Abstract
Gastrointestinal neuroendocrine tumors (GI-NETs) lack effective targeted options beyond somatostatin analogs and mTOR inhibitors. Spleen tyrosine kinase (SYK) is a non-receptor kinase with emerging roles in solid tumors and available small-molecule inhibitors. We explored whether SYK is a plausible therapeutic target in GI-NET using two human cell lines. SYK expression in GI-NET cells was confirmed by immunofluorescence. Cells were exposed to a selective SYK inhibitor (BI-1002494), and proliferation was quantified using both 2D and 3D models. Both GI-NET models expressed SYK and exhibited reduced growth upon SYK blockade, with dose-dependent suppression of viability and increased cytotoxicity relative to vehicle. In spheroid assays, morphologic changes and reduced size were observed. These pilot data suggest SYK as a targetable vulnerability in GI-NET and support formal dose–response studies, genetic validation, and combination strategies with standard-of-care agents. Given the clinical availability of SYK inhibitors, these findings provide a rationale for translational studies in GI-NET.
Similar content being viewed by others
Introduction
Gastrointestinal neuroendocrine tumors (GI-NETs) are rare and biologically heterogeneous, with a consistently increasing incidence. Despite this trend, therapies remain limited because resistance is common, and diagnosis often occurs at advanced disease stages [1,2,3].
Spleen tyrosine kinase (SYK) integrates immunoreceptor and integrin signaling. Although best studied in hematologic malignancies, in solid tumor SYK has been reported to play a major role in tumor cell survival and proliferation, underscoring its tumor-promoting function and therapeutic potential, including in endocrine related cancer, suggesting context-dependent oncogenic functions [4].
Currently the role of SYK in GI-NET biology has not been investigated. Our aim is to test whether inhibition of SYK suppresses growth of GI-NET cells, providing pilot evidence for SYK as a therapeutic target.
Brief descriptions of materials and methods
Cell lines and culture
Human GI-NET cell line GOT1, gift of Ola Nilsson Group, [5] and a colorectal adenocarcinoma cell line with neuroendocrine features COLO320DM were maintained under standard conditions as previously described. Cells were kept at low passage, routinely authenticated by morphology/growth characteristics, and regularly tested for mycoplasma [6].
Compound
The SYK inhibitor BI-1002494 was obtained from Boehringer Ingelheim via opnMe (https://www.opnme.com).
Antibodies
Rabbit anti-human SYK (ABclonal) and ABflo® 647–conjugated mouse anti-rabbit IgG (ABclonal) were used for immunofluorescence.
2D/3D viability
For 2D assays, 3 × 10³ cells/well were seeded in 96-well plates (low-serum medium), treated for 72 h, fixed in 4% paraformaldehyde, stained with 0.1% crystal violet, air-dried overnight, solubilized in 10% acetic acid, and read spectrophotometrically. For 3D assays, viability was quantified using CellTiter-Glo® 3D (Promega) according to the manufacturer’s protocol, with ATP-dependent luminescence measured on a plate reader [7].
Colony formation
COLO320DM cells (3 × 10³/well, 6-well plates; low-serum) were treated for 14 days with medium refreshed every 3–4 days, stained with crystal violet, and colonies quantified in ImageJ [8].
Caspase-3/7 activity
Cells were treated for 24 h and analysed using Caspase-Glo® 3/7 (Promega) per the manufacturer’s instructions; luminescence was recorded on a plate reader.
Invasion
Matrigel-coated transwells (8 µm pores) received 5 × 10⁵ cells/insert (low-serum) plus treatment; 20% FBS in the lower chamber served as chemoattractant. After 24 h, inserts were fixed (4% paraformaldehyde), stained with crystal-violet, and invading cells on the lower membrane surface were counted microscopically [9].
Immunofluorescence
Cells on coverslips were fixed (4% paraformaldehyde), permeabilized (0.1% Triton X-100), blocked (5% BSA), incubated with anti-SYK (overnight), then secondary antibody and Hoechst. Slides were mounted with anti-fade medium and imaged by confocal microscopy [10].
RNA and qPCR
RNA was isolated (PureLink™), reverse-transcribed (High-Capacity kit), and SYBR-based qPCR run on QuantStudio™ 1; β-ACTIN served as control. Primer sequences:
BACT-FW: CGCCGCCAGCTCACCATG
BACT-REV CACGATGGAGGGGAAGACGG
E-CADH-FW GGCAGCTAATACAGACTATAAG
E-CADH-REV TGTGAAGTTTGGATTGACAG
Statistics
Analyses were performed in GraphPad Prism 10; tests are specified in figure legends, with p < 0.05 considered significant. Unless noted, experiments used ≥3 biological replicates (qPCR: ≥2 biological, 3 technical replicates).
Results
SYK inhibition reduces cell viability and increases apoptosis in GI-NET cell lines
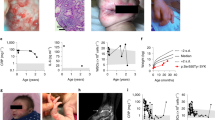
SYK expression was analysed by immunofluorescence, revealing a marked protein expression in both cell lines (Fig. 1A, B). To evaluate the impact of SYK inhibition on the viability of GI-NET cells, COLO320DM and GOT1 cells were treated with increasing concentrations of SYK inhibitor (0–10/20 µM range). In 2D cultures, a significant dose-dependent reduction in cell viability was observed in both COLO320DM and GOT1 cells, as assessed by the crystal violet assay (Fig. 1C). A 2.5 µM concentration of the compound was sufficient to significantly reduce cell viability. In 3D spheroid cultures of COLO320DM and GOT1, treatment with SYK inhibitor did not decrease its efficacy in reducing cell viability, with statistical significance requiring concentrations of 5–10 µM (Fig. 1E). In addition, the spheres of GI-NETs cell lines showed a reduction in volume upon treatment with SYK inhibitor (Fig. 1F).
SYK expression was detected in both COLO320DM (A) and in GOT1 (B) cells by immunofluorescence staining (SYK is stained magenta, while nuclei are shown in blue). Effects of SYK inhibition on 2D and 3D NETs cell culture. C, E COLO320DM and GOT1 cells were seeded in 96 well plates and incubated for 72 h with increasing concentration of SYK inhibitor, control cells were treated with a vehicle (DMSO). Cell viability in 2D assays (C) was assessed by crystal violet in three independent experiments with at least six replicates each, and it is expressed as the mean ± SEM. *P < 0.05 vs vehicle cells; ***P < 0.0001 vs vs vehicle cells. ****P < 0.00001 vs vs vehicle cells. Cell viability of 3D cells (E) was measured as luminescent output in three independent experiments with at least two replicates each, and it is expressed as the mean ± SEM **P < 0.01 vs vs vehicle cells, ****P < 0.00001 vs vs vehicle cells, significance was calculated by two-way ANOVA using Dunnett’s multiple comparison test. F Representative images of 3D spheroids at baseline, Day 1, and Day 3 of treatment. D Caspase activity was measured as luminescent output in two independent experiments with two replicates each, and it is expressed as the mean ± SEM. ***P < 0.01 vs vs vehicle cells, ****P < 0.00001 vs vehicle cells, significance was calculated by two-way ANOVA using Dunnett’s multiple comparison test.
Increased caspase activity confirmed apoptosis induction following treatment with SYK inhibitor (Fig. 1D). These results suggest that SYK inhibition activates apoptotic pathways contributing to the observed reduction in cell viability.
SYK inhibition decreases invasion, clonogenicity and increases E-cadherin expression in GI-NET cells
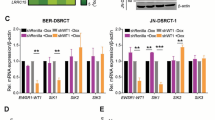
Invasion assays demonstrated that treatment with SYK inhibitor markedly reduced the invasive ability of COLO320DM (Fig. 2A) and GOT1 (Fig. 2B) cells compared to DMSO-treated controls, indicating that SYK activity is involved in maintaining invasive properties of GI-NET cells. Consistently, increased level of E-cadherin was detected in both cell lines upon treatment with SYK inhibitor (Fig. 2C, D). We next examined the effect of SYK inhibition on colony-forming potential. Treatment with SYK inhibitor reduced the colony-forming ability of COLO320DM cells (Fig. 2E), suggesting a role for SYK in sustaining their growth potential.
For the invasion assay of COLO320DM (A) and GOT1 (B), cells were seeded on the Matrigel-coated upper chamber of the transwell. Twenty-four hours later, migrated cells were fixed, stained, and counted (n = 3 independent experiments, minimum 2 technical replicates). qRT-PCR of E-cadherin expression levels in COLO320DM (C) and GOT1 (D) cells following treatment with SYK inhibitor. Significance was calculated using one-way ANOVA. Data are expressed as mean ± SEM; **P < 0.01, ****P < 0.0001 vs. DMSO, significance was calculated by Anova using Dunnett’s multiple comparison test. Changes in colony number and dimension of COLO320DM (E) after treatment with SYK inhibitor, with representative images of colony formation assays. Colonies were quantified using Image J Software and results show the percentage of colonies formed after treatment with the indicated concentrations of the drug (surviving fraction), corrected according to the plating efficiencies of the corresponding controls. Data are shown as mean ± SEM of at least three independent experiments, *P < 0.05 vs vehicle cells significance was calculated by two-way ANOVA using Šidák’s multiple comparison test.
Discussion and future perspectives
These data provide preliminary evidence that SYK supports growth and invasiveness in GI‑NET models. Pharmacologic SYK blockade reduces viability, invasion, and clonogenicity while increasing E‑cadherin expression, consistent with a less aggressive phenotype.
In solid tumors, SYK connects receptor and integrin cues to PI3K/AKT, NF-κB, and context-dependent STAT signaling that governs survival and invasion. This supports SYK inhibition as the basis of our observed effects and justify focused pathway mapping in GI-NETs [11]. Given the availability of clinically tested SYK inhibitors, key next steps include confirming target engagement (e.g., p‑SYK inhibition), genetic validation of SYK dependence (e.g. by siRNA/CRISPRi experiments), and testing combinations with somatostatin analogs or mTOR inhibitors in patient-derived models and in vivo to inform biomarker‑guided early‑phase trials.
References
Toffoli L, Ditsiou A, Gagliano T. Exploring emerging therapeutic targets and opportunities in neuroendocrine tumors: updates on receptor tyrosine kinases. Receptors. 2024;3:145–54.
Garcia-Carbonero R, Anton-Pascual B, Modrego A, Del Carmen Riesco-Martinez M, Lens-Pardo A, Carretero-Puche C, et al. Advances in the treatment of gastroenteropancreatic neuroendocrine carcinomas: are we moving forward?. Endocr Rev. 2023;44:724–36.
Di Domenico A, Wiedmer T, Marinoni I, Perren A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr Relat Cancer. 2017;24:R315–34.
Fu G, Somasundaram RT, Jessa F, Srivastava G, MacMillan C, Witterick I, et al. ER maleate is a novel anticancer agent in oral cancer: implications for cancer therapy. Oncotarget. 2016;7:17162–81.
Kölby L, Bernhardt P, Ahlman H, Wängberg B, Johanson V, Wigander A, et al. A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. Am J Pathol. 2001;158:745–55.
Toffoli L, Ditsiou A, Triboli L, Hamm V, Moschioni E, D’Este F, et al. FAK inhibition disrupts tumor growth, apoptosis, and transcriptional regulation in GI-NETs. Endocr Oncol. 2025;5:e250052.
Bresciani G, Ditsiou A, Cilibrasi C, Vella V, Rea F, Schiavon M, et al. EGF and IGF1 affect sunitinib activity in BP-NEN: new putative targets beyond VEGFR?. Endocr Connect. 2019;8:680–90.
Vella V, Ditsiou A, Chalari A, Eravci M, Wooller SK, Gagliano T, et al. Kinome-wide synthetic lethal screen identifies PANK4 as a modulator of temozolomide resistance in glioblastoma. Adv Sci. 2024;11:2306027.
Gagliano T, Shah K, Gargani S, Lao L, Alsaleem M, Chen J, et al. PIK3Cδ expression by fibroblasts promotes triple-negative breast cancer progression. J Clin Invest. 2020;130:3188–204.
Ditsiou A, Cilibrasi C, Simigdala N, Papakyriakou A, Milton-Harris L, Vella V, et al. The structure-function relationship of oncogenic LMTK3. Sci Adv. 2020;6:eabc3099.
Joshi S. New insights into SYK targeting in solid tumors. Trends Pharm Sci. 2024;45:904–18.
Funding
This work was supported by Departmental Strategic Plan (PSD) of the University of Udine-Interdepartmental Project on Healthy Ageing (2020-25). Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
TG conceived the idea, acquired funding, reviewed the manuscript and supervised the research group. AD performed QPCR, data analyses and wrote the original draft of the manuscript. LT performed invasion and colony assays and wrote the methods of manuscript. VV proofread the manuscript and prepared the figures. FD analysed and acquired immunofluorescence experiments.
Corresponding author
Ethics declarations
Competing interests
TG is Senior Associated Editor of CGT, while AD and VV are Editorial Board member of the journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ditsiou, A., Toffoli, L., Vella, V. et al. Spleen tyrosine kinase (SYK) inhibition suppresses growth of gastrointestinal neuroendocrine tumor cells: a pilot study in two cell lines. Cancer Gene Ther 32, 1303–1306 (2025). https://doi.org/10.1038/s41417-025-00979-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-025-00979-5