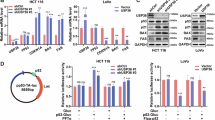
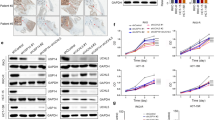
Abstract
Colorectal cancer (CRC) is the third malignant tumor in incidence rate and the second leading cause of cancer death worldwide. The progression of CRC is associated with both autophagy and ubiquitin-specific proteases (USPs). While USPs have been shown to regulate autophagy, their specific involvement in CRC autophagy remains largely unexplored. Herein, through the analysis of autophagy-related protein expression levels and proteomics, we found that high expression of USP48 was closely related to the inhibition of autophagy in CRC. And further analysis of CRC tissues showed that USP48 served as an independent risk factor for CRC patient prognosis. In vitro, we observed that USP48 significantly enhanced the proliferative, migration and invasion capacities of CRC cells. In vivo experiments showed that USP48 knockdown significantly inhibited the growth of subcutaneous tumors in nude mice, and the deletion of USP48 in intestinal epithelial cells reduced the number of intestinal tumors and mortality rate of mice. Mechanistically, USP48 was found to interact with the autophagy substrate protein sequestosome 1 (SQSTM1) and induce its deubiquitination at K420. This process inhibited autophagy, consequently maintaining the protein stability of SQSTM1. Rescue experiments demonstrated that SQSTM1 was the key target for USP48 to inhibit autophagy and promote CRC progression. Based on these findings, we constructed a tetrahedral DNA nanomaterial loaded with USP48 small interfering RNA (siRNA), which could effectively inhibited the progression of CRC in vivo while exhibiting excellent biocompatibility. In summary, our findings highlighted the role of USP48 in promoting CRC progression via the stabilization of SQSTM1, which leads to the suppression of autophagy. Thus, USP48 may be a potential therapeutic target for CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed in this study are available in this article and its Supplementary Files.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2023;41:678–700.
Luo XJ, Zhao Q, Liu J, Zheng JB, Qiu MZ, Ju HQ, et al. Novel genetic and epigenetic biomarkers of prognostic and predictive significance in stage II/III colorectal cancer. Mol Ther J Am Soc Gene Ther. 2021;29:587–96.
Zhang X, Sun H, Chen W, He X. Elevated expression of AGGF1 predicts poor prognosis and promotes the metastasis of colorectal cancer. BMC Cancer. 2019;19:1252.
Korver SK, Gibson RJ, Bowen JM, Coller JK. Toll-like receptor/interleukin-1 domain innate immune signalling pathway genetic variants are candidate predictors for severe gastrointestinal toxicity risk following 5-fluorouracil-based chemotherapy. Cancer Chemother Pharmacol. 2019;83:217–36.
Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: regulator of cell death. Cell Death Dis. 2023;14:648.
Eskelinen EL. Autophagy: supporting cellular and organismal homeostasis by self-eating. Int J Biochem Cell Biol. 2019;111:1–10.
Feng X, Zhang H, Meng L, Song H, Zhou Q, Qu C, et al. Hypoxia-induced acetylation of PAK1 enhances autophagy and promotes brain tumorigenesis via phosphorylating ATG5. Autophagy. 2021;17:723–42.
Marsh T, Debnath J. Autophagy suppresses breast cancer metastasis by degrading NBR1. Autophagy. 2020;16:1164–5.
Li X, Dai Z, Wu X, Zhang N, Zhang H, Wang Z, et al. The comprehensive analysis identified an autophagy signature for the prognosis and the immunotherapy efficiency prediction in lung adenocarcinoma. Front Immunol. 2022;13:749241.
Liu M, Sun T, Li N, Peng J, Fu D, Li W, et al. BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration. Nat Commun. 2019;10:4614.
Haruki K, Kosumi K, Hamada T, Twombly TS, Väyrynen JP, Kim SA, et al. Association of autophagy status with amount of Fusobacterium nucleatum in colorectal cancer. J Pathol. 2020;250:397–408.
Qureshi-Baig K, Kuhn D, Viry E, Pozdeev VI, Schmitz M, Rodriguez F, et al. Hypoxia-induced autophagy drives colorectal cancer initiation and progression by activating the PRKC/PKC-EZR (ezrin) pathway. Autophagy. 2020;16:1436–52.
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L, et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. 2024;20:114–30.
Hu M, Li P, Li M, Li W, Yao T, Wu JW, et al. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 2002;111:1041–54.
Kitamura H. Ubiquitin-specific proteases (USPs) and metabolic disorders. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms24043219.
Choi BJ, Park SA, Lee SY, Cha YN, Surh YJ. Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of snail: a potential role of Sox9. Sci Rep. 2017;7:15918.
Xiao Y, Jiang X, Yin K, Miao T, Lu H, Wang W, et al. USP35 promotes cell proliferation and chemotherapeutic resistance through stabilizing FUCA1 in colorectal cancer. Oncogenesis. 2023;12:12.
Kim JH, Seo D, Kim SJ, Choi DW, Park JS, Ha J, et al. The deubiquitinating enzyme USP20 stabilizes ULK1 and promotes autophagy initiation. EMBO Rep. 2018;19. https://doi.org/10.15252/embr.201744378.
Raimondi M, Cesselli D, Di Loreto C, La Marra F, Schneider C, Demarchi F. USP1 (ubiquitin specific peptidase 1) targets ULK1 and regulates its cellular compartmentalization and autophagy. Autophagy. 2019;15:613–30.
Thayer JA, Awad O, Hegdekar N, Sarkar C, Tesfay H, Burt C, et al. The PARK10 gene USP24 is a negative regulator of autophagy and ULK1 protein stability. Autophagy. 2020;16:140–53.
Gao Z, Li C, Sun H, Bian Y, Cui Z, Wang N, et al. N(6)-methyladenosine-modified USP13 induces pro-survival autophagy and imatinib resistance via regulating the stabilization of autophagy-related protein 5 in gastrointestinal stromal tumors. Cell Death Differ. 2023;30:544–59.
Kim MJ, Min Y, Jeong SK, Son J, Kim JY, Lee JS, et al. USP15 negatively regulates lung cancer progression through the TRAF6-BECN1 signaling axis for autophagy induction. Cell Death Dis. 2022;13:348.
Lee Y, Chou TF, Pittman SK, Keith AL, Razani B, Weihl CC. Keap1/Cullin3 modulates p62/SQSTM1 activity via UBA domain ubiquitination. Cell Rep. 2017;19:188–202.
Peng H, Yang J, Li G, You Q, Han W, Li T, et al. Ubiquitylation of p62/sequestosome1 activates its autophagy receptor function and controls selective autophagy upon ubiquitin stress. Cell Res. 2017;27:657–74.
Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9:1167–81.
Miller DR, Thorburn A. Autophagy and organelle homeostasis in cancer. Dev Cell. 2021;56:906–18.
Jing Z, He X, Jia Z, Sa Y, Yang B, Liu P. NCAPD2 inhibits autophagy by regulating Ca(2+)/CAMKK2/AMPK/mTORC1 pathway and PARP-1/SIRT1 axis to promote colorectal cancer. Cancer Lett. 2021;520:26–37.
Wei R, Xiao Y, Song Y, Yuan H, Luo J, Xu W. FAT4 regulates the EMT and autophagy in colorectal cancer cells in part via the PI3K-AKT signaling axis. J Exp Clin Cancer Res. 2019;38:112.
Jo YK, Kim SC, Park IJ, Park SJ, Jin DH, Hong SW, et al. Increased expression of ATG10 in colorectal cancer is associated with lymphovascular invasion and lymph node metastasis. PloS ONE. 2012;7:e52705.
Antao AM, Kaushal K, Das S, Singh V, Suresh B, Kim KS, et al. USP48 governs cell cycle progression by regulating the protein level of Aurora B. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22168508.
Velimezi G, Robinson-Garcia L, Muñoz-Martínez F, Wiegant WW, Ferreira da Silva J, Owusu M, et al. Map of synthetic rescue interactions for the Fanconi anemia DNA repair pathway identifies USP48. Nat Commun. 2018;9:2280.
Zhou A, Lin K, Zhang S, Ma L, Xue J, Morris SA, et al. Gli1-induced deubiquitinase USP48 aids glioblastoma tumorigenesis by stabilizing Gli1. EMBO Rep. 2017;18:1318–30.
Lei X, Li X, Chen H, Liu Z. USP48 sustains chemoresistance and metastasis in ovarian cancer. Current Cancer Drug Targets. 2020;20:689–99.
Nezis IP, Stenmark H. p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid Redox Signal. 2012;17:786–93.
Deng Z, Lim J, Wang Q, Purtell K, Wu S, Palomo GM, et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy. 2020;16:917–31.
Gou H, Chen X, Zhu X, Li L, Hou L, Zhou Y, et al. Sequestered SQSTM1/p62 crosstalk with Keap1/NRF2 axis in hPDLCs promotes oxidative stress injury induced by periodontitis. Free Radic Biol Med. 2022;190:62–74.
Pan JA, Sun Y, Jiang YP, Bott AJ, Jaber N, Dou Z, et al. TRIM21 ubiquitylates SQSTM1/p62 and suppresses protein sequestration to regulate redox homeostasis. Mol Cell. 2016;61:720–33.
Song P, Li S, Wu H, Gao R, Rao G, Wang D, et al. Parkin promotes proteasomal degradation of p62: implication of selective vulnerability of neuronal cells in the pathogenesis of Parkinson’s disease. Protein Cell. 2016;7:114–29.
Dong Y, Yao C, Zhu Y, Yang L, Luo D, Yang D. DNA functional materials assembled from branched DNA: design, synthesis, and applications. Chem Rev. 2020;120:9420–81.
Jiang Q, Liu S, Liu J, Wang ZG, Ding B. Rationally designed DNA-origami nanomaterials for drug delivery in vivo. Adv Mater. 2019;31:e1804785.
Wang D, Zhang X, Zhu X. Drug-grafted DNA for cancer therapy. J Phys Chem B. 2023;127:5379–88.
Guo W, Gao H, Li H, Ge S, Zhang F, Wang L, et al. Self-assembly of a multifunction DNA tetrahedron for effective delivery of aptamer PL1 and Pcsk9 siRNA potentiate immune checkpoint therapy for colorectal cancer. ACS Appl Mater Interfaces. 2022;14:31634–44.
Dai B, Hu Y, Duan J, Yang XD. Aptamer-guided DNA tetrahedron as a novel targeted drug delivery system for MUC1-expressing breast cancer cells in vitro. Oncotarget. 2016;7:38257–69.
Zhao Y, Xu J, Le VM, Gong Q, Li S, Gao F, et al. EpCAM aptamer-functionalized cationic liposome-based nanoparticles loaded with miR-139-5p for targeted therapy in colorectal cancer. Mol Pharm. 2019;16:4696–710.
Pishavar E, Yazdian-Robati R, Abnous K, Hashemi M, Ebrahimian M, Feizpour R, et al. Aptamer-functionalized mesenchymal stem cells-derived exosomes for targeted delivery of SN38 to colon cancer cells. Iran J Basic Med Sci. 2023;26:388–94.
Funding
This research was supported by grant from the National Natural Science Foundation of China (82122041, 82472357 and 82202604), the Key Research and Development Program of Shandong Province (2021ZLGX02, 2020CXGC011304 and 2022CXGC020507), Fundamental Research Funds for the Central Universities (2022JC002), Natural Science Foundation of Shandong Province (ZR2022QH044), Taishan Scholars Climbing Program of Shandong Province (NO.tspd20210323), Young Taishan Scholars Program of Shandong Province (NO.tsqn201909176, NO.tsqn202211322 and NO.tsqn202211323), Outstanding Young and Middle-aged Scholar of Shandong University and Tumor Biomarker Innovation Team Foundation of Jinan City (2021GXRC020).
Author information
Authors and Affiliations
Contributions
CW, PL, and LD designed and supervised this study; JL, AL, and WD executed the development of experiments, statistical analysis and writing; YL, XK, TW, DN, and SL participated in the experiments and collected data; PZ provided technical support. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ethics Committee of the Second Hospital of Shandong University and the Ethics Committee of Qutdo Biotech.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Liu, A., Duan, W. et al. Ubiquitin-specific protease 48 drives malignant progression of colorectal cancer by suppressing autophagy through stabilizing sequestosome 1. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01617-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41418-025-01617-1