Abstract
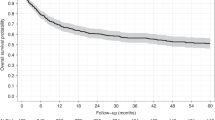
Allogeneic hematopoietic stem cell transplantation (HSCT) is still needed for many children with very high-risk acute leukemia. An HLA-haploidentical family donor is a suitable option for those without an HLA-matched donor. Here we present outcomes of a novel HLA-haploidentical HSCT (haplo-HSCT) strategy with adoptive immunotherapy with thymic-derived CD4+CD25+ FoxP3+ regulatory T cells (Tregs) and conventional T cells (Tcons) performed between January 2017 and July 2021 in 20 children with high-risk leukemia. Median age was 14.5 years (range, 4–21), 15 had acute lymphoblastic leukemia, 5 acute myeloid leukemia. The conditioning regimen included total body irradiation (TBI), thiotepa, fludarabine, cyclophosphamide. Grafts contained a megadose of CD34+ cells (mean 12.4 × 106/Kg), Tregs (2 × 106/Kg) and Tcons (0.5–1 × 106/Kg). All patients achieved primary, sustained full-donor engraftment. Only one patient relapsed (5%). The incidence of non-relapse mortality was 15% (3/20 patients). Five/20 patients developed ≥ grade 2 acute Graft versus Host Disease (aGvHD). It resolved in 4 who are alive and disease-free; 1 patient developed chronic GvHD (cGvHD). The probability of GRFS was 60 ± 0.5% (95% CI: 2.1–4.2) (Fig. 6), CRFS was 79 ± 0.9% (95% CI: 3.2–4.9) as 16/20 patients are alive and leukemia-free. The median follow-up was 2.1 years (range 0.5 months–5.1 years). This innovative approach was associated with very promising outcomes of HSCT strategy in pediatric patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Merli P, Algeri M, Del Bufalo F, Locatelli F. Hematopoietic stem cell transplantation in pediatric acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2019;14:94–105.
Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–85. https://doi.org/10.1182/blood-2008-01-132837.
Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel PG, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J 2020;10:77. https://doi.org/10.1038/s41408-020-00342-x.
Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2019;33:884–92. https://doi.org/10.1038/s41375-018-0265-z.
Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–24. https://doi.org/10.1182/bloodadvances.2020003092.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. https://doi.org/10.1056/NEJMoa1709866.
Curran E, O’Brien M. Role of blinatumomab, inotuzumab, and CAR T-cells: which to choose and how to sequence for patients with relapsed disease. Semin Hematol. 2020;57:157–63. https://doi.org/10.1053/j.seminhematol.2020.11.001.
Kruse A, Abdel-Azim N, Kim HN, Ruan Y, Phan V, Ogana H, et al. Minimal residual disease detection in acute lymphoblastic leukemia. Int J Mol Sci. 2020;21:1054. https://doi.org/10.3390/ijms21031054.
Segerink WH, de Haas V, Kaspers GJL. Measurable residual disease in pediatric acute myeloid leukemia: a systematic review. Expert Rev Anticancer Ther. 2021;21:451–9. https://doi.org/10.1080/14737140.2021.1860763.
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–14. https://doi.org/10.1182/blood-2009-10-248146.
Berger M, lanino E, Cesaro S, Zecca M, Vassallo E, Faraci M, et al. Feasibility and outcome of haploidentical hematopoietic stem cell transplantation with post-transplant high-dose cyclophosphamide for children and adolescents with hematologic malignancies: an AIEOP-GITMO retrospective multicenter study. Biol Blood Marrow Transplant. 2016;22:902–9. https://doi.org/10.1016/j.bbmt.2016.02.002.
Dufort G, Castillo S, Pisano S, Castiglioni M, Carolina P, Andrea I. Haploidentical hematopoietic stem cell transplantation in children with high-risk hematologic malignancies: outcomes with two different strategies for GvHD prevention. Ex vivo T-cell depletion and post-transplant cyclophosphamide: 10 years of experience at a single center. Bone Marrow Transplant. 2016;51:1354–60. https://doi.org/10.1038/bmt.2016.161.
Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood. 2017;130:677–85. https://doi.org/10.1182/blood-2017-04-779769.
Pierini A, Ruggeri L, Carotti A, Falzetti F, Saldi S, Terenzi A, et al. Haploidentical age-adapted myeloablative transplant and regulatory and effector T cells for acute myeloid leukemia. Blood Adv. 2021;5:1199–208. https://doi.org/10.1182/bloodadvances.2020003739.
Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–5. https://doi.org/10.1182/blood-2008-01-135285.
Kruchen A, Stahl T, Gieseke F, Binder TM, Özcan Z, Meisel R, et al. Donor choice in haploidentical stem cell transplantation: fetal microchimerism is associated with better outcome in pediatric leukemia patients. Bone Marrow Transpl. 2015;50:1367–70. https://doi.org/10.1038/bmt.2015.136.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100.
Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. https://doi.org/10.1182/blood-2010-10-311894.
Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–44. https://doi.org/10.1182/blood-2014-03-564401.
Bertaina A, Zecca M, Buldini B, Sacchi N, Algeri M, Saglio F, et al. Unrelated donor vs HLA-haploidentical alpha/beta T-cell and B-cell depleted HSCT in children with acute leukemia. Blood. 2018;132:2594–607. https://doi.org/10.1182/blood-2018-07-861575.
Lang P, Teltschik HM, Feuchtinger T, Muller I, Pfeiffer M, Schumm M, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165:688–98. https://doi.org/10.1111/bjh.12810.
Klein OR, Buddenbaum J, Tucker N, Chen AR, Gamper CJ, Loeb D, et al. Nonmyeloablative haploidentical bone marrow transplantation with post-transplantation cyclophosphamide for pediatric and young adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transpl. 2017;23:325–32. https://doi.org/10.1016/j.bbmt.2016.11.016.
Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority phase III study. J Clin Oncol. 2021;39:295–307. https://doi.org/10.1200/JCO.20.02529.
Aristei C, Carotti A, Palazzari E, Amico L, Ruggeri L, Perrucci E, et al. The total body irradiation schedule affects acute leukemia relapse after matched T cell-depleted hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2016;96:832–9. https://doi.org/10.1016/j.ijrobp.2016.07.025.
Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. https://doi.org/10.1038/nm915.
Perotti C, Del Fante C, Tinelli C, Viarengo G, Scudeller L, Zecca M, et al. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion. 2010;50:1359–69.
Gotoh M, Yoshizawa S, Katagiri S, Suguro T, Asano M, Kitahara T, et al. Human herpesvirus 6 reactivation on the 30th day after allogenic hematopoietic stem cell transplantation can predict grade 2-4 acute graft versus-host disease. Transpl Infect Dis. 2014;16:440–9.
Perruccio K, Sisinni L, Perez-Martinez A, Valentin J, Capolsini I, Massei MS, et al. High incidence of early human Herpesvirus-6 infection in children undergoing haploidentical manipulated stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2018. https://doi.org/10.1016/j.bbmt.2018.07.033.
Ogata M, Satou T, Kawano R, Takakura S, Goto K, Ikewaki J, et al. Correlations of HHV-6 viral load and plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem cell transplant recipients. Bone Marrow Transpl. 2010;45:129–36. https://doi.org/10.1038/bmt.2009.116.
Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: an update. Curr Opin Hematol. 2010;17:500–4. https://doi.org/10.1097/MOH.0b013e32833e5b2e.
Bansal D, Yadav AK, Kumar V, Minz M, Sakhuja V, Jha V. Deferred pre-emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of T regulatory cell population: a randomized, controlled trial. PLoS ONE. 2013;8:e75591. https://doi.org/10.1371/journal.pone.0075591.
Tkachev V, Furlan SN, Watkins B, Hunt DJ, Zheng HB, Panoskaltsis-Mortari A, et al. Combined OX40L and mTOR blockade controls effector T cell activation while preserving T reg reconstitution after transplant. Sci Transl Med. 2017;9:eaan3085. https://doi.org/10.1126/scitranslmed.aan3085.
Massei MS, Capolsini I, Cerri C, Gurdo G, Mastrodicasa E, Perruccio K, et al. SARS-CoV-2 infection in an adolescent after haploidentical stem cell transplantation. Clin Oncol. 2021;6:1787.
Theil A, Tuve S, Oelschlägel U, Maiwald A, Döhler D, Oβmann D, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy. 2015;17:473–86. https://doi.org/10.1016/j.jcyt.2014.11.005.
Acknowledgements
The authors thank Geraldine Anne Boyd and Fabio De Paola for editorial assistance, the nonprofit charity associations Comitato per la Vita Daniele Chianelli and nurses and the multidisciplinary group of the hematopoietic stem cell transplantation program. The authors also thank the patients and their caregivers for participating in this study.
Author information
Authors and Affiliations
Contributions
MSM provided clinical care, performed research, and wrote the manuscript; IC performed research and evaluated suitability of donors; EM, KP, FA, CC, GG, SS and MB provided clinical care; FF, TZ and RIO processed the graft; SiSa designed and performed; RT performed HLA typing; OM evaluated suitability of donors; BMP and SS performed radiation treatment; MM collected clinical data; MaMa and OM evaluated suitability of donors and performed leukaphereses and photophereses; CA supervised radiation therapy and reviewed the manuscript; AV, AP, LR, MFM and AC reviewed the manuscript; MC provided clinical care, supervised the pediatric transplantation program, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Massei, M.S., Capolsini, I., Mastrodicasa, E. et al. HLA-haploidentical hematopoietic stem cells transplantation with regulatory and conventional T-cell adoptive immunotherapy in pediatric patients with very high-risk acute leukemia. Bone Marrow Transplant 58, 526–533 (2023). https://doi.org/10.1038/s41409-023-01911-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-023-01911-x
This article is cited by
-
Outcomes of hematopoietic stem cell transplantation in pediatric acute lymphoblastic leukemia: a multicenter Brazilian cohort study
Bone Marrow Transplantation (2025)