Abstract
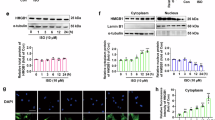
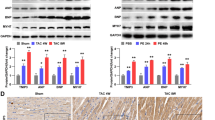
Sorting nexins (SNXs), the retromer-associated cargo binding proteins, have emerged as critical regulators of the trafficking of proteins involved in the pathogenesis of diverse diseases. However, studies of SNXs in the development of cardiovascular diseases, especially cardiac hypertrophy and heart failure, are lacking. Here, we ask whether SNX3, the simplest structured isoform in the SNXs family, may act as a key inducer of myocardial injury. An increased level of SNX3 was observed in failing hearts from human patients and mice. Cardiac-specific Snx3 knockout (Snx3-cKO) mice and Snx3 transgenic (Snx3-cTg) mice were generated to evaluate the role of Snx3 in myocardial hypertrophy, fibrosis, and heart function by morphology, echocardiography, histological staining, and hypertrophic biomarkers. We report that Snx3-cKO in mice significantly protected against isoproterenol (ISO)-induced cardiac hypertrophy at 12 weeks. Conversely, Snx3-cTg mice were more susceptible to ISO-induced cardiac hypertrophy at 12 weeks and showed aggravated cardiac injury even heart failure at 24 weeks. Immunoprecipitation-based mass spectrometry, immunofluorescent staining, co-immunoprecipitation, localized surface plasmon resonance, and proximity ligation assay were performed to examine the direct interaction of SNX3-retromer with signal transducer and activator of transcription 3 (STAT3). We discovered that STAT3 was a new interacting partner of SNX3-retromer, and SNX3-retromer served as an essential platform for assembling gp130/JAK2/STAT3 complexes and subsequent phosphorylation of STAT3 by direct combination at EE. SNX3-retromer and STAT3 complexes were transiently imported into the nucleus after hypertrophic stimuli. The pharmacological inhibition or knockdown of STAT3 reversed SNX3 overexpression-induced myocardial injury. STAT3 overexpression blunts the beneficial function of SNX3 knockdown on hypertrophic cardiomyocytes. We show that SNX3-retromer promoted importin α3-mediated STAT3 nuclear trafficking and ultimately leading to cardiac injury. Taken together, our study reveals that SNX3 plays a key role in cardiac function and implicates SNX3 as a potential therapeutic target for cardiac hypertrophy and heart failure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, et al. Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–50.
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Amer Heart Assoc Stat, S. Stroke Stat, Heart disease and stroke statistics-2012 update a report from the American Heart Association. Circulation. 2012;125:E2–E220.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600.
Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, et al. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2000;97:315–9.
Kovtun O, Leneva N, Bykov YS, Ariotti N, Teasdale RD, Schaffer M, et al. Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Nature. 2018;561:561–4.
Lucas M, Gershlick DC, Vidaurrazaga A, Rojas AL, Bonifacino JS, Hierro A. Structural mechanism for cargo recognition by the retromer complex. Cell. 2016;167:1623–35.e14.
Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z, et al. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med. 2014;20:1165–73.
Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2011;14:29–37.
Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–82.
Zhou C, You Y, Shen W, Zhu YZ, Peng J, Feng HT, et al. Deficiency of sorting nexin 10 prevents bone erosion in collagen-induced mouse arthritis through promoting NFATc1 degradation. Ann Rheum Dis. 2016;75:1211–8.
Li J, Li CM, Zhang DS, Shi D, Qi M, Feng J, et al. SNX13 reduction mediates heart failure through degradative sorting of apoptosis repressor with caspase recruitment domain. Nat Commun. 2014;5:5177.
Okada H, Zhang W, Peterhoff C, Hwang JC, Nixon RA, Ryu SH, et al. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 2010;24:2783–94.
Zhang JL, Li K, Zhang YG, Lu R, Wu SP, Tang JR, et al. Deletion of sorting nexin 27 suppresses proliferation in highly aggressive breast cancer MDA-MB-231 cells in vitro and in vivo. BMC Cancer. 2019;19:555.
McGough IJ, de Groot REA, Jellett AP, Betist MC, Varandas KC, Danson CM, et al. SNX3-retromer requires an evolutionary conserved MON2:DOPEY2:ATP9A complex to mediate Wntless sorting and Wnt secretion. Nat Commun. 2018;9:3737.
Feng S, Streets AJ, Nesin V, Tran U, Nie H, Onopiuk M, et al. The sorting nexin 3 retromer pathway regulates the cell surface localization and activity of a Wnt-activated polycystin channel complex. J Am Soc Nephrol. 2017;28:2973–84.
Cui Y, Carosi JM, Yang Z, Ariotti N, Kerr MC, Parton RG, et al. Retromer has a selective function in cargo sorting via endosome transport carriers. J Cell Biol. 2019;218:615–31.
Patel D, Xu C, Nagarajan S, Liu Z, Hemphill WO, Shi R, et al. Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum Mol Genet. 2018;27:1514–32.
Wang L, Li Z, Tan Y, Li Q, Yang H, Wang P, et al. PARP1 interacts with STAT3 and retains active phosphorylated-STAT3 in nucleus during pathological myocardial hypertrophy. Mol Cell Endocrinol. 2018;474:137–50.
Zhang W, Qu X, Chen B, Snyder M, Wang M, Li B, et al. Critical roles of STAT3 in beta-adrenergic functions in the heart. Circulation. 2016;133:48–61.
Zhang HX, Xu ZS, Lin H, Li M, Xia T, Cui K, et al. TRIM27 mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis. Nat Commun. 2018;9:3441.
Liu L, McBride KM, Reich NC. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha 3. Proc Natl Acad Sci USA. 2005;102:8150–5.
Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–12.
Ohara R, Fujita Y, Hata K, Nakagawa M, Yamashita T. Axotomy induces axonogenesis in hippocampal neurons through STAT3. Cell Death Dis. 2011;2:e175.
Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–63.
Di Liberto V, Cavalli V. Ready, STAT, go: transcription factors on the move. EMBO J. 2012;31:1331–3.
Mukhopadhyay S, Shah M, Xu F, Patel K, Tuder RM, Sehgal PB. Cytoplasmic provenance of STAT3 and PY-STAT3 in the endolysosomal compartments in pulmonary arterial endothelial and smooth muscle cells: implications in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L449–68.
Bild AH, Turkson J, Jove R. Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO J. 2002;21:3255–63.
German CL, Sauer BM, Howe CL. The STAT3 beacon: IL-6 recurrently activates STAT 3 from endosomal structures. Exp Cell Res. 2011;317:1955–69.
Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J Cell Biol. 2008;182:855–63.
Li J, Huang J, Lu J, Guo Z, Li Z, Gao H, et al. Sirtuin 1 represses PKC-zeta activity through regulating interplay of acetylation and phosphorylation in cardiac hypertrophy. Br J Pharmacol. 2019;176:416–35.
Hashimoto T, Kim GE, Tunin RS, Adesiyun T, Hsu S, Nakagawa R, et al. Acute enhancement of cardiac function by phosphodiesterase type 1 inhibition: translational study in the dog and rabbit. Circulation. 2018;138:1974–87.
Zhang N, Zhang Y, Qian H, Wu S, Cao L, Sun Y. Selective targeting of ubiquitination and degradation of PARP1 by E3 ubiquitin ligase WWP2 regulates isoproterenol-induced cardiac remodeling. Cell Death Differ. 2020;27:2605–19.
Li Y, Lv Z, He L, Huang X, Zhang S, Zhao H, et al. Genetic tracing identifies early segregation of the cardiomyocyte and nonmyocyte lineages. Circ Res. 2019;125:343–55.
Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Investig. 1997;100:169–79.
Kunisada K, Tone E, Fujio Y, Matsui H, Yamauchi-Takihara K, Kishimoto T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation. 1998;98:346–52.
Frias MA, Rebsamen MC, Gerber-Wicht C, Lang U. Prostaglandin E2 activates Stat3 in neonatal rat ventricular cardiomyocytes: a role in cardiac hypertrophy. Cardiovasc Res. 2007;73:57–65.
Chen C, Garcia-Santos D, Ishikawa Y, Seguin A, Li L, Fegan KH, et al. Snx3 regulates recycling of the transferrin receptor and iron assimilation. Cell Metab. 2013;17:343–52.
Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:715–21.
Nothwehr SF, Bruinsma P, Strawn LA. Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol Biol Cell. 1999;10:875–90.
Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–81.
Gallon M, Clairfeuille T, Steinberg F, Mas C, Ghai R, Sessions RB, et al. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc Natl Acad Sci USA. 2014;111:E3604–13.
Zhang P, Wu YH, Belenkaya TY, Lin XH. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res. 2011;21:1677–90.
Lorenowicz MJ, Macurkova M, Middelkoop TC, de Groot R, Betist MC, Korswagen HC. Inhibition of late endosomal maturation restores Wnt secretion in Caenorhabditis elegans vps-29 retromer mutants. Cell Signal. 2014;26:19–31.
Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol. 2011;13:914–23.
Strochlic TI, Schmiedekamp BC, Lee J, Katzmann DJ, Burd CG. Opposing activities of the Snx3-retromer complex and ESCRT proteins mediate regulated cargo sorting at a common endosome. Mol Biol Cell. 2008;19:4694–706.
Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specic adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–25.
Xu WJ, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 2015;13:533–45.
Gross JC, Zelarayán LC. The mingle-mangle of Wnt signaling and extracellular vesicles: functional implications for heart research. Front Cardiovasc Med. 2018;5:10.
Lakhal-Littleton S. Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radic Biol Med. 2019;133:234–37.
Yin CR, Zhang T, Qu XY, Zhang YG, Putatunda R, Xiao X, et al. In vivo excision of HIV-1 provirus by saCas9 and multiplex single-guide RNAs in animal models. Mol Ther. 2017;25:1168–86.
Tao Z, Yang H, Shi Q, Fan Q, Wan L, Lu X. Targeted delivery to tumor-associated pericytes via an affibody with high affinity for PDGFRbeta enhances the in vivo antitumor effects of human TRAIL. Theranostics. 2017;7:2261–76.
Cottrell GS, Soubrane CH, Hounshell JA, Lin H, Owenson V, Rigby M, et al. CACHD1 is an α2δ-Like protein that modulates Ca(V)3 voltage-gated calcium channel activity. J Neurosci. 2018;38:9186–201.
Lu J, Zhang RW, Hong HQ, Yang ZL, Sun DP, Sun SY, et al. The poly(ADP-ribosyl)ation of FoxO3 mediated by PARP1 participates in isoproterenol-induced cardiac hypertrophy. Biochim Biophys Acta. 2016;1863:3027–39.
Lu J, Sun DP, Liu ZP, Li M, Hong HQ, Liu C, et al. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl Res. 2016;172:96–112.
de Araujo ME, Lamberti G, Huber LA. Isolation of early and late endosomes by density gradient centrifugation. Cold Spring Harb Protoc. 2015;2015:1013–6.
Nakatsuka S, Hayashi M, Muroyama A, Otsuka M, Kozaki S, Yamada H, et al. D-aspartate is stored in secretory granules and released through a Ca2+-dependent pathway in a subset of rat pheochromocytoma PC12 cells. J Biol Chem. 2001;276:26589–96.
Luo WW, Wang Y, Yang HW, Dai CM, Hong HL, Li JY, et al. Heme oxygenase-1 ameliorates oxidative stress-induced endothelial senescence via regulating endothelial nitric oxide synthase activation and coupling. Aging. 2018;10:1722–44.
Horvath CM, Wen Z, Darnell JEA. STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–94.
Acknowledgements
We thank all patients who participated in this study for their cooperation. We also thank Jiantao Ye, Min Li, and Zhiping Liu for their excellent technical assistance.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81803521, 81872860, 82003710, 82070464), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y093), National Major Special Projects for the Creation and Manufacture of New Drugs (2019ZX09301104), National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province, 2017B090903004), Special Program for Applied Science and Technology of Guangdong Province (2015B020232009), Natural Science Foundation of Guangdong Province (2019A1515010273, 2021B1515020100), and Fundamental Research Funds for the Central Universities (19ykpy131).
Author information
Authors and Affiliations
Contributions
JL and PQL conceived the project. JL, ZKW, YHH, JJW, and XLZ designed and performed majority of the experiments and data analyses. SWX, YQH, and PQL provided scientific advice. DPS, PXW, ZML, and MYL performed several in vitro experiments. JL, ZKW, and PQL wrote the manuscript. JL, SWX, YQH, and DPS. critically revised this paper. All authors critically evaluated the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All the sample collection and experimental protocols were approved by the Research Ethics Committee of Sun Yat-sen University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by R. Kitsis
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, J., Xu, S., Huo, Y. et al. Sorting nexin 3 induces heart failure via promoting retromer-dependent nuclear trafficking of STAT3. Cell Death Differ 28, 2871–2887 (2021). https://doi.org/10.1038/s41418-021-00789-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-021-00789-w
This article is cited by
-
Targeting sorting nexin 3 to treat pulmonary fibrosis by dual modulating Wnt/β-catenin signaling
Cell Death & Disease (2026)
-
SNX3 mediates heart failure by interacting with HMGB1 and subsequently facilitating its nuclear-cytoplasmic translocation
Acta Pharmacologica Sinica (2025)
-
The functional role of m6A demethylase ALKBH5 in cardiomyocyte hypertrophy
Cell Death & Disease (2024)
-
EGF-SNX3-EGFR axis drives tumor progression and metastasis in triple-negative breast cancers
Oncogene (2022)