Abstract
The effect of involved margins after breast cancer surgery on distant recurrence (DR) is unknown. We determined the association between margin width or involvement, DR and cancer deaths.
Patients and methods
Greater Manchester (GM) and the National Cancer Registry (NCRAS) cohorts were analysed. Margin status after curative surgery was measured. Cox-proportional hazards investigated factors associated with LR, DR and breast cancer deaths.
Results
In GM (2010–2014), 2295 (70.2%) patients had clear margins ( > 2 mm), 302 (9.2%) close (1–2 mm) and 673 (20.6%) involved ( < 1 mm) margins. 2030 patients underwent breast conservation surgery (BCS). After multivariable adjustment in BCS patients, involved margins had an increased hazard of DR (HR 1.73, 95% CI:1.03, 2.88, p = 0.037) and LR (HR 2.16, 95% CI:1.31, 3.58, p = 0.003). NCRAS data from 2010–2013 in 16,420 BCS patients included 3,913 patients (23.9%) with final margins <1 mm. There were 642 deaths (3.9%) after 80.2 months median follow-up: 5.6% in patients with final margins <1 mm and 3.4% with margins >1 mm. After BCS, in 5246 patients who underwent chemotherapy after BCS, involved margins <1 mm had a HR of 1.33 (CI 1.10–1.60, p = 0.003) for cancer death.
Conclusions
Margins >1 mm were associated with lower DR and cancer deaths. Guidelines should recommend a minimum margin clearance of 1 mm.
Similar content being viewed by others
Introduction
Breast cancer causes 11,400 deaths annually in the UK. Most women with early breast cancer receive conservation surgery (BCS). If cancer cells remain present at the edges (of the cut) there is an increased risk of cancer returning at the same site [1, 2]. Previous studies have shown an association between the width of tumour from the margin in colorectal cancer and subsequent outcomes [1, 2].
Removing cancers without leaving tumour at a surgical margin reduces local recurrence (LR) [3,4,5,6,7,8] but the effects of margin involvement on distant recurrence (DR) are unclear. How far the tumour should be from the margin is controversial. After BCS, adjuvant treatment including radiotherapy to the breast [3, 7, 9]. and endocrine therapy reduce LR [8, 10, 11].
American Society of Clinical Oncology (ASCO) 2014 guidelines [12], state that negative margins reduced LR but recommended “a margin of no tumour on ink” was sufficient clearance around invasive cancer after BCS [12]. The effect of margin width on DR or breast cancer mortality is unclear [13].
Tumour at the margin (ToI) led to an increased risk of DR (HR 1.75 (1.17–2.62)) in two trials [14]. UK Guidelines require a final cancer margin clearance of 1 mm or more [7, 9] but a prospective study POSH found that the 21% of patients left with margins ( < 1 mm) involved, suffered increased DR and worse survival [8].
Our recent metanalysis of margin status after BCS found a 1 mm margin clearance at the edge of a BCS specimen reduced DR and LR [4]. Randomized trials no longer consider women with involved margins as eligible for study participation.
If DR and cancer deaths are associated with involved surgical margins after treatment, changes to current international practice are required. The aim of this study was to use two large UK breast cancer patient datasets to: 1) determine if margin involvement was associated with DR and death from breast cancer, 2) determine the optimal margin width to reduce DR, LR and breast cancer deaths after BCS.
Methods
Greater Manchester (GM) cohort
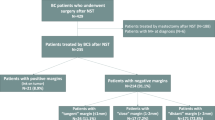
An audit in 5 Breast Units of patients diagnosed with early invasive primary breast cancer(T1–3) was undertaken between January 2010 and December 2014. Ethical approval was obtained (IRAS Nos 275022 and 248313) for the research. Patients undergoing neoadjuvant therapy or not undergoing curative surgery, with inoperable, T4, inflammatory or metastatic cancer were excluded (Fig. 1:Supplementary Table 1a). Pathological data (type, size, tumour grade and node status), oestrogen (ER) and progesterone (PR), HER2 receptor status were prospectively recorded on all patients using the National Health Service Breast Screening Pathology (NHSBSP) reporting standards. Final margin status and exact width(mm) were prospectively recorded after surgery (including re-excision) according to NHSBSP standards [15]. Final margins more than 1 mm clearance were considered clear as per local GM and ABS guidelines [9].
Women underwent BCS or mastectomy with either sentinel node biopsy or clearance and then adjuvant radiotherapy, endocrine and chemotherapy according to local guidelines. Patients excluded from radiotherapy included those over 75 years of age, with a cancer <1 mm, low grade or ER positive.
NCRAS cohort
National Cancer Registry (NCRAS) data analysis received ethics and Public Health England approvals. Data for the years 2010–2013(Fig. 1) included final margin distance (after any re-excision) recorded prospectively from NHSBSP pathology reports as margins >1 mm clear or margins <1 mm. Validation and verification of a subset of NCRAS data were performed. Patients not undergoing surgery (mastectomy or BCS) as primary treatment were not included in the analysis. Other variables available for analysis were age, tumour size, grade, stage, nodal involvement, mode of detection and treatment.
Outcome definitions (GM & NCRAS cohorts)
The outcomes were LR and DR in the GM data, and breast cancer death in NCRAS data. Within the GM data, LR was defined as histopathological evidence of any recurrence of breast cancer (invasive or DCIS) in the breast, chest wall, or adjacent lymph nodes. DR was defined as a clinically, radiologically, or morphologically verified recurrence (any recurrence in the supraclavicular nodes or beyond) and deaths from breast cancer. Recurrence outcomes were confirmed by review of electronic patient notes and histopathology reports for all patients. Simultaneous LR and DR was defined as pathologically proven LR and DR developing within a 3-month period.
Within NCRAS, cause and timing of death are obtained from death certification (1 A/B) for England. Follow-up was defined from the date-of-surgery in all cases.
Statistical analysis
Baseline characteristics as a full cohort, and across groups of final margin status, were summarised using the mean, standard deviation and range for continuous variables, and frequencies of occurrence for categorical data.
We fitted a logistic regression model, with margins (clear vs. close/involved) as the outcome, and all the other variables in the dataset as covariates. Associations between the covariates and margin status were summarised through odds ratios (ORs) and 95% confidence intervals.
Within the GM data time-to-LR and time-to-DR analyses were conducted under a competing risk framework (where all-cause death was a competing risk) [16–18]. Univariable analysis of time-to-event outcomes were visually explored using cumulative incidence plots, summarised across margin status. LR and DR rates were cross tabulated by presentation route and by margin status.
To explore whether margin status was associated with recurrence, we fitted cause-specific Cox-proportional hazards models. Associations between patient, tumour, and surgical characteristics on recurrence were estimated both as a whole cohort and in a subset of symptomatic only patients. These associations were quantified using hazard ratios (HR) and associated 95% confidence intervals. The cause-specific Cox proportional hazards models included margin status (close (1.1–2 mm), involved < 1 mm, or clear > 2 mm) and other variables recorded in the dataset. The proportional hazards assumption for the primary variable of interest (margin status) was checked in all Cox models by examining the Schoenfeld residuals. Sensitivity analysis was undertaken for BCS only patients to address margin width according to ASCO and ABS guidelines and for symptomatic patients after analysis demonstrated it as a strong predictive factor for relapse.
Similar analyses were undertaken in the NCRAS data, but outcome changed to death from cancer. Death from any other cause was taken as a competing risk, again employing cause-specific Cox-proportional hazards models to explore the association between margin status and cancer mortality [17, 18]. Statistical analysis was performed using SPSS software, R version 4.0.2 [16, 19] and Stata version 16.1. For R, the following packages were used: tidyverse, MICE [19], survival [20,21,22] and cmprsk [23].
Patient and public involvement
Independent Patient Cancer Voice patient representatives felt that our findings were clear, to avoid technical terms and to enable wide dissemination of the results given the implications for research and clinical practice
Results
Greater Manchester (GM) cohort
Across GM hospitals, 3281 cases of invasive breast cancer treated (Fig. 1) either by BCS (n = 2030) or mastectomy (n = 1240) with 11(0.34%) patients excluded due to no follow-up or incorrect patient identifiers leaving 3270 patients, with 1817 (55.6%) presenting symptomatically and 1453 (44.4%) via breast screening.
Median age was 61 (range 24–100) years. Median follow up was 64.4 months (range 0.0–126.6). Most patients underwent adjuvant radiotherapy (71%) and/or hormone therapy (84%). Out of 2033 patients undergoing BCS, 1873 (92.1%) received radiotherapy, but 160 (7.9%) didn’t, mainly due to older age. Radiotherapy was given to 37% patients post-mastectomy. Overall, 3231 (98.8%) patients had adjuvant systemic therapy. Most patients were T-stage 1 (58%) or 2 (37%), with 73% N-stage N0 (Supplementary Table 1) with ER positive (84%), PR positive (73%), and 12% HER2 positive cancers.
Margin involvement
Overall, 2295 patients (70.2%) had clear ( > 2 mm), 302 (9.2%) close ( > 1–2 mm) and 673 (20.6%) involved final margins ( < 1 mm). Only 193(6.2%) of cancers had “Tumour on ink” (ToI) margins.
Women presenting with symptoms more often had margin involvement than screening presentation (OR 1.39,95% CI: 1.12, 1.72, p = 0.002). Amongst BCS patients, mode of presentation and grade predicted margin involvement (Supplementary Tables 4). Margins were potentially re-excisable in 64% of patients (radial or anterior) (Supplementary Table 6a, b).
Local (LR) and distant (DR) recurrence
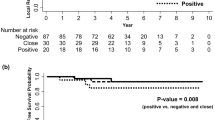
LR developed in 160 (4.9%) and DR in 231 patients (7.1%). Thirty (1.3%) patients experienced simultaneous LR and DR. Table 2 and Fig. 2a show the cumulative incidence functions for LR and DR. Time-to-DR (p = 0.017) and time-to-LR (p = 0.083) differed by margin status. At 5-years, the probability of DR was 4.84%, 5.9% and 7.14%, for clear>2 mm, close (1–2 mm) and involved margins<1 mm, respectively. For LR, the corresponding 5-year probabilities were 3.1%, 3.53% and 11% respectively. Time to recurrence for involved margins was median 33.8 months for DR and 42.0 months for LR.
Cumulative incidence functions by prospectively measured margin status for (a) distant and (b) local recurrence in patients presenting with symptomatic breast cancer in Greater Manchester 2011–2103, each adjusted for the competing risks of death. c Involved margin < 1 mm (ToI excluded,0 vs close 1–2 mm versus 2 mm or greater) in GM. d National Cancer Registry (NCRAS) 2010–2013 data prospectively assessed surgical widths and Breast Cancer Mortality in 24962 women aged 25–70 years in England. Mortality according to Death Certification (1 A/B) Margin Status. Clear>1 mm; Clear distance not stated compared to involved margins <1 mm. Between 2010 and 2013, in 24962 breast cancers treated surgically on the NCRAS database, 22.6% cancer patients had involved margins <1 mm, 67.8% margins >1 mm and 9.7% were classed as “clear margins” by their MDT as margins>1 mm but exact margin width was not reported. The patients with clear margins were defined by their MDT are shown separately in (d, e) and it can be seen that Clear margins had a similar survival to Margins>1 mm and significantly different to margins<1 mm. e Effect of margins<1 mm (red lines) compared Margins > 1 mm (green) on cancer mortality in 16,420 Breast Conservation Surgery treated NCRAS patients aged 25–70 years in England. f NCRAS mortality data of 5624 Breast Conservation Surgery patients who underwent post-op chemotherapy and margin status. Mortality according to Death Certification (1 A/B) Margin Status. Clear>1 mm; Clear distance not stated compared to involved margins <1 mm.
In univariable analysis, involved margins had higher DR rates compared with clear margins (HR 1.38, 95% CI: 1.03, 1.86, p = 0.032). In a cause-specific Cox proportional hazards model, involved margins < 1 mm had an increased risk of DR compared with clear margins>1 mm (HR 1.39 (95% CI: 1.02, 1.89: p = 0.037), taking into account tumour stage, grade and number of involved nodes (Fig. 2a, b; Table 1)
Involved (compared to clear>1 mm) margins were associated with higher LR (HR 1.61, 95% CI: 1.14, 2.27, p = 0.007). Cox proportional hazards models for LR showed involved margins<1 mm (compared to clear) were associated with higher LR with an adjusted HR 1.69 (95% CI: 1.18, 2.44, p = 0.005).
Rates of LR (HR 1.96, 95% CI: 1.24, 3.10, p = 0.004). and DR (HR 1.77, 95% CI: 1.12, 2.61, p = 0.004) were higher in patients presenting with symptomatic cancer compared with a screening diagnosis (p = <0.001 (Table 1: Supplementary Table 2).
Sensitivity analysis: GM breast conservation surgery cases (Table 2)
In Cox Regression analysis of BCS patients, margins <1 mm were associated with increased DR, HR 1.73 (1.03–2.89) and LR, HR 2.16 (1.31–3.58) as were node positivity, grade 3 tumours, HR 8.71 (1.91–39.62), use of endocrine therapy HR 0.25 (0.01–0.63) and radiotherapy HR 0.50 (0.25–0.99) (Table 2).
Involved margins were independently associated with LR even when ToI cases were excluded from the analysis HR 1.56 (1.10–2.38, p = 0.038) (Fig. 2c: Supplementary Tables 7, 8). Restricting analysis to BCS patients who underwent chemotherapy found margins <1 mm had a HR of 1.71 (1.17–2.50: p = 0.005) for DR and for margins 1–2 mm a HR of 1.78 (1.03–3.09: p = 0.041) compared to margins<2 mm. Increased LR was associated with margins <1 mm, HR 1.84 (1.15–2.94: p = 0.041). The majority of breast cancer deaths in GM patients occurred in Stage 1 or 2 cancers (Supplementary Table 5).
NCRAS cohort
Between 2010 and 2013, out of 40,794 breast cancers treated surgically on the NCRAS database aged 20–112 years old, 9,210 (22.6%) cancer patients had involved margins <1 mm, 27,644 margins >1 mm (67.8%) and 3,940 (9.7%) were classed as “clear margins” by their MDT but exact margin width was not reported. Median follow-up of patients was 79.9 months (range 0.0–109.2) with 3,176 (7.8%) breast cancer deaths. Margins were involved in 23.9% patients whose first treatment was BCS and 16.9% of patients when it was mastectomy (Supplementary Table 9).
An unadjusted excess cancer mortality occurred in the 22.6% of patients whose pathology indicated margins<1 mm (HR 1.17 (95% CI 1.08–1.27: p < 0.001)) compared to either clear margins>1 mm or margins coded as “clear” without a specific margin width recorded. Absolute cancer death rates were 7.5% with clear to 8.7% with involved margins 5 years after surgery. In multivariate analysis margins remained associated with cancer mortality (HR 1.15 (1.06–1.25: p = 0.001)). Combining margins >1 mm and “clear” margins groups compared to patients with involved margins, the HR was 1.17 (1.08–1.27 (p < 0.001)). Restricting analysis to patients aged 18–65 years or 18–50 years reduced patient numbers but 1617 cancer deaths occurred and involved margins remained in multivariate analysis (HR 1.21 (1.08–1.35), p < 0.001: Fig. 2d).
NCRAS BCS patients
Overall, 18,991 patients aged 25–70 years in England underwent BCS, 4534 of whom had final margins <1 mm (23.9%). In multivariate analysis, patients with final margins <1 mm were associated with a worse survival (HR 1.22 (1.05–1.42: p = 0.009)).
Analysing the 16,420 patients aged 25–70 years old whose first cancer treatment was BCS, there were 3,696 patients with final margins <1 mm and 642 deaths (3.9%) by the end of follow-up: 5.6% in patients with final margins <1 mm and 3.4% with margins >1 mm, respectively. Margins<1 mm remained significant in multivariate Cox regression analysis (Fig. 2e: Supplementary Table 9). Absolute cancer death rates increased from 7.5% with clear margins to 8.7% with involved margins 5 years after surgery.
Multivariate analysis showed margins<1 mm (HR 1.28 (1.08–1.52): p = 0.004) were associated with increased cancer deaths along with tumour stage (stage 3 HR 5.18 (4.05–6.64, p < 0.001)), Grade 3, HR 8.91 (5.86–13.56, p < 0.001), age (HR 1.03 (1.02–1.04, p < 0.001), symptomatic detection (HR 1.91 (1.61–2.27, p < 0.001) and lower socio-economic status HR 1.33 (1.02–1.73, p < 0.001) (Table 3).
Restricting analysis to 5246 patients aged 25–70 years whose first treatment was BCS followed by adjuvant chemotherapy, there were 1457 patients with final margins <1 mm.
After follow-up 520 deaths (9.9%) had occurred: 12.5% in patients with final margins <1 mm and 9.1% in patients with margins >1 mm. Margins <1 mm were associated with increased breast cancer mortality in multivariate analysis (HR 1.33 (1.10–1.60): p = 0.003) (Fig. 2f) (Table 4).
In 5,900 symptomatic patients aged 25–70 years treated by BCS first: 1673 patients had final margins <1 mm and 408 deaths (6.9%) occurred on follow-up: 9.1% in patients with final margins <1 mm and 6.1% in patients with margins >1 mm. Margins <1 mm remained in the multivariate Cox analysis (HR 1.33 (1.08–1.63): p = 0.007) (Supplementary Table 10).
Discussion
BCS patients with a margin of less than 1 mm after their final surgery had higher subsequent DR and cancer death in comparison to patients with clear margins >1 mm. This association was independent of tumour stage, grade, receptor status and use of adjuvant chemotherapy. Involved margins were associated with LR after GM patients with ToI margins were excluded from the analysis. These findings support evidence from a published metanalysis in which margins<1 mm were associated with 4.9% more LR and higher DR [4].
Strengths and limitations
This is the largest cohort study addressing surgical margins with final margin data assessed prospectively using standard pathology reporting criteria. Several limitations should be noted when interpreting the results from this study. Firstly, this was not randomised trial data and therefore the presence of unobserved confounding remains and the results cannot be confirmed as causal. Margin width data was missing from some NCRAS registered patients and NCRAS does not have LR data. Similar levels of margin involvement (1 mm) were found in a prospective UK study [8] and we verified Coding from NHSBSP pathology reports in a large sample of NCRAS patients. The nature of the margin involvement (invasive cancer or DCIS) could not be ascertained, but previous studies found both DCIS or invasive cancer at any margin and any location of involvement increased the development of LR [24, 25]. An association with cancer-related mortality beyond ten years could not be studied. Although the absolute effect of involved margins appears small, it was associated with 3% more DR and 3.4% higher cancer mortality. The development of DR, even with modern therapeutic approaches, results in incurable disease. The adverse impact of margin involvement on LR and DR after BCS persisted despite the use of adjuvant chemotherapy.
Interpretation of findings with existing literature
One in four women who develop LR within 5 years of surgery die within 15 years from breast cancer [6]. Although re-excision of involved margins is advised [7, 9, 12], the definition of involved margins varies internationally [7, 9, 12]. LR ranged between 3.8–8% at 5 years in symptomatic cancers treated in GM. NCRAS does not have LR data, although rates of LR have fallen in clinical trials; randomised trials exclude recruitment of patients with involved margins [11]. DR was more frequent and occurred without LR after 64 months followup (Supplementary Table 5), implying margin involvement can contribute to metastatic disease without causing LR first. Persistence of incompletely removed breast cancer in the breast is a potential source of post-surgery metastatic dissemination [26, 27]. Residual margin involvement <1 mm should lead to re-excision because up to 60% of patients with DCIS and invasive cancer and 36% with invasive disease have further cancer in the re-excision specimen [27]. Boost dose radiotherapy after BCS reduced LR but did not reduce distant recurrence from breast cancer after 10 years of follow-up [28]. Surgical margins were associated with increased risks of DR, independently of whether LR developed and LR occurred less frequently than DR. In BCS patients with margins<1 mm given adjuvant chemotherapy, cancer mortality remained higher than those with clear margins>1 mm, demonstrating adjuvant chemotherapy does not fully compensate for incomplete excision of a cancer.
The margin width necessary to ensure complete surgical removal remains controversial [3, 4, 7, 8, 29]. ASCO guidelines state a “margin of no tumour on the edge of the inked” specimen is sufficient clearance after BCS to reduce LR [12] based on a metanalysis of 28,000 patients undergoing BCS and radiotherapy in 33 studies from 1979–1985. Most patients in the ASCO metanalysis did not receive systemic adjuvant therapy [12], and the study did not use a specific definition of close or negative margins, instead considering any margin greater than ToI was negative. However, our recent metanalysis [4] and this data suggest that at least a 1 mm margin is required to reduce DR, LR and cancer mortality even with current adjuvant therapies. Indeed, involved margins (excluding ToI) were associated with increased LR in this study. The current best evidence for ASCO guidelines needs updating.
An EBCTCG metanalysis [11] found reductions in DR in early breast cancer between 1990 and 2009, was explained by a greater proportion of women with lower-risk disease(node negative) entering trials and adjuvant treatment. The absolute benefit of adjuvant therapy on DR was 2.8% in ER positive cancers. In the USA and UK, the majority of breast cancer deaths now occur in Stage 1/2 ER positive, node negative patients [30] (as in the GM data), a group already prescribed adjuvant endocrine therapy, indicating the potential extra therapeutic benefit for complete surgical excision leading to reductions in DR and cancer deaths.
Both BCS cohorts used measured margin data from a standardised NHSBSP minimum dataset in patients treated with systemic adjuvant therapy to demonstrate that a final margin <1 mm is associated with an increased risk of DR and cancer mortality. The margin width used to ensure tumour clearance should minimise DR. Decisions about re-excision to clear margins should be a consensual decision between clinicians and patients [31]. A previous IPCV member survey [4] indicated patients prefer full information about margins and that decisions about margin widths and re-excision must be a consensual decision between clinicians and patients with full disclosure of the risks of increased DR associated with close margins [3, 4, 26, 28].
BCS patients with symptomatic cancers had a worse prognosis than screen detected cancers as expected but involved margins independently produced worse outcomes.
Countries which do not audit margin status have higher rates of margin involvement and LR [10, 11] which may reflect poor surgical quality.
International guidelines should aim to minimise DR and cancer deaths by recommending complete cancer excision( > 1 mm) after BCS [4, 5, 8, 24].
Conclusion
In early breast cancer, where surgery is the main driver of cure, accepting the possibility of unobserved confounding in our analysis, incomplete excision may contribute to breast cancer mortality in good prognosis cancers.
Research in context
Evidence before this study
Guidelines for women undergoing breast conserving surgery (BCS) for early invasive cancer recommend avoiding tumour on ink to reduce local recurrence (LR) risk, the effect of margin clearance on cancer deaths is unknown.
Added value of this study
In cohort studies using prospectively assessed margin data, BCS patients with a final margin of <1 mm post-surgery had an increased risk of Distant Recurrence (HR 1.73), LR (HR 2.16) and cancer death (HR1.28), independent of tumour stage, grade, receptor status and adjuvant therapy.
Implications for practice
International guidelines should minimise DR and cancer deaths by complete cancer excision ( > 1 mm) after BCS.
Data availability
The data and materials are all available through writing to NJB or GM.
References
McArdle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ. 1991;302:1501–5.
Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. https://doi.org/10.1016/s0140-6736(94)92206-3.
Houssami N, Macaskill P, Marinovich ML, Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46:3219–32. https://doi.org/10.1016/j.ejca.2010.07.043.
Bundred JR, Michael S, Stuart B, Cutress RI, Beckmann K, Holleczek B, et al. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. BMJ. 2022;378:e070346 https://doi.org/10.1136/bmj-2022-070346.
Bundred J, Michael S, Bowers S, Barnes N, Jauhari Y, Plant D, et al. Do surgical margins matter after mastectomy? A systematic review. Eur J Surg Oncol. 2020. https://doi.org/10.1016/j.ejso.2020.08.015.
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), Darby S, McGale P, Taylor C, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10801 women in 17 randomised trials. Lancet. 2011;378:1707–16. https://doi.org/10.1016/S0140-6736(11)61629-2.
Early and locally advanced breast cancer: diagnosis and treatment | Guidance and guidelines | NICE. https://www.nice.org.uk/guidance/CG80/chapter/1-Guidance#surgery-to-the-breast (accessed 18 Apr 2018).
Maishman T, Cutress RI, Hernandez A, et al. Local Recurrence and Breast Oncological Surgery in Young Women With Breast Cancer. Ann Surg. 2017;266:165–72. https://doi.org/10.1097/SLA.0000000000001930.
Association of Breast Surgery: Surgical guidelines for the management of breast cancer. Eur Journal of Surgical Oncology (EJSO) 2009;35:S1–22. https://doi.org/10.1016/j.ejso.2009.01.008.
Holleczek B, Stegmaier C, Radosa JC, Solomayer EF, Brenner H. Risk of loco-regional recurrence and distant metastases of patients with invasive breast cancer up to ten years after diagnosis – results from a registry-based study from Germany. BMC Cancer. 2019;19:520. https://doi.org/10.1186/s12885-019-5710-5.].
EBCTCG.. Reductions in recurrence in women with early breast cancer entering clinical trials between 1990 and 2009: a pooled analysis of 155 746 women in 151 trials. Lancet. 2025;ume 404:1407–18.
Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32:1502–6. https://doi.org/10.1200/JCO.2014.55.1572.
Aalders KC, van Bommel ACM, van Dalen T, et al. Contemporary risks of local and regional recurrence and contralateral breast cancer in patients treated for primary breast cancer. Eur J Cancer. 2016;63:118–26. https://doi.org/10.1016/j.ejca.2016.05.010.
Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer. J Clin Oncol. 2001;19:1688–97.
Pathology Reporting of Breast Disease. NHS Cancer Screening Programmes. NHSBSP Publication No. 58. January 2005.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99.
Therneau T (2020). _A Package for Survival Analysis in R_. R package version 3.2-3, <URL: https://CRAN.R-project.org/package=survival>.
Terry M. Therneau, Patricia M. Grambsch (2000). _Modeling Survival Data: Extending the Cox Model_. Springer, New York. ISBN 0-387-98784-3.
Rubin DB Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 1987. Available from: http://doi.wiley.com/10.1002/9780470316696.
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
van Buuren Stef, Groothuis-Oudshoorn Karin (2011). MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software, 45, 1-67. URL https://www.jstatsoft.org/v45/i03.
Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. https://doi.org/10.1002/sim.2712.
Gray B (2020). Cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-10. https://CRAN.R-project.org/package=cmprsk.
Behm EC, Beckmann KR, Dahlstrom JE, Zhang Y, Cho C, Stuart-Harris R, et al. Surgical margins and risk of locoregional recurrence in invasive breast cancer: An analysis of 10-year data from the Breast Cancer Treatment Quality Assurance Project. Breast. 2013;22:839–44.
Goldstein NS, Kestin L, Vicini F. Factors associated with ipsilateral breast failure and distant metastases in patients with invasive breast carcinoma treated with breast-conserving therapy. Am J Clin Pathol. 2003;120:500–27.
Vos EL, Gaal J, Verhoef C, Brouwer K, van Deurzen CHM, Koppert LB. Focally positive margins in breast conserving surgery: Predictors, residual disease, and local recurrence. Eur J Surg Oncol. 2017;43:1846–54. https://doi.org/10.1016/j.ejso.2017.06.007.
Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer. 1995;31A:1574–9. https://doi.org/10.1016/0959-8049(95)00271-j.
Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–65.
Tyler S, Truong P, Lesperance M, Nichol A, Balsiki C, Warburton R, et al. Close Margins less than 2mmm are not associated with higher risks of 10year local recurrence and breast cancer mortality compared with negative margins in women treated with Breast Conserving therapy. Int J Rad Oncol. 2018;101:661–70.
Marczyk M, Kahn A, Silber A, Rosenblit M, Digiovanna MP, Lustberg M, et al. Trends in breast cancer-specific death by clinical stage at diagnoses between 2000 and 2017. J Natl Cancer Inst. 2025;117:287–95. https://doi.org/10.1093/jnci/djae241.
Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019;14:103–10. https://doi.org/10.1159/000499931.
Acknowledgements
We received funding for the GM Audit fom the Manchester Academic Health Science Centre Cancer Board. PHE kindly approved the NCRAS analysis and Institutional review of the protocol came from the University of Manchester and Public Health England (now NHS England). Ethical approval was gained for the work.
Funding
Funding for the GM Audit and research was obtained from Manchester AHSC.
Author information
Authors and Affiliations
Contributions
NJB conceived and designed this study. SM, SB, AA, JO, MA, SE, ND, JB and N.J.B. and JG were involved in patient data audit and project administration. JG arranged the IPCV survey. NB, SM, and SB collected and assembled data and GM analysed and interpreted the GM data. JB analysed the NCRAS data. NJB, SM, AA, GM and JB drafted the paper, and all authors critically reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have provided their consent for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michael, S., Broggio, J., Bowers, S. et al. Distant recurrence and margin involvement in invasive breast cancer. Br J Cancer (2026). https://doi.org/10.1038/s41416-025-03275-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41416-025-03275-z