Abstract
Radiotherapy is widely used in cancer treatment in both curative and palliative care due to its good safety profile and broad clinical availability. It not only directly destroys tumor cells by damaging their DNA but also plays a critical immunomodulatory role, making it a potential combination partner for immunotherapy. Radiotherapy-induced immune effects are complex. They could enhance antitumor immunity by releasing tumor antigens but also promote tumor immune evasion by adaptively regulating immunosuppressive molecules, such as phagocytosis checkpoints. However, the effects of radiotherapy on phagocytosis checkpoints are not fully elaborated compared to T cell-associated immune checkpoints. Phagocytosis checkpoints are regulated by a series of receptor-ligand binding molecules, respectively on the tumor cells and phagocytes, which mediate pro-phagocytosis or anti-phagocytosis signals, modulate tumor antigen presentation, and further determine the infiltration of tumor-specific cytotoxic T cells in the tumor microenvironment. Radiotherapy regulates the different phagocytosis checkpoints on the tumor cells and phagocytes to modulate phagocytic clearance and reshape the irradiated tumor microenvironment. Therefore, radiotherapy in combination with phagocytosis checkpoints-associated immunotherapy can be a promising antitumor approach by considering the type, dose, and sequence of this combinatory regimen as well as the biomarkers for patient selection. This review attempts to summarize the cross-effects of radiotherapy and phagocytosis checkpoints and their combination strategies to enhance the efficiency of radiotherapy and improve the survival of cancer patients. Opportunities built on the roles of the phagocytosis checkpoint in radiotherapy are duly warranted.
Similar content being viewed by others
Facts
-
As a bridge between innate and adaptive immunity, the phagocytosis checkpoints play important roles both in antitumor immunity and in cancer immune evasion.
-
Radiotherapy can impact phagocytosis checkpoints through diverse molecular mechanisms.
-
Radiotherapy-induced changes in phagocytosis checkpoints influence the anti-tumor effects of radiotherapy.
-
Radiotherapy combined with phagocytosis checkpoints-associated immunotherapy is a novel and promising treatment strategy.
Open Questions
-
What are the detailed mechanisms by which radiotherapy regulates phagocytosis checkpoints?
-
What is the impact of radiotherapy on the tumor immune microenvironment after radiotherapy-induced changes in phagocytosis checkpoints?
-
What is the effect of phagocytosis checkpoints-associated immunotherapy on the anti-tumor efficacy of radiotherapy?
-
How to combine radiotherapy and phagocytosis checkpoint-associated immunotherapy to boost antitumor effect?
Introduction
As a well-established therapeutic modality, radiotherapy plays an important role in the local treatment of various cancers, whether it is used alone or in conjunction with other treatments [1]. Radiotherapy directly leads to tumor tissue apoptosis by causing lethal DNA damage [2]. Radiotherapy also regulates complicated immune responses, including both innate and adaptive immune responses. For example, radiotherapy promotes innate immunity via MHC-I-dependent mechanisms [3], cell surface death receptor FAS [4], death receptor DR5 [4], damage-associated molecular patterns (DAMPs) [5], and calreticulin [6, 7]. Radiotherapy also enhances adaptive immunity through interferon γ (IFN-γ) [8], tumor necrosis factor α (TNF-α) [9], and activation of tumor-specific T cells [5, 8, 9]. Furthermore, radiotherapy has a systemic abscopal effect, leading to anti-tumor response for non-irradiated tumor tissues [10, 11]. However, radiotherapy can also induce immunosuppressive effects, such as systemic and intratumoral lymphopenia [12], transforming growth factor β(TGFβ) [13], immunosuppressive M2-phenotype macrophages [14], Treg cells [15], and myeloid-derived suppressor cells (MDSCs) [16], resulting in radioresistance.
Immunotherapy is a powerful systemic antitumor treatment by activating patients’ immune systems to kill tumor cells [17]. Especially, the immune checkpoint blockade represented by targeting programmed death 1 ligand 1 (PD-L1)-programmed cell death 1 (PD-1) [18] and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [19] is broadly applied in different tumors by activating antitumor T cells. Recently, innate immunotherapy has also been gradually applied in diverse tumors, especially phagocytosis checkpoints-associated immunotherapy [20,21,22]. Phagocytosis checkpoints are emerging as key mechanisms with the ability to inhibit or promote phagocytosis [23]. By suppressing or promoting these phagocytosis checkpoints helps cancer cells to escape or succumb to immune surveillance. In particular, they regulate the cytoplasmic regions of phagocyte receptors, such as immune receptor tyrosine-based inhibitor motifs (ITIMs) or immune receptor tyrosine-based activating motifs (ITAMs), and modulate their downstream signals [21]. The cluster of differentiation 47(CD47)–signal regulatory protein α (SIRPα) axis is the first identified anti-phagocytosis signal and is widely studied in numerous preclinical studies [24]. Moreover, increasing clinical trials targeting the CD47/SIRPα axis have been either finished or are ongoing and have achieved certain antitumor curative effects [25, 26]. Consequently, phagocytosis checkpoints as a crucial immune component can become novel diagnostic or prognostic biomarkers and promising therapeutic targets for cancer patients [27].
As mentioned above, T cell checkpoint-related immunotherapy is currently an essential pillar of tumor immunotherapy by reversing T cell exhaustion [28]. Therefore, in the field of radiotherapy combined with immunotherapy, numerous studies mainly focus on radiotherapy and T cell-mediated adaptive immunotherapy [29, 30]. However, radiotherapy can reduce the production of peripheral blood T cells because of the intrinsic sensitivity of the bone marrow and T cells themselves, which leads to lymphopenia and reduction of T cell infiltration in the tumor microenvironment [31, 32], resulting eventually in T-cell checkpoint-associated immunotherapy failure. This makes the combination treatment of radiotherapy and T cell-associated immunotherapy limited and suggests the need to find novel immunotherapy methods combined with radiotherapy [33]. Fortunately, radiotherapy has less impairment on macrophages because of the higher tolerance of macrophages to radiotherapy compared to T cells. Radiotherapy could also promote the recruitment of macrophages in the tumor microenvironment [34,35,36]. Furthermore, an increasing number of phagocytic checkpoints are being discovered [21], allowing for more selective targets to rely on the macrophages that remain after radiotherapy. Also, phagocytosis checkpoints are associated with dual immune responses, including innate and adaptive immunity. Therefore, as an emerging immunotherapy with both innate and adaptive immune functions, phagocytosis checkpoints-associated immunotherapy is a promising treatment option combined with radiotherapy. Importantly, accumulating evidence suggests that radiotherapy could regulate the expression of phagocytosis checkpoints [37,38,39].
Therefore, considering the promising application of phagocytosis checkpoints-associated immunotherapy combined with radiotherapy, we provide an overview of the regulatory mechanisms of diverse phagocytosis checkpoints upon radiotherapy. Then, we analyze the clinical application of phagocytosis checkpoints-associated immunotherapy and radiotherapy combination strategies in tumor patients. Altogether, this review aims to provide a molecular insight into the exploitation of phagocytosis checkpoints-associated immunotherapy combined with radiotherapy.
The phagocytosis checkpoints in radiotherapy
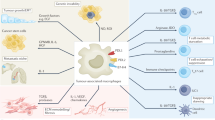
Phagocytosis checkpoints have been gradually identified in recent years because of the fast development of high-throughput technologies allowing a deep understanding of cancer immunology and molecular bases. For example, the CD47/SIRPα axis was identified in the late 1990s as the first tumor phagocytosis checkpoint [20]. Next, others phagocytosis checkpoints including PD-L1-PD-1 axis (in 2017) [40], major histocompatibility complex class I (MHC-1)-leukocyte immunoglobulin-like receptor subfamily B member 1(LILRB1) axis (in 2018) [41], cluster of differentiation 24(CD24)-sialic acid binding Ig like lectin 10(Siglec-10) (in 2019) [42], cluster of differentiation 22 (CD22) (in 2019) [43], signaling lymphocytic activation molecule family 3/4 (SLAMF3/4) (in 2022) [44] and ganglioside 2 (GD2)-sialic acid binding Ig like lectin 7 (Siglec-7) (in 2022) [45] were followingly demonstrated in the tumor microenvironment. Radiotherapy can influence the phagocytosis checkpoints including the ligands on tumor cells, the receptors on the phagocytes and their downstream phagocytosis function signals (Table 1) (Figs. 1, 2).
In irradiated tumor cells, radiotherapy upregulates CD47 expression by DNA damage response-associated signals, HER2-PI3K/AKT-NF-κB axis, fatty acids oxidation-Acetyl-COA-NF-κB axis, AMPK-Histone modification signals, TAK2-STAT1/3 axis, AREG-STAT3 axis and ATM-ATR-JAK/STAT1/3 axis. At the post-transcriptional level, radiotherapy-suppressed Has-miR-222 and DNMT-TTP axis promotes CD47 expression. Also, the radiotherapy-induced PD-L1 upregulation is associated with DNA damage response-associated BRCA1/KU70/80-Chk-1-JAK-STAT1/3 axis, EGFR-JAK-STAT1/3 axis, cGAM-Cgas-STING-TBK1-IRF1/3 axis and KPNA/KPNB1-IRF1/3 axis. Furthermore, irradiated tumor cells-derived extracellular vesicles promote MHC-Iexpression through NBS1-ATM-ATR-JAK/STAT1/3 axis. Unfortunately, detailed regulation mechanisms of radiotherapy are unclear both on CD24, SLAMF3/4, GD2, FC-IgG and α2,6-linked sialic acid in irradiated tumor cells and on ligands of anti-phagocytosis in irradiated phagocytes according to current research. (Created with Microsoft Office PowerPoint).
Radiotherapy promotes calreticulin translocation to the tumor cell surface by increasing endoplasmic reticulum (ER) stress in irradiated tumor cells. Radiotherapy-induced Caspase8 also promotes calreticulin translocation to irradiated tumor cell surface. However, regulation mechanisms of radiotherapy on other “Eat me” signals including Phosphatidylserine (PS)-TIM4/CD300b/BAI1/ Stabilin-2 axis, SLAMF7-SLAMF7 axis and Fc-FcγRs axis are still lacking. (Created with Microsoft Office PowerPoint).
“Don’t eat me” signals
CD47-SIRPα axis
Immune-related characteristics of the CD47/SIRPα axis
The CD47 protein is universally expressed on cancer cells [46, 47]. CD47 conveys inhibitory “don’t eat me” signals to its receptor SIRPα on the phagocytes (such as macrophages, DCs [47], neutrophils [48]) to activate inhibitory anti-phagocytosis signals [49, 50]. Various transcription factors can upregulate CD47 on the surface of cancer cells, such as MYC [51], nuclear factor kappa B (NFκB) [52], signal transducer and activators of transcription 3 (STAT3) [53], and hypoxia-inducible factor-1(HIF-1) [54]. Targeting CD47/SIRPα not only enhances the innate response through macrophage-mediated phagocytosis [48, 55] but also promotes adaptive immunity, which activates antitumor-specific T cells [56]. Therefore, CD47/SIRPα-targeted therapy is a promising antitumor therapy [47, 48].
Radiotherapy effects on the CD47/SIRPα axis
The CD47/SIRPα axis is usually overexpressed in diverse radioresistant tumors, such as head and neck cancer [57], glioblastoma multiforme [58], and breast cancer cells [59]. Radiotherapy mainly upregulates CD47 [59,60,61] and SIRPα [62] in the most diverse tumor microenvironments. For example, radiotherapy upregulates CD47 on tumor cells [61] and SIRPα expression on myeloid cells in colorectal cancer [62]. Radiotherapy-induced upregulation of amphiregulin (AREG) not only promotes CD47 upregulation via STAT3 activation in tumor cells but also reprograms EGFR+ mononuclear phagocytes into an immunosuppressive phenotype, resulting in impaired mononuclear phagocyte phagocytosis [63]. However, radiotherapy downregulates CD47 in oropharyngeal squamous cell carcinoma (OSSC), resulting in increased phagocytosis by dendritic cells (DCs) [64]. The molecular mechanisms underlying radiotherapy-induced CD47/SIRPα expression changes have been at least partly elucidated in different tumor types and are summarized in Table 1.
The immune responses after radiotherapy-induced CD47/SIRPα expression
Radiotherapy-induced effects are mainly related to radioresistance by upregulation of CD47 or SIRPα, but other studies [64, 65] demonstrated that it can also induce tumor cells sensitivity to radiotherapy by inhibiting CD47 expression. For example, radiotherapy-induced upregulation of CD47 inhibits phagocytosis function of macrophages, promotes M2 polarization of macrophages and inhibits activation of CD8 + T cells to induce radioresistance of GSCs [66]. However, radiotherapy-induced microRNA can inhibit CD47 expression to promote tumor cells sensitivity to radiotherapy in cervical carcinoma, kidney carcinoma and human alveolar adenocarcinoma [65]. Therefore, radiotherapy combined with CD47/SIRPα axis-associated immunotherapy can further promote anti-tumor efficiency, which is mainly associated with reshaped immune-activated irradiated tumor microenvironments (Table 2).
CD47/SIRPα-induced abscopal effects after radiotherapy
Furthermore, the anti–CD47/SIRPα axis not only inhibits local irradiated tumor cells but also inhibits distant non-irradiated tumor cells [60, 67, 68]. CD47 blockade combined with radiotherapy promotes abscopal effects to inhibit distant, non-irradiated small cell lung cancer. The radiotherapy-induced secretion of cytokines from tumor cells, such as CSF1, CCL2, and MCP3, promotes macrophage recruitment and activation from the irradiated tumor microenvironment to the non-irradiated tumor site [67]. Therefore, CD47/SIRPα is a novel mechanism of abscopal response independent of CD8+ T cells [68]. Previous studies have shown that the abscopal effects are mainly associated with tumor-draining lymph nodes(TDLN) [69] and CD8+ T cells [70] because radiotherapy could impair T cells in these TDLN. For example, elective nodal irradiation(ENI) inhibits abscopal responses by decreasing active CD8+ T cells in non-irradiated head and neck tumors [71]., whereas the delayed TDLN irradiation has more effective antitumor effects in metastatic melanoma compared to simultaneous radiotherapy for lymph nodes and tumors [72]. However, in clinical practice, it is difficult to distinguish which TDLNs have not metastasized and therefore avoid their irradiation to retain function of T cells. Therefore, macrophages, as the immune cells with a relatively large residual amount after radiotherapy, can compensate for the weakened abscopal effect caused by the reduction of T cells due to lymph node injury after radiotherapy.
Anti-tumor effects with phagocytosis-independent mechanism in radiotherapy
Beyond phagocytosis-related antitumor immune response, the anti-CD47/SIRPα axis also enhances tumor cells sensitivity to radiotherapy through phagocytosis-independent mechanisms. Anti-CD47 enhanced tumor cells sensitivity to radiotherapy in oral squamous cell carcinoma (OSCC) by suppressing cancer stem cell-like phenotype [73]. Also, anti-CD47 can enhance tumor cells sensitivity to radiotherapy by inhibiting tumor pluripotency capabilities and reducing the expression of DNA repair enzymes in OSCC [74].
Radioprotection effects of CD47 blockade in normal cells
However, some studies indicated that CD47 blockade can have different radiotherapy effects between normal and tumor cells [75, 76]. Blocking CD47 combined with radiotherapy has radioprotection effects in normal tissues and organs, which contribute to normal cell survival involving various tissues (muscle, skin, vascular, endothelial tissue) and bone marrow [77, 78], whereas it increases tumor cells sensitivity to radiotherapy [79]. The main mechanism underlying such selective radiotherapy reaction is that CD47 not only serves as a receptor of SIRPα on phagocytosis but also as a receptor of the secreted matricellular glycoprotein thrombospondin-1(TSP-1) [80]. Therefore, radiotherapy combined with anti-CD47 not only promotes tumor cells sensitivity to radiotherapy but also has radioprotective effects on normal tissues and organs.
PD-L1/PD-1 axis
Immune-related characteristics of PD-L1/PD-1 axis
The PD-L1/PD-1 interaction between tumor cell and T cell contributes to escape from T cell-mediated tumor immune surveillance by acting as a “don’t find me” signal [81, 82]. However, PD-L1/PD-1 axis is also a phagocytosis checkpoint. PD-L1 is expressed on tumor cells, and its receptor PD-1, which is an inhibitory transmembrane protein, is expressed on phagocytes to activate anti-phagocytosis signals [40, 83]. PD-1(+) tumor-associated macrophages (TAMs) have lower phagocytosis function compared to PD-1(-) TAMs and PD-L1 knockout promotes phagocytosis function of PD-1(+) macrophages [40]. High expression of PD1 on macrophages predicts poor outcomes [84]. Therefore, PD-L1/PD-1 blockade not only improves adaptive immune responses associated with activated T cells but also promotes innate immune responses associated with macrophages phagocytosis.
Radiotherapy effects on the PD-L1/PD-1 axis
In radioresistant head and neck squamous cell carcinoma cells, PD-L1 expression is upregulated in nuclear and cytoplasmic cell fractions [85]. Also, high PD-L1 expression is positively correlated with radioresistance in NSCLC [86]. Furthermore, radiotherapy increases the tyrosine phosphatase SHP-2 of PD-1 cytoplasmic domain in the M1 TAMs in NSCLC [87]. The radiotherapy-induced mechanisms of PD-L1 expression are mainly associated with DNA damage and repair signaling pathway [88], cGAS-STING pathway [89], IFN-γ signaling [90] and epidermal growth factor receptor (EGFR) pathway [91]. However, most researchers mainly study how radiotherapy-induced PD-L1/PD-1 expression changes influence adaptive antitumor immune responses mediated by CD8+ T cells but not innate immune responses mediated by phagocytosis.
The immune responses after radiotherapy combined with PD-L1/PD-1 blockade
There is great research associated with radiotherapy combined with PD-L1/PD-1 blockade to activate antitumor CD8+ T cells [92, 93]. However, innate antitumor immune responses also may play a significant role in the combination treatment. Radiotherapy combined with anti-PD-1 promotes tumor cell phagocytosis by DCs, increases tumor-associated antigen presentation, and further promotes tumor-specific CD8+ T cells priming in colorectal cancer. Furthermore, radiotherapy combined with anti-PD-1 also increases abscopal effects [60]. Anyway, although the anti-PD-L1/PD-1 axis as “don’t find me” signal in combination with radiotherapy has been studied extensively, its role as a phagocytosis checkpoint combined with radiotherapy needs to be further elucidated (Table 2).
MHC-1-LILRB1 axis
Immune-related characteristics of the MHC-1-LILRB1 axis
MHC-1 is a transmembrane polymorphic glycoprotein which can process and present tumor antigen fragments to TCR on the surface of CD8+ T cells [94]. However, MHC-I is also expressed on the surface of various cancer cells and its component β2M binds with its receptor LILRB1 on macrophages to inhibit phagocytosis function [41] by activating four ITIM sequences [95]. LILRB1 antibody blocks the interaction of MHC-I and LILRB1 to increase the phagocytosis capability of macrophages, increase M1/M2 ratio and also improve the cytotoxic capability of both NK and T cells to inhibit tumor growth [96]. Therefore, anti-MHC-I/LILRB1 axis is a promising immunotherapy strategy, which is associated with the phagocytosis function of macrophages [41].
Radiotherapy effects on the MHC-1-LILRB1 axis
Radiotherapy can upregulate MHC-I on the surface of various cancers, including glioblastoma [97, 98], cervical cancer [99], colon, lung, prostate cancer [100], ovarian carcinoma [101], melanoma [3] and tongue and mobile tongue squamous cell carcinoma [102]. However, many studies mainly focus on its antigen presentation function to cytotoxic T cells to augment the anti-tumor adaptive immune response but do not mention its negative effects on phagocytosis function.
CD24-Siglec-10 axis
Immune-related characteristics of the CD24-Siglec-10 axis
CD24, as a cancer stem cell marker [103], has recently been confirmed to be a phagocytosis checkpoint and highly expressed on tumor cells. CD24 interacts with its inhibitory transmembrane protein receptor Siglec-10 on macrophages, resulting in the activation of an inhibitory phagocytosis signaling cascade [42, 104]. High expression of CD24 is associated with short survival and both anti-CD24 on tumor cells and anti-Siglec-10 on tumor-associated macrophages effectively promote tumor cell phagocytosis by macrophages [42]. Furthermore, high expression of CD24 is correlated with more lymph node metastases, more advanced pathological stage, and shorter survival in breast cancer [105]. Therefore, targeting CD24/Siglec-10 axis can improve prognosis of cancer patients both as anti-CD24/Siglec-10 monotherapy or in combination with other therapy approaches.
Radiotherapy effects on the CD24-Siglec-10 axis
The effect of radiotherapy on CD24/Siglec-10 expression is scarcely known, according to current research. However, Shen, W. et al. indicated that radiotherapy combined with CD24/Siglec-10 blockade has a synergistic anti-tumor efficiency compared with their treatment as single agents by increasing the percentage of IFNγ-expressing CD8+ T cells, ratio of M1/M2 macrophages, and proportion of monocytic MDSCs in colon cancer [106] (Table 2).
CD24-Siglec-10 axis as cancer stem cell marker in radiotherapy
While high expression of CD24 is associated with tumor cells sensitivity to radiotherapy because CD24(-)/CD44(+) is a tumor stem marker, its low expression or loss in stem-like breast cancer generates radioresistance by inhibiting radiotherapy-induced tumor cell death, because loss of CD24 leads to low level of radiation-induced ROS and decreased genomic instability [107].
Other anti-phagocytosis checkpoints
α2-6-linked sialic acid-CD22 axis
CD22 (siglec-2) is also an anti-phagocytosis molecule expressed on the surface of phagocytes but traditionally expressed on B-cells to inhibit B-cell receptor signaling [108]. Pluvinage, J. V. et al. demonstrated that CD22 is upregulated on aged microglia and CD22 binding with α 2,6-linked sialic acid inhibits the phagocytic capacity of microglia by activating CD22 downstream inhibitory SHP-1 signaling [43].
Fc-FcγR IIB axis
Fc receptors are a series of classical and important phagocytosis-related cell surface receptors expressed on the macrophages, which mediate both anti-phagocytosis and pro-phagocytosis processing by interacting with their ligand IgG immune complexes, especially type I Fc common gamma receptors(FcγR) [109, 110]. In these Fcγ receptors, FcγRIIB mediates anti-phagocytosis on the surface of macrophages to activate inhibitory phagocytosis signals [111, 112].
SLAMF3/SLAMF4
Li, D. et al. demonstrated that signaling lymphocytic activation molecule (SLAM) family receptors, particularly SLAMF3 (CD229) and SLAMF4 (CD244), are also “don’t eat me” receptors on macrophages. They confirmed these receptors decrease the phagocytosis function of macrophages by inhibiting low density lipoprotein receptor-related protein 1(LRP1)-mediated activation of mTOR and Syk signaling to inhibit “eat me” signals [44].
GD2-Siglec-7 axis
The disialoganglioside GD2 is a sialic acid-linked glycolipid and is widely expressed on diverse tumor cells, especially neuroblastoma [113]. Recently, Theruvath, J. et al. demonstrated that combination treatment of anti-GD2 and anti-CD47 has potent antitumor synergy by promoting macrophages to phagocytose tumor cells in neuroblastoma, osteosarcoma and small-cell lung cancer. Siglec-7 is the ligand for GD2 to mediate “don’t eat me” signals. Interestingly, anti-GD2 not only inhibits anti-phagocytosis signals but also promotes “eat me” signals by upregulating surface calreticulin on tumor cells [45].
However, the above-mentioned anti-phagocytosis checkpoints have not been studied with radiotherapy according to current research.
“Eat me” signals
Calreticulin (CRT)-LRP1 axis
Immune-related characteristics of CRT-LRP1 axis
Calreticulin(CRT) is a multifunctional protein in the endoplasmic reticulum(ER) [114]. Under ER stress induced by chemotherapy [115] and radiotherapy [7], CRT can translocate from the lumen of ER to the surface of tumor cells. The low-density lipoprotein receptor-related protein 1 (LRP1, CD91) [116] is the ligand for CRT and is expressed on the surface of phagocytes to promote phagocytosis of tumor cells [114]. Chao, M. P. et al. suggested that CRT is highly expressed both on hematologic malignancies and solid tumors whereas it is less expressed on normal cells. Furthermore, high CRT expression is associated with increased CD47 expression and CRT-LRP1 blockage abrogates anti-CD47 antibody-mediated phagocytosis [117]. Different molecular mechanisms, including stanniocalcin1 (STC1) [118] and hormone glucocorticoid (GC) [119] regulate CRT and LRP1 expression.
Radiotherapy effects on the CRT-LRP1 axis
Radiotherapy is also a regulator of CRT-LRP1 signal axis for which radiation-induced ER stress facilitates CRT translocation to the tumor cells surface, resulting in promoting immunogenic cell death by increasing antigen-specific CD8+ cytotoxic T lymphocytes [6, 120]. Radiotherapy upregulates CRT in cervical cancer patients’ tumor biopsy specimens [39]. Moreover, different radiotherapy types, including photon, proton, and carbon-ion, all can increase CRT membrane exposure in lung adenocarcinoma, glioma, tongue squamous carcinoma, and nasopharyngeal carcinoma in a dose-dependent manner [121]. These different radiotherapy types can upregulate CRT under normoxic conditions, especially carbon-ion radiation. However, under hypoxic conditions, the baseline expression level of CRT is high enough, and radiotherapy could not further increase the expression of CRT [122].
The immune responses after radiotherapy combined with the CRT-LRP1 targeting therapy
Radiotherapy-induced CRT upregulation can improve antitumor effects of anti-PD-L1 treatment in caspase-8 knockout tumors because the knockout of caspase-8 suppresses the translocation of CRT to the surface of tumor cells, which impairs phagocytosis function and antigen presentation of DCs and the infiltration of tumor-specific CD8+ T cells [123]. Furthermore, radiotherapy-induced upregulation of CRT inhibits tumor neurospheroid formation and tumor stemness to increase tumor cells sensitivity to radiotherapy in neuroblastoma [124] (Table 2).
The negative role of CRT-LRP1 axis in tumors
Interestingly, high expression of CRT is associated with worse clinical outcomes in different tumors [117]. Also, LRP1 is highly expressed in radioresistant colorectal cancer and higher expression of LRP1 is associated with poor clinical outcomes [125]. The high expression of mutated CRT possibly can explain this interesting phenomenon. Soluble exon-9-mutated CRT is highly released from ER of tumor cells to further inhibit phagocytosis by DCs and induce immunosuppressive effects in osteosarcoma, cervix adenocarcinoma, fibrosarcoma and NSCLC [126].
Phosphatidylserine (PS)-TIM4/CD300b/BAI1/ Stabilin-2
Immune-related characteristics of PS-associated axis
Phosphatidylserine (PS), as an “eat me” signal, is an inner cell membrane molecule at normal physiological environment but can also translocate to the surface of apoptotic cells under different molecular signals [127,128,129]. PS has different receptors [130] on the surface of phagocytes to promote phagocytosis, such as T-cell immunoglobulin mucin-4 (TIM4), single Ig-domain type I transmembrane protein CD300b, brain-specific angiogenesis inhibitor 1 (BAI1), Rage, Scarf1, CD36, Trem2 and transmembrane protein Stabilin-2 [130,131,132].
The effects of radiotherapy on PS-associated axis
Radiotherapy can upregulate PS on tumor cells by inducing caspase activity, and high expression of PS is associated with radioresistance [133]. However, radiotherapy-induced PS upregulation promotes immunosuppressive signals and targeting PS antibody combination with radiotherapy can enhance anti-tumor efficiency in melanoma by increasing M1 phenotype macrophages and tumor antigen-specific CD8+ T cells [134]. However, it is needed to further study the influence of radiotherapy-induced PS upregulation on phagocytosis function in future studies.
Other pro-phagocytosis axis
SLAMF7-SLAMF7 axis
SLAMF7 is expressed exclusively on the surface of hematologic tumor cells, which promotes tumor cells phagocytosis by binding with its equal receptor SLAMF7 on phagocytes [135]. Chen, J. et al. demonstrate that SLAMF7 on macrophages promotes phagocytosis by increasing polarization of actin associated with key step of phagocytosis process [135]. Interestingly, another study suggested that SLAMF7 can express highly on solid breast cancer, and high SLAMF7 expression is associated with poor clinical outcomes, for which SLAMF7 mediates “don’t eat me” signals [136].
Fc-FcγRs axis
FcγRI, FcγRIIB, FcγRIII, and FcγRIV on macrophages mediates pro-phagocytosis signals by binding with IgG Fc domain of target cells [110, 137]. The binding of Fc and pro-phagocytosis FcγRs also promotes phosphorylation of ITAMs by activating tyrosine kinase and further increases downstream phagocytosis signals in macrophages [138].
Unfortunately, there are no studies on revealing the effects of radiotherapy on the expression of SLAMF7 and Fc-FcγRs axis according to present literature.
Clinical application of phagocytosis checkpoints in tumor radiotherapy
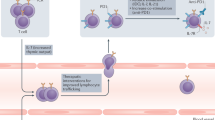
Given that numerous preclinical studies as previously described, the potential combination treatment of radiotherapy and phagocytosis checkpoints-associated immunotherapy is a promising treatment strategy for cancer patients (Fig. 3). Besides, these phagocytosis checkpoints molecules have other functions which make the novel combination treatment have stronger anti-tumor effects compared to T cell-mediated immune checkpoint-related immunotherapy (Table 3). In clinical applications, phagocytosis checkpoints can be putative biomarkers to predict tumor cells sensitivity to radiotherapy to realize individualized treatment but not blind treatment (Fig. 4). Also, targeting these molecules in radiotherapy would have synergistic efficiency by using feasible and optimal therapy strategies.
Radiotherapy-induced reduction of phagocytosis function and lymphopenia promote tumor immune evasion leading to radioresistance. Combination of radiotherapy and phagocytosis checkpoints-associated immunotherapy could convert radiotherapy-resistant tumors into radiotherapy-sensitive ones by promoting phagocytosis function and further activating antitumor immune response. In the combination treatment, innate immunity could be enhanced by directly lysing tumor cells by increasing infiltration of M1 macrophages and dendritic cells but decreasing infiltration of M2 macrophages. Also, phagocytes as antigen presentation cells could process and present tumor antigen to increased CD8+ T cells and further promote the release of IFN-γ, TNF-α and granzyme B from activated CD8+ T cells. Furthermore, the combination treatment could enhance abscopal effects in non-irradiated tumor sites through more macrophages remaining after radiotherapy in tumor-draining lymph nodes. (Created with Microsoft Office PowerPoint).
Detecting and evaluating biomarkers is a wise choice for the combination of radiotherapy and immunotherapy to select an appropriate immunotherapy regimen for tumor patients who are suitable for radiotherapy. Given that radiotherapy regulates phagocytosis checkpoints, it is a wise choice to select phagocytosis checkpoints as new biomarkers in combination with radiotherapy and immunotherapy. Patients with high infiltration of antitumor immune cells (such as macrophages, DCs, CD8+ T cells) or high activation of phagocytosis checkpoints (such as CD47, PD-L1, CD24, CRT) could be selected to the combination of radiotherapy and phagocytosis checkpoints-associated immunotherapy. In the combination treatment, it is needed to consider radiotherapy plans, immunotherapy schemes, sequence of combination treatment and tumor types for enhancing antitumor effects of the novel combination treatment. According to current studies, tumor patients with lung cancer, breast cancer, liver cancer, melanoma, glioma, colorectal cancer or cervical cancer might acquire benefits from the novel combination treatment. However, for patients with low infiltration of immune cells or low activation of phagocytosis checkpoints, other treatments need to be selected, such as chemotherapy, targeted therapy and other types of immunotherapy and so on. (Created with Microsoft Office PowerPoint).
Phagocytosis checkpoints as potential biomarkers for prediction of tumor cells sensitivity to radiotherapy
Radiotherapy is not always an effective antitumor manner with low radiotherapy curative effect or radioresistance because of various and complicated tumor and patient characteristics [139]. Therefore, it is necessary to find assessment methods for evaluating whether tumor patients are suitable for radiotherapy. The main assessment method includes biomarkers that can predict the efficacy of radiotherapy in diverse tumors [140,141,142] (Table 4).
Potential strategies for targeting the phagocytosis checkpoints combined with radiotherapy
Radiotherapy has different antitumor responses for diverse tumor characteristics. For example, in CURB clinical trial, stereotactic body radiotherapy (SBRT) targeting oligometastatic sites could effectively improve PFS of NSCLC patients after resistance to systemic therapy, but not improve outcomes in breast cancer with oligometastatic sites [143]. Similarly, in EXTEND trials, metastasis-directed therapy via SBRT could also improve PFS in oligometastatic pancreatic ductal adenocarcinoma [144] and prostate cancers [145]. However, nivolumab(anti-PD-1) plus SBRT does not enhance abscopal effects compared to nivolumab alone in patients with metastatic head and neck squamous cell carcinoma [146]. Although SBRT has limited antitumor effects in metastatic breast cancer and HNSCC, radiotherapy plus phagocytosis checkpoints-associated immunotherapy might be an effective therapy because radiotherapy upregulates phagocytosis checkpoints and targeting these molecules could enhance tumor cells sensitivity to radiotherapy in these tumor cells as mentioned in section 2 [59, 147]. Furthermore, we also need to consider the types of tumors that would benefit from the combination of radiotherapy and phagocytosis checkpoints-associated immunotherapy.
However, only two ongoing clinical trials are studying the combination treatment of phagocytosis checkpoint-associated immunotherapy and radiotherapy. One (NCT02890368) is based on the intratumoral injection of TTI-621 (anti-CD47 antibody) combined with different antitumor treatments including radiotherapy in solid tumors. The other one (NCT05967416) is based on the autologous SIRPα-low macrophages (SIRPant-M) administration to confirm the efficiency of SIRPant-M alone or in combination with radiotherapy in relapsed or refractory Non-Hodgkin lymphoma. Although there are few clinical applications in combination therapy, the efficiency of this combination treatment may be improved with different potential strategies by referring to previous immune checkpoint inhibitor treatments in radiotherapy [148] (Table 4).
The influence of diverse radiotherapy plans on the novel combination therapy
Radiotherapy dose fraction in the novel combination therapy
The radiotherapy dose fraction includes hypo-fractionation (3–20 Gy/fraction), conventional fractionation schemes (1.8–2.2 Gy/fraction) and hyper-fractionation (0.5–2.2 Gy/fraction) [149]. Diverse radiotherapy dose fraction could regulate macrophage-associated innate immune response. The low dose irradiation (2 Gy) promotes the polarization of irradiated tumor-associated macrophages to M1 macrophages in pancreatic cancer [150, 151]. The stereotactic body radiotherapy (SBRT) (6.5 to 7.25 Gy), as a precise, high-dose, hypofractionated radiation treatment technique delivered in few sessions to extracranial targets with maximal tumor control and minimal damage to healthy tissues, activates innate immunity by enhancing proinflammatory M1 macrophages-mediated metabolite elevations of tumor cells in mitigatory prostate cancers [152]. Therefore, in the novel combination treatment, radiotherapy dose fraction may play a crucial role by referring to radioimmunotherapy studies that have been published.
Radiotherapy types in the novel combination therapy
At present, the wide application of radiation source types is mainly light photon radiation including X-ray and γ-ray. However, heavy-particle radiation including proton and carbon ion radiation, as new types of radiotherapy, are gradually starting to be applied to tumor treatment with less damage to normal tissues because of their unique Bragg peak [153]. The heavy-particle radiation combination with immunotherapy also has stronger antitumor efficiency compared with light photon radiation.
The effects of phagocytosis checkpoints-associated immunotherapy schemes on the combination therapy
Immunotherapy types in the novel combination therapy
Given that radiotherapy influences immunity molecule expression, targeting these diverse immune molecules may induce different antitumor efficiency in radiotherapy. A preclinical study indicated that anti-PD-1 or anti-CTLA4 combined with radiotherapy has opposite antitumor effects [154]. Therefore, it is needed to verify and choose which optimal phagocytosis checkpoints-associated immunotherapy scheme is better combined with radiotherapy.
Immunotherapy forms in the novel combination therapy
The forms of phagocytosis checkpoints-associated drugs also influence antitumor effects in radiotherapy, such as monoclonal antibodies, small molecule inhibitors, antibody fusion protein and nanoparticles. Targeting the CD47/SIRPα is the most popular phagocytosis checkpoint-associated immunotherapy. For example, anti-CD47 antibody Hu5F9-G4 has a well-tolerated anti-tumor efficiency both in solid tumors and hematologic tumors [26] Also, the anti-SIRPα antibody is a promising antitumor drug with fewer hematologic toxic side effects because of the confined expression of SIRPα on normal cells compared with CD47 [49]. CD47/SIRPα-associated small molecule inhibitors have some advantages including oral administration, shorter half-life, low cost and no immunogenicity compared with CD47/SIRPα antibodies [155]. What’s more, the novel peptide pep-20 also blocks the CD47/SIRPα interaction by binding to the human CD47-IgV domain and inhibiting SIRPα tyrosine phosphorylation of ITIMs. Also, pep-20-D12 in combination with radiotherapy has synergistic antitumor effects [38]. Besides, nanoparticles (gCM-MNs) not only inhibit the CD47-SIRPα axis but also repolarize tumor-associated macrophages to M1 macrophages [156].
Multiple immunotherapy regimens in combination with radiotherapy
The three-treatment strategy combining two types of immunotherapy drugs and radiotherapy is also a wise choice for radioimmunotherapy. For example, the application of anti-SIRPα and anti-PD-1 combined with radiotherapy activates more robust adaptive antitumor immune responses in colorectal cancer [60].
The sequence of radiotherapy in combination with phagocytosis checkpoints-associated immunotherapy
Concurrent therapy in the novel combination therapy
Concurrent therapy is a widely applied combination treatment based on the radiotherapy-induced immune response. However, in the concurrent treatment, toxicity overlap is a big challenge for which both radiotherapy and immunotherapy can produce adverse reactions, and toxicity may increase [157]. Administering radiotherapy immediately after immunotherapy may exacerbate these toxicity events, leading to treatment interruption or dose modification [158]. Hence, when designing treatment plans, it is crucial to arrange the sequence of radiotherapy and immunotherapy reasonably based on the patient’s specific conditions to minimize toxicity risks.
Sequential therapy in the novel combination therapy
Likewise, sequential therapy is also a common combination treatment manner, such as radiotherapy before immunotherapy or immunotherapy before radiotherapy. Especially, radiotherapy before immunotherapy is the main order according to current studies for diverse reasons. Firstly, radiotherapy can shift tumor immune microenvironment from an immunosuppressive “cold” tumor state to an immunostimulatory “hot” one by increasing the release of proinflammatory mediators and chemokines and infiltration of immune cells [8, 9]. Therefore, administering radiotherapy first can create a more favorable immune microenvironment for subsequent immunotherapy. Secondly, radiotherapy could induce immunogenic cell death to release tumor-associated antigens [5]. Immunotherapy, in turn, enhances the ability of immune cells to recognize and attack these antigens. Hence, as a first-line treatment, radiotherapy provides more targets for immunotherapy and improves therapeutic outcomes. Thirdly, radiotherapy can upregulate the expression of immune checkpoints on tumor cell surfaces, such as CD47 and PD-L1 [60]. Initiating radiotherapy before immunotherapy enables immunotherapy to work more effectively and inhibits immune evasion. However, the optimal time for immunotherapy after radiotherapy may be in a few days to weeks after radiotherapy (such as 1 to 14 days after radiation therapy) [159,160,161]. Also, immunotherapy before radiotherapy is another option. If radiotherapy is given first, it may have a certain inhibitory effect on the immune system [13, 15, 16], affecting the safety of subsequent immunotherapy. Immunotherapy could increase tumor-vascular normalization and decrease tumor hypoxia. Hence, if immunotherapy is given first, it could promote subsequent tumor cells sensitivity to radiotherapy [162]. Therefore, when determining the treatment sequence, it is necessary to consider the balance between therapeutic efficacy and toxicity.
Biomarkers and patient selection with different tumor types in combination with radiotherapy and phagocytosis checkpoints-associated immunotherapy
Biomarkers in the novel combination treatment
A reliable biomarker is essential to select the appropriate patient populations who are suitable for treatment with radiotherapy in combination with phagocytosis checkpoints-associated treatments. There are various biomarkers including molecular genes, immune cells, clinical models, and radiomic models to predict the antitumor efficiency of radioimmunotherapy and help to make optimal clinical decisions in different tumors. However, at present, many types of biomarkers are mainly associated with PD-1/PD-L1 or CTLA4 immunotherapy in radiotherapy, not phagocytosis checkpoint-associated immunotherapy. Therefore, it is urgent to explore novel biomarkers able to predict the antitumor effects of the novel combination.
Tumor selection for novel combination therapy
It is important to select appropriate and responsive patients eligible for the combination therapy to maximize the antitumor effects by considering characteristics of tumors and patients. The phagocytosis checkpoints-associated immunotherapy is mainly dependent on phagocytes or expression level of phagocytosis checkpoints molecules to realize its antitumor effects [21]. Therefore, tumor types with more macrophage infiltration and high expression of phagocytosis checkpoint molecules might be more sensitive to the novel combination therapy. These particular tumor types mainly include breast cancer [59, 163], glioma [58, 164], hepatocellular carcinoma [89, 165], colorectal cancer [60, 166], and NSCLC [167, 168]. Furthermore, as summarized in section 2 of this review, there are numerous preclinical studies suggesting that radiotherapy combined with phagocytosis checkpoints-associated immunotherapy has effective antitumor effects in these tumor types.
Conclusions
Radiotherapy, as a complicated tumor immune effector, is widely applied in combination with immunotherapy. The effects of radiotherapy on phagocytosis checkpoints are an emerging and developing research interest that promises to lead to new immunotherapy in radiotherapy. Moreover, in the irradiated tumor microenvironment, phagocytosis checkpoints not only involve innate immunity to phagocytose tumor cells but also involve the adaptive immune response because macrophages can present tumor antigens to CD8+ T cells to further kill tumor cells. Furthermore, phagocytosis checkpoints also involve systemic abscopal effects by increasing migratory macrophages in the distant, non-irradiated tumor. Therefore, phagocytosis checkpoint molecules can become potential biomarkers or promising targeting immune molecules in radiotherapy to predict or regulate tumor cells sensitivity to radiotherapy and further to enhance the antitumor response of radiotherapy.
Nevertheless, there are still some problems that need to be resolved in this research area, according to current research results. Firstly, the molecular mechanisms of radiotherapy-induced phagocytosis checkpoint expression still need to be further explored, especially other molecule types except for the CD47-SIRPα axis in future studies. Secondly, it is not clear whether the balance between pro-phagocytosis and anti-phagocytosis signals caused by radiotherapy can engulf or not tumor cells under different conditions, resulting in tumor cells sensitivity to radiotherapy. Therefore, it is needed to confirm the balance between these two adverse phagocytosis signals. Thirdly, phagocytosis checkpoint-associated immunotherapy has annoying hematological toxicities, such as anemia, thrombocytopenia, and other immune-associated side effects resulting in limitation of drug dose. Therefore, it is an advisable choice to combine phagocytosis checkpoints-associated immunotherapy with other treatments to reduce these side effects, such as radiotherapy. Fourthly, although there have been some phagocytosis checkpoints associated immunotherapy in clinical patients, there are only two ongoing clinical trials related to combining treatment of radiotherapy and phagocytosis checkpoint-associated immunotherapy one with TTI-621(anti-CD47) (NCT02890368) and the other one with autologous SIRPα-low macrophages (SIRPant-M) (NCT05967416). In the future, researchers maybe should carry out more clinical trials to explore the synergistic effects of radiotherapy and phagocytosis checkpoint-associated immunotherapy.
In conclusion, this review summarizes the influence of radiotherapy on phagocytosis checkpoints in the tumor microenvironment and suggests the optimal modes of combination treatment of radiotherapy and phagocytosis checkpoint-associated immunotherapy by considering diverse therapy regimens to improve antitumor efficacy and tumor patients’ outcomes.
References
Citrin DE. Recent Developments in Radiotherapy. N Engl J Med. 2017;377:1065–75.
Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5:60.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71.
Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M, Galluzzi L. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Investig. 2021;131:e138740.
Krombach J, Hennel R, Brix N, Orth M, Schoetz U, Ernst A, et al. Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology. 2019;8:e1523097.
Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–16.
Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50.
Wu SY, Chen CL, Tseng PC, Chiu CY, Lin YE, Lin CF. Fractionated ionizing radiation facilitates interferon-γ signaling and anticancer activity in lung adenocarcinoma cells. J Cell Physiol. 2019;234:16003–10.
Weichselbaum RR, Kufe DW, Hellman S, Rasmussen HS, King CR, Fischer PH, et al. Radiation-induced tumour necrosis factor-alpha expression: clinical application of transcriptional and physical targeting of gene therapy. Lancet Oncol. 2002;3:665–71.
Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. 2017;280:249–79.
Liu J, Zhou J, Wu M, Hu C, Yang J, Li D, et al. Low-dose total body irradiation can enhance systemic immune related response induced by hypo-fractionated radiation. Front Immunol. 2019;10:317.
Koukourakis MI, Giatromanolaki A. Lymphopenia and intratumoral lymphocytic balance in the era of cancer immuno-radiotherapy. Crit Rev Oncol/Hematol. 2021;159:103226.
Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75:2232–42.
Jones KI, Tiersma J, Yuzhalin AE, Gordon-Weeks AN, Buzzelli J, Im JH. et al. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol Med. 2018;10:e9342.
Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res. 2017;5:992–1004.
Yang X, Lu Y, Hang J, Zhang J, Zhang T, Huo Y, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol Res. 2020;8:1440–51.
Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends cancer. 2022;8:9–20.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol cancer. 2022;21:28.
Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67.
Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–86.
Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. 2023;8:104.
Li Z, Han B, Qi M, Li Y, Duan Y, Yao Y. Modulating macrophage-mediated programmed cell removal: An attractive strategy for cancer therapy. Biochim Biophys Acta Rev cancer. 2024;1879:189172.
Brown GC. Cell death by phagocytosis. Nat Rev Immunol. 2024;24:91–102.
Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J, et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci. 2021;17:3281–7.
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med. 2018;379:1711–21.
Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, et al. First-in-Human, first-in-class Phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol: J Am Soc Clin Oncol. 2019;37:946–53.
Schürch CM, Forster S, Brühl F, Yang SH, Felley-Bosco E, Hewer E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology. 2017;7:e1373235.
Oliveira G, Wu CJ. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 2023;23:295–316.
Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2:1124–35.
Wang J, Gai J, Zhang T, Niu N, Qi H, Thomas DL, et al. Neoadjuvant radioimmunotherapy in pancreatic cancer enhances effector T cell infiltration and shortens their distances to tumor cells. Sci Adv. 2024;10:eadk1827.
Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol: Publ Int Assoc Study Lung Cancer. 2020;15:1624–35.
Reijmen E, De Mey S, De Mey W, Gevaert T, De Ridder K, Locy H, et al. Fractionated radiation severely reduces the number of CD8+ T cells and mature antigen presenting cells within lung tumors. Int J Radiat Oncol, Biol, Phys. 2021;111:272–83.
Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, Kao KZ, Lako A, Tsuji J, et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2022;23:279–91.
Rafat M, Aguilera TA, Vilalta M, Bronsart LL, Soto LA, von Eyben R, et al. Macrophages promote circulating tumor cell-mediated local recurrence following radiotherapy in immunosuppressed patients. Cancer Res. 2018;78:4241–52.
Stary V, Wolf B, Unterleuthner D, List J, Talic M, Laengle J, et al. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J Immunother Cancer. 2020;8(2):e000667.
Wang H, Zhai M, Li M, Han C, Liu L, Huang C, et al. Phenotypic plasticity and increased infiltration of peripheral blood-derived TREM1(+) mono-macrophages following radiotherapy in rectal cancer. Cell Rep Med. 2025;6:101887.
Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74:6771–83.
Wang H, Sun Y, Zhou X, Chen C, Jiao L, Li W, et al. CD47/SIRPα blocking peptide identification and synergistic effect with irradiation for cancer immunotherapy. J Immunothe Cancer. 2020;8:e000905.
Okada K, Sato H, Kumazawa T, Mori Y, Permata TBM, Uchihara Y, et al. Calreticulin upregulation in cervical cancer tissues from patients after 10 Gy radiation therapy. Adv Radiat Oncol. 2023;8:101159.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9.
Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76–84.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–6.
Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019;568:187–92.
Li D, Xiong W, Wang Y, Feng J, He Y, Du J, et al. SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Sci Immunol. 2022;7:eabj5501.
Theruvath J, Menard M, Smith BAH, Linde MH, Coles GL, Dalton GN, et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat Med. 2022;28:333–44.
Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50.
Liu X, Kwon H, Li Z, Fu YX. Is CD47 an innate immune checkpoint for tumor evasion?. J Hematol Oncol. 2017;10:12.
Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145–64.
Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα Immune Checkpoint. Immunity. 2020;52:742–52.
Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Investig. 2016;126:2610–20.
Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Sci (NY). 2016;352:227–31.
Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatol (Balt, Md). 2015;62:534–45.
Lu J, Li J, Lin Z, Li H, Lou L, Ding W, et al. Reprogramming of TAMs via the STAT3/CD47-SIRPα axis promotes acquired resistance to EGFR-TKIs in lung cancer. Cancer Lett. 2023;564:216205.
Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA. 2015;112:E6215–23.
Li Z, Li Y, Gao J, Fu Y, Hua P, Jing Y, et al. The role of CD47-SIRPα immune checkpoint in tumor immune evasion and innate immunotherapy. Life Sci. 2021;273:119150.
van Duijn A, Van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer. 2022;10:e004589.
Lee WH, Kim SH, An JH, Kim TK, Cha HJ, Chang HW, et al. Tristetraprolin regulates phagocytosis through interaction with CD47 in head and neck cancer. Exp Ther Med. 2022;24:541.
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y, et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13:1511.
Candas-Green D, Xie B, Huang J, Fan M, Wang A, Menaa C, et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat Commun. 2020;11:4591.
Hsieh RC, Krishnan S, Wu RC, Boda AR, Liu A, Winkler M, et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci Immunol. 2022;7:eabl9330.
Rostami E, Bakhshandeh M, Ghaffari-Nazari H, Alinezhad M, Alimohammadi M, Alimohammadi R, et al. Combining ablative radiotherapy and anti CD47 monoclonal antibody improves infiltration of immune cells in tumor microenvironments. PloS One. 2022;17:e0273547.
Ji K, Zhang Y, Jiang S, Sun L, Zhang B, Hu D, et al. SIRPα blockade improves the antitumor immunity of radiotherapy in colorectal cancer. Cell Death Discov. 2023;9:180.
Piffkó A, Yang K, Panda A, Heide J, Tesak K, Wen C, et al. Radiation-induced amphiregulin drives tumour metastasis. Nature. 2025;643:810–19.
Vermeer DW, Spanos WC, Vermeer PD, Bruns AM, Lee KM, Lee JH. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer. 2013;133:120–9.
Shi L, Wang X, Hu B, Wang D, Ren Z. miR-222 enhances radiosensitivity of cancer cells by inhibiting the expression of CD47. Int J Clin Exp Pathol. 2019;12:4204–13.
Sun T, Liu B, Cao Y, Li Y, Cai L, Yang W. AMPK-mediated CD47 H3K4 methylation promotes phagocytosis evasion of glioma stem cells post-radiotherapy. Cancer Lett. 2024;583:216605.
Nishiga Y, Drainas AP, Baron M, Bhattacharya D, Barkal AA, Ahrari Y, et al. Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect. Nat Cancer. 2022;3:1351–66.
Guilbaud E, Yamazaki T, Galluzzi L. T cell-independent abscopal responses to radiotherapy. Trends Cancer. 2023;9:93–5.
Buchwald ZS, Nasti TH, Lee J, Eberhardt CS, Wieland A, Im SJ, et al. Tumor-draining lymph node is important for a robust abscopal effect stimulated by radiotherapy. J Immunother Cancer. 2020;8:e000867.
Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39:644–55.
Darragh LB, Gadwa J, Pham TT, Van Court B, Neupert B, Olimpo NA, et al. Elective nodal irradiation mitigates local and systemic immunity generated by combination radiation and immunotherapy in head and neck tumors. Nat Commun. 2022;13:7015.
Telarovic I, Yong CSM, Kurz L, Vetrugno I, Reichl S, Fernandez AS, et al. Delayed tumor-draining lymph node irradiation preserves the efficacy of combined radiotherapy and immune checkpoint blockade in models of metastatic disease. Nat Commun. 2024;15:5500.
Pai S, Bamodu OA, Lin YK, Lin CS, Chu PY, Chien MH, et al. CD47-SIRPà signaling induces epithelial-mesenchymal transition and cancer stemness and links to a poor prognosis in patients with oral squamous cell carcinoma. Cells. 2019;8:1658.
Pai S, Yadav VK, Kuo KT, Pikatan NW, Lin CS, Chien MH, et al. PDK1 Inhibitor BX795 improves Cisplatin and radio-efficacy in oral squamous cell carcinoma by downregulating the PDK1/CD47/Akt-mediated glycolysis signaling pathway. Int J Mol Sci. 2021;22:11492.
Soto-Pantoja DR, Stein EV, Rogers NM, Sharifi-Sanjani M, Isenberg JS, Roberts DD. Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther targets. 2013;17:89–103.
Boerma M, Freeman ML. Radiation biology: targeting CD47 in cancer growth inhibition and normal tissue protection. Int J Radiat Oncol, Biol, Phys. 2016;96:245–7.
Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, et al. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008;173:1100–12.
Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7.
Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep. 2013;3:1038.
Soto-Pantoja DR, Isenberg JS, Roberts DD. Therapeutic targeting of CD47 to modulate tissue responses to ischemia and radiation. J Genet Syndromes Gene Ther. 2011;2:1000105.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9.
Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–78.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Yang H, Yan M, Li W, Xu L. SIRPα and PD1 expression on tumor-associated macrophage predict prognosis of intrahepatic cholangiocarcinoma. J Transl Med. 2022;20:140.
Schulz D, Streller M, Piendl G, Brockhoff G, Reichert TE, Menevse AN, et al. Differential localization of PD-L1 and Akt-1 involvement in radioresistant and radiosensitive cell lines of head and neck squamous cell carcinoma. Carcinogenesis. 2020;41:984–92.
Zhang H, Zhou F, Wang Y, Xie H, Luo S, Meng L, et al. Eliminating radiation resistance of non-small cell lung cancer by Dihydroartemisinin through abrogating immunity escaping and promoting radiation sensitivity by inhibiting PD-L1 Expression. Front Oncol. 2020;10:595466.
Chen D, Barsoumian HB, Yang L, Younes AI, Verma V, Hu Y, et al. SHP-2 and PD-L1 inhibition combined with radiotherapy enhances systemic antitumor effects in an Anti-PD-1-resistant model of non-small cell lung cancer. Cancer Immunol Res. 2020;8:883–94.
Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751.
Du SS, Chen GW, Yang P, Chen YX, Hu Y, Zhao QQ, et al. Radiation Therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol, Biol, Phys. 2022;112:1243–55.
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68.
Song X, Shao Y, Jiang T, Ding Y, Xu B, Zheng X, et al. Radiotherapy upregulates programmed death ligand-1 through the pathways downstream of epidermal growth factor receptor in glioma. EBioMedicine. 2018;28:105–13.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Investig. 2014;124:687–95.
Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res : J Am Assoc Cancer Res. 2017;23:5514–26.
Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–36.
Zeller T, Münnich IA, Windisch R, Hilger P, Schewe DM, Humpe A, et al. Perspectives of targeting LILRB1 in innate and adaptive immune checkpoint therapy of cancer. Front Immunol. 2023;14:1240275.
Mandel I, Haves Ziv D, Goldshtein I, Peretz T, Alishekevitz D, Fridman Dror A, et al. BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression. J Immunother Cancer. 2022;10:e004859.
Klein B, Loven D, Lurie H, Rakowsky E, Nyska A, Levin I, et al. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg. 1994;80:1074–7.
Hrbac T, Kopkova A, Siegl F, Vecera M, Ruckova M, Kazda T, et al. HLA-E and HLA-F Are Overexpressed in Glioblastoma and HLA-E Increased After Exposure to Ionizing Radiation. Cancer Genomics Proteom. 2022;19:151–62.
Santin AD, Hermonat PL, Hiserodt JC, Chiriva-Internati M, Woodliff J, Theus JW, et al. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM-1 in human cervical cancer. Int J Radiat Oncol, Biol, Phys. 1997;39:737–42.
Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94.
Santin AD, Hiserodt JC, Fruehauf J, DiSaia PJ, Pecorelli S, Granger GA. Effects of irradiation on the expression of surface antigens in human ovarian cancer. Gynecol Oncol. 1996;60:468–74.
Haeggblom L, Nordfors C, Tertipis N, Bersani C, Ramqvist T, Näsman A, et al. Effects of irradiation on human leukocyte antigen class I expression in human papillomavirus positive and negative base of tongue and mobile tongue squamous cell carcinoma cell lines. Int J Oncol. 2017;50:1423–30.
Yang Y, Zhu G, Yang L, Yang Y. Targeting CD24 as a novel immunotherapy for solid cancers. Cell Commun Signal: CCS. 2023;21:312.
Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66.
Kwon MJ, Han J, Seo JH, Song K, Jeong HM, Choi JS, et al. CD24 Overexpression Is Associated with Poor Prognosis in Luminal A and Triple-Negative Breast Cancer. PloS One. 2015;10:e0139112.
Shen W, Shi P, Dong Q, Zhou X, Chen C, Sui X, et al. Discovery of a novel dual-targeting D-peptide to block CD24/Siglec-10 and PD-1/PD-L1 interaction and synergize with radiotherapy for cancer immunotherapy. J Immunother Cancer. 2023;11:e007068.
Bensimon J, Biard D, Paget V, Goislard M, Morel-Altmeyer S, Konge J, et al. Forced extinction of CD24 stem-like breast cancer marker alone promotes radiation resistance through the control of oxidative stress. Mol Carcinogenesis. 2016;55:245–54.
Müller J, Obermeier I, Wöhner M, Brandl C, Mrotzek S, Angermüller S, et al. CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc Natl Acad Sci USA. 2013;110:12402–7.
Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20:633–43.
Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–99.
Nagelkerke SQ, Dekkers G, Kustiawan I, van de Bovenkamp FS, Geissler J, Plomp R, et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood. 2014;124:3709–18.
Huang ZY, Hunter S, Kim MK, Indik ZK, Schreiber AD. The effect of phosphatases SHP-1 and SHIP-1 on signaling by the ITIM- and ITAM-containing Fcgamma receptors FcgammaRIIB and FcgammaRIIA. J Leukoc Biol. 2003;73:823–9.
Dobrenkov K, Cheung NK. GD2-targeted immunotherapy and radioimmunotherapy. Semin Oncol. 2014;41:589–612.
Fucikova J, Spisek R, Kroemer G, Galluzzi L. Calreticulin and cancer. Cell Res. 2021;31:5–16.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34.
Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94.
Lin H, Kryczek I, Li S, Green MD, Ali A, Hamasha R, et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer cell. 2021;39:480–93.e6.
Wu Y, Luo X, Zhou Q, Gong H, Gao H, Liu T, et al. The disbalance of LRP1 and SIRPα by psychological stress dampens the clearance of tumor cells by macrophages. Acta Pharm Sin B. 2022;12:197–209.
Malamas AS, Gameiro SR, Knudson KM, Hodge JW. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget. 2016;7:86937–47.
Huang Y, Dong Y, Zhao J, Zhang L, Kong L, Lu JJ. Comparison of the effects of photon, proton and carbon-ion radiation on the ecto-calreticulin exposure in various tumor cell lines. Ann Transl Med. 2019;7:542.
Huang Y, Huang Q, Zhao J, Dong Y, Zhang L, Fang X, et al. The impacts of different types of radiation on the CRT and PDL1 Expression in tumor cells under Normoxia and Hypoxia. Front Oncol. 2020;10:1610.
Gong Z, Jia Q, Guo J, Li C, Xu S, Jin Z, et al. Caspase-8 contributes to an immuno-hot microenvironment by promoting phagocytosis via an ecto-calreticulin-dependent mechanism. Exp Hematol Oncol. 2023;12:7.
Chen CJ, Hu YC, Chien Y, Huang WC, Wu CS, Tsai CY, et al. Calreticulin Expression controls cellular redox, stemness, and radiosensitivity to function as a novel adjuvant for radiotherapy in neuroblastoma. Oxid Med Cell Longev. 2023;2023:8753309.
Lee KJ, Ko EJ, Park YY, Park SS, Ju EJ, Park J, et al. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials. 2020;255:120151.
Liu P, Zhao L, Loos F, Marty C, Xie W, Martins I, et al. Immunosuppression by mutated calreticulin released from malignant cells. Mol cell. 2020;77:748–60.e9.
Vallabhapurapu SD, Blanco VM, Sulaiman MK, Vallabhapurapu SL, Chu Z, Franco RS, et al. Variation in human cancer cell external phosphatidylserine is regulated by flippase activity and intracellular calcium. Oncotarget. 2015;6:34375–88.
Elnemr A, Ohta T, Yachie A, Fushida S, Ninomiya I, Nishimura GI, et al. N-ethylmaleimide-enhanced phosphatidylserine externalization of human pancreatic cancer cells and immediate phosphatidylserine-mediated phagocytosis by macrophages. Int J Oncol. 2000;16:1111–6.
Chang GH, Barbaro NM, Pieper RO. Phosphatidylserine-dependent phagocytosis of apoptotic glioma cells by normal human microglia, astrocytes, and glioma cells. Neuro-Oncol. 2000;2:174–83.
Lemke G. How macrophages deal with death. Nat Rev Immunol. 2019;19:539–49.
Chang W, Fa H, Xiao D, Wang J. Targeting phosphatidylserine for Cancer therapy: prospects and challenges. Theranostics. 2020;10:9214–29.
Vorselen D. Dynamics of phagocytosis mediated by phosphatidylserine. Biochem Soc Trans. 2022;50:1281–91.
Davis HW, Vallabhapurapu SD, Chu Z, Vallabhapurapu SL, Franco RS, Mierzwa M, et al. Enhanced phosphatidylserine-selective cancer therapy with irradiation and SapC-DOPS nanovesicles. Oncotarget. 2019;10:856–68.
Budhu S, Giese R, Gupta A, Fitzgerald K, Zappasodi R, Schad S, et al. Targeting Phosphatidylserine enhances the anti-tumor response to tumor-directed radiation therapy in a preclinical model of Melanoma. Cell Rep. 2021;34:108620.
Chen J, Zhong MC, Guo H, Davidson D, Mishel S, Lu Y, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493–7.
Wang SH, Chou WC, Huang HC, Lee TA, Hsiao TC, Wang LH, et al. Deglycosylation of SLAMF7 in breast cancers enhances phagocytosis. Am J cancer Res. 2022;12:4721–36.
Bruhns P, Jönsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268:25–51.
Getahun A, Cambier JC. Of ITIMs, ITAMs, and ITAMis: revisiting immunoglobulin Fc receptor signaling. Immunol Rev. 2015;268:66–73.
Suwa T, Kobayashi M, Nam JM, Harada H. Tumor microenvironment and radioresistance. Exp Mol Med. 2021;53:1029–35.
Chang H, Wei JW, Tao YL, Ding PR, Xia YF, Gao YH, et al. CCR6 is a predicting biomarker of radiosensitivity and potential target of radiosensitization in rectal cancer. Cancer Res Treat. 2018;50:1203–13.
Li K, Zhu X, Li L, Ning R, Liang Z, Zeng F, et al. Identification of non-invasive biomarkers for predicting the radiosensitivity of nasopharyngeal carcinoma from serum microRNAs. Sci Rep. 2020;10:5161.
Liao F, Chen X, Peng P, Dong W. RWR-algorithm-based dissection of microRNA-506-3p and microRNA-140-5p as radiosensitive biomarkers in colorectal cancer. Aging. 2020;12:20512–22.
Tsai CJ, Yang JT, Shaverdian N, Patel J, Shepherd AF, Eng J, et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet. 2024;403:171–82.
Ludmir EB, Sherry AD, Fellman BM, Liu S, Bathala T, Haymaker C, et al. Addition of metastasis-directed therapy to systemic therapy for Oligometastatic Pancreatic Ductal Adenocarcinoma (EXTEND): A multicenter, randomized Phase II trial. J Clin Oncol. 2024;42:3795–805.
Tang C, Sherry AD, Haymaker C, Bathala T, Liu S, Fellman B, et al. Addition of Metastasis-directed therapy to intermittent hormone therapy for oligometastatic prostate cancer: The EXTEND Phase 2 randomized clinical trial. JAMA Oncol. 2023;9:825–34.
McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized Phase II Trial of Nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol: J Am Soc Clin Oncol. 2021;39:30–7.
Song A, Wu L, Zhang BX, Yang QC, Liu YT, Li H, et al. Glutamine inhibition combined with CD47 blockade enhances radiotherapy-induced ferroptosis in head and neck squamous cell carcinoma. Cancer Lett. 2024;588:216727.
Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose?. J Immunother Cancer. 2021;9:e002038.
Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104.
Nadella V, Singh S, Jain A, Jain M, Vasquez KM, Sharma A, et al. Low dose radiation primed iNOS + M1macrophages modulate angiogenic programming of tumor derived endothelium. Mol Carcinogenesis. 2018;57:1664–71.
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer cell. 2013;24:589–602.
Cheema AK, Li Y, Ventimiglia M, Kowalczyk K, Hankins R, Bandi G, et al. Radiotherapy Induces Innate Immune Responses in Patients Treated for Prostate Cancers. Clin Cancer Res. 2023;29:921–9.
Lehrer EJ, Prabhu AV, Sindhu KK, Lazarev S, Ruiz-Garcia H, Peterson JL, et al. Proton and heavy particle intracranial radiosurgery. Biomedicines. 2021;9:31.
Frijlink E, Bosma DM, Busselaar J, Battaglia TW, Staal MD, Verbrugge I, et al. PD-1 or CTLA-4 blockade promotes CD86-driven Treg responses upon radiotherapy of lymphocyte-depleted cancer in mice. J Clin Investig. 2024;134:e171154.
Yu WB, Ye ZH, Chen X, Shi JJ, Lu JJ. The development of small-molecule inhibitors targeting CD47. Drug Discov today. 2021;26:561–8.
Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32:e2004853.
Wang Y, Liu ZG, Yuan H, Deng W, Li J, Huang Y, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25:1709–17.
Louvel G, Bahleda R, Ammari S, Le Péchoux C, Levy A, Massard C, et al. Immunotherapy and pulmonary toxicities: can concomitant immune-checkpoint inhibitors with radiotherapy increase the risk of radiation pneumonitis?. Eur Respir J. 2018;51:1701737.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-small-cell Lung Cancer. N Engl J Med. 2017;377:1919–29.
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC Trial: Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. J Clin Oncol: J Am Soc Clin Oncol. 2022;40:1301–11.
Bestvina CM, Pointer KB, Karrison T, Al-Hallaq H, Hoffman PC, Jelinek MJ, et al. A Phase 1 trial of concurrent or sequential Ipilimumab, Nivolumab, and stereotactic body radiotherapy in patients with Stage IV NSCLC Study. J Thorac Oncol. 2022;17:130–40.
Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–4.
Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer cell. 2019;35:588–602.e10.
Ochocka N, Segit P, Walentynowicz KA, Wojnicki K, Cyranowski S, Swatler J, et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun. 2021;12:1151.
Qiu X, Zhou T, Li S, Wu J, Tang J, Ma G, et al. Spatial single-cell protein landscape reveals vimentin(high) macrophages as immune-suppressive in the microenvironment of hepatocellular carcinoma. Nat Cancer. 2024;5:1557–78.
Matusiak M, Hickey JW, van IDGP, Lu G, Kidziński L, Zhu S, et al. Spatially segregated macrophage populations predict distinct outcomes in colon cancer. Cancer Discov. 2024;14:1418–39.
Casanova-Acebes M, Dalla E, Leader AM, LeBerichel J, Nikolic J, Morales BM, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595:578–84.
Wan X, Fang M, Chen T, Wang H, Zhou Q, Wei Y, et al. The mechanism of low-dose radiation-induced upregulation of immune checkpoint molecule expression in lung cancer cells. Biochem Biophys Res Commun. 2022;608:102–7.
Ghantous L, Volman Y, Hefez R, Wald O, Stern E, Friehmann T, et al. The DNA damage response pathway regulates the expression of the immune checkpoint CD47. Commun Biol. 2023;6:245.
Zhang P, Rashidi A, Zhao J, Silvers C, Wang H, Castro B, et al. STING agonist-loaded, CD47/PD-L1-targeting nanoparticles potentiate antitumor immunity and radiotherapy for glioblastoma. Nat Commun. 2023;14:1610.
Yoshino H, Sato Y, Nakano M. KPNB1 Inhibitor Importazole Reduces Ionizing Radiation-Increased Cell Surface PD-L1 Expression by Modulating Expression and Nuclear Import of IRF1. Curr issues Mol Biol. 2021;43:153–62.
Wang J, Xu Y, Rao X, Zhang R, Tang J, Zhang D, et al. BRD4-IRF1 axis regulates chemoradiotherapy-induced PD-L1 expression and immune evasion in non-small cell lung cancer. Clin Transl Med. 2022;12:e718.
Azad A, Yin Lim S, D’Costa Z, Jones K, Diana A, Sansom OJ, et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med. 2017;9:167–80.
Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J Immunother Cancer. 2019;7:160.
Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12:4730–7.
Peters HL, Yan Y, Solheim JC. APLP2 regulates the expression of MHC class I molecules on irradiated Ewing’s sarcoma cells. Oncoimmunology. 2013;2:e26293.
Zebertavage LK, Alice A, Crittenden MR, Gough MJ. Transcriptional upregulation of NLRC5 by radiation drives STING- and Interferon-independent MHC-I Expression on cancer cells and T Cell Cytotoxicity. Sci Rep. 2020;10:7376.
Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PloS One. 2012;7:e32542.
Bian Z, Shi L, Kidder K, Zen K, Garnett-Benson C, Liu Y. Intratumoral SIRPα-deficient macrophages activate tumor antigen-specific cytotoxic T cells under radiotherapy. Nat Commun. 2021;12:3229.
Gholamin S, Youssef OA, Rafat M, Esparza R, Kahn S, Shahin M, et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun. 2020;26:130–7.
Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–45.
Schwartz AL, Nath PR, Allgauer M, Lessey-Morillon EC, Sipes JM, Ridnour LA, et al. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol Immunother. 2019;68:1805–17.
Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6:46.
Ene CI, Kreuser SA, Jung M, Zhang H, Arora S, White Moyes K, et al. Anti-PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro-Oncol. 2020;22:639–51.
Li X, Chen Y, Wang R, Lu E, Luo K, Sha X. Enhancement of cancer immunotherapy using CRT valgus tumor cell membranes coated bacterial whole peptidoglycan combined with radiotherapy. Int J Pharm. 2023;646:123430.
Boreel DF, Sandker GGW, Ansems M, van den Bijgaart RJE, Peters JPW, Span PN, et al. MHC-I and PD-L1 Expression is associated with decreased tumor outgrowth and is radiotherapy-inducible in the murine head and neck squamous cell carcinoma model MOC1. Mol Imaging Biol. 2024;26:835–46.
Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol. 2021;12:636568.
Soni P, Qayoom S, Husain N, Kumar P, Chandra A, Ojha BK, et al. CD24 and Nanog expression in Stem Cells in Glioblastoma: Correlation with response to chemoradiation and overall survival. Asian Pac J Cancer Prev. 2017;18:2215–9.
Huang S, Zhang X, Wei Y, Xiao Y. Checkpoint CD24 function on tumor and immunotherapy. Front Immunol. 2024;15:1367959.
Zaib T, Cheng K, Liu T, Mei R, Liu Q, Zhou X, et al. Expression of CD22 in triple-negative breast cancer: a novel prognostic biomarker and potential target for CAR therapy. Int J Mol Sci. 2023;24:2152.
Kim MH, Lee JH, Lee JS, Kim DC, Yang JW, An HJ. et al. Fc receptor expression as a prognostic factor in patients with non-small-cell lung cancer. In vivo. 2022;36:2708–13.
Farhangnia P, Ghomi SM, Mollazadehghomi S, Nickho H, Akbarpour M, Delbandi AA. SLAM-family receptors come of age as a potential molecular target in cancer immunotherapy. Front Immunol. 2023;14:1174138.
Gunes M, Rosen ST, Shachar I, Gunes EG. Signaling lymphocytic activation molecule family receptors as potential immune therapeutic targets in solid tumors. Front Immunol. 2024;15:1297473.
Machy P, Mortier E, Birklé S. Biology of GD2 ganglioside: implications for cancer immunotherapy. Front Pharm. 2023;14:1249929.
Eggleton P, De Alba J, Weinreich M, Calias P, Foulkes R, Corrigall VM. The therapeutic mavericks: Potent immunomodulating chaperones capable of treating human diseases. J Cell Mol Med. 2023;27:322–39.
Han A, Li C, Zahed T, Wong M, Smith I, Hoedel K, et al. Calreticulin is a critical cell survival factor in malignant neoplasms. PLoS Biol. 2019;17:e3000402.
Guilbaud E, Kroemer G, Galluzzi L. Calreticulin exposure orchestrates innate immunosurveillance. Cancer cell. 2023;41:1014–6.
Pulica R, Aquib A, Varsanyi C, Gadiyar V, Wang Z, Frederick T, et al. Dys-regulated phosphatidylserine externalization as a cell intrinsic immune escape mechanism in cancer. Cell Commun Signal: CCS. 2025;23:131.
Sharma B, Kanwar SS. Phosphatidylserine: A cancer cell targeting biomarker. Semin cancer Biol. 2018;52:17–25.
Lingel H, Fischer L, Remstedt S, Kuropka B, Philipsen L, Han I, et al. SLAMF7 (CD319) on activated CD8(+) T cells transduces environmental cues to initiate cytotoxic effector cell responses. Cell Death Differ. 2025;32:561–72.
Deng Y, Zhang L, Dai C, Xu Y, Gan Q, Cheng J. SLAMF7 predicts prognosis and correlates with immune infiltration in serous ovarian carcinoma. J Gynecol Oncol. 2024;35:e79.
Skinner HD, Giri U, Yang LP, Kumar M, Liu Y, Story MD, et al. Integrative analysis identifies a novel AXL-PI3 kinase-PD-L1 signaling axis associated with radiation resistance in head and neck cancer. Clin Cancer Res. 2017;23:2713–22.
Li S, Xie Y, Zhou W, Zhou Q, Tao D, Yang H, et al. Association of long noncoding RNA MALAT1 with the radiosensitivity of lung adenocarcinoma cells via the miR-140/PD-L1 axis. Heliyon. 2023;9:e16868.
Fiedler M, Weber F, Hautmann MG, Haubner F, Reichert TE, Klingelhöffer C, et al. Biological predictors of radiosensitivity in head and neck squamous cell carcinoma. Clin Oral Investig. 2018;22:189–200.
Lyu X, Zhang M, Li G, Jiang Y, Qiao Q. PD-1 and PD-L1 expression predicts radiosensitivity and clinical outcomes in head and neck cancer and is associated with HPV infection. J Cancer. 2019;10:937–48.
Uckun FM, Song CW. Lack of CD24 antigen expression in B-lineage acute lymphoblastic leukemia is associated with intrinsic radiation resistance of primary clonogenic blasts. Blood. 1993;81:1323–32.
Bensimon J, Altmeyer-Morel S, Benjelloun H, Chevillard S, Lebeau J. CD24(-/low) stem-like breast cancer marker defines the radiation-resistant cells involved in memorization and transmission of radiation-induced genomic instability. Oncogene. 2013;32:251–8.
Wang L, Li P, Hu W, Xia Y, Hu C, Liu L, et al. CD44(+)CD24(+) subset of PANC-1 cells exhibits radiation resistance via decreased levels of reactive oxygen species. Oncol Lett. 2017;14:1341–6.
Okunaga T, Urata Y, Goto S, Matsuo T, Mizota S, Tsutsumi K, et al. Calreticulin, a molecular chaperone in the endoplasmic reticulum, modulates radiosensitivity of human glioblastoma U251MG cells. Cancer Res. 2006;66:8662–71.
Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. 2020;8:e000537.
Yin L, Xue J, Li R, Zhou L, Deng L, Chen L, et al. Effect of low-dose radiation therapy on abscopal responses to hypofractionated radiation therapy and anti-PD1 in mice and patients with non-small cell lung cancer. Int J Radiat Oncol, Biol Phys. 2020;108:212–24.
Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol, Biol Phys. 2016;95:120–30.
Zhou H, Tu C, Yang P, Li J, Kepp O, Li H, et al. Carbon ion radiotherapy triggers immunogenic cell death and sensitizes melanoma to anti-PD-1 therapy in mice. Oncoimmunology. 2022;11:2057892.
Bertho A, Iturri L, Prezado Y. Radiation-induced immune response in novel radiotherapy approaches FLASH and spatially fractionated radiotherapies. Int Rev Cell Mol Biol. 2023;376:37–68.
Kuo TC, Chen A, Harrabi O, Sockolosky JT, Zhang A, Sangalang E, et al. Targeting the myeloid checkpoint receptor SIRPα potentiates innate and adaptive immune responses to promote anti-tumor activity. J Hematol Oncol. 2020;13:160.
Logtenberg MEW, Jansen JHM, Raaben M, Toebes M, Franke K, Brandsma AM, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPα axis and a target for cancer immunotherapy. Nat Med. 2019;25:612–9.
Nigro A, Ricciardi L, Salvato I, Sabbatino F, Vitale M, Crescenzi MA, et al. Enhanced expression of CD47 Is associated with off-target resistance to Tyrosine Kinase inhibitor Gefitinib in NSCLC. Front Immunol. 2019;10:3135.
Hazama D, Yin Y, Murata Y, Matsuda M, Okamoto T, Tanaka D, et al. Macrocyclic peptide-mediated blockade of the CD47-SIRPα interaction as a potential cancer immunotherapy. Cell Chem Biol. 2020;27:1181–91.e7.
Tang L, Yin Y, Cao Y, Fu C, Liu H, Feng J, et al. Extracellular vesicles-derived hybrid nanoplatforms for amplified CD47 blockade-based cancer immunotherapy. Adv Mater. 2003;35:e2303835.
Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y, et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12:763–9.
Zhan Z, Zeng W, Liu J, Zhang L, Cao Y, Li P, et al. Engineered biomimetic copper sulfide nanozyme mediates “Don’t Eat Me” signaling for photothermal and chemodynamic precision therapies of breast cancer. ACS Appl Mater interfaces. 2023;15:24071–83.
Lu K, He C, Guo N, Chan C, Ni K, Lan G, et al. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2:600–10.
Chen Q, Chen J, Yang Z, Xu J, Xu L, Liang C, et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv Mater. 2019;31:e1802228.
Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7.
Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122:3051–8.
Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol, Phys. 2015;92:368–75.
Jokimäki A, Hietala H, Lemma J, Karhapää H, Rintala A, Kaikkonen JP, et al. Previous radiotherapy improves treatment responses and causes a trend toward longer time to progression among patients with immune checkpoint inhibitor-related adverse events. Cancer Immunol Immunother. 2023;72:3337–47.
Pomeranz Krummel DA, Nasti TH, Izar B, Press RH, Xu M, Lowder L, et al. Impact of sequencing radiation therapy and immune checkpoint inhibitors in the treatment of melanoma brain metastases. Int J Radiat Oncol, Biol, Phys. 2020;108:157–63.
Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Ebstein S, Cortez C, et al. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol. 2021;6:eabg0117.
Qin R, Olson A, Singh B, Thomas S, Wolf S, Bhavsar NA, et al. Safety and efficacy of radiation therapy in advanced melanoma patients treated with Ipilimumab. Int J Radiat Oncol, Biol, Phys. 2016;96:72–7.
Han L, Shi H, Luo Y, Sun W, Li S, Zhang N, et al. Gene signature based on B cell predicts clinical outcome of radiotherapy and immunotherapy for patients with lung adenocarcinoma. Cancer Med. 2020;9:9581–94.
Dai YH, Wang YF, Shen PC, Lo CH, Yang JF, Lin CS, et al. Radiosensitivity index emerges as a potential biomarker for combined radiotherapy and immunotherapy. NPJ Genom Med. 2021;6:40.
Ohri N, Jolly S, Cooper BT, Kabarriti R, Bodner WR, Klein J, et al. Selective Personalized RadioImmunotherapy for Locally Advanced Non-Small-Cell Lung Cancer Trial (SPRINT). J Clin Oncol: J Am Soc Clin Oncol. 2024;42:562–70.
van Hooren L, Handgraaf SM, Kloosterman DJ, Karimi E, van Mil L, Gassama AA, et al. CD103(+) regulatory T cells underlie resistance to radio-immunotherapy and impair CD8(+) T cell activation in glioblastoma. Nat Cancer. 2023;4:665–81.
Zafra J, Onieva JL, Oliver J, Garrido-Barros M, González-Hernández A, Martínez-Gálvez B, et al. Novel blood biomarkers for response prediction and monitoring of stereotactic ablative radiotherapy and immunotherapy in metastatic oligoprogressive lung cancer. Int J Mol Scii. 2024;25:4533.
Ratnayake G, Reinwald S, Edwards J, Wong N, Yu D, Ward R, et al. Blood T-cell profiling in metastatic melanoma patients as a marker for response to immune checkpoint inhibitors combined with radiotherapy. Radiother Oncol: J Eur Soc Ther Radio Oncol. 2022;173:299–305.
Sun R, Sundahl N, Hecht M, Putz F, Lancia A, Rouyar A, et al. Radiomics to predict outcomes and abscopal response of patients with cancer treated with immunotherapy combined with radiotherapy using a validated signature of CD8 cells. J Immunother Cancer. 2020;8:e001429.
Grambozov B, Kalantari F, Beheshti M, Stana M, Karner J, Ruznic E, et al. Pretreatment 18-FDG-PET/CT parameters can serve as prognostic imaging biomarkers in recurrent NSCLC patients treated with reirradiation-chemoimmunotherapy. Radiother Oncol: J Eur Soc Ther Radio Oncol. 2023;185:109728.
Wang X, Gong G, Sun Q, Meng X. Prediction of pCR based on clinical-radiomic model in patients with locally advanced ESCC treated with neoadjuvant immunotherapy plus chemoradiotherapy. Front Oncol. 2024;14:1350914.
Peng H, Moore C, Zhang Y, Saha D, Jiang S, Timmerman R. An AI-based approach for modeling the synergy between radiotherapy and immunotherapy. Sci Rep. 2024;14:8250.
Oliveira G, Stromhaug K, Klaeger S, Kula T, Frederick DT, Le PM, et al. Phenotype, specificity and avidity of antitumour CD8(+) T cells in melanoma. Nature. 2021;596:119–25.
Gueguen P, Metoikidou C, Dupic T, Lawand M, Goudot C, Baulande S, et al. Contribution of resident and circulating precursors to tumor-infiltrating CD8(+) T cell populations in lung cancer. Sci Immunol. 2021;6:eabd5778.
Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. 2018;564:268–72.
Tietscher S, Wagner J, Anzeneder T, Langwieder C, Rees M, Sobottka B, et al. A comprehensive single-cell map of T cell exhaustion-associated immune environments in human breast cancer. Nat Commun. 2023;14:98.
Wang S, Zota V, Vincent MY, Clossey D, Chen JJ, Cieslewicz M, et al. Assessing CD36 and CD47 expression levels in solid tumor indications to stratify patients for VT1021 treatment. NPJ Precis Oncol. 2024;8:278.
Soltani M, Abbaszadeh M, Fouladseresht H, Sullman MJM, Eskandari N. PD-L1 importance in malignancies comprehensive insights into the role of PD-L1 in malignancies: from molecular mechanisms to therapeutic opportunities. Clin Exp Med. 2025;25:106.
Cao X, Ren X, Song Y, Sun Q, Mao F, Shen S, et al. High Expression of Calreticulin affected the tumor microenvironment and correlated with worse prognosis in patients with triple-negative breast cancer. J Immunother. 2025;48:173–82.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 82303831 to Jian Wang, 82273323 to Xiaorong Dong).
Author information
Authors and Affiliations
Contributions
Yulan Kui wrote the draft manuscript and made the Figures and Tables; Fan Tong and Ruiguang Zhang revised the draft manuscript; Jian Wang and Xiaorong Dong conceived and funded the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Francesca Pentimalli
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kui, Y., Tong, F., Zhang, R. et al. Roles of the phagocytosis checkpoint in radiotherapy. Cell Death Dis 16, 630 (2025). https://doi.org/10.1038/s41419-025-07921-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41419-025-07921-5