Abstract
This single-center, retrospective study analyzed vaccine responses in patients who received post-Chimeric Antigen Receptor (CAR) T-cell therapy vaccination between 2018 and 2024. Vaccinations were administered according to EBMT/CIBMTR recommendations and pathogen-specific IgG responses to 12 vaccine-preventable infections were assessed. Seroprotection was defined by established cut-offs or a significant fold increase in titers. A total of 73 patients that had not received intravenous immunoglobulins within the eight weeks prior to pre- or post titer were included. The median time to vaccination initiation was 13 months (range 6–66) post-CAR T. Pre and post-vaccination titers were available for 49 patients. Pre-vaccination seroprotection was high (> 85%) for tetanus and poliovirus. Among patients not seroprotected prior to vaccination, vaccine response rates were high for tetanus and polio (100%), moderate for diphtheria (75%) and haemophilus influenzae type b (62%), and lower for pertussis (48%), hepatitis A (43%), hepatitis B (44%), and pneumococcal disease (33%). CD19 CAR T recipients had higher pre-vaccination seroprotection rates than BCMA recipients, but vaccine responses did not differ significantly between groups. Pre-vaccination IgA levels were significantly associated with vaccine response, and absolute B-cell counts trended higher among responders (p = 0.054). Our findings highlight the importance of immune reconstitution in vaccine responses post-CAR T.
Similar content being viewed by others
Introduction
Infections are frequent, associated with significant morbidity and are the leading cause of non-relapse mortality following CAR T-cell therapy [1,2,3,4,5,6]. Patients treated with CAR T-cell therapy have a multifactorial immune deficiency resulting from the underlying disease, preceding antitumor therapies, and CAR T-cell therapy-related factors including lymphodepleting chemotherapy and “on-target/off-tumor” depletion of non-malignant B cells expressing the CAR T-cell targets [7]. After CD19 CAR T-cell therapy, CD19 + B cell aplasia is nearly universal and may persist for years [8]. CD19 is highly expressed in naïve and memory B cells, whereas expression is absent or reduced in certain types of plasma cells. After BCMA CAR T-cell therapy, there is a specific depletion of plasma cells expressing BCMA, however BCMA is not expressed on earlier B cell subsets. Thus, CD19 versus BCMA CAR T-cell recipients have distinct humoral deficiencies.
Vaccination is one of the key tools for infectious prevention, but there are limited data on vaccine immunogenicity post CAR T and the best strategy or timing for initiating vaccination after treatment are not well established. The main data published refers to the mRNA vaccines against COVID, with response rates from 27 to 34% following primary vaccination (three doses) among recipients of CD19 CAR [9, 10]. One study examined responses to influenza vaccine in 26 recipients of CAR T-cell therapy showing response rates of 35% [11].
Data on vaccine responses in patients receiving BCMA-targeted therapies are limited to subsets in larger vaccine cohorts of myeloma patients, where BCMA-directed therapies, mainly bispecific antibody treatments, have been found to be predictors of poor vaccine responses to COVID-19 mRNA vaccines [12,13,14]. Differences in immune defects resulting from CD19- and BCMA-targeted therapy were elegantly illustrated by Walti et al. in a study of 30 patients, where BCMA recipients were half as likely to have preserved seroprotection rates against 12 common vaccine-preventable infections post-CAR T-cell therapy compared with CD19 CAR T-cell recipients [15]. However, when comparing vaccine responses between CD19 and BCMA-targeted CAR T-cells recipients, a recent study found superior COVID-19 mRNA-vaccine response among recipients of BCMA-directed CAR T compared to recipients of CD-19-directed CAR T [16].
While vaccinations have been recommended to reduce infection risk among CAR T-cell therapy recipients since the approval of these therapies, few studies have systematically assessed immune responses or evaluated the clinical efficacy of vaccination among CAR T-recipients. A recent study found that 31% of infections post BCMA CAR T-cell therapy are so called vaccine preventable [17], highlighting the importance of studying vaccine responses post CAR.
The aim of the current study was to investigate humoral immunity and vaccine responses to 12 vaccine-preventable infections in patients treated with CAR T-cell therapy.
Methods
Patients and study design
All consecutive patients with lymphoma and myeloma treated with CAR T-cell therapy at MSKCC from 2018 to 2024 were reviewed for study inclusion. Patients eligible for this retrospective analysis included recipients of a commercial CAR T product who had been vaccinated with one of the vaccines of interest and had a pre-vaccine titer drawn. Patients who had received intravenous immunoglobulin (IVIG) within eight weeks prior to the pre- or post-vaccination titer were excluded. The pre-titer was drawn prior to the first vaccine dose, no samples were available prior to CAR T.
All serological assessments included in this study were obtained as part of routine clinical care. These results were retrospectively reviewed and compiled for the purpose of the current analysis. As such, the number of available samples varies between vaccine antigens, reflecting differences in clinical testing practices, vaccine eligibility, and timing of patient follow-up.
Serological titers post vaccination were rechecked after the patient’s last dose of each vaccine for a subgroup of patients. The vaccine schedule used at MSKCC is shown in Supplementary Table 1. Permission to use clinical and laboratory information was obtained from the Institutional Review Board.
Vaccines and definitions of response
Institutional definitions of protective levels and thresholds for response are defined in Supplemental Table 2 and are based on a combination of published thresholds and manufacturer recommendations. All definitions were set prior to study start and have been used in previous publications [18,19,20] with minor modifications: removal of poliovirus 2 and since 2017, and using 1.3 µg/mL, instead of 2.4 µg/mL, as the protective level against serotypes of pneumococci.
The vaccination schedule used included three doses of pneumococcal conjugate vaccines (13-valent, 15-valent, and or 20-valent), followed by a booster dose 6-12 months after the primary series with either conjugate vaccine or polysaccharide vaccine. Tetanus, diphtheria, pertussis (given as Boostrix) three doses, vaccine against haemophilus influenza b (Hib) (three doses), and three doses of inactivated polio vaccine (IPV). Patients were also offered hepatitis B vaccines given as Twinrix (hepatitis A and B combination) or hepatitis B recombinant vaccine, two doses, and inactivated recombinant zoster-vaccine (Shingrix), two doses (Supplemental Table 1).
Commercially available serological tests from accredited laboratories were implemented
and utilized at our institution, shown in Supplemental Table 3. For analysis, patients were categorized as having a pneumococcal vaccine response if they had a > 2-fold increase in over 70% of the shared serotypes between the pneumococcal vaccines received. Baseline seroprotection against pneumococci was based on reaching >1.3 mcg/mL in 70% of the tested serotypes at baseline. The two different antibody assays used to assess pneumococcal immunity, and an overview of what serotypes are included in the different pneumococcal vaccines are shown in Supplemental Table 4.
To facilitate analysis across the multiple vaccines administered to each patient, we introduced a composite measure of vaccine response. Rather than evaluating predictors of response to each antigen individually—which would be limited by small sample sizes and multiple comparisons—we categorized patients based on their overall pattern of serological responses. Specifically, we defined global responders as patients who mounted or retained immunity to all antigens tested, and non-responders as those who failed to respond to two or more vaccines. Patients with limited serological data (e.g., only one vaccine or incomplete data), or who responded to all but one vaccine, were assigned a “no call” designation. This classification allowed us to identify meaningful trends in immune recovery without overinterpreting sparse or inconsistent data.
Immune parameters
Immune parameters were evaluated to characterize immune reconstitution at the time of vaccination. We included values for total lymphocyte count (ALC), CD4 + T cells, CD8 + T cells, CD19 + B cells, and IgA, G and M levels, all of which were obtained as part of routine clinical care within the two months prior to the first vaccine dose. If multiple measurements were available within this window, we selected the one closest to the first vaccination. These immune parameters were not derived from the same blood samples used for vaccine serologies.
Statistics
Wilcoxon rank sum tests and Fisher’s exact tests were used to compare BCMA and CD-19 groups in terms of patient characteristics, baseline seroprotection, vaccine responses, geometric fold rise (GMFR), and post geometric mean titers (GMTs). Wilcoxon signed rank tests and McNemar’s tests were used to compare pre and post-GMTs, and binary results, respectively. Wilcoxon rank sum tests and Fisher’s exact tests were used to compare global responders vs. non –responders in terms of patient’s characteristics.
Bonferroni correction was used for multiple testing. All analyses were conducted using R version 4.4.2 with the tidyverse (v2.0.0) and gtsummary (v2.0.4) packages 1-3.
Results
Seroprotection pre-vaccination
Detailed patient characteristics of the 73 patients are shown in Table 1. Pre-vaccination seroprotection rates were particularly low for pertussis 20/68 (29%), haemophilus influenzae type b 16/69 (23%), and pneumococcal disease 0/66 (0%). Only 2/8 (25%) had antibodies against measles, but titers were only performed in 8 patients. Pre-vaccination seroprotection was significantly better in CD19 recipients compared to BCMA recipients for most antigens tested, as shown in Fig. 1 and Table 2. In the BCMA group, 14/21 (67%) of patients were seroprotected against tetanus, 11/18 (61%) against poliovirus type I, 12/18 (67%) against poliovirus type II, and only 8/21 (38%) were seroprotected against diphtheria.
Vaccine responses to individual vaccines
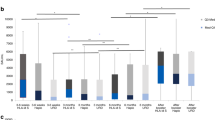
Clinical characteristics of patients (n = 49) in whom before and after titers are available were similar to the overall cohort (Supplemental Table 5). Patients received a median of 3 (range 1–6) doses of vaccine between the pre- and post-titer, depending on the antigen. Pre and post-vaccine titers are shown in Fig. 2. The post titer was obtained at a median of 29 days (IQR 28–41) after the last vaccine dose. Geometric mean titers (GMTs) were significantly higher after vaccination compared to before, for most tested antigens where GMT could be calculated (diphtheria, haemophilus b, and pneumococcus) (Supplemental Table 6).
An overview of vaccine responses by antigen are shown in Table 3.
The range in number of vaccine doses received reflects variation in clinical management, as some patients who remained seronegative after the initial vaccine series received additional booster doses. For each antigen, the post-vaccination titer was based on the final serology obtained after the last documented dose for each antigen.
The response rates among patients that were not protected pre-vaccination varied between antigens, for polio: 20/20 (100%), tetanus: 4/4 (100%), diphtheria: 9/12 (75%), pertussis: 11/23 (48%), Haemophilus type b: 16/26 (62%), Pneumococcal: 7/21 (33%), Hepatitis A: 4/7 (57%), Hepatitis B: 7/16 (44%) and varicella: 6/12 (50%). An overview of vaccine responses to different antigens is shown in Fig. 3.
Post-vaccination GMTs or the GMFR were not significantly different between CD19 and BCMA patients, for any antigen (Supplemental Table 7).
As multiple pneumococcal conjugate vaccines (PCV13, PCV15, and PCV20) were administered over time—often in combination within the same patient—vaccine response analyses were limited to serotypes shared across the vaccines received, to allow for consistent interpretation of serotype-specific antibody responses across the cohort.
Global responses
Most patients evaluated for vaccine responses received vaccinations against several different antigens. Patients were defined as “global responders” if responding or having “retained immunity” to all antigens tested (n = 18). Patients were defined as “non-responders” if not responding to at least two of the vaccines given (n = 17). Patients who received only one vaccine or did not fit into the above groups (n = 14) were removed from the comparison. No significant differences were observed in responders (n = 18) vs non-responders (n = 17) in terms of age, gender, CAR T-target, or months from CAR T to first vaccination. For the immune reconstitution data, IgA-level prior to first vaccination was significantly associated with vaccine responses (p = 0.041). The absolute number of B-cells at baseline was higher among responders compared to non-responders, but the difference did not reach statistical significance after multiple testing corrections (p = 0.054). CD4 counts at baseline were not associated with vaccine responses. For an overview of immune reconstitution in global responders vs. non-responders, see Table 4.
Discussion
We found higher seroprotection rates pre-vaccination among CD19-directed CAR T-cell therapy recipients compared to those receiving BCMA-targeted therapy, in accordance with previous studies [15]. However, vaccine response rates did not differ significantly between CD19 and BCMA recipients. A substantial proportion of patients were seroprotected pre-vaccination, complicating the assessment of vaccine-induced responses. Most patients with pre-existing immunity retained their immunity following vaccination. Among those non-protected, humoral vaccines response rates varied considerably between different antigens, but for some antigens (tetanus, hepatitis A and B) the number of evaluable patients was relatively low. The particularly low response rate to conjugate pneumococcal vaccines (7/21 = 33%) is concerning, as pneumococcal vaccination is a key component of post-CAR T immunization strategies. While alternative approaches, such as penicillin prophylaxis and IVIG exist, they have distinct disadvantages, and their efficacy in this population remains unproven.
Among the immune markers assessed, IgA was the only parameter significantly associated with vaccine response status. Although absolute B-cell counts were numerically higher among global responders, this difference was not statistically significant. Notably, most clinicians defer vaccination until patients reach a CD4+ count above 200 cells/µL and IgG above 500 mg/dL, which may have led to a more selectively immunoreconstituted cohort at the time of vaccination, potentially impacting the observed associations.
The significant association with IgA may reflect its role as a marker of broader B-cell recovery and functional immune reconstitution. Unlike serum IgG, which can be influenced by passive immunoglobulin replacement, IgA is primarily produced by reconstituting plasma cells and may serve as a more dynamic indicator of vaccine readiness. Notably, expert recommendations have suggested using IgA levels to guide vaccination timing post-CAR T [21], as the presence of IgA implies successful class switching and B-cell maturation. Class switching from IgM to IgG and IgA is a key feature of humoral recovery, indicating not only B-cell presence but also functional differentiation, necessary for vaccine responsiveness.
No patient in the study was seroprotected against pneumococcus pre-vaccination, despite some patients (n = 20) having received a full vaccination schedule, including several pneumococcal conjugate vaccine doses, following prior autologous hematopoietic cell transplantation (auto-HCT). Since most of these patients had myeloma and had been vaccinated before CAR T therapy, it is conceivable that the lack of immunity may be specifically due to the depletion of plasma cells by BCMA CAR T-treatment. However, samples collected prior to CAR T-therapy, which were unfortunately not available, could have provided further clarification on this issue. Another potential explanation for this observation may be the immunoparesis observed in myeloma patients. Alternatively, the specific types of previous treatment lines may contribute to the disease-specific differences in seroprotection rates, as more myeloma patients had received CD38-targeting antibodies, autologous HCT, or BCMA/GPRC5D-directed bispecific antibodies.
As BCMA CAR T-recipients were less likely to be seroprotected against most antigens prior to vaccination, earlier or more intensive vaccination strategies may be justified in this population. This consideration is particularly relevant for patients with travel plans or in other settings with increased exposure risk, such as a local outbreak of vaccine-preventable disease.
Given the lack of data, practices for vaccination post-CAR T vary between centers, with some centers adapting the schedule from the stem cell population, some centers awaiting immunological milestones, some centers checking serology pre and post-vaccination and some centers not offering basic immunization [22]. Thus, different approaches to vaccination post-CAR T have been proposed. According to the recent ASTCT guidelines, it is recommended to assess serological responses both pre and post-vaccination to tailor immunization strategies [23], while Reynolds et al. propose an abbreviated vaccination schedule modeled on that used following hematopoietic stem cell transplantation [21]. Both approaches offer distinct advantages and disadvantages, with the former focusing on individualized monitoring and the latter prioritizing a simplified implementation. The retrospective nature of our study precludes us from providing definitive clinical recommendations. However, the particularly low seroprotection rates pre-vaccination in BCMA recipients, as well as in both groups for pneumococcus, warrant clinical attention.
The timing of vaccination post-CAR T-cell therapy remains a clinical challenge. On one hand, delaying vaccination until full immune reconstitution may optimize vaccine responsiveness. On the other hand, postponing immunization increases the period during which patients remain vulnerable to severe infections. The urgency of vaccination may also vary depending on the pathogen. For infections such as tetanus, diphtheria, polio and pertussis where the primary goal is long-term protection and herd immunity provides an additional layer of defense, a more cautious approach to vaccination timing may be justified. However, for pathogens like Streptococcus pneumoniae, Influenza, SARS-CoV-2, Respiratory syncytial virus (RSV) and Varicella-zoster, where the risk of severe infection is high [24,25,26] and protection relies primarily on individual immunity, it may be beneficial to vaccinate earlier, even if responses are suboptimal. Striking a balance between these factors is crucial, and identifying immune markers predictive of vaccine responsiveness, such as IgA levels and the extent of B-cell aplasia, could aid in determining the optimal timing of vaccination.
The current study has some limitations. T-cell-mediated immunity was not assessed, which may play an essential role in vaccine responses, particularly in B-cell-depleted patients. Given that humoral responses are often impaired following CAR T therapy, T-cell immunity could provide an alternative mechanism of protection. Previous studies have suggested that T-cell responses may compensate for diminished antibody production in immunocompromised individuals [9, 27]. However, evaluating T-cell responses remains challenging due to the high cost, labor-intensive nature, and lack of standardized assays for functional T-cell testing. Further limitations of the present study were its retrospective nature with relatively small patient numbers. The study cohort only consisted of patients who were considered eligible for vaccination by their treating clinician. Patients with ongoing severe infections, relapse, immunomodulating therapy, or those who had not achieved immunological milestones were often excluded from vaccination. Therefore, our data are most applicable to CAR T recipients who are clinically stable and progressing well.
Furthemore, the VZV serological testing was performed using commercially available IgG assays commonly used in clinical practice, which do not specifically measure glycoprotein E (gE)-directed antibodies. As a result, the reported seroprotection rates may reflect immunity from either prior infection or vaccination, as the assay does not distinguish between the two.
To our knowledge, this is the first study to evaluate vaccine responses post-CAR T beyond COVID-19 and influenza, providing novel insights into immunity against a broader range of pathogens. The availability of detailed immune reconstitution parameters allowed for a comprehensive analysis of factors influencing vaccine responses in this population.
Conclusions
Pre-vaccination seroprotection was higher among CD19 CAR T-recipients for nearly all antigens; however, vaccine responses did not differ significantly between BCMA and CD19 CAR T-recipients. The timing of vaccination post-CAR T therapy was not associated with response status; instead, immune reconstitution played a key role. Higher IgA levels were associated with better vaccine responses, and B-cell counts trended higher among responders (p = 0.054). Our findings highlight the importance of immune recovery in vaccine efficacy post-CAR T. Further prospective studies are warranted, ideally incorporating T-cell assays to better define cellular immune responses in this population.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Cordas Dos Santos DM, Tix T, Shouval R, Gafter-Gvili A, Alberge JB, Cliff ERS, et al. A systematic review and meta-analysis of nonrelapse mortality after CAR T cell therapy. Nat Med. 2024;30:2667–78.
Kampouri E, Little JS, Rejeski K, Manuel O, Hammond SP, Hill JA. Infections after chimeric antigen receptor (CAR)-T-cell therapy for hematologic malignancies. Transpl Infect Dis. 2023;25:e14157.
Wudhikarn K, Perales MA. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2022;57:1477–88.
Rejeski K, Perez A, Iacoboni G, Penack O, Bücklein V, Jentzsch L, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. 2022;10:e004475.
Arya S, Shahid Z. Overview of infectious complications among CAR T- cell therapy recipients. Front Oncol. 2024;14:1398078.
Little JS, Tandon M, Hong JS, Nadeem O, Sperling AS, Raje N, et al. Respiratory infections predominate after day 100 following B-cell maturation antigen-directed CAR T-cell therapy. Blood Adv. 2023;7:5485–95.
Luan D, DeWolf S, Fei T, Raj S, Shah GL, Lareau CA, et al. Dynamics of Immune Reconstitution and Impact on Outcomes Across CAR-T Cell Products in Large B-Cell Lymphoma. Blood Cancer Discov. 2024;6:119–30.
Cordeiro AC, Durisek G, Batista MV, Schmidt J, de Lima M, Bezerra E. Late events after anti-CD19 CAR T-cell therapy for relapsed/refractory B-cell non-Hodgkin lymphoma. Front Oncol. 2024;14:1404351.
Hill JA, Martens MJ, Young JH, Bhavsar K, Kou J, Chen M, et al. SARS-CoV-2 Vaccination in the First Year After Hematopoietic Cell Transplant or Chimeric Antigen Receptor T-Cell Therapy: A Prospective, Multicenter, Observational Study. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2024;79:542–54.
Wiedmeier-Nutor JE, Iqbal M, Rosenthal AC, Bezerra ED, Garcia-Robledo JE, Bansal R, et al. Response to COVID-19 Vaccination Post-CAR T Therapy in Patients With Non-Hodgkin Lymphoma and Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2023;23:456–62.
Walti CS, Loes AN, Shuey K, Krantz EM, Boonyaratanakornkit J, Keane-Candib J, et al. Humoral immunogenicity of the seasonal influenza vaccine before and after CAR-T-cell therapy: a prospective observational study. J Immunother Cancer. 2021;9:e003428.
Ramasamy K, Sadler R, Jeans S, Weeden P, Varghese S, Turner A, et al. Immune response to COVID-19 vaccination is attenuated by poor disease control and antimyeloma therapy with vaccine driven divergent T-cell response. Br J Haematol. 2022;197:293–301.
Van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–30.
Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11:138.
Walti CS, Krantz EM, Maalouf J, Boonyaratanakornkit J, Keane-Candib J, Joncas-Schronce L, et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight. 2021;6:e146743.
Abid MB, Rubin M, Szabo A, Longo W, Fenske TS, McCoy C, et al. Efficacy of Multiple SARS-CoV-2 Vaccine Doses in Patients with B Cell Hematologic Malignancies Receiving Chimeric Antigen Receptor T Cell Therapy: A Contemporary Cohort Analysis. Transpl Cell Ther. 2024;30:285–97.
Richardson T, Schütte D, Kobbe G, Baermann BN, Holderried TAW, Schmitz F, et al. Characteristics of Infections after BCMA-directed CAR-T cells therapy for Multiple Myeloma - a real-world analysis. Blood Adv. 2025;9:1370–5.
Shah GL, Shune L, Purtill D, Devlin S, Lauer E, Lubin M, et al. Robust Vaccine Responses in Adult and Pediatric Cord Blood Transplantation Recipients Treated for Hematologic Malignancies. Biol Blood Marrow Transpl. 2015;21:2160–6.
Palazzo M, Shah GL, Copelan O, Seier K, Devlin SM, Maloy M, et al. Revaccination after Autologous Hematopoietic Stem Cell Transplantation Is Safe and Effective in Patients with Multiple Myeloma Receiving Lenalidomide Maintenance. Biol Blood Marrow Transpl. 2018;24:871–6.
Melica G, Preston E, Palazzo M, Seier K, Malard F, Cho C, et al. Immune reconstitution, vaccine responses, and rituximab use after ex-vivo CD34-selected myeloablative allogenic hematopoietic cell transplantation. Bone marrow Transplant. 2024;59:625–9.
Reynolds G, Hall VG, Teh BW. Vaccine schedule recommendations and updates for patients with hematologic malignancy post-hematopoietic cell transplant or CAR T-cell therapy. Transpl Infect Dis. 2023;25:e14109.
Penack O, Dreger P, Ajib S, Ayuk F, Baermann BN, Bug G, et al. Management of Patients Undergoing CAR-T Cell Therapy in Germany. Oncol Res Treat. 2024;47:65–75.
Shahid Z, Jain T, Dioverti V, Pennisi M, Mikkilineni L, Thiruvengadam SK, et al. Best Practice Considerations by The American Society of Transplant and Cellular Therapy: Infection Prevention and Management After Chimeric Antigen Receptor T Cell Therapy for Hematological Malignancies. Transpl Cell Ther. 2024;30:955–69.
Shigayeva A, Rudnick W, Green K, Chen DK, Demczuk W, Gold WL, et al. Invasive Pneumococcal Disease Among Immunocompromised Persons: Implications for Vaccination Programs. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2016;62:139–47.
Grant LR, Meche A, McGrath L, Miles A, Alfred T, Yan Q, et al. Risk of Pneumococcal Disease in US Adults by Age and Risk Profile. Open forum Infect Dis. 2023;10:ofad192.
McKay SL, Guo A, Pergam SA, Dooling K. Herpes Zoster Risk in Immunocompromised Adults in the United States: A Systematic Review. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2020;71:e125–e34.
Zonozi R, Walters LC, Shulkin A, Naranbhai V, Nithagon P, Sauvage G, et al. T cell responses to SARS-CoV-2 infection and vaccination are elevated in B cell deficiency and reduce risk of severe COVID-19. Sci Transl Med. 2023;15:eadh4529.
Acknowledgements
This research was supported in part by National Institutes of Health award numbers P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the Celine and Anthony Larocci Fund for providing research support for this study. Dr. Einarsdottir has received postdoc grants from The Swedish Foundation for International Cooperation in Research and Higher Education (STINT), The Swedish Society of Medicine (SLS), Gothenburg Medical Society (GLS), The Iris-grant and the Swedish state under the agreement between the Swedish government and the county councils (ALF).
Author information
Authors and Affiliations
Contributions
ZS and GuS designed the study and collected the data. PS extracted and curated the dataset for analysis. ZS, GuS, SE, SL and SD analyzed the data. SE wrote the first draft of the manuscript. SL and SD performed the statistical analyses. ZS, DC, DL, SES, MLP, PD, LF, SG, HL, AL, RL, JL, SM, MP, JP, GiS, JS, MGL, SU, KR, RS and MS critically reviewed the manuscript drafts, approved the last version, and made the decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
Stephanie Lobaugh, Padmapriya Supramanian, Sean Devlin, Parastoo Dahi, Alexander Lesokhin, Maria Palomba, Gilles Salles, Lorenzo Falchi, Heather Landau, Jae Park, Sham Mailankody - Nothing to Declare. Sigrun Einarsdottir: research funding (institutional) from MSD. Michael Scordo served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc., Omeros Corporation, Amgen Inc., Bristol Myers Squibb, and Sanofi; served on ad hoc advisory boards for Kite – A Gilead Company, and Miltenyi Biotec; and received honoraria from i3Health, Medscape, CancerNetwork, and IDEOlogy. Gunjan Shah: research funding to the institution from Janssen, Amgen, BMS, Beyond Spring, GPCR, and Recordati, and is on the DSMB for ArcellX. Dr. Perales reports honoraria from Adicet, Allogene, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, Takeda, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune, Omeros and OrcaBio. He has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. Roni Shouval reports speaker honoraria from Incyte and Sanofi. Kai Rejeski: Kite/Gilead: Research funding, Consultancy, Honoraria and travel support; BMS/Celgene: Consultancy, Honoraria; Pierre-Fabre; travel support. CSL Baehring: Consultancy. Saad Usmani: Research funding (past 2 years): Abbvie, BMS/Celgene, GSK, Gilead, Gracell Biotechnologies, Janssen. Consulting (past 2 years): Abbvie, BMS/Celgene, Genentech, Gilead, GSK, Janssen, Kite/Arcellx, Oricell Therapeutics, Pfizer, Regeneron, Sanofi. Sergio Giralt: Research Funding: Amgen, J&J, Takeda, Celgene, Actinuum, Sanofi, Miltenyi, Kite, EUSA. Consultancy: Amgen, J&J, Takeda, Celgene, Actinuum, Sanofi. Miltenyi, Novartis, Kite, Novartis, Jazz, BMS, Crisper, EUSA Omeros.
Ethical approval and consent to participate
All methods were performed in accordance with relevant guidelines and regulations. Approval from Institutional Review Board (IRB)/Privacy Board at Memorial Sloan Kettering Cancer Center (MSK) was obtained, reference number: 23-070. Informed consent was waived by the Ethics committee due to the retrospective nature of the study and minimal risk to participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Einarsdottir, S., Lobaugh, S., Luan, D. et al. Humoral vaccine responses following Chimeric Antigen Receptor T-cell therapy for hematological malignancies. Blood Cancer J. 15, 114 (2025). https://doi.org/10.1038/s41408-025-01321-w
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-025-01321-w