart.servier.com. K, L βarr2 and miniGαq recruitment assays. Assays were conducted in duplicate. %E was normalized relative to the maximum response of 5-HT. Data are expressed as means ± SEM for N ≥ 3 for all in vitro experiments.
DMT and the tested 5-halo-DMT derivatives possess high affinity (nanomolar range) for the h5-HT receptors tested. Affinity for h5-HT receptors and hSERT increased with the addition of F, Cl or Br at the 5-position (Table 1; Fig. 1A-E). Moreover, increasing the volume of the halogen resulted in improved affinity (Ki) at 5-HT1AR (p < 0.01; R2 = 0.9858; Table 1; Fig. 1F). Additionally, 5-Br-DMT presented the highest selectivity for h5-HT1AR over h5-HT2AR (lowest 5-HT 2A/1 A ratio) among the tested compounds. DMT and 5-Br-DMT exhibited 3- to 4-fold greater selectivity for h5-HT2AR over h5-HT2BR (highest 5-HT 2 A/2B ratios). Despite the structural modifications, the tested compounds showed similar selectivity for both h5-HT2AR and h5-HT2CR (5-HT 2A/2C ratio close to 1).
The tested compounds reached equal maximal effects compared to the ones induced by 5-HT in cAMP accumulation inhibition (h5-HT1AR) and calcium mobilization (h5-HT2AR) assays, respectively (Table 1; Fig. 1G, H). All tested compounds were capable of inhibiting 5-HT uptake at micromolar concentrations (Table 1; Fig. 1I). Notably, all halogenated derivatives exhibited greater potency in inhibiting 5-HT uptake compared to DMT. Larger substituents at the 5-position also increased potency for h5-HT1AR-mediated cAMP accumulation inhibition (Supplementary Material, Figure S3; p < 0.01, R2 = 0.9662).
To further characterize the mechanism of action of the 5-halo-DMT derivatives at the h5-HT2AR, we assessed the potential bias between two complementary pathways (βarr2 vs. miniGαq recruitment, Fig. 1J). The EC50, Emax values (normalized to the Emax of 5-HT and LSD) and bias factors are summarized in Table 1. Data for the reference agonists (5-HT and LSD) are shown in Supplementary Material, Table S1. Concentration-response curves and bias plots are shown in Supplementary Material, Figure S4 and S5 relative to 5-HT and LSD, respectively). 5-halo-DMT derivatives showed higher potency and efficacy at the h5-HT2AR in the βarr2 assay compared to the miniGαq assay. In both assays, the addition of a halogen atom at the 5-position enhanced potency by 7-fold for –F and 3-fold for –Cl and –Br. As shown in Fig. 1K, L, there is a trend to a decreased Emax as the size of the halogen atom increases. However, all compounds showed a similar ligand bias, whether expressed relative to the endogenous agonist 5-HT (bias factor = -0.27 to -0.15), or expressed relative to LSD (bias factor = 0.40 to 0.58).
Molecular docking reveals that larger substituents improve affinity at 5-HT1AR
The molecular modeling of 5-HT receptors in complex with halogenated ligands is useful to explain the observed Ki values (Fig. 2). Across the evaluated systems, a consistent trend emerges: larger halogens, such as those in 5-Cl-DMT and 5-Br-DMT, exhibit distinct interaction profiles compared to molecules with smaller substituents, namely 5-F-DMT and DMT.
Visualization of ligand-receptor interactions for (A-D) 5-HT1AR, (E-H) 5-HT2AR, and (I-L) 5-HT2CR each set of panels (left to right) showing interactions with DMT, 5-F-DMT, 5-Cl-DMT, and 5-Br-DMT, respectively. Each panel illustrates the binding pose of the ligand and its surrounding residues, highlighting key regions of interaction. The electron density isosurfaces are displayed with coloration based on the second eigenvalue of the ED Hessian (λ2) on a BGR color scale: blue = bonding region, green = very weak interactions (van der Waals), red = non-bonding.
In the 5-HT1AR (Fig. 2A-D), the equatorial plane of larger halogens engages with aliphatic and aromatic residues, promoting hydrophobic interactions that enhance ligand stability. The electronic overlap values are marginally higher for 5-Br-DMT compared to 5-Cl-DMT, aligning with the Ki values and suggesting stronger binding (Table 1). An alternative interaction profile is observed for 5-HT2AR (Fig. 2E-H), where halogen binding involves an asparagine residue along with aliphatic residues like leucine and valine. In the 5-HT2CR (Fig. 2I-L), the halogen-binding environment includes both hydrophobic residues (e.g., L209 and I301) and a nearby amide group.
In contrast to the 5-HT1AR, the presence of nitrogen-containing groups in the 5-HT2A/2CR facilitate specific interactions with fluorine, which, due to its properties as a hydrogen bond acceptor, effectively forms hydrogen bonds [59]. Conversely, larger halogens like chlorine and bromine predominantly engage in hydrophobic interactions. 5-F-DMT may benefit from hydrogen bonding, while 5-Br-DMT can maximize hydrophobic interactions, leaving 5-Cl-DMT underperforming in leveraging either interaction type. These findings underscore the influence of the receptor’s heterogeneous environment on ligand binding specificity.
5-Br-DMT does not induce HTR and blocks the HTR induced by 5-F-DMT
DMT, 5-F-DMT and 5-Cl-DMT elicited HTR in mice with varying potencies (ED50) and maximal effects (Emax) (Table 1; Fig. 3A-D). The head twitch dose-response profile followed an inverted U-shape or reached a plateau. Dose-response curves are shown in Supplementary Material, Figure S6 and statistical data are shown in Supplementary Material, Table S2. Interestingly, both potency and maximum efficacy decrease with bulkier halogen substituents at the 5-position, with 5-Br-DMT failing to elicit a significant HTR compared to saline administration.
A-D Number of head twitch events during a 10-min period upon i.p. administration of DMT, 5-F-DMT, 5-Cl-DMT and 5-Br-DMT. N = 10 mice/group. E Pearson correlation (p < 0.05, R2 = 0.0313) between calcium mobilization potency (EC50) and HTR maximal effects. F Pearson correlation (p < 0.01, R2 = 9835) between the ratio EC50 (Ca2+ mobilization)/EC50 (cAMP accumulation inhibition) and HTR potency (ED50). G Scored head twitches in 10 min upon administration of 5-Br-DMT (10 mg/kg, i.p.), 5-F-DMT (1 mg/kg, i.p.), or 5-Br-DMT (10 mg/kg, i.p.) pretreatment followed by 5-F-DMT (1 mg/kg, i.p.). N = 7 mice/group. H-K Change in core body temperature 60 min after injection. L-O Total distance traveled in a 30-min period following i.p. administration. N = 10 mice/group. Data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group (One-way ANOVA followed by Dunnett’s test or Kruskal-Wallis followed by Dunn’s test).
The in vivo HTR maximal effects were correlated with in vitro calcium mobilization potency (EC50) (p < 0.05, R2 = 0.9313; Fig. 3E). Additionally, HTR potency (ED50) was positively correlated with the EC50 ratio between calcium mobilization (5-HT2AR-mediated) and cAMP accumulation inhibition (5-HT1AR-mediated) (Fig. 3F).
To confirm that 5-Br-DMT can cross the blood brain barrier and interact with the central 5-HT2A receptors necessary for the HTR, we pretreated mice with 5-Br-DMT (10 mg/kg, i.p.) and subsequently administered the most powerful halogenated compound in inducing HTR, the 5-F-DMT (1 mg/kg, i.p.) (Fig. 3G). The corresponding statistical analysis (Kruskal-Wallis followed by Dunn’s test; Supplementary Material, Table S3) revealed that 5-Br-DMT administration was able to block the HTR induced by 5-F-DMT in mice.
All 5-halo-DMT derivatives tested decrease both core body temperature and locomotion in mice
All the tested compounds induced a dose-dependent hypothermic response measured 60 min after injection (Table 1, Fig. 3H-K, Supplementary Material, Table S2). Also, all of them induced hypolocomotion (Table 1, Fig. 3L-O, Supplementary Material, Table S2).
5-Halo-DMT derivatives induce neuroplasticity-related gene expression in vivo, and 5-Br-DMT promotes dendritogenesis in vitro
In order to evaluate the ability of the 5-halogenated-DMT derivatives to induce the expression of plasticity-related genes (Fig. 4A-L), mRNA levels of Bdnf, Arc, Egr-1, Egr-2 and Egr-3 were determined in the mouse PFC (Fig. 4B, E-H) and HIP (Fig. 4C, I-L) 30 min after i.p. administration (10 mg/kg) of the corresponding DMT derivative.
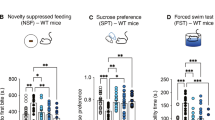
A Experimental setup for in vivo gene expression experiments after 5-halo-DMT administration to mice (10 mg/kg, i.p.). B-D Effects on mRNA Bdnf levels in the PFC, HIP and mPFC, respectively. E-H Effects on mRNA levels of Arc, Egr-1, Egr-2 and Egr-3 in the PFC; and I-L HIP. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group (One-way ANOVA with Dunnett’s test or Kruskal-Wallis with Dunn’s test). The discontinuous line represents the corresponding saline levels. Data are expressed as means ± SEM of fold changes in mRNA levels vs. the corresponding saline group. Samples were tested in duplicate. N = 6 mice/group. M Cell culture and experimental scheme of mouse primary mixed cortical cells for dendritogenesis experiments. N Representative images of cortical neurons without treatment (control) and exposed to 5-Br-DMT (5 µM). O Number of crossings, primary dendrites and branches assessed by Sholl analysis; and P total dendritic length of control vs. 5-Br-DMT-treated (5 μM) cortical cells. Data are represented as means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, vs. control (Student’s t-test, two-sided). N = 3 cell cultures (40 neurons per culture). Q Representation of the TST protocol. Images source: adapted from Servier Medical Art, CC BY 3.0, via https://smart.servier.com and [52]. R Immobility time in the TST in the first day (baseline) and test day of saline-treated animals and 5-Br-DMT-treated (10 mg/kg, i.p.) animals 24 h after administration. ns = not significant, *p < 0.05, **p < 0.01 vs. control group; mixed-effects analysis with Bonferroni’s test. N = 20 mice/group.
One-way ANOVA followed by Dunnett’s post-hoc test (Supplementary Material, Table S4) revealed a significant increase in the expression of Arc for all DMT derivatives tested, in both the PFC and HIP. Moreover, DMT, 5-F-DMT and 5-Br-DMT administration also induced a significantly elevated expression of Egr-2 in both brain areas tested. On the other hand, 5-F-DMT and 5-Cl-DMT were also able to induce a significant increase in the gene expression of Egr-1 in HIP. Notably, 5-Br-DMT was the only compound able to induce a significant increase in the expression of all genes tested (Arc, Egr-1/2/3) in PFC. However, no significant differences were observed when analyzing Bdnf mRNA levels in PFC, HIP. Additionally, mRNA levels of Bdnf were tested in the mouse mPFC 120 min post-injection (Fig. 4D), as previously reported [43], although no upregulation of Bndf mRNA was detected.
Building on previous results, we next assessed the capacity of 5-Br-DMT to produce structural neuroplasticity. Thus, we incubated mixed neuronal cortical cultures with 5-Br-DMT (10 µM) for a period of 24 h at DIV4 (Fig. 4M). After, an immunocytochemistry against MAP-2 was performed to measure the resulting changes in various morphological features of the dendritic arbor at DIV7 (Fig. 4N) using Sholl analysis. Unpaired t-test (Supplementary Material, Table S5) indicated a significant increment in number of crossings, number of primary dendrites, number of branches and total dendritic length (Fig. 4O-P), resulting in an increased arbor complexity.
5-Br-DMT induces antidepressant effects 24 h after administration
Since 5-Br-DMT induced little to no HTR in mice and yet it promoted an overexpression of plasticity-related IEGs in vivo and structural neuroplasticity in vitro, we selected this halogenated derivative to assess its potential antidepressant effects.
No differences were observed between male and female mice either in the first day (baseline) (Student’s t-test, two-sided) or after treatment (Two-way ANOVA; Supplementary Material, Table S6). Therefore, males and females were grouped. Figure 4Q shows the experimental setup of the TST experiment. First, mice were subjected to daily tail suspension (for 6 min) over four consecutive days to cause stress-induced depression. A mixed-effects analysis with Bonferroni’s post-hoc test (Supplementary Material, Table S7) revealed a significant increase in the immobility time in the test day compared to the first day (baseline) of saline-treated animals, confirming that the repeated exposure to tail suspension induced depressive-like behavior. Most importantly, on the test day, 5-Br-DMT-treated animals had a significantly lower immobilization time compared to saline-treated mice (Bonferroni’s test, p < 0.05, Figure 4R), revealing an antidepressant-like response [56].
Discussion
Given the urgent need for antidepressants with faster onset and improved effectiveness, especially in major depressive disorder, psychedelics have emerged as promising alternatives due to their ability to enhance neuroplasticity. However, their hallucinogenic effects remain a major obstacle for widespread therapeutic use. In this study, we investigated the neuropharmacological properties of halogenated DMT analogs, whose substitutions can improve potency, target selectivity, tolerability and/or may have implications for developing novel psychedelic-based treatments.
Firstly, our structure-activity relationship (SAR) study revealed that addition of a halogen atom at position five of the indole ring can increase affinity for 5-HT receptors and transporter compared to DMT, with the size and type of halogen substituents differently influencing the ligand-receptor interaction and binding affinities. In 5-HT1AR, larger halogens, such as chlorine and bromine, interact with aliphatic and aromatic residues, promoting hydrophobic interactions that enhance ligand stability. In fact, the molecular volume of the halogenated derivative is positively correlated with the affinity for 5-HT1AR and the 5-HT1AR-mediated cAMP inhibition potency (the higher the volume of the 5-substituted atom, the lower the Ki and EC50 values). Contrarily to 5-HT1AR, whose binding site favors mainly hydrophobic interactions, 5-HT2AR and 5-HT2CR enable fluorine to form hydrogen bonds with nitrogen-containing residues, resulting in a higher affinity of 5-F-DMT. On the other hand, 5-Br-DMT maximizes hydrophobic interactions with 5-HT2AR, which renders 5-Cl-DMT underperforming in leveraging either interaction type. This distinction is reflected in the affinity constant values: 5-F-DMT and 5-Br-DMT exhibited comparable binding affinities, approximately double the affinity of 5-Cl-DMT at 5-HT2AR.
Although all tested compounds exhibited nanomolar affinities for the 5-HT receptors (1 A, 2 A, 2B and 2 C), their selectivity differed among them. For instance, 5-Cl- and 5-Br-DMT were the most selective tested compounds for 5-HT1AR over 5-HT2AR (~10-fold), while the fluorinated derivative was nearly a non-selective 5-HT2A/1AR ligand. Moreover, both DMT and 5-F-DMT exhibited lower affinity for 5-HT1AR than their previously reported congeners 5-MeO-DMT derivatives (Ki = 0.577 – 24.8 nM) [53]. Following similar comparisons, all halogenated compounds tested exhibited affinities for 5-HT2AR comparable to those of most of the 5-MeO-DMT derivatives (Ki = 105 – 399 nM). However, the halogenated-DMT analogs tested demonstrated lower potency than 5-MeO-DMT derivatives in inducing calcium mobilization through 5-HT2AR activation (EC50 = 4.46 – 23.6 nM) [53]. A comparative analysis of receptor binding affinities suggests that halogenated DMT derivatives share a broadly similar pharmacological profile to 5-HT. According to Kozell and coworkers [60], 5-HT binds to h5-HT2AR and h5-HT1AR with Ki values of 19.5 nM and 3.26 nM, respectively. In our study, 5-halo-DMT compounds displayed affinity values within the same nanomolar range, although generally showing lower affinity at 5-HT1AR compared to 5-HT.
Importantly, agonism at the 5-HT2BR has been linked to adverse cardiovascular effects [23, 61, 62]. In line with these concerns, the FDA has recently issued guidance for industry on clinical investigations for psychedelics, strongly recommending the evaluation of binding to 5-HT2BR subtype due to its established link to heart valvulopathy in humans [63]. In this sense, among the halogenated-DMT derivatives, 5-Br-DMT exhibited the highest selectivity for 5-HT2AR over 5-HT2BR ( ~ 3-fold), whereas 5-F-DMT showed the highest affinity and selectivity for 5-HT2BR among all compounds tested. Conversely, none of the tested compounds displayed notable selectivity for 5-HT2AR over 5-HT2CR, although retaining nanomolar affinities. Given that 5-HT2AR, and specially 5-HT2CR, are promising targets for treating substance use disorders [64, 65], this observation could open an intriguing area for future investigation. On the other hand, adding a halogen group resulted in an approximately 4-fold increased SERT affinity by approximately four times compared to DMT, also resulting in improved potency at inhibiting 5-HT uptake. Although SERT interaction and 5-HT uptake inhibition might not be the main mechanism of the tested compounds, it could still play a supportive role in their pharmacological properties [40, 66].
Our HTR studies in mice demonstrated that DMT derivatives with smaller halogen substituents elicited a higher response. In contrast, 5-Br-DMT induced little to no HTR, despite exhibiting nanomolar affinity and potency at 5-HT2AR. To assess whether 5-Br-DMT partitions into the brain, binds to and engages central 5-HT2AR required for HTR, mice were pretreated with this non--HTR-inducing compound. Our results revealed that 5-Br-DMT blocks/attenuates the HTR elicited by 5-F-DMT, indicating blood-brain barrier penetration by 5-Br-DMT and binding to/engaging 5-HT receptors, but eliciting an opposite response probably due to stronger 5-HT1AR activation induced by 5-Br-DMT. However, competitive binding of 5-Br-DMT at 5-HT2AR and subsequent differences in downstream signaling cascades cannot be fully ruled out as contributing factors to the attenuation of the HTR induced by 5-F-DMT, as observed for some non-hallucinogenic phenethylamines [15].
Although 5-HT2AR activation is largely known to induce the HTR in mice and hallucinogenic effects in humans, typical of psychedelics [6, 7, 20], the mechanisms underlying these responses remain elusive [12, 13]. Among the proposed mechanisms, the occurrence of a biased agonism for the βarr2 versus Gq pathways in the 5-HT2AR signaling cascade stands as a possible explanation for the varied hallucinogenic and/or therapeutic properties that psychedelics may elicit [15, 67]. In our study, although all compounds tested induced differentiated HTR, DMT and its halogenated counterparts have a similar biased agonism profile, showing a higher efficacy and potency for βarr2 than miniGαq recruitment, with bias factors similar to that of the endogenous ligand 5-HT. Moreover, all compounds tested showed a tendency towards 5-HT2AR biased agonism in the βarr2 recruitment assay when compared to LSD, a ligand that displays equal potency for both βarr2 and miniGαq recruitment in these assays [45] and is widely known for its hallucinogenic effects. Thus, our findings reveal that biased agonism may not play an important role at inducing HTR. Furthermore, a recent study [15] also proposed Gq recruitment efficacy as a predictor of the hallucinogenic potential in mice, whereas βarr2-biased agonists would lack psychedelic effects. However, the aforementioned study is only focused on highly selective 5-HT2AR agonists (i.e., psychedelic phenethylamines) and does not consider the interaction with other 5-HT receptor subtypes that could plausibly modulate the hallucinogenic response (i.e., 5-HT1AR, 5-HT2CR,…) [20, 68]. Interestingly, our results demonstrated that although the EC50 values of HTR do not correlate with either the potency of 5-HT2AR-mediated calcium mobilization or 5-HT1A-mediated cAMP accumulation inhibition assays, they do correlate with the ratio of potencies of both parameters (calcium mobilization EC50/cAMP accumulation inhibition EC50). Similarly, Klein and co-workers [69] found that HTR potency was simultaneously dependent on activity at both 5-HT2AR and 5-HT1AR, with a positive relationship between HTR potency and 5-HT2AR affinity and a negative relationship between HTR potency and 5-HT1AR affinity. Considering the opposite roles of 5-HT2AR and 5-HT1AR in the HTR (in which 5-HT2AR activation elicits the HTR [6, 7, 20]; whereas 5-HT1AR activation blocks the HTR [17, 53]) these findings may explain that the higher preference for 5-HT1AR receptor over 5-HT2AR could translate into a lower hallucinogenic-like response. Although we did not directly assess the role of 5-HT2CR in this study, previous research indicates that 5-HT2CR plays a modulatory role in the HTR. Studies using 5-HT2CR knockout mice [70] have shown a reduction in HTR elicited by serotonergic psychedelics, suggesting that this receptor may contribute to the intensity of hallucinogenic-like behaviors. However, since all compounds tested here exhibited similar affinity for 5-HT2CR, it is unlikely that differential engagement of this receptor accounts for the differences observed in HTR across compounds. Furthermore, as 5-HT1AR agonists, DMT and its halogenated derivatives also induced a decrease in the core body temperature as well as a reduction in the locomotor activity of mice, two behavioral responses mediated by the same receptor [71,72,73]. Moreover, while all 5-halo-DMT compounds exhibited increased affinity and potency at hSERT compared to DMT, their values remain in the micromolar range—well above the nanomolar concentrations required for 5-HT receptor activation and for clinically relevant SERT inhibition. Therefore, under our experimental conditions, it is unlikely that SERT inhibition plays a major role in the observed behavioral or physiological effects. However, we cannot rule out a modulatory contribution, as even moderate SERT occupancy may increase extracellular 5-HT levels. This could synergize with 5-HT2AR activation to enhance downstream signaling and/or neuroplasticity. Conversely, elevated 5-HT may also engage 5-HT1AR-mediated feedback inhibition or compete with agonist binding at 5-HT2AR, potentially dampening hallucinogenic-like effects. Thus, depending on the regional distribution and interaction of serotonergic targets, SERT inhibition could enhance, reduce, or have no impact on the behavioral and physiological outcomes observed. Finally, it must be pointed out that in vitro assays were conducted using human receptor/transporter-expressing cell lines, while behavioral experiments were performed in mice. The use of human receptor/transporter-expressing cell lines is a widely used strategy that facilitates comparisons not only with existing literature but also with human pharmacology. Although 5-HT receptors are highly conserved between species, subtle variations may exist [74]. While our in vitro and in vivo results are largely consistent, caution is warranted when extrapolating findings across systems.
Exposure to chronic stress has shown to cause atrophy and loss of neurons in some brain areas such as the PFC and HIP [27]. Fortunately, certain psychedelics have been reported to revert or attenuate those effects by upregulating plasticity-related transcripts and inducing neuroplasticity [28, 75]. Consistent with previous studies [76, 77], most of the tested compounds increased Arc, Egr-1, Egr-2 and Egr-3 mRNA levels in mouse PFC and/or HIP. Importantly, these IEGs are rapidly expressed upon neuronal activity and are essential for synaptic plasticity, i.e., by encoding growth factors, cytoskeletal proteins and transcription factors, and triggering further transcription of late response genes [78]. It is worth noting that 5-Br-DMT was the only compound capable of significantly increasing Egr-3 mRNA levels in the mouse PFC, as well as most of the tested IEGs (in turn, EGR-3 is believed to indirectly induce expression of BDNF [79]). However, none of the tested compounds showed upregulation of Bdnf mRNA. Similarly, psychedelics like DOI, DMT, and LSD have been reported to have no effect on Bdnf mRNA levels [4]. However, the absence of mRNA changes at 30 min or 2 h post-administration does not exclude the possibility of transient transcriptional peaks at other time points. Future studies including multiple sampling intervals and parallel measurement of protein levels will be important to more fully understand BDNF signaling in response to 5-Br-DMT. Alternatively, psychedelics could influence BDNF signaling by binding directly to the BDNF receptor TrkB and thereby acting through its pathway without altering Bdnf mRNA levels, as proposed in previous studies [77, 80].
Our findings highlight unique characteristics of 5-Br-DMT compared to its counterparts. This tryptamine activated the 5-HT receptors, and induced expression of most of the IEGs tested that are reportedly related to structural plasticity. Thus, we aimed to investigate its potential to promote structural neuroplasticity. Specifically, we examined the dendritic growth of cortical neurons because of its critical role in synaptic connectivity and neural circuit function. Our results showed that 5-Br-DMT effectively induced dendritogenesis, by increasing the number of primary dendrites, branches, crossings and total dendritic length, thereby enhancing arbor complexity. Consistently, previous studies have shown that other tryptamine derivatives induce structural plasticity in vitro [4, 9, 81,82,83], a key aspect of psychedelic and dissociative drugs in rebuilding neural circuitry and restoring normal brain function in depression [4].
While the necessity of inducing a subjective experience as a requirement for the antidepressant effects of psychedelics has been long debated [26, 84], recent studies point out that non-hallucinogenic psychedelic derivatives may hold therapeutic potential [25, 38, 82]. Therefore, we investigated the antidepressant potential of the non-hallucinogenic and psychoplastogenic halogenated-DMT derivative, 5-Br-DMT, in a mouse (of both sexes) model of repeated stress-exposure. Our results demonstrated a significant reduction of the immobilization time in the TST 24 h after a single 5-Br-DMT administration to stress-induced depressive mice, which translates into a rapid antidepressant effect. In agreement, prior studies in rodents have demonstrated antidepressant effects of other tryptamine derivatives such as psilocin or DMT in rodent models [32, 85, 86]. While our findings provide new hints into the ongoing search for non-hallucinogenic 5-HT2AR agonists with therapeutic properties, further studies involving other preclinical models of depression, as well as other related mood disorders (i.e., anxiety, substance abuse,…) are warranted to fully assess the therapeutic potential of this novel compound and related analogs. Furthermore, while 5-HT1AR activation may contribute to therapeutic effects such as anxiolysis and possibly neuroplasticity [87, 88], this does not exclude a coexisting role for 5-HT2AR activation in driving psychoplastogenic responses, as supported by recent studies [9, 83]. Nonetheless, the relative contribution of these receptor subtypes remains under debate. Further research is warranted to clarify the exact mechanisms by which 5-Br-DMT – and other psychedelics – induce neuroplasticity.
To sum up, the neuropharmacological profile of 5-halo-DMT derivatives presented in this study highlights their distinct activation patterns across key 5-HT receptors involved in the HTR in mice and hallucinogenic response in humans. These differences lead to varying behavioral effects and further support the negative influence of 5-HT1AR interaction on this response. Additionally, our findings underscore the modulation of plasticity-related IEG expression induced by 5-halo-DMT analogs as well as the structural plasticity induced by 5-Br-DMT. Notably, the unique pharmacological profile of 5-Br-DMT, including its antidepressant effects, highlights its potential as a non-hallucinogenic psychoplastogen with therapeutic potential. Finally, this study opens new avenues for the design of next-generation antidepressants and provides valuable insights for future drug discovery research.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Kovich H, Kim W, Quaste AM. Pharmacologic treatment of depression. Am Fam Physician. 2023;107:173–81.
Montgomery SA. Fast-onset antidepressants. Int Clin Psychopharmacol. 1997;12:S1–6.
Thompson C. Onset of action of antidepressants: results of different analyses. Human Psychopharmacology: Clinical and Experimental. 2002;17:S27–32.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
Nichols DE. Structure-activity relationships of serotonin 5-HT2A agonists. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:559–79.
Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB. The role of 5-HT2A, 5-HT2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl). 2015;232:275–84.
Shahar O, Botvinnik A, Esh-Zuntz N, Brownstien M, Wolf R, Lotan A, et al. Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 receptors in the head twitch response induced by 5-hydroxytryptophan and psilocybin: translational implications. Int J Mol Sci. 2022;23:14148.
Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E. The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J Psychopharmacol. 2018;32:779–92.
Cameron LP, Patel SD, Vargas MV, Barragan EV, Saeger HN, Warren HT, et al. 5-HT2ARs mediate therapeutic behavioral effects of psychedelic tryptamines. ACS Chem Neurosci. 2023;14:351–8.
Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–65.
Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933.
Canal CE. Serotonergic psychedelics: experimental approaches for assessing mechanisms of Action. Handb Exp Pharmacol. 2018;252:227–60.
López-Giménez JF, González-Maeso J. Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr Top Behav Neurosci. 2017;36:45–73.
Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–16.
Wallach J, Cao AB, Calkins MM, Heim AJ, Lanham JK, Bonniwell EM, et al. Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential. Nat Commun. 2023;14:8221.
Puigseslloses P, Nadal-Gratacós N, Ketsela G, Weiss N, Berzosa X, Estrada R, et al. structure-activity relationships of serotonergic 5-MeO-DMT Derivatives: Insights 1 into psychoactive and thermoregulatory properties. Mol Psychiatry. 2024;29:2346–58.
Glatfelter GC, Clark AA, Cavalco NG, Landavazo A, Partilla JS, Naeem M, et al. Serotonin 1A receptors modulate serotonin 2A receptor-mediated behavioral effects of 5-methoxy- N,N-dimethyltryptamine analogs in mice. ACS Chem Neurosci. 2024;15:4458–77. https://doi.org/10.1021/acschemneuro.4c00513.
Kaltschmidt B, Kaltschmidt C. NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Front Mol Neurosci. 2015;8:69.
Masson J, Emerit MB, Hamon M, Darmon M. Serotonergic signaling: multiple effectors and pleiotropic effects. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:685–713.
Erkizia-Santamaría I, Alles-Pascual R, Horrillo I, Meana JJ, Ortega JE. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed Pharmacother. 2022;154:113612.
Custodio RJP, Ortiz DM, Lee HJ, Sayson LV, Kim M, Lee YS, et al. Serotonin 2C receptors are also important in head-twitch responses in male mice. Psychopharmacology (Berl). 2023. https://doi.org/10.1007/s00213-023-06482-9.
Zhu H, Wang L, Wang X, Yao Y, Zhou P, Su R. 5-hydroxytryptamine 2C/1A receptors modulate the biphasic dose response of the head twitch response and locomotor activity induced by DOM in mice. Psychopharmacology (Berl). 2024. https://doi.org/10.1007/s00213-024-06635-4.
Ayme‐Dietrich E, Lawson R, Côté F, de Tapia C, Da Silva S, Ebel C, et al. The role of 5‐HT2B receptors in mitral valvulopathy: bone marrow mobilization of endothelial progenitors. Br J Pharmacol. 2017;174:4123–39.
Duan W, Cao D, Wang S, Cheng J. Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants. Chem Rev. 2024;124:124–63.
Olson DE. The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol Transl Sci. 2021;4:563–7.
Yaden DB, Griffiths RR. The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol Transl Sci. 2021;4:568–72.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Calder AE, Hasler G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology. 2023;48:104–12.
de Vos CMH, Mason NL, Kuypers KPC. Psychedelics and neuroplasticity: a systematic review unraveling the biological underpinnings of psychedelics. Front Psychiatry. 2021;12:724606.
Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci. 2016;8:78.
Brito-da-costa AM, Dias-da-silva D, Gomes NGM, Dinis-oliveira RJ, Madureira-carvalho Á. Toxicokinetics and toxicodynamics of ayahuasca alkaloids N,N-dimethyltryptamine (DMT), harmine, harmaline and tetrahydroharmine: clinical and forensic impact. Pharmaceuticals. 2020;13:1–39.
Cameron LP, Benson CJ, Dunlap LE, Olson DE. Effects of N, N-dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci. 2018;9:1582–90.
Reckweg J, Mason NL, van Leeuwen C, Toennes SW, Terwey TH, Ramaekers JG. A Phase 1, dose-ranging study to assess safety and psychoactive effects of a vaporized 5-methoxy-N,N-dimethyltryptamine formulation (GH001) in healthy volunteers. Front Pharmacol. 2021;12:760671.
Reckweg JT, van Leeuwen CJ, Henquet C, van Amelsvoort T, Theunissen EL, Mason NL, et al. A phase 1/2 trial to assess safety and efficacy of a vaporized 5-methoxy-N,N-dimethyltryptamine formulation (GH001) in patients with treatment-resistant depression. Front Psychiatry. 2023;14:1133414.
Ibrahim MA, El-Alfy AT, Ezel K, Radwan MO, Shilabin AG, Kochanowska-Karamyan AJ, et al. Marine inspired 2-(5-halo-1H-indol-3-yl)-N,N-dimethylethanamines as modulators of serotonin receptors: an example illustrating the power of bromine as part of the uniquely marine chemical space. Mar Drugs. 2017;15:248.
Gerebtzoff G, Li-Blatter X, Fischer H, Frentzel A, Seelig A. Halogenation of drugs enhances membrane binding and permeation. Chembiochem. 2004;5:676–84.
Simoens A, Dejaegere A, Vandevelde M, Stevens CV. Continuous flow synthesis of N,N-dimethyltryptamine (DMT) analogues with therapeutic potential. RSC Med Chem. 2024;16:367–72.
Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, et al. Psychedelic-inspired drug discovery using an engineered biosensor. Cell. 2021;184:2779–92.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 2020;40:1769–77.
Mayer FP, Wimmer L, Dillon-Carter O, Partilla JS, Burchardt NV, Mihovilovic MD, et al. Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters. Br J Pharmacol. 2016;173:2657–68.
Duart-Castells L, Nadal-Gratacós N, Muralter M, Puster B, Berzosa X, Estrada-Tejedor R, et al. Role of amino terminal substitutions in the pharmacological, rewarding and psychostimulant profiles of novel synthetic cathinones. Neuropharmacology. 2021;186:108475.
Nadal-Gratacós N, Lleixà E, Gibert-Serramià M, Estrada-Tejedor R, Berzosa X, Batllori X, et al. Neuropsychopharmacology of emerging drugs of abuse: meta-and para-halogen-ring-substituted α-PVP (“flakka”) derivatives. Int J Mol Sci. 2022;23:2226.
Nadal-Gratacós N, Alberto-Silva AS, Rodríguez-Soler M, Urquizu E, Espinosa-Velasco M, Jäntsch K, et al. Structure–activity relationship of novel second-generation synthetic cathinones: mechanism of action, locomotion, reward, and immediate-early genes. Front Pharmacol. 2021;12:749429.
Pottie E, Cannaert A, Van Uytfanghe K, Stove CP. Setup of a serotonin 2A receptor (5-HT2AR) bioassay: demonstration of its applicability to functionally characterize hallucinogenic new psychoactive substances and an explanation why 5-HT2AR bioassays are not suited for universal activity-based screening of biofluids for new psychoactive substances. Anal Chem. 2019;91:15444–52.
Poulie CBM, Pottie E, Simon IA, Harpsøe K, D’Andrea L, Komarov IV, et al. Discovery of β-arrestin-biased 25CN-NBOH-derived 5-HT2Areceptor agonists. J Med Chem. 2022;65:12031–43.
Pottie E, Dedecker P, Stove CP. Identification of psychedelic new psychoactive substances (NPS) showing biased agonism at the 5-HT2AR through simultaneous use of β-arrestin 2 and miniGαq bioassays. Biochem Pharmacol. 2020;182:114251.
Pottie E, Cannaert A, Stove CP. In vitro structure–activity relationship determination of 30 psychedelic new psychoactive substances by means of β-arrestin 2 recruitment to the serotonin 2A receptor. Arch Toxicol. 2020;94:3449–60.
Arcon JP, Modenutti CP, Avendaño D, Lopez ED, Defelipe LA, Ambrosio FA, et al. AutoDock Bias: improving binding mode prediction and virtual screening using known protein–ligand interactions. Bioinformatics. 2019;35:3836–8.
Case DA, Aktulga HM, Belfon K, Cerutti DS, Cisneros GA, Cruzeiro VWD, et al. AmberTools. J Chem Inf Model. 2023;63:6183–91.
Lefebvre C, Klein J, Khartabil H, Boisson J‐C, Hénon E. IGMPlot: A program to identify, characterize, and quantify molecular interactions. J Comput Chem. 2023;44:1750–66.
Stierand K, Maaß PC, Rarey M. Molecular complexes at a glance: automated generation of two-dimensional complex diagrams. Bioinformatics. 2006;22:1710–6.
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd edn. San Diego: Elsevier Academic Press; 2004.
Puigseslloses P, Nadal-Gratacós N, Ketsela G, Weiss N, Berzosa X, Estrada-Tejedor R, et al. Structure-activity relationships of serotonergic 5-MeO-DMT derivatives: insights into psychoactive and thermoregulatory properties. Mol Psychiatry. 2024;29:2346–58. https://doi.org/10.1038/s41380-024-02506-8.
Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, et al. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–9.
Ruan C-S, Guo Y, Bobrovskaya L, Zhou X-F, Zeng Y-Q. A pilot study in modeling mood disorders in mice by chronic tail-suspension stress. Neuropsychiatry. 2018;8:1586–92.
Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85:367–70.
Ehlert FJ. On the analysis of ligand-directed signaling at G protein-coupled receptors. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:549–77.
Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–77.
Shinada NK, de Brevern AG, Schmidtke P. Halogens in protein–ligand binding mechanism: a structural perspective. J Med Chem. 2019;62:9341–56.
Kozell LB, Eshleman AJ, Swanson TL, Bloom SH, Wolfrum KM, Schmachtenberg JL, et al. Pharmacologic activity of substituted tryptamines at 5-hydroxytryptamine (5-HT)2A receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and serotonin transporter. J Pharmacol Exp Ther. 2023;385:62–75.
Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease—It was meant 2B. Pharmacol Ther. 2011;132:146–57.
McIntyre RS. Serotonin 5-HT2B receptor agonism and valvular heart disease: implications for the development of psilocybin and related agents. Expert Opin Drug Saf. 2023;22:881–3.
Food and Drug Administration (FDA) & Center for Drug Evaluation and Research (CDER). Psychedelic Drugs: Considerations for Clinical Investigations Guidance for Industry (Draft Guidance). 2023.
Campbell EJ, Bonomo Y, Pastor A, Collins L, Norman A, Galettis P, et al. The 5‐HT2C receptor as a therapeutic target for alcohol and methamphetamine use disorders: A pilot study in treatment‐seeking individuals. Pharmacol Res Perspect. 2021;9:e00767.
Bubar M, Cunningham K. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–85.
Mayer FP, Niello M, Cintulova D, Sideromenos S, Maier J, Li Y, et al. Serotonin-releasing agents with reduced off-target effects. Mol Psychiatry. 2023;28:722–32.
Cao D, Yu J, Wang H, Luo Z, Liu X, He L, et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science. 2022;375:403–11.
Glatfelter GC, Naeem M, Pham DNK, Golen JA, Chadeayne AR, Manke DR, et al. Receptor binding profiles for tryptamine psychedelics and effects of 4-Propionoxy- N,N -dimethyltryptamine in mice. ACS Pharmacol Transl Sci. 2023;6:567–77.
Klein LM, Cozzi NV, Daley PF, Brandt SD, Halberstadt AL. Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology. 2018;142:231–9.
Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl). 2010;209:163–74.
Cryan JF, Kelliher P, Kelly JP, Leonard BE. Comparative effects of serotonergic agonists with varying efficacy at the 5-HT(1A) receptor on core body temperature: Modification by the selective 5-HT(1A) receptor antagonist WAY 100635. J Psychopharmacol. 1999;13:278–83.
Newman-Tancredi A, Depoortère R, Carilla-Durand E, Tarayre JP, Kleven M, Koek W, et al. NLX-112, a highly selective 5-HT1A receptor agonist: Effects on body temperature and plasma corticosterone levels in rats. Pharmacol Biochem Behav. 2018;165:56–62.
Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl). 2006;189:319–29.
Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213.
Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD. Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chem Neurosci. 2020;11:864–71.
González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52.
Nogueira M, Ferreira Golbert DC, Menezes R, Nóbrega de Almeida R, Galvão-Coelho NL, Siroky AN, et al. Serotonergic psychedelic 5-MeO-DMT alters plasticity-related gene expression and generates anxiolytic effects in stressed mice. Mol Psychiatry. 2024;30:50–60.
Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV. Immediate early genes, memory and psychiatric disorders: Focus on c-Fos, Egr1 and Arc. Front Behav Neurosci. 2018;12:79.
Pfaffenseller B, Kapczinski F, Gallitano AL, Klamt F. Egr3 immediate early gene and the brain-derived neurotrophic factor in bipolar disorder. Front Behav Neurosci. 2018;12:15.
Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci. 2023;26:1032–41.
Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–44.e4.
Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 2021;589:474–9.
Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science. 2023;379:700–6.
Roseman L, Nutt DJ, Carhart-Harris RL. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol. 2018;8:974.
Pic-Taylor A, da Motta LG, de Morais JA, Junior WM, Santos A, de FA, et al. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav Processes. 2015;118:102–10.
Takaba R, Ibi D, Yoshida K, Hosomi E, Kawase R, Kitagawa H, et al. Ethopharmacological evaluation of antidepressant-like effect of serotonergic psychedelics in C57BL/6J male mice. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:3019–35.
Aguiar RP, Soares LM, Varney M, Newman-Tancredi A A, Milani H, Prickaerts J, et al. NLX-101, a 5-HT1A receptor-biased agonist, improves pattern separation and stimulates neuroplasticity in aged rats. Neurobiol Aging. 2023;124:52–59.
Aguiar RP, Soares LM, Meyer E, da Silveira FC, Milani H, Newman-Tancredi A, et al. Activation of 5-HT1A postsynaptic receptors by NLX-101 results in functional recovery and an increase in neuroplasticity in mice with brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109832.
Acknowledgements
This study was supported by Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación MICIU/AEI/10.13039/ 501100011033 and by ERDF/EU (PID2022-137541OBI00), Plan Nacional sobre Drogas (2020I051) and European Union (EU) Home Affairs Funds, NextGenPS project (number: 101045825). DP, RLA, and EE belong to 2021SGR00090 from Generalitat de Catalunya. PP received a doctoral scholarship grant from Generalitat de Catalunya (AGAUR), 2022 FISDU 00004. CRC received a doctoral scholarship grant from Generalitat de Catalunya (AGAUR), 2024 FI-1 00088. EP acknowledges funding from Ghent University’s Bijzonder Onderzoeksfonds (BOF23/PDO/073). We are grateful to Dr. Gemma Navarro and her staff for providing access to their Nivo plate reader and for their advice on its use.
Author information
Authors and Affiliations
Contributions
RLA conceptualized and designed the study with the assistance of PP, CPM, CS and EE. PP, NNG, BF, EP, JRO, APQ, CRC and DP collected and analyzed the data. PP, NNG, EP, DP, CPM and RLA developed the methodology. PP and XB synthetized the compounds. PP, ME, CS, CPM and RLA interpreted the data. XB, CPM, ME, CS and RLA supervised research activities. PP and RLA wrote the original draft. PP, NNG, EP, XB, DP, ME, CPM, CS, EE and RLA revised and edited the manuscript. RLA, DP, EE and CS acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Puigseslloses, P., Nadal-Gratacós, N., Fumàs, B. et al. Neuropharmacology of halogenated DMT analogs: psychoplastogenic and antidepressant properties of 5-Br-DMT, a psychedelic derivative with low hallucinogenic potential. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03308-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03308-2