Abstract
Exposure to traumatic life events may compromise physical and mental health of specific subjects. While some individuals extinguish fear appropriately, others exhibit an inefficient and persistent fear response, with remarkable differences between sexes. Understanding the heterogeneity in fear extinction responses is essential for elucidating the underlying mechanisms of fear-related disorders. We used a multidisciplinary approach analyzing the hypothalamic-pituitary-adrenal (HPA) axis tone, the microbiota composition, and the transcriptome of the amygdala (primary brain region involved in fear regulation) in adult male and female mice that were exposed to the Pavlovian fear conditioning and extinction paradigm. This model allowed us to stratify the mice population into two extreme phenotypic subgroups (resilient and susceptible), based on their individual fear extinction behavior. Characterization of some components of the HPA axis revealed strong disturbances in vulnerable males (e.g., increased hypothalamic CRF mRNA and corticosterone plasma levels), whereas softer changes were found in female animals. Several bacterial groups such as the genera Parvibacter, Alloprevotella and Limosilactobacillus and the family Christensenellaceae were enriched in the microbiota of resilient males, as well as relevant bacterial taxa enrichment was also observed in resilient (genus Muribaculum) and susceptible (family Eggerthellaceae) female mice. We also identified clear differences in the transcriptomic profile of the amygdala (31 differentially expressed genes) in male animals. These findings underscore the intricate involvement of multiple factors shaping the inter-individual variability of fear extinction response in a sex-dependent manner, thus paving the way for new potential targets for fear-related disorders.
Similar content being viewed by others
Introduction
Fear entails an evolutionary-preserved emotional response that allows individuals to recognize threats in order to avoid or, at least, reduce damage, thus ensuring survival. However, exposure to severe acute or long-lasting stress may give rise to the onset of fear-related disorders, such as posttraumatic stress disorder (PTSD), phobias, and panic disorder [1]. These disabling illnesses are characterized by persevering recollections of trauma, hyperarousal, and negative consequences on mood and cognition [2]. Antidepressants, anxiolytics, antipsychotics, and beta-blockers are the most prescribed drugs [3, 4], usually combined with behavior-based strategies like exposure therapy [5]. Nevertheless, such psychopharmacological treatments present limited efficacy and a clear retention of fear dysregulation diagnosis later in life, even among those who do respond [6]. At the crux of this issue lies a lack of neurobiological understanding of these disorders.
Although we humans are all exposed to traumatic events throughout life, around 1 out of every 15 people are not able to correctly extinguish fear memories and display a persistent somatization of such response [7, 8]. In parallel, animal models that undergo behavioral paradigms recapitulating key features from fear-related diseases also present such inter-individual variability [9,10,11]. This highlights individual neurobehavioral differences that contribute to the resilience (maintenance or quick recovery of mental health during and after adversity) or susceptibility (vulnerability to be influenced or harmed by a traumatic event) to the long-term negative effects of stress. Identification of the core mechanisms that explain such inter-individual differences can be addressed from different views. One of the most popular and studied components of fear response are hormones [12]. Specifically, hypothalamic-pituitary-adrenal (HPA) axis-related hormones and the several components that modulate this axis have been reported to be a crucial driver of fear response in animal models and humans [13]. Despite incongruent results, several studies have observed dysregulated HPA axis functionality in PTSD patients [14]. Reciprocally, HPA axis disturbances generated in animal models have revealed clear changes in stress sensitivity, fear and anxiety-like behavior [15, 16]. In addition, increasing evidence also warns us about the critical role of the microbiota-gut-brain axis in the regulation of emotional processes and behavior. A key study demonstrated that fear extinction learning might be regulated by deleting the gut microbiota through antibiotic administration or in germ-free adult mice [17]. Finally, analyzing the transcriptomic profile in fear-related brain structures including the amygdala, prefrontal cortex, and hippocampus, has revealed specific genes involved in the resistance to extinguish fear memories and, in turn, new potential targets for the treatment of these neuropsychiatric disorders [10, 18].
The main goal of our research was to describe potential factors contributing to the variability in the extinction of fear memories among individuals, considering male and female animals. To this end, we isolated two extreme subpopulations in both sexes based on their extinction behavior, thus identifying resilient and susceptible animals. This approach enabled the identification of substantial inter-individual factors that influence fear extinction, including stress markers, microbiota and transcriptome of the amygdala.
Materials and methods
Animals
Adult C57BL/6 J male and female mice at the age of 8–10 weeks (Charles River) were used in these experiments. In contrast to outbred lines, this inbred strain was selected to address the challenge of isolating epigenetic contributors, which are expected to be highly relevant to individual variability in the extinction of fear memories. Mice were housed 4 per cage in a temperature (21 ± 1 °C)- and humidity (55 ± 10%)-controlled room under a 12 h light/dark cycle (lights on at 8:00 AM). All behavioral studies were performed during the light phase. Mice were acclimated to the facility for 1 week and tests were conducted in alternate weeks in male and female mice. Environmental factors were carefully controlled in order to preserve the same experimental conditions among the different batches. Food and water were available ad libitum. All behavioral data were obtained by experimental observers blinded to the experimental conditions. Experimental procedures were conducted in accordance with the guidelines of the European Communities Directive 2010/63/EU and Spanish Regulations RD 1201/2005 and 53/2013 regulating animal research and approved by the local ethical committee (CEEA-UFV).
Cued fear conditioning and extinction
Mice were individually placed in the test chamber (LE116, Panlab, Harvard Instruments) made of black methacrylate walls with a transparent front door. The box (25 × 25 × 25 cm) was located inside a soundproof module with a ventilation fan to provide background noise and attenuate nearby sounds. The chamber floor was formed by parallel stainless-steel bars of 2 mm of diameter spaced at 6 mm and connected to a shock generator (LE100-26 module, Panlab, Harvard Instruments). A high-sensitivity weight transducer (load cell unit) was used to record the signal generated by the animal movement intensity. Experimental software PACKWIN V2.0 automatically calculated the percentage of immobility time for each experimental phase. Mice were individually conditioned after a 180 s habituation with 3 cue tones (3 kHz, 80 dB) of 20 s long (2 min interval). Each cue tone (conditioned stimulus, CS) co-terminated with a 0.7 mA foot-shock of 1 s duration (unconditioned stimulus, US). Fear extinction sessions (E1-E3) took place 24, 48 and 72 h after the conditioning day in a novel environment (white walls, transparent cylinder, and smooth floor), and after an acclimatation period (120 s in E1 and 60 s in E2 and E3), 20 cue tones (CS) were presented with an interval period of 10 s in absence of electric foot-shock. Freezing behavior, a rodent’s natural response to fear, was automatically evaluated and defined as complete lack of movement, except for breathing for more than 800 ms. This time was chosen based on previous studies [19], and considering the manufacturer’s recommendations of the chamber used in our experiments (LE116, Panlab, Harvard Instruments). The chamber was cleaned with 70% ethanol between each animal trial in order to avoid olfactory cues. Data were expressed as percentage of freezing behavior during the time the sound was active and area under the curve (AUC), which was calculated by using a standard trapezoid method: AUC = [0.5 × (B1 + B2) × h] + [0.5 × (B2 + B3) × h] + … [0.5 × (Bn+ Bn+1) × h], where Bn were de freezing percentages for each mouse and h the time between two consecutive measurements.
Criteria for categorization in resilient and susceptible mice
Two criteria were applied to identify resilient (good extinguishers) and susceptible (poor extinguishers) mice. These criteria were applied independently in the group of male and female mice. The first criterion was based on the freezing in the last extinction session (E3), which threshold was calculated as the freezing average in E3 ± 40% of the mean. The second criterion comprised the percentage of decrease in the freezing behavior between E1 and E3 and the threshold value was fixed in ± 50%. Therefore, resilient mice were those scoring under the low threshold in the first criterion and above the 50% in the second criterion. On the contrary, susceptible mice scored above the top threshold in the first criterion and under 50% in the second criterion.
Experimental design
Sixty male and 62 female mice were exposed to cued fear conditioning and extinction. Animals selected as resilient (n = 8 for females; n = 11 for males) or susceptible (n = 9 for both males and females) were chosen for further experiments. Immediately after E3, fecal samples were collected and frozen at −80 °C until DNA extraction for the microbiota analysis. Fifteen minutes after E3, blood samples were obtained from trunk blood following decapitation. Mice were spaced at short time intervals and all the samples were collected in the morning to avoid variability in corticosterone analysis. The samples were centrifuged at 15000 g for 5 min and the supernatants were collected and frozen at −80 °C until assayed for corticosterone. Amygdala and hypothalamus tissues were also extracted immediately after euthanasia and frozen at −80 °C until used for transcriptome and qPCR analysis. The final number of animals for each biochemical experiment varied, given the different requirements for each one. However, regardless of the experiment, all animals included were obtained from those categorized as resilient or susceptible, as previously detailed. Each experimental sequence was performed once.
Plasma corticosterone measurement
Plasma corticosterone levels were measured in resilient (n = 8 for females; n = 9 for males) and susceptible (n = 7 for females; n = 9 for males) mice. A competitive ELISA with a sensitivity of 2.5 ng/mL was performed according to the manufacturer’s instructions (DRG Instruments GmbH, Germany).
Quantitative RT-PCR analysis
Total RNA was purified from hypothalamic tissues of resilient (n = 8 for females; n = 10 for males) and susceptible (n = 9 for males and females) mice with the RiboPureTM Kit (Invitrogen). Reverse transcription was performed with 1 µg of total RNA and the SuperscriptTM II Reverse Transcriptase (Invitrogen). PCR reactions were conducted using PrimePCRTM Probe Assay (Bio-Rad) to quantify levels of: CRH (ID: qMmuCEP0032005), AVP (qMmuCEP0057424), OX (ID: qMmuCEP0027871), NR3C1 (ID: qMmuCIP0033656), NR3C2 (ID: qMmuCEP0055661), FKBP5 (ID: qMmuCEP0054766), ADCYAP1R1 (ID: qMmuCIP0030184), OX1R (ID: qMmuCIP0030311), OX2R (ID: qMmuCIP0036671), CB1R (ID: qMmuCEP0038879), CB2R (ID: qMmuCEP0039299), CX3CR1 (ID: qMmuCEP0058111) and SGK1 (ID: qMmuCEP0053869). Additionally, GAPDH (ID: qMmuCEP0039581) expression was used as endogenous control gene for normalization. PCR assays were carried out with the CFX Connect RealTime PCR Detection System (Bio-Rad). The fold changes in gene expression of susceptible animals in comparison with resilient mice were calculated using the 2−ΔΔCt method.
Gut microbiome sequencing and bioinformatics
DNA was extracted from resilient (n = 7 for males and females) and susceptible (n = 7 for males; n = 8 for females) mice’s fecal samples using QIAmp Powerfecal DNA kit (Qiagen) following manufacturer’s instructions. The V3-V4 region of the bacteria 16S ribosomal RNA genes was amplified by PCR using primers 341 F (5’-ACACTGACGACATGGTTCTACACCTACGGGNGGCWGCAG-3’) and 785 R (5’- TACGGTAGCAGAGACTTGGTCTGACTACHVGGGTATCTAATCC-3’). Amplicons were validated and quantified by TapeStation (Aligent). An Illumina NextSeq 2000 was used to sequence the DNA to generate paired end 300 bp reads. Sequence analyses and data quality filtering were conducted using QIIME2 version 2023.7 [20], with reads assigned to Amplicon Sequence Variants (ASVs) employing DADA2 [21]. Taxonomy was attributed to ASVs utilizing the q2-feature-classifier plugin [22]. The weighted classifier was constructed from Silva Database version 138.1, encompassing the V3-V4 region, and weighted information was obtained from the Qiita database (https://qiita.ucsd.edu/) by extracting the ‘Animal distal gut’ information based on EMPO samples classification. Sequences lacking matches with any reference were excluded. Data normalization was achieved through rarefaction. ASVs belonging to the same genus were merged. Alpha (Faith’s PD) and beta (Jaccard and Unweighted Unifrac) indices were calculated. Abundance of functional pathways was predicted using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) using a Nearest Sequenced Taxon Index (NSTI) value of 2.0 [23]. Statistical analyses were conducted using QIIME2 version 2023.7 and the R statistical package version 4.3.2, with Phyloseq and microbiomeMarker packages. Differences in alpha diversity between groups were assessed using the Kruskal–Wallis test. The p-values were corrected for multiple testing using the Benjamini-Hochberg false discovery rate (FDR) procedure, with results considered significant for q-values < 0.05. Beta diversity differences between groups were evaluated using both the ADONIS permutation-based statistical test with a significance threshold of Pr(>F) < 0.05 and Permutation-Based Analysis of Variance (PERMANOVA), adjusting p-values with Benjamini-Hochberg FDR. Linear discriminant analysis coupled with effect size (LEfSe v1.0) was employed to identify differences in bacterial abundance between groups and to identify pathways associated with bacteria that exhibited differential representation between groups, using default settings for both analyses. Differential abundance was further assessed with Analysis of Compositions of Microbiomes with Bias Correction (ANCOMBC) [24].
RNA sequencing
Total RNA was purified from amygdala tissues of resilient (n = 4 for males and females) and susceptible (n = 4 for males and females) mice with the RiboPureTM Kit (Invitrogen). RNA integrity > 7 was confirmed by TapeStation (Aligent). Sequencing libraries were prepared using TruSeq Stranded mRNA Sample Prep Kit (Illumina) following manufacturer’s instructions. Libraries were validated by using KAPA Library Quantification Kit for Illumina according to the qPCR Quantification Protocol Guide (KAPA Biosystems) and quantified by TapeStation (Aligent). Libraries were submitted to an Illumina NovaSeq and sequencing was performed using a 2 × 150 bp paired end configuration. Pseudo-alignment and quantification were then made with Salmon algorithm (reference genome GRCh38) (PMID: 28263959). Correlation analysis, principal component study and differential expression analysis were performed with DESeq2 package (PMID: 25516281). Differential gene expression analyses were done using the parametric Wald test, with Benjamini-Hochberg adjustment method (padj). Genes with padj < 0.05 and a cutoff of 1.5 fold change were considered significantly DEGs. Disease-gene association between the DEGs and fear related disorders (“Post-traumatic stress disorder”, “Anxiety”, “Fear”, “Stress”) was performed using the DISEASES database. The z-score in this database is a statistical measure based on the frequency of literature citations linking a gene with a specific disease, highlighting associations that are significantly stronger than what would be expected by chance [25].
Statistical analysis
Before the analysis, all data were checked for normality (Kolmogorov-Smirnov test). Statistical analysis was carried out using unpaired Student t-test, one-way ANOVA and two-way ANOVA of repeated measures followed by Sidak post hoc comparisons after significant interactions between factors. When parametric normality test was violated, Mann-Whitney and Kuskal-Wallis nonparametric tests were used. For contingency graphs, one-tailed chi-square tests were performed. Outliers were excluded if they were > 2 standard deviations from the mean. A p value < 0.05 was used to determine statistical significance. The statistical analysis was performed using GraphPad Prism 9 for each behavioral and molecular experiment as detailed in Table S1.
Results
Individual differences in the extinction of fear in male and female mice
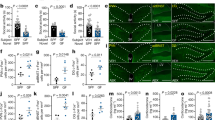
Male and female C57BL/6 J mice were exposed to an auditory fear conditioning paradigm and subsequent extinction protocol for 3 days (Fig. 1A). Freezing levels differed between sexes over extinction (Fig. 1B), although no modifications between males and females were observed during the previous conditioning phase (Fig. S1). Females were more resistant to fear extinction as revealed the increase of freezing behavior (interaction: F11,1320 = 2.271, p < 0.01; sex: F1,120 = 15.34, p < 0.001) and AUC (p < 0.001) in comparison with male animals (Fig. 1B). As expected, male (p < 0.001) and female (p < 0.001) mice froze less by the end of the extinction day 3 (interaction: F1,120 = 10.35, p < 0.01) (Fig. 1C). Notably, freezing behavior was higher in females with regards to male mice (p < 0.001) when comparing the last bin of conditioned stimulus presentation during extinction (Fig. 1C). To further examine the extinction dynamics between sexes, we performed a distribution analysis of males and females according to their freezing levels during the last extinction session (day 3) (Fig. 1D and E). Interestingly, although both clusters of animals showed a normal distribution, there was a clear shift to the right in the female group (Fig. 1E). In fact, when applying the resilience/susceptibility criteria to fear extinction (see Materials and Methods) (Fig. 1A) to the entire population of female and male mice, the percentage of female mice susceptible (bad extinguishers) was significantly higher than in males (11.48% vs 3,28%, x2 = 6.14, p < 0.05) (Fig. S2). As a whole, these results are in agreement with epidemiological studies demonstrating that the prevalence of PTSD is higher in women than in men [26], as PTSD patients often show impairment of fear extinction [27].
A Schematic representation of the experimental design. B Time course of the freezing levels and AUC values during cued fear extinction trials (n = 60–62 mice per group). Four bins (5 tones each) of CS presentations are shown in each extinction session. C Measure of the extinction (freezing levels) using the first bin (5 tones, extinction day 1) and the last bin (5 tones, extinction day 3) of CS presentations. D, E Distribution analysis of average freezing levels in E3 in male D and female E mice. F, I Schematic representation of the subpopulations of resilient and susceptible male F and female I mice considering the classification criteria used. G, J Time course of the freezing levels and AUC values during cued fear extinction trials in male G and female J mice considering the classification criteria. Four bins (5 tones each) of CS presentations are shown in each extinction session. H, K Measure of the extinction (freezing levels) using the first bin (5 tones, extinction day 1) and the last bin (5 tones, extinction day 3) of CS presentations in resilient and susceptible male H and female K mice considering the classification criteria (n = 8–11 mice per group). Data are expressed as mean + SEM. $$$p < 0.001 (comparison between male and female mice); *p < 0.05, ***p < 0.001 (comparison between the first bin (CS 1–5) and the last bin (CS 56–60) in (C), (H) and (K)); **p < 0.01, ***p < 0.001 (comparison between intermediate and susceptible mice in (G) and (J)); &&p < 0.01, &&&p < 0.001 (comparison between intermediate and resilient mice in (G) and (J)). &&&p < 0.001 (comparison of the last bin (CS 56–60) between male and female mice in (C); comparison of the last bin (CS 56–60) between resilient and susceptible mice in (H) and (K)). #p < 0.05; ##p < 0.01; ###p < 0.001 (comparison between resilient and susceptible mice). AUC, area under the curve; CS, conditioned stimulus; E3, extinction day 3.
Given the normal distribution in the fear extinction behavior, we isolated two distinct extreme phenotypic subgroups (resilient and susceptible) in both male and female mice by applying specific criteria on the extinction profile (Fig. 1F and I). As shown in Fig. 1G, freezing levels during extinction presented clear differences between phenotypes in male mice revealed by two-way ANOVA (phenotype effect: F2,57 = 33.72, p < 0.001: interaction: F22,627 = 2.43, p < 0.001) and AUC values (p < 0.001, resilient versus susceptible mice). Accordingly, freezing behavior was higher in susceptible in comparison with resilient male mice (p < 0.001) when analyzing the last cluster of cues presentation the day 3 of extinction (interaction: F1,18 = 14.40, p < 0.01) (Fig. 1H). Similarly, fear extinction was different in resilient and susceptible female mice as revealed two-way ANOVA (phenotype effect: F2,59 = 13.98, p < 0.001; interaction: F22,649 = 3.89, p < 0.001) and AUC values (p < 0.001, resilient versus susceptible mice) (Fig. 1J). The analysis of the last bin of conditioned stimulus presentation also showed a clear difference between phenotypes (interaction: F1,15 = 5.53, p < 0.05) in female mice (p < 0.001) (Fig. 1K). A significant increase in the freezing behavior of all the experimental groups during the first conditioned stimulus in comparison with the previous habituation was observed, thus highlighting the discrimination between the absence and the presence of the conditioned stimulus (Fig. S3).
To explore resilience and vulnerability factors underlying inter-individual differences in fear extinction, we performed corticosterone, hypothalamic gene expression, microbiome and transcriptome analysis in the selected population of resilient/susceptible mice.
Corticosterone and hypothalamic gene expression analysis in resilient and susceptible male and female mice
Stress has a critical role in the development and expression of many psychiatric disorders including those related to the presence of pathological fear [28]. The hypothalamus is critical in initiating hormonal responses to stressful stimuli via the HPA axis (Fig. 2A), and consequently, hypothalamic functions have been attributed to the pathophysiology of fear-related disorders [29]. Notably, plasma corticosterone levels were higher in susceptible male mice in comparison with resilient animals (p < 0.01) (Fig. 2B). Moreover, corticotropin-releasing hormone (CRH) mRNA level was also higher (p < 0.05) (Fig. 2C) in susceptible male mice. Congruent with this, NR3C1 gene expression, encoding the glucocorticoid receptor, decreased in susceptible animals (p < 0.05) (Fig. 2D) suggesting the existence of an impaired negative feedback on the HPA axis. No modification was found in the mineralocorticoid receptor gene expression (NR3C2) (Fig. 2E). We next analyzed the expression of other hypothalamic neuropeptides involved in stress response. An interesting increase of prepro-orexin mRNA level was observed in susceptible male mice (p < 0.01) (Fig. 1G), while no changes were found between groups in the expression of arginine vasopressin (AVP) (Fig. 1F). Other receptors and factors involved in fear regulation such as cannabinoid receptors (CB1R and CB2R), orexin receptors (OX1R and OX2R), pituitary adenylate cyclase-activating polypeptide type 1 receptor (ADCYAP1R1), CX3C motif chemokine receptor 1 (CX3CR1), serum/glucocorticoid regulated kinase 1 (SGK1) and FK506 binding protein 5 (FKBP5) were also evaluated. An almost statistically significant increase in the expression of CB2R (p = 0.06) was observed in susceptible mice (Fig. 2I), while CX3CR1 mRNA level was also enhanced (p < 0.05) in this group of animals (Fig. S3C). No changes were observed in the rest of receptors/factors analyzed (Fig. 2 and Fig. S4).
A Schematic representation of the HPA axis. B, K Plasma corticosterone levels in resilient and susceptible male B and female K mice (n = 7–9 mice per group). C–S mRNA levels of CRH, NR3C1, NR3C2, AVP, OX, CB1R, CB2R, and ADCYAP1R1 in resilient and susceptible male C–J and female L–S mice (n = 7–10 mice per group). Data are represented as mean + SEM. *p < 0.05, **p < 0.01 (comparison between resilient and susceptible mice). HPA, hypothalamic-pituitary-adrenal; CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone; AVP arginine vasopressin; NR3C1, glucocorticoid receptor; NR3C2, mineralocorticoid receptor; OX prepro-orexin; CB1R, cannabinoid receptor-1; CB2R, cannabinoid receptor-2; ADCYAP1R1 pituitary adenylate cyclase-activating polypeptide type I receptor; E3, extinction day 3.
Regarding female mice, CRH expression was also higher in susceptible in comparison with resilient animals (p < 0.05) (Fig. 2L). However, this enhancement of CRH mRNA was not associated with alterations in plasma corticosterone concentration (Fig. 2K). We also observed a clear trend of increase in the expression of ADCYAP1R1 in susceptible female animals (p = 0.07) (Fig. 2S). No modifications were found in the other receptors/factors analyzed (Fig. 2 and Fig. S4). These results suggest a sex-dependent alteration in hypothalamic factors contributing to individual differences in fear extinction.
Gut microbiome analysis in resilient and susceptible male and female mice
Gut-brain communication is implicated in cognition, social behavior, fear expression, and stress response [30], and probiotic supplementation has been widely investigated for its potential use improving mood and anxiety [31]. The analysis of the alpha-diversity (Faith’s PD, fraction of a phylogenetic tree represented in each sample), revealed an interesting decrease in susceptible male mice in comparison with resilient animals (p < 0.05) (Fig. 3A), while no changes were observed in female mice (Fig. 3I). Regarding beta-diversity assessment, we also found significant differences in Unweighted UniFrac distance (p < 0.05) (Fig. 3B) and Jaccard distance (p < 0.05) (Fig. 3C) between susceptible and resilient male mice. Principal coordinate analysis plot based on these indices revealed a clear separation between groups (Fig. 3D and E). However, no differences were found in beta-diversity in the case of females, as showed the analysis of Unweighted UniFrac and Jaccard metrics (Fig. 3J and K) and the principal coordinate analysis (Fig. 3L and M). Aside from general differences in the microbiome diversity, we also analyzed the microbiota composition in the different groups of male and female mice by representing the distribution of relative abundance of gut microbiota at the family level (Fig. 3F and N). To further identify which specific taxa is enriched and/or decreased in resilient and susceptible male and female mice, we carried out linear discriminant effect size (LEfSe) assessment. This analysis showed some bacterial groups enriched in resilient male animals (Fig. 3G), including the genera Parvibacter, Alloprevotella and Limosilactobacillus, the family Christensenellaceae and the order Christensenellales. ANCOM-BC (analysis of compositions of microbiomes with bias correction) confirmed the presence of the genus Parvibacter in resilient, but not in susceptible male animals (Fig. 3H). Details of the relative abundance of the taxa enriched in resilient and susceptible male mice is shown in Fig. S5. Moreover, abundance of functional metabolic pathways in these subpopulations of male mice (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, PICRUSt2) is included in Fig. S6.
A, I Alpha diversity of resilient and susceptible male (n = 7 mice per group) A and female (n = 7–8 mice per group) I mice using Faith’s PD metric. B, J Beta diversity of resilient and susceptible male B and female J mice using unweighted UniFrac distance. C, K Beta diversity of resilient and susceptible male C and female K mice using Jaccard distance. D–M Three-dimensional principal coordinate analysis based on unweighted UniFrac and Jaccard distances in resilient and susceptible male D, E and female L, M mice. F, N Microbial distribution of relative abundance at the family level in resilient and susceptible male F and female N mice. G, O Bacterial groups enriched in resilient and susceptible male G and female O mice by using LEfSe analysis. H Enriched bacterial group in susceptible and resilient male mice by using ANCOM-BC analysis. *p < 0.05 (comparison between resilient and susceptible male mice).
Regarding female mice, LEfSe analysis revealed an enrichment of the genus Muribaculum and the family Eggerthellaceae in, respectively, resilient and susceptible animals (Fig. 3O). Taken together, these results indicate the existence of alterations in the gut microbiota diversity and composition between good and bad extinguishers of fear, which were more substantial in male subjects.
Transcriptome analysis in resilient and susceptible male and female mice
Given the importance of the amygdala as the central regulator of fear extinction, we used RNA-Seq to examine the molecular profile of this brain region in resilient and susceptible males and females (Fig. 4A). Resilient animals were used as controls in these experiments. RNA-Seq identified 31 differentially expressed genes (DEGs) (adjusted p < 0.05 and cutoff of 1.5 fold change) between resilient and susceptible male animals (Fig. 4B). Fourteen were increased and 17 were decreased in susceptible male mice (Fig. 4B) in comparison with resilient mice. Surprisingly, only one gene was upregulated in susceptible females (Fig. 4B). Further principal component analysis (PCA) was sufficient to separate male mice (Fig. 4C), but not females (Fig. 4G), into groups corresponding to their ability for fear extinction. The PC1 variances, the amount of variability in a data set that can be attributed to first principal component, were 54% for males and 39% for females (Fig. 4C and G). Volcano plots (p value by fold changes, in log2 scale) of DEGs are shown in Fig. 4D (males) and Fig. 4H (females), confirming the existence of a different pattern of gene expression in males resistant to fear extinction. DEGs in each resilient and susceptible male mouse were clustered with a heat map (Fig. 4E). A disease-gene association analysis using the DISEASES database revealed that 14 DEGs in susceptible male mice were previously associated with PTSD, anxiety, fear, or stress disorders (Fig. 4F). In summary, a sex-dependent gene expression profile, in a key region of the fear circuit, was associated with a fear extinction vulnerability phenotype.
A Schematic representation of the experimental design. B DEGs between resilient and susceptible male and female mice (n = 4 mice per group). C, G Principal component analysis in resilient and susceptible male C and female G mice. D, H Volcano plot summarizing DEGs of resilient and susceptible male D and female H mice. E DEGs in each resilient and susceptible male mouse clustered with a heat map. F Disease-gene association analysis using the DISEASES database reveals that 14 DEGs in susceptible male mice were previously associated with PTSD, anxiety, fear, or stress disorders. DEGs, differentially expressed genes.
Discussion
The knowledge of the neurobiological factors involved in inter-individual differences in the ability to extinguish fear is crucial to understand the vulnerability to develop fear-related disorders, as well as to discover new potential targets for the treatment of these diseases. Here, we describe important inherent group differences in male and female mice considering their individual variation in cued-fear extinction performance.
The behavioral model herein proposed represents a powerful and reliable tool to study individual differences in fear extinction. Using an auditory-cued fear extinction paradigm, we clearly obtained two different subpopulations of good (resilient) and poor (susceptible) extinguishers in male and female mice considering their extinction patterns. In this model, animals with similar fear memory consolidation show individual differences in the extinction process that were inherent to the animals and observed in the absence of any genetic or environmental manipulation. Interestingly, female mice presented an overall higher resistance to extinguish fear in comparison with males, in agreement with previous studies in rodents [32, 33]. Several factors seem to contribute to this effect including sex-specific differences in brain structures and neuronal circuits, molecular mechanisms, or gonadal hormones [26, 34]. Importantly, women have a twofold likelihood of developing PTSD compared to men [26, 35]. Despite the clear clinical relevance of studying changes associated with inter-individual differences in the extinction of fear memories in male and female mice, most of the pre-clinical research has exclusively used male animals. We used male and female mice to analyze potential differences in individual susceptibility to fear extinction within sexes.
Fear memories processing is greatly influenced by the HPA axis through its effects on stress-related hormones. For example, impairment of the HPA axis with dexamethasone leads to enhanced fear extinction in adult male mice [36]. Notably, we observed here that CRH expression, the first activator of the HPA axis rapidly synthesized and released during stress events, was substantially enhanced in susceptible male mice which may probably give rise to the observed increase in the corticosterone tone in these animals. Consistently, hypothalamic NR3C1 gene expression, encoding the glucocorticoid receptor, decreased in susceptible males possibly leading to impaired negative feedback control of the HPA-axis [37]. In vulnerable female mice, the increased expression of CRH was not associated with changes in plasma corticosterone levels, suggesting that gonadal hormones could have an influence in the modulation of the HPA axis [38]. Moreover, corticosterone levels in resilient female mice were higher in comparison with resilient males. Therefore, a possible ceiling effect could also mask an increase in corticosterone levels in susceptible female mice. Alterations of the HPA axis in individuals with PTSD are thought to contribute to the persistent and chronic nature of the disorder, although its activity can vary since it is not always consistently hyperactive or hypoactive [39]. On the other hand, current evidence indicates that glucocorticoid receptor mRNA levels remain stable or decrease after stress exposure in a sex-specific manner [40, 41] which, in turn, could be related to a pro-inflammatory tone given the important role of these receptors in the modulation of inflammation and immune responses [42]. Interestingly, the reduced glucocorticoid receptor expression revealed in susceptible male mice was associated with increased hypothalamic mRNA levels of CX3CR1, a general microglial marker usually related to neuroinflammatory states [43]. We also found a robust increase in the expression of the common precursor gene for both orexin-A (OXA) and orexin-B (OXB) neuropeptides in susceptible male, but not female, animals. In agreement, orexins seem to play a key role in the regulation of fear memory and anxiety [44]. OXA administration impaired fear extinction [45], while OX1R antagonism facilitated this response in male rodents [45, 46]. Accordingly, the activity of orexin neurons was negatively correlated with successful extinction of conditioned fear in male rats [9]. Most of the studies in orexin research have been conducted only in male individuals which is a clear limitation, as constitutive sex differences in the orexin system have already been reported [47]. CB2R expression showed a clear trend to increase in susceptible males, which is interesting due to the recent involvement of this cannabinoid receptor subtype in fear extinction deficits induced by OXA in male mice [19]. A single nucleotide polymorphism in ADCYAP1R1 gene has been suggested as a specific biomarker of PTSD in women but not men [48], and female mice subjected to acute stress immobilization show fear extinction impairments related to ADCYAP1R1 mRNA upregulation in the hypothalamus [49]. Compatible with this, we found a trend of increased ADCYAP1R1 expression in the hypothalamus of susceptible female, but not male, mice.
In recent years, there have been major advances in elucidating the involvement of the gut-brain axis in the regulation of fear memories, with special attention to the extinction process [17]. Indeed, most of the studies examine the neurobehavioral consequences of modifying the gut microbiome through the administration of either antibiotics [17, 50] or probiotics [51]. We analyzed the gut microbiota of male and female naïve mice with opposite response in the extinction of fear memories, without adding any experimental condition (e.g., pharmacological treatment, early stress or genetic alteration). Notably, our results revealed clear differences in the diversity and structure of the gut microbiota between good- and poor-extinguishers male mice. In this sense, we found bacterial groups enriched in resilient mice belonging to the Firmicutes phylum, including Limosilactobacillus genus and Christensenellaceae family. Limosilactobacillus species are described to present beneficial properties and have been used as probiotics [52], while Christensenellaceae is considered as a signature of a healthy gut, and its abundance is reduced in patients with general anxiety disorder [53]. Also, Parvibacter (Actinobacteria phylum) and Alloprevotella (Bacteroidetes phylum) genera, both enriched in resilient male mice, have been related to a positive reduction of low-density lipoprotein-cholesterol and alleviation of metabolic liver damage during oxidative stress [54]. Parvibacter, which has not previously been directly linked to psychiatric illness, requires special attention since it was the only group significantly enriched in resilient males by using additional ANCOM-BC statistical methodology, a more restrictive test with bias correction. Related to our data, decreased total abundance of the phylum Actinobacteria, in which is included the genus Parvibacter, was associated with PTSD status [55]. Further research will be necessary to confirm the lack of Parvibacter as a susceptibility factor to develop fear-related disorders. On the other hand, given the bidirectional modulation of gut microbiome and sex hormones, mainly estrogen [56], females present significant variation between microbial communities, and this may probably hide engaging differences in the microbiota composition between resilient and susceptible female mice. However, despite the lack of significant differences in the overall microbiota composition, we could detect relevant bacterial taxa enrichment in female animals. Muribaculum genus (Bacteroidetes phylum), whose reduction has recently been associated with anxiety-like behaviors induced by sleep deprivation [57], was enriched in resilient female mice, whereas significant increase of the enteritis-related Eggerthellaceae family (Actinobacteria phylum) [58] was observed in susceptible females.
The third key element that might shed light on the unrevealed fear extinction variability is the transcriptomic profile, even more challenging considering both sexes. In male mice, transcriptomic analyses highlighted the existence of a different transcriptional footprint in the amygdala of resilient and susceptible animals, in agreement with previous studies [10, 59]. Taking these results into account, a very recurring question emerges: genetics or environment? The development of a “traumatic” memory and the subsequent recovery depends on the pressure exerted by the environmental conditions on our genome expression, thus arising epigenetics. In our study, we used identical subjects (same strain, origin, and experimental conditions) that were supposed to encode very similar genome and transcriptome, although this question was not addressed. Hence, experience-dependent epigenetic changes may have accurately influenced the expression of specific genes and, in turn, fear extinction behavior. Increasing evidence points to histone modifications like methylation [60], as a hallmark to provide resilience or susceptibility for the extinction of aversive memories, given the monitoring role over the expression of numerous fear-related genes [61]. Indeed, RNA-seq revealed several DEGs in resilient and susceptible male mice, some of them previously involved in fear-related disorders (e.g., PTSD, panic attacks, phobias), as confirmed using DISEASES database. It is also noteworthy that some of the identified DEGs are expressed in the dendritic tree (Ntrk1, Slc17a8, Ppp1r1b, Grik1, Pura and Epha6), which adequate branching and arborization are essential to develop efficient synapsis to correctly extinguish fear episodes [62]. Particularly, the strongest DEG Ntrk1 (negligible expression in resilient animals and highly increased in the susceptible group), which is involved in the regulation of proliferation, differentiation, and survival of sympathetic and nervous neurons, may become an interesting target to further explore within fear-related disorders. This gene participates in the creation of new synapsis and has been recently reported as a key player in hippocampal neuronal damage [63]. In contrast to male mice, females did not present remarkable differences in their transcriptomic profile related to their fear extinction behavior. As previously discussed, female mice were included without regard to estrus cycle phase, which is known to alter the expression of different microRNA in the amygdala [64], thus probably increasing the variability among individuals. Additionally, it is important to mention the reduced number of studies with female subjects, and more specifically analyzing the transcriptomic signatures in the extinction of fear memories. Recent research suggests sex-specific differences in the mouse amygdala transcriptional response during fear acquisition [18], thus involving the necessity of including female subjects in further fear extinction-related studies to fill this gap of knowledge. However, it is worth noting the challenges related to research conducted with females given the considerable variability – especially regarding behavior experiments – compared to male subjects, which gives rise to marked difficulties to achieve significant results and, in turn, clear conclusions [65, 66]. This is most likely one of the main reasons for the limited number of studies involving females, although some reviews and meta-analysis point in the opposite direction [67, 68]. In addition, the increased costs associated with the use of both sexes may also limit the inclusion of female individuals. Despite these issues, our study includes male and female mice, which underwent exactly the same procedure in the behavioral experiments and were subjected to the same criteria in all the biochemical analysis, aiming to achieve a greater translational relevance in the obtained results.
In summary, the integrated approach of our study advances in the understanding of the substrates that support differences between adaptive or maladaptive fear extinction behaviors, with the goal of identifying targets that may be suitable for alleviating the persistence of traumatic memories in both sexes.
Data availability
Raw data corresponding to gut microbiome analyses were deposited at the NCBI Sequence Read Archive (SRA), ID number: PRJNA1104437. Raw data corresponding to RNA sequencing analyses were also deposited at the NCBI SRA, ID number: PRJNA1104832.
References
De Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci. 2017;18:7–19.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed., text rev. 2013. https://doi.org/10.1176/appi.books.9780890425596.
Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, et al. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Front Psychiatry. 2020;11:595584.
Thakur A, Choudhary D, Kumar B, Chaudhary A. A review on post-traumatic stress disorder (PTSD): symptoms, therapies and recent case studies. Curr Mol Pharmacol. 2022;15:502–16.
Rauch SAM, Eftekhari A, Ruzek JI. Review of exposure therapy: a gold standard for PTSD treatment. J Rehabil Res Dev. 2012;49:679–88.
Shalev AY, Ankri Y, Gilad M, Israeli-Shalev Y, Adessky R, Qian M. et al. Long-term outcome of early interventions to prevent posttraumatic stress disorder. J Clin Psychiatry. 2016;77:e580–e587.
Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51:1137–48.
Kessler RC, Wai TC, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27.
Sharko AC, Fadel JR, Kaigler KF, Wilson MA. Activation of orexin/hypocretin neurons is associated with individual differences in cued fear extinction. Physiol Behav. 2017;178:93–102.
Sillivan SE, Joseph NF, Jamieson S, King ML, Chévere-Torres I, Fuentes I, et al. Susceptibility and resilience to posttraumatic stress disorder-like behaviors in inbred mice. Biol Psychiatry. 2017;82:924–33.
Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31.
Merz CJ, Wolf OT. How stress hormones shape memories of fear and anxiety in humans. Neurosci Biobehav Rev. 2022;142:104901.
Stockhorst U, Antov MI. Modulation of fear extinction by stress, stress hormones and estradiol: a review. Front Behav Neurosci. 2016;9:359.
Speer KE, Semple S, Naumovski N, D’Cunha NM, McKune AJ. HPA axis function and diurnal cortisol in post-traumatic stress disorder: a systematic review. Neurobiol Stress. 2019;11:100180.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA. 2008;105:12004–9.
Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, et al. Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Biol Psychiatry. 2016;80:345–55.
Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM. et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574:543–8.
Reis ALM, Hammond JM, Stevanovski I, Arnold JC, McGregor IS, Deveson IW, et al. Sex-specific transcriptomic and epitranscriptomic signatures of PTSD-like fear acquisition. iScience. 2022;25:104861.
Ten-Blanco M, Flores A, Pereda-Pérez I, Piscitelli F, Izquierdo-Luengo C, Cristino L, et al. Amygdalar CB2 cannabinoid receptor mediates fear extinction deficits promoted by orexin-A/hypocretin-1. Biomed Pharmacother. 2022;149:112925.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:1091.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:90.
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685–8.
Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020;11:3514.
Pletscher-Frankild S, Pallejà A, Tsafou K, Binder JX, Jensen LJ. DISEASES: text mining and data integration of disease-gene associations. Methods. 2015;74:83–89.
Velasco ER, Florido A, Milad MR, Andero R. Sex differences in fear extinction. Neurosci Biobehav Rev. 2019;103:81–108.
Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–63.
Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79.
Raise-Abdullahi P, Meamar M, Vafaei AA, Alizadeh M, Dadkhah M, Shafia S, et al. Hypothalamus and post-traumatic stress disorder: a review. Brain Sci. 2023;13:1010.
Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-gut-brain axis: new therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2020;60:477–502.
Berding K, Cryan JF. Microbiota-targeted interventions for mental health. Curr Opin Psychiatry. 2022;35:3–9.
Clark JW, Drummond SPA, Hoyer D, Jacobson LH. Sex differences in mouse models of fear inhibition: Fear extinction, safety learning, and fear-safety discrimination. Br J Pharmacol. 2019;176:4149–58.
Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem. 2014;21:55–60.
Bauer EP. Sex differences in fear responses: neural circuits. Neuropharmacology. 2023;222:109298.
Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204.
Sawamura T, Klengel T, Armario A, Jovanovic T, Norrholm SD, Ressler KJ, et al. Dexamethasone treatment leads to enhanced fear extinction and dynamic Fkbp5 regulation in amygdala. Neuropsychopharmacology. 2016;41:832–46.
van der Knaap LJ, Oldehinkel AJ, Verhulst FC, van Oort FVA, Riese H. Glucocorticoid receptor gene methylation and HPA-axis regulation in adolescents. The TRAILS study. Psychoneuroendocrinology. 2015;58:46–50.
Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20:476–94.
Skolariki K, Vrahatis AG, Krokidis MG, Exarchos TP, Vlamos P. Assessing and modelling of post-traumatic stress disorder using molecular and functional biomarkers. Biology (Basel). 2023;12:1050.
Mifsud KR, Saunderson EA, Spiers H, Carter SD, Trollope AF, Mill J, et al. Rapid down-regulation of glucocorticoid receptor gene expression in the dentate gyrus after acute stress in vivo: role of DNA methylation and microRNA activity. Neuroendocrinology. 2017;104:157–69.
Karandrea D, Kittas C, Kitraki E. Forced swimming differentially affects male and female brain corticosteroid receptors. Neuroendocrinology. 2002;75:217–26.
Fadel L, Dacic M, Fonda V, Sokolsky BA, Quagliarini F, Rogatsky I, et al. Modulating glucocorticoid receptor actions in physiology and pathology: Insights from coregulators. Pharmacol Ther. 2023;251:108531.
Subbarayan MS, Joly-Amado A, Bickford PC, Nash KR. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol Ther. 2022;231:107989.
Flores Á, Saravia R, Maldonado R, Berrendero F. Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci. 2015;38:550–9.
Flores Á, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 2014;39:2732–41.
Salehabadi S, Abrari K, Elahdadi Salmani M, Nasiri M, Lashkarbolouki T. Investigating the role of the amygdala orexin receptor 1 in memory acquisition and extinction in a rat model of PTSD. Behav Brain Res. 2020;384:112455.
Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol Psychiatry. 2017;81:683–92.
Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7.
Velasco ER, Florido A, Flores A, Senabre E, Gomez-Gomez A, Torres A, et al. PACAP-PAC1R modulates fear extinction via the ventromedial hypothalamus. Nat Commun. 2022;13:4374.
Geary CG, Wilk VC, Barton KL, Jefferson PO, Binder T, Bhutani V, et al. Sex differences in gut microbiota modulation of aversive conditioning, open field activity, and basolateral amygdala dendritic spine density. J Neurosci Res. 2021;99:1780–801.
Cui X, Zhou S, Xia G, Chen J, Jiang L, Huang J, et al. A multispecies probiotic accelerates fear extinction and inhibits relapse in mice: role of microglia. Neuropharmacology. 2021;193:108613.
Abuqwider J, Altamimi M, Mauriello G. Limosilactobacillus reuteri in health and disease. Microorganisms. Microorganisms. 2022;10:522.
Dong Z, Shen X, Hao Y, Li J, Li H, Xu H, et al. Gut microbiome: a potential indicator for differential diagnosis of major depressive disorder and general anxiety disorder. Front Psychiatry. 2021;12:651536.
Wu L, Liu X, Hu R, Chen Y, Xiao M, Liu B, et al. Prebiotic Agrocybe cylindracea crude polysaccharides combined with Lactobacillus rhamnosus GG postpone aging-related oxidative stress in mice. Food Funct. 2022;13:1218–31.
Hemmings SMJ, Malan-Müller S, Van Den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: An exploratory study. Psychosom Med. 2017;79:936–46.
Maeng LY, Beumer A. Never fear, the gut bacteria are here: Estrogen and gut microbiome-brain axis interactions in fear extinction. Int J Psychophysiol. 2023;189:66–75.
Yang DF, Huang WC, Wu CW, Huang CY, Yang YCSH, Tung YT. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol Res. 2023;268:127292.
Li Y, Yan H, Zhang Y, Li Q, Yu L, Li Q, et al. Alterations of the gut microbiome composition and lipid metabolic profile in radiation enteritis. Front Cell Infect Microbiol. 2020;10:541178.
Laricchiuta D, Gimenez J, Sciamanna G, Termine A, Fabrizio C, Della Valle F, et al. Synaptic and transcriptomic features of cortical and amygdala pyramidal neurons predict inefficient fear extinction. Cell Rep. 2023;42:113066.
Barchiesi R, Chanthongdee K, Petrella M, Xu L, Söderholm S, Domi E, et al. An epigenetic mechanism for over-consolidation of fear memories. Mol Psychiatry. 2023;28:963.
Ell MA, Schiele MA, Iovino N, Domschke K. Epigenetics of fear, anxiety and stress - Focus on histone modifications. Curr Neuropharmacol. 2024;22:843–65.
Luchkina NV, Bolshakov VY. Mechanisms of fear learning and extinction: synaptic plasticity-fear memory connection. Psychopharmacology (Berl). 2019;236:163–82.
Yang K, Wu J, Li S, Wang S, Zhang J, Wang YP, et al. NTRK1 knockdown induces mouse cognitive impairment and hippocampal neuronal damage through mitophagy suppression via inactivating the AMPK/ULK1/FUNDC1 pathway. Cell Death Discov. 2023;9:404.
Hirsch MM, Brusco J, Vaccaro T, Margis R, Moreira JE, Gottfried C, et al. Sex differences and estrous cycle changes in synaptic plasticity-related microRNA in the rat medial amygdala. Neuroscience. 2018;379:405–14.
Bridgewater LC, Zhang C, Wu Y, Hu W, Zhang Q, Wang J, et al. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci Rep. 2017;7:10776.
Saravia R, Ten-Blanco M, Julià-Hernández M, Gagliano H, Andero R, Armario A, et al. Concomitant THC and stress adolescent exposure induces impaired fear extinction and related neurobiological changes in adulthood. Neuropharmacology. 2019;144:345–57.
Beery AK. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci. 2018;23:143–9.
Smarr B, Kriegsfeld LJ. Female mice exhibit less overall variance, with a higher proportion of structured variance, than males at multiple timescales of continuous body temperature and locomotive activity records. Biol Sex Differ. 2022;13:41.
Acknowledgements
We thank Alba Blesa for her advice in microbiota experiments, Víctor Sánchez-Arévalo for his help with transcriptomics, and Dreamgenics (Oviedo, Spain) for helping with the bioinformatics analyses.
Funding
This work was supported by MICIU/AEI/10.13039/501100011033 and FEDER, UE/ Grant [PID2023-151223OB-I00] and MICIU/AEI/10.13039/501100011033/ Grant [PID2020-116579RB-I00], and “Proyectos de Investigación Universidad Francisco de Vitoria (2024–2025)”. C.I-L is supported by a predoctoral fellowship from Universidad Francisco de Vitoria. M.P-R is supported by a predoctoral fellowship from MICIU.
Author information
Authors and Affiliations
Contributions
MT-B, MP-R, and FB designed research; MT-B, MP-R, IP-P, CI-L, and RMT performed behavioral and biochemical experiments; OZ and CB performed computational analysis; MT-B, MP-R, and FB analyzed data; MT-B, MP-R, and FB wrote the paper, with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ten-Blanco, M., Ponce-Renilla, M., Pereda-Pérez, I. et al. Exploring individual differences in fear extinction in male and female mice: insights from HPA axis, microbiota, and transcriptomics. Transl Psychiatry 15, 195 (2025). https://doi.org/10.1038/s41398-025-03400-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03400-9