Abstract
Diabetes mellitus is a metabolic disorder with persistent hyperglycemia caused by a variety of underlying factors. Chronic hyperglycemia can lead to diverse serious consequences and diversified complications, which pose a serious threat to patients. Among the major complications are cardiovascular disease, kidney disease, diabetic foot ulcers, diabetic retinopathy, and neurological disorders. Heme oxygenase 1 (HO-1) is a protective enzyme with antioxidant, anti-inflammatory and anti-apoptotic effects, which has been intensively studied and plays an important role in diabetic complications. By inducing the expression and activity of HO-1, it can enhance the antioxidant, anti-inflammatory, and anti-apoptotic capacity of tissues, and thus reduce the degree of damage in diabetic complications. The present study aims to review the relationship between HO-1 and the pathogenesis of diabetes and its complications. HO-1 is involved in the regulation of macrophage polarization and promotes the M1 state (pro-inflammatory) towards to the M2 state (anti-inflammatory). Induction of HO-1 expression in dendritic cells inhibits them maturation and secretion of pro-inflammatory cytokines and promotes regulatory T cell (Treg cell) responses. The induction of HO-1 can reduce the production of reactive oxygen species, thereby reducing oxidative stress and inflammation. Besides, HO-1 also has an important effect in novel programmed cell death such as pyroptosis and ferroptosis, thereby playing a protective role against diabetes. In conclusion, HO-1 plays a significant role in the occurrence and development of diabetic complications and is closely associated with a variety of complications. HO-1 is anticipated to serve as a novel target for addressing diabetic complications, and it holds promise as a potential therapeutic agent for diabetes and its associated complications. We hope to provide inspiration and ideas for future studies in the mechanism and targets of HO-1 through this review.
Similar content being viewed by others
Facts
-
Diabetic complications are various and the pathological mechanism is complex.
-
HO-1 is involved in a variety of pathological mechanisms of diabetic complications, especially inflammation and cell death caused by oxidative stress.
-
HO-1 induction also brings negative effects, inducing ferroptosis, CO toxicity.
-
Targeting HO-1 may provide new strategies for the prevention and treatment of diabetes.
Open questions
-
What are the therapeutic targets or strategies suitable for the various diabetic complications?
-
How does HO-1 interact or cross-regulation in different modes of cell death?
-
How to regulate the dual character of HO-1 in ferroptosis?
-
How to develop precise strategies for the toxicity of HO-1 targeted therapy?
Introduction
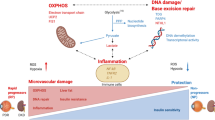
Diabetes mellitus (DM, abbreviations are defined in Table 1) is a major public health problem worldwide, which imposes heavy economic and medical burdens on patients and healthcare systems [1, 2]. The International Diabetes Federation predicted that the number of individuals inflicted with DM is slated to surpass 578 million globally by 2030, including both developed and developing countries. By 2045, the number of DM patients could soar to a staggering 783.2 million [3, 4]. The development of DM related complications has raised mortality rates and resulted in significant medical expenses. Diabetes patients frequently have a wide range of complications encompassing diabetic cardiovascular diseases, diabetic neuropathy, diabetic foot ulcers, diabetic retinopathy and diabetic nephropathy, which have a substantial negative influence on the physical and psychological health of these individuals (Fig. 1) [5]. Consequently, it is crucial to investigate the underlying causes and effective treatments for diabetes and its associated complications.
Heme oxygenase (HO) has three distinct isoenzymes, respectively namely heme oxygenase 1 (HO-1), heme oxygenase 2 (HO-2) and heme oxygenase 3 (HO-3) [6]. HO-1 is found in various tissue cells throughout the human body, which has the potential to regulate the onset and advancement of diabetic complications, such as cardiovascular disease, neuropathy, and renal disease [7,8,9]. HO-1 expression is mainly regulated by the activation of nuclear factor-erythroid2-related factor 2 (Nrf2). In the normal state of cells, Nrf2 binds to kelch-like ECH-associated protein-1 (Keap1) to form a complex, which exists in the cytoplasm. When cells are subjected to oxidative stress, Nrf2 dissociates from Keap1 and moves to the nucleus to bind to the promoter of HO-1, promoting the transcription and expression of HO-1 [10]. Its main function is to catalyze the combination of heme molecules with oxygen, resulting in the formation of oxyhemoglobin. Subsequently, it breaks down oxyhemoglobin into ferric ions, carbon monoxides (CO) and bilirubin (BR) in a process known as heme metabolism. Among them, heme, being essential for all oxygen-demanding organisms, is synthesized from protoporphyrin IX and ferrous ions [11]. Heme produces signaling molecules, such as CO, biliverdin (BV), BR and Fe2+, through the catalysis of HO-1. Each of these molecules exerts distinct effects on cellular function, both locally and distally, through individual molecular targets [12, 13]. BV, a breakdown product of HO-1, can potentially enhance the production of BR, a compound with the potential to prevent diabetes. This effect is achieved by increasing HO-1 activity and signaling to biliverdin reductase (BVR). Bilirubin is produced through a reaction catalyzed by both HO-1 and HO-2, which also simultaneously releases CO and Fe2+. NADH and NADPH serve as electron donors in this process. The conversion of bilirubin to BRHO-1, catalyzed by BVR-A, plays a role in defending the body against oxidative stress, diseases and injuries [14]. HO-1 and HO-2 are the products of two distinct genes, which are located in chromosome 22 for HO-1 and chromosome 16 for HO-2, respectively. HO-1 and HO-2 degrade heme in the same pattern, releasing biliverdin, CO, and Fe2+. In contrast to HO-1, the HO-2 isozyme is constitutively expressed, highly detectable in brain, testis, endothelial and smooth muscle cells from cerebral vessels. Most studies have shown that the presence of HO-2 indicates a crucial role in male reproductive system and brain related diseases, although some studies have found HO-2 in the prevention of kidney injury and diabetes. However, the HO-3 is thought to be a pseudogene processed from HO-2 transcripts, and its function is not known [15, 16].
Research has established an abnormal HO-1 expression among diabetic individuals, especially in those with complications like cardiovascular disease, neuropathy, nephropathy and diabetic foot ulcers [17, 18]. Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized by impaired insulin secretion, while type 2 diabetes mellitus (T2DM) is mainly caused by insufficient insulin secretion or insulin resistance. HO-1 in β cells and immature dendritic cells can delay autoimmune damage in pancreatic islet transplants and effectively maintain immune tolerance, which helps to delay the onset of T1DM in NOD mice model [19]. In type 2 diabetes, the reduced expression of HO-1 will increase the production of ROS, leading to oxidative stress and inflammatory reaction, further aggravating cell damage (Table 2). The studies mentioned above propose the possibility of the involvement of HO-1 in the control of both diabetes and its related complications.
The function of HO-1 in inflammatory reaction
In various experimental models of inflammation, HO-1 induction produces favorable effects. Inflammatory pathways have been implicated as potential pathogenic mediators of many diseases, including obesity, immune diseases, metabolic diseases and infections [20,21,22]. Indeed, inflammation has emerged as a crucial determining factor of the evolution of DM, as well as major complications and cardiovascular disease [23, 24]. The human inflammatory response is an intricate biological process that entails the interaction of various immune cells and molecules. The persistence of hyperglycemia in diabetes triggers a sequence of local metabolic, vascular, and neurochemical modifications, which ultimately culminate in vascular disease and microangiopathy [25]. In this process, a vital role in causing considerable harm to the Schwann cells and neurons in the peripheral nervous system is played by pro-inflammatory cytokines produced by cells that are resident or have infiltrated [26]. Macrophages regulate the inflammatory process depending on their differentiation status. Classical macrophages (M1) are responsible for triggering the inflammatory response via the secretion of pro-inflammatory cytokines and reactive oxygen species (ROS). Conversely, alternatively activated macrophages (M2) work towards inflammation resolution and promote tissue remodeling via the discharge of interleukin-10 (IL-10) and transforming growth factor β (TGF-β) [27, 28]. For instance, in a basic inflammatory response like infected tissues, macrophages typically undergo a sequential transition between the M1 and M2 phases. M1 macrophages play a key role in the initial stages of the inflammatory response by releasing considerable amounts of inflammatory agents such as tumor necrosis factor α (TNF-α), IL-6 and IL-12, which contribute to aggravating inflammation. Subsequently, M2 phenotype is polarized from macrophages and primarily responsible for tissue repair and anti-inflammatory pathways via producing mediators such as IL-10 and TGF-β [29]. However, the response is distinct in chronic inflammatory diseases such as atherosclerosis in diabetic mice and diabetic nephropathy [30, 31]. In these disorders, the coexistence of M1 and M2 macrophages results in the formation of complex cellular networks, which exacerbate persistent inflammation and fibrosis, further accelerating disease progression. Studies have demonstrated that induction of HO-1 expression in macrophages yields amplified antioxidant efficacy and mitigates the inflammatory aspect of atherosclerotic lesions. The lowered or nonexistent HO-1 expression within macrophages is linked to an increase in pro-inflammatory cytokine expression, namely monocyte chemotactic protein 1 (MCP-1) and IL-6. Furthermore, this association is also observed in scavenger receptor A expression, leading to the formation of foam cells [32,33,34]. Interestingly, polarization of M2 macrophages is also associated with HO-1 expression in diabetes [35, 36]. M2 macrophage polarization reduces the apoptosis of interstitial cells of Cajal in diabetic rats, which is mediated by the activation of Nrf2/HO-1 pathway [37]. At the same time, the activation of HO-1-related pathways promotes the polarization of M2 macrophages to improve diabetic-inflammatory response, oxidative stress, and cell proliferation have been verified by various of studies [38,39,40]. Although studies on the relationship between HO-1 and macrophages in diabetes or its complications are limited, the mechanisms by which macrophages maintain the microenvironment stability of the body and HO-1’s excellent antioxidant and anti-inflammatory properties suggest that targeting HO-1 with macrophages may be a treatment choice for diabetes (Fig. 2A).
A Induction of HO-1 facilitated the differentiation of macrophages into anti-inflammatory M1 and the release of IL-10. And inhibitedthe production of reactive oxygen species to reduce inflammation. B HO-1 expression in dendritic cells promotedtheir anti-inflammatory activity and inhibited differentiation of cytotoxic T cells. Induced expression of HO-1 proteindecreases the expression of pro-inflammatory TH1 cytokines shifted to a beneficial TH2 pattern and promotesantigen-specific Treg cell differentiation.
Other studies have confirmed that HO-1 obstructs the initiation of inflammation and ROS by directly regulating the activation of immune cells, including the antigen-presenting cells (APCs) and lymphocytes [41,42,43]. HO-1 was first demonstrated to block the maturation process of dendritic cells to exert a suppressive effect on inflammatory response in 2005 [44]. HO-1 expression was significantly reduced during dendritic cell maturation in vitro, which corresponds to the absence of HO-1 expression by mature dendritic cells in human tissues. Induction of HO-1 expression in dendritic cells can maintain their immature state, further preventing polarization of T cells towards to inflammatory phase (TH1 cells and TH17 cells) and promoting Treg cell responses [45,46,47]. Related findings have also been found in the treatment of diabetes. Compared with uninduced mice, HO-1 expression induced in dendritic cells showed a lower incidence of T1DM and reduced the risk of insulitis (Fig. 2B). Induction of HO-1 expression in dendritic cells also prevented further hyperglycemia in recently diabetic NOD mice [19]. HO-1 regulated immune balance by reducing the expression of pro-inflammatory TH1 cytokines towards a beneficial TH2 pattern, restoring Treg cell responses in diabetic mice and T2DM patients [48, 49]. These observations suggested that induction of HO-1 ameliorates detrimental inflammation in a variety of diseases, which also exhibited the preventive therapeutic approaches and management strategies of HO-1 expression for diabetes.
Another curative function of the HO-1 is to facilitate the conversion of heme into BV, Fe2+ and CO in equimolar quantities, thus promoting efficient antioxidant and anti-inflammatory effects [50]. The BV produced during these metabolic processes is rapidly transformed into BR through the action of BV reductase, which has a beneficial impact on numerous biological processes [51, 52]. HO-1 has been demonstrated to inhibit the inflammatory response via the simultaneous production of two anti-inflammatory molecules, CO and BR, as well as the removal of the pro-inflammatory factor heme [53]. The administration of hemin results in the boost of HO-1 expression and mitigates inflammatory responses in retinal ganglion cell injury in diabetic retinopathy, as well as diabetic wound healing, diabetic kidney function and [54,55,56]. CO can selectively impede the functioning of TNF-α, IL-1β and macrophage inflammatory proteins, which are pro-inflammatory cytokines, triggered by LPS [57, 58]. Meanwhile, CO actively promoted the production of the anti-inflammatory cytokine class IL-10 [59]. CO also suppressed the function of early growth response protein 1 (EGR-1), a crucial transcription factor in the inflammatory response initiated by macrophages [60]. Inflammation plays a pivotal role in exploring the pathophysiology of diabetic complications. The damage induced by hyperglycemia was generally regarded as results from a series of chain reactions triggered by inflammatory responses, leading to increased activity of the transcription factor NF-κB. This in turn enhances the activity of pro-inflammatory cytokines like TNF-α and leukocytes. Numerous studies have demonstrated that HO-1 has a significant effect on diabetic complications, with induction of HO-1 protein expression enhancing antioxidant defense and reducing inflammatory factors (Table 2). Notably, induction of HO-1 expression alleviated diabetic neuropathic pain and facilitated modulation of the diabetic nerve functions [61, 62]. The crucial role of HO-1 in safeguarding neurons against inflammation is also supported by related experiments with the Nrf2 inhibitor ML385 and si-HO-1 [63]. Therefore, HO-1, as a key factor in the inflammatory response, is expected to provide effective strategies for the prevention and treatment of diabetic complications and can provide novel ideas and drug targets for the prevention and treatment of clinical diseases.
The role of HO-1 in programmed cell death in diabetes mellitus
Cell death is a fundamental physiological process in living organisms, which can be programmed or triggered by accident. Programmed cell death is an active and orderly way of death determined by genes. When host cells are attacked by exogenous and endogenous stimulating factors, different signaling pathways lead to lysis or non-lytic morphology [64]. Apoptosis is a widely studied mild non-lytic mode of cell death characterized by a decrease in cell volume and fragmentation of the nucleus and involved in many diseases including diabetes [65]. Conversely, lytic cell death is highly inflammatory, such as pyroptosis, necroptosis, and necrotic extracellular traps (NETosis) [66].
Pyroptosis, a pro-inflammatory programmed cell death triggered by activated Caspase-1/Caspase-11, which can lead to inflammatory cytokines IL-18/IL-1β release to activate pro-inflammatory immune cell mediators. NLRP3 is an inflammasome sensor protein that can perceive various DAMPs (danger-associated molecular patterns) and PAMPs (pathogen-associated molecular patterns). DAMPs including mitochondrial dysfunction, ionic flux confusion, and the active oxygen generation, which trigger pyroptosis and have been implicated in the kidney pathogenesis of many diseases including diabetes. The ASC as a small intermediary protein between Caspase-1 and most inflammasomes; the PYD domain of ASC interacts with the PYD of inflammasomes, and its caspase activation domain and CARD domain interact with the CARD of Caspase-1 to form the NLRP3 complex. After the inflammatory complex activation of NLRP3, Caspase-1 is markedly activated by the NLRP3 complex to form active Caspase-1. The precursors of IL-18 and IL-1β are cleaved into biologically active mature IL-18 and IL-1β by activated Caspase-1 and secreted through the cell membrane pyrototic pores. On the other hand, gasdermin D is cleaved into N-terminus and C-terminus by activated Caspase-1. N-terminal fragments promote the formation of cell membrane pyroptotic pores, which further leads to pyroptosis [67, 68]. Pyroptosis plays an important role in the progression of many diseases, including diabetes and its complications. Studies have shown that the protein level of HO-1 was downregulated and NLRP3/Caspase-1 was significantly activated, while HO-1 induction could reverse the activation of these pyroptosis-related proteins. This mechanism was validated by dapagliflozin (hypoglycemic agents) and atorvastatin (hypolipidemic agents) treatment in diabetic mice kidney and PA/high glucose-induced podocytes [69, 70]. Syringaresinol is a natural plant-derived polyphenolic compound, triptolide as a complex triepoxide diterpene natural product, similar protective effects have been observed in diabetic kidney-related studies [71, 72]. HO-1 was also found to be involved in a protective effect by inhibiting pyroptosis in diabetic cardiomyopathy [73].
Ferroptosis is an innovative mechanism of cell death that is mostly caused by the deposition of lipid peroxides and unevenness in the regulation of iron levels due to disruption of intracellular metabolic pathways. Ferroptosis is significantly different from the previously known apoptosis or autophagy in cell morphology, in which cell membrane rupture does not occur during ferroptosis. The mitochondrial membrane became denser and the outer membrane appeared ruptured, the structure of the cristae disappeared and the volume decreased. However, the volume of the nucleus does not change significantly, there is a lack of chromatin condensation but the chromosome structure does not disappear, and the rupture of the cell membrane does not occur in the process of ferroptosis. Ferroptosis is biochemically characterized by the generation of ROS and iron overloading. ROS and intracellular iron accumulation are important processes that trigger the mitogen-activated protein kinase (MAPK) pathway, decrease intracellular glutathione (GSH) depletion, and limit cystine absorption. Meanwhile, the activity of the cysteine-glutamate transporter protein system XC- activity is inhibited, further exacerbating the process. Glutathione peroxidase 4 (GPx4), is the only enzyme in cells that can reduce lipid peroxides to normal phospholipid molecules, its activity is crucial for maintaining normal cell function and ferroptosis [74]. Inhibition of cysteine-glutamate transporter protein system (e.g., Erastin) will deplete intracellular GSH, eventually resulting in the inactivation of GPx4 and the accumulation of lipid peroxidation, which can induce cell ferroptosis to a certain extent [75]. Furthermore, cellular antioxidant capability is also decreased by suppressed GPx4 expression (e.g., RSL3), which results in lipid peroxidation and ferroptosis [76]. The molecular regulation and pharmacological mechanisms of ferroptosis in disease and therapy are of great significance, and increasing evidence suggests that ferroptosis is associated with the pathogenesis of diabetes-related complications (Table 2). Quercetin is an important flavonoid with a variety of protective properties, it has been shown that quercetin inhibits ferroptosis in renal tubular epithelial cells by activating the HO-1 signaling pathway to regulate the expression level of antioxidant enzymes and by reducing intracellular ROS production and iron overloading [77]. However, another study showed the process of ferroptosis may exacerbate proteinuria, damaging renal tubules and promoting renal fibrosis via the HIF-1α/HO-1 pathway in diabetes models. The levels of HIF-1α and HO-1 were raised in diabetic kidney tissue exacerbated tubular iron accumulation, enhanced the lipid peroxidation response, and augmented the generation of ROS. Ferrostatin-1 significantly alleviates renal tubular injury and renal scar formation in db/db mice by preventing ferroptosis and reducing HIF-1α and HO-1 [78]. Numerous studies have shown that vitamin D receptor plays a positive role by inhibiting ferroptosis [79,80,81]. Paricasitol as a VDR agonist can inhibit ferroptosis by activating VDR/Nrf2/HO‐1 signaling pathway in DN, which effectively attenuates iron deposition in renal tubular epithelial cells and renal damage after diabetic injury [82]. At the same time, the elevated HO-1 expression helps to scavenge free radicals such as peroxynitrite, which is achieved through the overexpression of BV, thus ultimately inhibiting the process of lipid peroxidation [83]. In conclusion, the relationship between HO-1 and programmed cell death in diabetes deserves further investigation (Fig. 3).
Heme is catalyzed by HO-1 to form CO, Fe2+, and BV, and BV forms BR under the catalysis of biliverdin reductase. Both BV and BR have cellular antioxidant activities. Iron can act as a pro-oxidant or can trigger the synthesis of ferritin and play a role as a synergistic cytoprotective agent. CO can regulate a variety of cellular processes such as inflammation, apoptosis. In addition, HO-1 is also involved in the process of pyroptosis and ferroptosis.
Role of HO-1 in regulating diabetic cardiomyopathy
Diabetic cardiovascular disease is the microvascular and macrovascular disease of the cardiovascular system caused by diabetes, which includes coronary heart disease, diabetic cardiovascular autonomic neuropathy, and diabetic cardiomyopathy [84]. Compared with non-diabetic patients, the incidence and mortality of cardiovascular disease in diabetic patients are higher [85]. The key risks of cardiovascular disease in diabetes are vascular endothelial dysfunction and impaired angiogenesis [86, 87]. Vascular dysfunction serves as the primary underlying cause of cardiovascular illness occurrence in patients experiencing anomalous glucose metabolism. The majority of diabetic fatalities arise from vascular impairments, including myocardial infarction and cerebrovascular afflictions. Insulin resistance, as well as the conditions of hyperinsulinemia, hyperglycemia, and elevated free fatty acids, play a substantial role in causing injury to cardiomyocytes, deteriorating their functionality, and leading to myocardial lipotoxicity in individuals with diabetes [88]. Diabetic cardiomyopathy increases mortality in diabetic patients by causing heart failure and is characterized by a multitude of interrelated systems that are influenced, including calcium homeostasis, renin-angiotensin system, protein kinase C signaling pathway, metabolism, mitochondria, fibrosis, and oxidative stress [89]. Interstitial fibrosis occurs after cardiomyocyte sclerosis, and collagen cross-links through late glycosylation products, ultimately leading to contractile dysfunction [90].
Induction of HO-1 expression is critical for preventing vascular dysfunction, endothelial cell death, and elevated ROS levels [91]. Moreover, research conducted in vitro has indicated the remarkable antioxidant properties of the bilirubin, which is derived from HO-1. Bilirubin has demonstrated the ability to enhance the protection of vascular endothelial cells, even when the concentration of this compound is in the nanomolar range. Bilirubin prohibits oxidative stress injury by directly scavenging reactive oxygen radicals and indirectly by restraining the activity of nitrogen compounds [92, 93]. HO-1 plays a significant function in vascular tissues by safeguarding endothelial cells from various apoptotic stimuli, thereby regulating endothelial cell cycle control, proliferation, vascular endothelial growth factor (VEGF) secretion, and angiogenesis [94, 95]. Furthermore, the increased expression of HO-1 in individuals suffering from heart failure serves to mitigate the adverse effects of pathological left ventricular remodeling, diminish myocardial hypertrophy, minimize oxidative stress, and abate inflammatory activation [96]. HO-1–/– mice were used to prove that the deletion of HO-1 would aggravate myocardial injury in ischemia/reperfusion, especially related to diabetes. Compared with the HO-1+/+ mice (21.4 ± 1.8%), the myocardial infarct area significantly reached at 36.4 ± 20% in HO-1 deficient mice. Furthermore, the specific induction of HO-1 had a protective effect in diabetic mice suffering from myocardial ischemic injury [97]. Research in mice lacking HO-1 has revealed the overall function of HO-1 in restoring functional macrophages, maintaining iron balance within tissues, and increasing tolerance to oxidative stress conditions [98,99,100]. The protective effects of HO-1 induction in cardiac tissues can be further demonstrated by prolonging the survival time of allogeneic cardiac grafts, decreasing the mortality rate, and preserving the left ventricle [101, 102]. Generally, HO-1 levels remain low across diverse tissues except for the spleen, but diverse stimuli (like hydrogen peroxide, UV radiation, endotoxins, and hypoxia) can cause a strong inducible response for shielding cells from damaging oxidative and inflammatory agents [34]. Though limited, research data proposes activation of HO-1 as a prospective approach to amplify endothelial cell longevity with HO-1 byproducts CO and bilirubin, which could postpone the emergence of diverse cardiovascular complications related to diabetes. However, these studies are still in the preliminary stage, and the specific mechanism of action and effects need further research and validation [91, 103]. Therefore, we can expect more studies to reveal the specific role of HO-1 in diabetic cardiomyopathy in the future.
In addition to its direct effects on the cardiovascular system, HO-1 may also indirectly affect the occurrence and development of cardiovascular diseases in diabetes by regulating other biological processes. For example, HO-1 can affect insulin sensitivity and glucose metabolism, thereby improving glycemic control in diabetic patients [104]. Furthermore, HO-1 can affect biological mechanisms such as inflammatory response and oxidative stress, both of which significantly contribute to the onset of cardiovascular diseases precipitated by diabetes. Besides, HO-1 improved cardiovascular function by regulating cellular signaling pathways and promoting angiogenesis, etc (Table 2).
AMPK is an energy receptor that is activated when cells are low on energy and regulate multiple metabolic pathways through phosphorylation to maintain energy homeostasis. In diabetic cardiomyopathy, energy metabolism in cardiomyocytes may be impaired due to factors such as hyperglycemia and insulin resistance. Therefore, induction of HO-1 may activate AMPK pathway by enhancing the phosphorylation, thereby improving energy metabolism in cardiomyocytes [105, 106]. Autophagy is an intracellular degradation process that provides energy and nutrition by degrading damaged organelles and macromolecules [107]. In diabetic cardiomyopathy, the autophagy pathway may be inhibited, which leads to the accumulation of harmful substances in cardiomyocytes. LC3-II and Beclin-1 are key molecules in the autophagy pathway, induction of HO-1 activates the autophagy pathway by increasing the expression of LC3-II and Beclin-1, thereby removing harmful substances from cardiomyocytes [108]. Cardiomyocyte apoptosis and cardiac fibrosis were increased in the mouse model of diabetic cardiomyopathy, the protein expression of Nrf2 and HO-1 was reduced in left ventricular cardiomyocytes. After treatment with salidroside, Nrf2, and HO-1 were significantly restored, cardiac apoptosis, hypertrophy, and fibrosis were also improved [109]. Myricetin is a naturally occurring flavonol with a strong antioxidant effect. Studies have shown that myricetin significantly increased the activity of the Nrf2/HO-1 pathway to enhance the resistance to oxidative stress, demonstrated the reversal of glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities, and reduced malondialdehyde (MDA) production [110]. High glucose-induced accumulation of persistent peroxidation in cardiomyocytes triggers ferroptosis and results in cell damage in H9C2 cells and New Zealand rabbits. Curcumin is a natural phenolic antioxidant, can increases Nrf2 transfer into the nucleus and promote HO-1 expression, reducing the excessive downregulation of GPx4 [111]. Besides, HO-1 was also involved in the protective effects of curcumin against pyroptosis, apoptosis, and oxidative stress in diabetic cardiomyopathy [73, 112].
In summary, the utilization of HO-1 as a multifunctional molecule shows remarkable promise in improving cardiovascular dysfunction, especially in diabetes-related cardiovascular diseases (Fig. 4). Its superior properties in terms of shielding cardiovascular well-being stem from its potential as both an antioxidant and anti-inflammatory agent. Despite the immense potential of HO-1 in mitigating the manifestation of diabetes-related cardiovascular disorders, there exists a dearth of studies that explore its precise action mechanism and applicability. By reviewing deeper into the mechanism of HO-1 functions, we hope to devise innovative therapeutic measures that are aimed at preventing and curing diabetes-associated cardiovascular complications, ultimately enhancing the well-being and prospects of the patients concerned.
HO-1 is involved in diabetic wound healing
Diabetes mellitus has a range of complications, among which diabetic foot ulcer (DFU) stands as a significant one. The diabetic environment often results in impaired wound healing processes for DFU, mainly attributed to excess oxidative stimulation, prolonged inflammation, dysfunction of immune cells, delayed reinnervation, and reduced angiogenesis at the wound site [113, 114]. The significance of neovascularization, inflammation, and apoptosis might imply that HO-1 plays a role in governing the process of wound healing [115].
Several studies have shown that hyperglycemia affects skin wound healing in diabetic rats [116, 117]. Induction of HO-1 reduced inflammatory cytokines, such as TNF-α and IL-6, increased antioxidants, and improved angiogenesis in wound tissues of diabetic rats, thereby wound healing was accelerated in the diabetic state [118]. Along with its angiogenic and cytoprotective enzyme functions, HO-1 performed crucial biological activities, making it highly significant in the wound healing process. Its enzymatic activity generates bioactive end products like CO, BV, and BR, which have a mediating effect on wound healing [119,120,121]. It was shown that increased HO-1-derived CO production has a vascular protective effect on T1DM, reduces endothelial cell fragmentation, and decreases ICAM-1, VCAM-1 expression, and Caspase-3 activity [122].
Diabetes patients exist in excessive oxidative stress and inflammation, these factors impair the response to skin damage [123]. HO-1 serves as a strong antioxidant that possesses anti-inflammatory as well as cell proliferation-promoting abilities. When the skin is damaged, the process of hemolysis releases hemoglobin (a pro-oxidant), which can further contribute to the induction of HO-1 [124]. Furthermore, HO-1 expression can be triggered by oxidative stress, inflammation, and hypoxia, and HO-1 and its products of enzymatic activity have the effect of reducing oxidative stress and pro-inflammatory factors. More importantly, they also promote cell viability, production of anti-inflammatory factors, as well as cell migration, proliferation, and angiogenesis, thus contributing to the healing process of diabetic wounds. A major factor contributing to chronic wound healing failure in diabetic patients is ROS overproduction and decreased antioxidant defenses [125]. The activation of HO-1 serves as a vital cellular defense mechanism against oxidative stress and possesses robust antioxidant properties. Hemin acts as a potent inductor of HO-1, which promotes the healing process through the reduction of oxidative stress as is evident by the diminished level of lipid peroxidation, and elevated levels of various antioxidant defense mechanisms, including GSH, SOD, GPx, and catalase [55, 120]. The presence of HO-1 significantly reduces the levels of inflammatory markers associated with diabetic wounds such as TNF-α, IL-1β, IL-6, iNOS, COX2, MCP-11, and MIP-1, while simultaneously boosting the levels of IL-10 [126].
HO-1 also plays an immunomodulatory effect in a variety of immune cells. In macrophages, increased HO-1 expression shows the ability to convert mobilized M1 macrophages into alternately activated M2 macrophages with anti-inflammatory effects, which is essential for the wound healing phase [127]. The process of wound healing requires the presence of angiogenesis. It is a commonly accepted fact that wounds in diabetic patients exhibit decreased levels of angiogenic factors, resulting in impairment of angiogenesis [128, 129]. HO-1 has the potential to stimulate the angiogenesis by elevating the expression of several pro-angiogenic factors including VEGF, translational growth factor-1 (TGF-1), and stromal-derived factor-1 (SDF-1) [130, 131]. As a methoxyindole secreted by the pineal gland, melatonin can increase the number of EPCs and enhance EPCS-mediated angiogenesis by induction HO-1 [132]. The anti-apoptotic properties of HO-1 have also been fully confirmed in several studies. Gynura divaricata (L.) DC. (GD), a traditional Chinese herbal medicine with hypoglycemic effects promotes angiogenesis and granulation tissue growth, which contribute to faster wound healing in diabetic SD rats. At the same time, GD improves HUVECs cells survival efficiency, reduces ROS generation and cell apoptosis, restores MMP, improves migration ability, and increases VEGF expression. These beneficial effects were abolished when inhibition was performed by using Nrf2-siRNA. It suggested that GD activates Nrf2 signaling pathway to increase HO-1 expression to regulate the expression of related proteins [133]. As a glucagon-like peptide-1 (GLP-1) receptor agonist used clinically to treat type 2 diabetes mellitus, liraglutide improves endothelial dysfunction caused by hyperglycemia, thereby preventing angiogenesis disorders in C57BLKs/J mice of wound model. Liraglutide also induction HO-1 expression by activating AMPK and promoting HIF-1α protein export from cytoplasm to nucleus, which significantly increases the expression of anti-apoptotic protein Bcl-2 and decreases the expression of pro-apoptotic Bax and Caspase-3 in HUVECs cells [134]. Turmeric-derived nanoparticles functionalized aerogel (TDNPs) can regulate the polarization balance between pro-inflammatory M1 and anti-inflammatory M2 in RAW 264.7 and restore macrophage-fibroblast communication network in L929 cells to ameliorate diabetes wound healing. Moreover, TDNPs have been verified to activate the Nrf2/HO-1 signaling pathway to promote the proliferation and migration of fibroblasts by improving their endogenous antioxidant capacity and reducing cell apoptosis [40]. Pyroptosis has also been confirmed to be involved in diabetic wounds, and cold atmospheric plasma can reduce major mediators NLRP3, Caspase-1, and IL-1β [58, 135]. However, there is no conclusive evidence to associate pyroptosis with HO-1 in diabetic wounds. Chronic hyperglycemia can induce circulating accumulation of lipid peroxidation products and impaired iron metabolic pathways, which results in the presence of a variety of free iron in plasma. Intracellular iron overload and accumulation of lipid peroxides are the characteristics of ferroptosis, so ferroptosis is considered as one of the potential mechanisms of delayed wound healing in diabetics [136, 137]. The mechanism of HO-1 in ferroptosis pathway of diabetic wounds has been rarely explored, and we believe that this is a work worthy of further investigation. We can excavate the value and significance of diabetic wound healing through a comprehensive and systematic study.
Significant progress has been made in improving tissue healing and regeneration, but treatment options for untreated patients with chronic diabetes remain limited. Regulation of the complex wound healing process has proved to be a challenging task in diabetic patients due to impaired cellular function due to an excessive oxidative and inflammatory environment. HO-1 is considered as a promising therapeutic approach because of its crucial role as a marker in wound healing phases. Regulating HO-1 activity could be an effective strategy in treating diabetic foot ulcers, given its significance in every stage of wound healing. This highlights the potential to exploit HO-1 as a valuable target for therapeutic intervention in DFU cases (Fig. 5).
Development and role of HO-1 in regulating diabetic nephropathy
Diabetic nephropathy (DN) is a widespread factor contributing to the morbidity and mortality in patients with T1DM and T2DM. End-stage diabetes is increasingly becoming one of the main causes of chronic renal failure, timely intervention of DN to prevent its development into end-stage renal disease has become a priority. DN is pathologically characterized by mesangial matrix thickening, progressive destruction of glomerular and tubulointerstitial, and loss of functioning glomeruli, ultimately leading to chronic renal failure [138, 139]. Various factors are involved in the progression of DN, such as disorder of oxygen metabolism and lipid metabolism, hyperglycemia, advanced glycosylation products, and inflammatory response [140, 141]. What makes sense is that persistent HO-1 stimulation diminishes hyperglycemia and enhances glucose metabolism while shielding renal tissues, partially or entirely, against hyperglycemic injuries. It is expected that HO-1’s antioxidant capabilities are accountable for this protection [142]. With multiple studies considering HO as a feasible target, the treatment of DN seems to have new prospects. There exist three distinct isoforms of HO referred to as HO-1, HO-2, and HO-3. HO-1 is renowned for its positive impacts including anti-apoptotic, antioxidant, anti-nitrosative, as well as anti-inflammatory effects against iNOS among three isoforms.
In various diseases, reduction of reactive oxygen species (ROS) accumulation by HO-1 induction. DM and its complications are significantly influenced by ROS. The role of ROS in the pathogenesis of DM, specifically in the development of DN, has been significantly highlighted [143]. When ROS accumulates excessively, it will cause oxidative stress damage to cells. ROS can cause oxidative damage to unsaturated fatty acids, proteins, DNA, and other important molecules in cells. In turn, these damaged cells are the targets of elimination by the immune system, which results in the production of endogenous injury-related molecular patterns and the release of cytokines. Inflammation is usually triggered by these dangerous molecular patterns and factors [144]. A variety of drugs have been reported to alleviate renal tissue damage by reducing ROS and inflammation, most of which is achieved through the Nrf2/HO-1 signaling pathway (Table 2). Shenkang injection is an active ingredient extracted from four medicinal plants: Radix et Rhizoma Salviae Miltiorrhizae, Radix Astragali, Flos Carthami, Radix et Rhizoma Rhei. Shenkang injection can improve the kidney function of diabetic SD rats by increasing the expression of antioxidant enzyme GPx4 and promoting the activation of antioxidant system. The molecular mechanism may be via the Keap1/Nrf2/HO-1 axis modulation [10]. Baicalin, a flavonoid, effectively increases the levels of GSH-PX, SOD, and catalase, inhibits the infiltration of inflammatory cells such as T-lymphocytes, T-helper cells, neutrophils, and macrophages, as well as the mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, MCP-1, and TNF-α) [145]. The relief of oxidative stress and inflammation in kidney of C57BLKs/J mice is due to the activation of Nrf2/HO-1 and MAPK signaling pathways. Notoginsenoside R1 promoted HO-1 expressions to reduce oxidative stress-induced apoptosis and kidney fibrosis in HK-2 cells and C57BL/6J mice [146]. As described above, AMPK are sensors and protectors of cellular energy needs. Echinochrome A, a natural bioproduct extracted from sea urchin, protects mitochondrial function against oxidative stress damage by activating AMPK phosphorylation and regulating the AMPKα/Nrf2/HO-1 pathway [147].
Diabetic renal fibrosis is stimulated by multiple factors such as inflammation, which forms a large amount of improper proliferation and excessive deposition of collagen fibers. In the process of development, there will be renal tubular atrophy, glomerular sclerosis, and microvascular sparse, forming a vicious circle and finally, the kidney completely loses its organ function [148]. HO-1 plays an important role in diabetic kidney fibrosis (Table 2). By regulating the activity or expression of HO-1, it may provide a new strategy for the treatment of diabetic renal fibrosis. Astaxanthin reduces the expression of FN, ICAM-1, and TGF-β1 induced by HG to reduce ECM deposition, and significantly promotes the nuclear translocation and transcriptional activity of Nrf2, and induction the expression of HO-1 [149, 150]. In another in vitro and in vivo study, Nrf2/HO-1 was also demonstrated to play an important inhibitory role in the pro-fibrotic pathway activated by TGF-β1, which was verified by oligo-fucoidan in NRK-52E cells and kidney tissues of C57BL/6 mice [151].
Current studies have unraveled that programmed cell death has a notable effect on DN progression. Apoptosis is a widely explored mechanism of programmed cell death, and apoptosis of renal tissue is a key feature of diabetic kidney injury [152]. HO-1 as an important regulator of oxidative stress and inflammation, also plays an important role in diabetic kidney apoptosis. HO-1 was involved in the protective effect of telmisartan against DN, and the mRNA levels regulation of Nrf2 and HO-1 indicated that telmisartan had a regulatory effect on apoptosis besides anti-inflammation and anti-oxidation [153]. In addition to the excellent therapeutic effect on diabetic cardiomyopathy, sinapic acid also ameliorated the expression of apoptosis-related proteins in STZ-induced Wistar rats’ diabetic kidneys via Nrf2/HO-1 mediated pathways [154]. Pyroptosis is an important programmed cell death pattern that can be activated by DAMPs and PAMPs. Dapagliflozin can decrease podocyte pyroptosis mediated through the miR-155-5p/HO-1/NLRP3 signaling pathway, and induction of HO-1 can promote the inhibitory effect of dapagliflozin on pyroptosis [69]. Similar protective effects on podocyte pyroptosis mediated by HO-1 were proved by multiple drugs, including atorvastatin, triptolide. A study based on Nrf2-KO (knockout of Nrf2) diabetic mice showed that syringaresinol inhibits pyroptosis caused by NLRP3/Caspase-1 in DN by activating the Nrf2/HO-1 signaling pathway [72]. Ferroptosis is a programmed cell death with iron-dependent accumulation of lipid peroxides, which has recently been identified in animal models of DN [155]. After ferroptosis was inhibited by ferrostatin-1 treatment in C57BLKs/J mice, urinary albumin to creatinine ratio was decreased, kidney tubular injury and kidney fibrosis were improved, HIF-1α and HO-1 expression were suppressed [78]. In HK-2 cells and C57BL/KsJ mice, the kidney jury restoration after ferroptosis inhibition via the Nrf2/HO-1 signaling pathway has been sufficiently confirmed in the research on the therapeutic mechanism of quercetin, vitamin D receptor, umbelliferone, etc [77, 82, 156]. In SV40-MES 13 cells, HMGB1 (high-mobility group box-1) has been recognized as a valuable factor of ferroptosis based on the Nrf2/HO-1 signaling pathway, proposing that HMGB1 is a new target for the treatment of DN [157]. This indicated that HO-1 exhibits excellent benefits in ferroptosis of DN since it is an important downstream target affected by HGBM1.
Thus, HO-1 plays significant part in regulating the progression and application of DN (Fig. 6). Through a deeper knowledge of the biological function and regulatory mechanism of HO-1, it can provide groundbreaking ideas and strategies for the therapy of diabetic nephropathy. Nonetheless, the utilization of HO-1 target in the treatment of diabetic nephropathy is still in the examination stage and requires further clinical trials for its efficacy and safety. We hope that in the future, more researchers will focus on more basic research on HO-1 in DN, including its expression regulation, mechanism of action, and interaction with other molecules. Based on these researches, clinical trials targeting HO-1 should be carried out to evaluate its application in the treatment of DN, so as to provide evidence for clinical application.
Protective role of HO-1 in other complications of diabetes
Diabetic retinopathy (DR) is a series of retinal microvascular diseases caused by DM, which has a great impact on vision and even blindness in the late stage. An increasing number of clinical and laboratory studies have revealed the pathophysiological changes of DR [158]. The protective effect of HO-1 in diabetic retinopathy is not only reflected in the inhibition of inflammation and oxidative stress but also promoted microglia polarization from the M1 state (pro-inflammatory) towards the M2 state (anti-inflammatory) [159]. And HO-1 related to programmed cell death such as apoptosis and ferroptosis in DR was also revealed by hydroxysafflor yellow A(a single chalcone glycoside), maresin-1 (pro-resolving lipid mediator), amygdalin (natural cyanogenic glycoside) [160, 161]. Multiple studies have shown that diabetes is associated with cognitive impairment, in which HO-1 as an important antioxidant enzyme, plays an important role in protecting against apoptosis, inflammation, and oxidative stress in diabetic cognitive dysfunction [162,163,164]. HO-1 is also involved in the protection of mitochondrial dysfunction and oxidative stress in diabetic Alzheimer's [165, 166]. What’s more, HO-1 can halt ferroptosis related to diabetes, such as diabetes with sepsis, diabetes-induced liver injury, diabetic encephalopathy, and diabetic islet transplantation [167,168,169,170]. Through in-depth study of the mechanism of HO-1, it is expected to provide new strategies and methods for the treatment of diabetes complications.
Conclusion
DM and its associated complications are significant maladies that affect human health, which pose a serious threat to the wellness of the global population. The persistent intricacies of diabetes stem from various metabolic pathway disorders, ultimately resulting in substantial health risks and danger of death associated with the condition. The HO-1 plays a crucial role in process of triggering and advancing diabetes and its associated complications. In this investigation, the function and correlation that HO-1 with diabetes and its complications have been comprehensively examined. Studies have shown that HO-1 may be involved in the development of diabetic complications like diabetic kidney, diabetic cardiovascular disease, and diabetic wound healing. Most studies have focused on HO-1 affecting diabetic complications through its antioxidant, anti-apoptotic, and anti-inflammatory effects, but HO-1 has also shown a role in programmed cell death that cannot be ignored. Further research is essential to thoroughly investigate HO-1’s regulatory mechanisms, its interaction with other transcription factors, and its functions across varying kinds and phases of diabetes. Further, there is a need to concentrate on examining the function and correlation of HO-1 in diabetes and its complications, which will pave the way for innovative approaches to avoid and treat diabetes. However, HO-1 induction also brings negative effects. Induction of HO-1 causes abnormal accumulation of ROS due to Fe2+ accumulation, inducing ferroptosis. It also causes cellular CO toxicity and bilirubin encephalopathy. These findings suggest that HO-1 is not merely protective in disease and requires a comprehensive understanding.
In conclusion, exploring the new molecular mechanism of HO-1 in the treatment of diabetes can provide new methods and ideas for the diagnosis and treatment of diabetes and related diseases, and offer the molecular theoretical foundation for the development of new hypoglycemic drugs.
References
Balooch Hasankhani M, Mirzaei H, Karamoozian A. Global trend analysis of diabetes mellitus incidence, mortality, and mortality-to-incidence ratio from 1990 to 2019. Sci Rep 2023;13:21908.
Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol 2020;18:104–9.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diab Res Clin Pr 2022;183:109119.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diab Res Clin Pr 2019;157:107843.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88–98.
Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biophys Biochem Res Commun 2005;338:558–67.
Negi G, Nakkina V, Kamble P, Sharma SS. Heme oxygenase-1, a novel target for the treatment of diabetic complications: focus on diabetic peripheral neuropathy. Pharm Res. 2015;102:158–67.
Dou Y, Ai G, Huang R, Huang Z, Li Y, Liu Y, et al. In vitro and in vivo hypoglycemia effect of oxyberberine, a novel HO-1 agonist: A renewed evidence linking HO-1 to diabetes mellitus. Phytomedicine 2022;101:154135.
Ryter SW. Heme oxygenase-1: an anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants. 2022;11:555.
Liu Y, Wang S, Jin G, Gao K, Wang S, Zhang X, et al. Network pharmacology-based study on the mechanism of ShenKang injection in diabetic kidney disease through Keap1/Nrf2/Ho-1 signaling pathway. Phytomedicine 2023;118:154915.
Gell DA. Structure and function of haemoglobins. Blood Cell Mol Dis. 2018;70:13–42.
Rochette L, Zeller M, Cottin Y, Vergely C. Redox functions of heme oxygenase-1 and biliverdin reductase in diabetes. Trends Endocrinol Met 2018;29:74–85.
Takeda T-A, Sasai M, Adachi Y, Ohnishi K, Fujisawa J-i, Izawa S, et al. Potential role of heme metabolism in the inducible expression of heme oxygenase-1. BBA-Gen Subj 2017;1861:1813–24.
Sassa S. Why heme needs to be degraded to iron, biliverdin IXα, and carbon monoxide? Antioxid Redox Signal 2004;6:819–24.
Intagliata S, Salerno L, Ciaffaglione V, Leonardi C, Fallica AN, Carota G, et al. Heme oxygenase-2 (HO-2) as a therapeutic target: activators and inhibitors. Eur J Med Chem. 2019;183:111703.
Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, et al. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004;336:241–50.
Facchinetti MM. Heme-oxygenase-1. Antioxid Redox Signal 2020;32:1239–42.
Pittala V, Vanella L, Salerno L, Romeo G, Marrazzo A, Di Giacomo C, et al. Effects of polyphenolic derivatives on heme oxygenase-system in metabolic dysfunctions. Curr Med Chem 2018;25:1577–95.
Pogu J, Tzima S, Kollias G, Anegon I, Blancou P, Simon T. Genetic restoration of heme oxygenase-1 expression protects from type 1 diabetes in NOD mice. Int J Mol Sci 2019;20:1676.
Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–35.
Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med 2019;18:121–6.
Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol 2020;320:C375–C91.
Lee SH, Lee TW, Ihm CG, Kim MJ, Woo JT, Chung JH. Genetics of diabetic nephropathy in type 2 DM: candidate gene analysis for the pathogenic role of inflammation. Nephrology 2005;10:S32–S6.
Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ 2018;27:1327–34.
Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab 2001;12:225–30.
Kornelius E, Tsou SH, Chang CC, Ho YJ, Lin SC, Chen WL, et al. Liraglutide attenuates glucolipotoxicity-induced RSC96 schwann cells’ inflammation and dysfunction. Biomolecules 2022;21:1338.
Wang S, Cao M, Xu S, Shi J, Mao X, Yao X, et al. Luteolin alters macrophage polarization to inhibit inflammation. Inflammation 2020;43:95–108.
Faas M, Ipseiz N, Ackermann J, Culemann S, Grüneboom A, Schröder F, et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity 2021;54:2531–46.e5.
Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol 2020;877:173090.
Landis CR, Quimby RK, Greenidge RA. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr Pharm Des 2018;24:2241–9.
Josefs T, Barrett TJ, Brown EJ, Quezada A, Wu X, Voisin M, et al. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight 2020;5:e134796.
Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, et al. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res 2007;100:1703–11.
Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY, Yang LY, et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients 2021;14:67.
Wu ML, Ho YC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. Am J Cardiovasc Dis 2011;1:150–8.
Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys 2014;564:83–8.
Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis—the missing link? Neurogastroent Motil 2015;27:7–18.
Wang H, Zhao K, Ba Y, Gao T, Shi N, Niu Q, et al. Gastric electrical pacing reduces apoptosis of interstitial cells of Cajal via antioxidative stress effect attributing to phenotypic polarization of M2 macrophages in diabetic rats. Oxid Med Cell Longev. 2021;2021:1298657.
Oh YJ, Jin SE, Shin HK, Ha H. Daeshiho-tang attenuates inflammatory response and oxidative stress in LPS-stimulated macrophages by regulating TLR4/MyD88, NF-κB, MAPK, and Nrf2/HO-1 pathways. Sci Rep 2023;13:18891.
Sha W, Zhao B, Wei H, Yang Y, Yin H, Gao J, et al. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway. Phytomedicine 2023;112:154667.
Wu B, Pan W, Luo S, Luo X, Zhao Y, Xiu Q, et al. Turmeric-derived nanoparticles functionalized aerogel regulates multicellular networks to promote diabetic wound healing. Adv Sci 2024;11:2307630.
Li BZ, Guo B, Zhang HY, Liu J, Tao SS, Pan HF, et al. Therapeutic potential of HO-1 in autoimmune diseases. Inflammation 2014;37:1779–88.
Campbell NK, Williams DG, Fitzgerald HK, Barry PJ, Cunningham CC, Nolan DP, et al. Trypanosoma brucei secreted aromatic ketoacids activate the Nrf2/HO-1 pathway and suppress pro-inflammatory responses in primary murine glia and macrophages. Front Immunol 2019;10:2137.
Franchina DG, Dostert C, Brenner D. Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol 2018;39:489–502.
Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer SV, Hubert FO-X, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005;106:1694–702.
George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, et al. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Dance Ther 2008;173:154–60.
Campbell NK, Fitzgerald HK, Malara A, Hambly R, Sweeney CM, Kirby B, et al. Naturally derived heme-oxygenase 1 inducers attenuate inflammatory responses in human dendritic cells and T cells: relevance for psoriasis treatment. Sci Rep 2018;8:10287.
Listopad J, Asadullah K, Sievers C, Ritter T, Meisel C, Sabat R, et al. Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Exp Derm 2007;16:661–70.
Mahmoud FF, Haines D, Dashti AA, El-Shazly S, Al-Najjar F. Correlation between heat shock proteins, adiponectin, and T lymphocyte cytokine expression in type 2 diabetics. Cell Stress Chaperones 2018;23:955–65.
Song F, Chen W, Jia W, Yao P, Nussler AK, Sun X, et al. A natural sweetener, Momordica grosvenori, attenuates the imbalance of cellular immune functions in alloxan-induced diabetic mice. Phytother Res 2006;20:552–60.
Ali MAM, Heeba GH, El-Sheikh AAK. Modulation of heme oxygenase-1 expression and activity affects streptozotocin-induced diabetic nephropathy in rats. Fund Clin Pharmacol 2017;31:546–57.
Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol 2010;49:186–95.
Yao Q, Lan QH, Jiang X, Du CC, Zhai YY, Shen X, et al. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics 2020;10:11719–36.
Ahanger, Prawez AA, Kumar S, Prasad D, Amarpal R, Tandan SK, et al. Wound healing activity of carbon monoxide liberated from co-releasing molecule (CO-RM). Naunyn Schmiedeberg Arch Pharmacol 2011;384:93–102.
Fan J, Xu G, Jiang T, Qin Y. Pharmacologic Induction of Heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Investig Ophth Vis Sci 2012;53:6541–56.
Kumar D, Jena GR, Ram M, Lingaraju MC, Singh V, Prasad R, et al. Hemin attenuated oxidative stress and inflammation to improve wound healing in diabetic rats. Naunyn Schmiedeberg Arch Pharmacol 2019;392:1435–45.
Ndisang JF, Jadhav A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology 2014;155:215–29.
Kwak HJ, Song JS, No ZS, Song JH, Yang SD, Cheon HG. The inhibitory effects of roflumilast on lipopolysaccharide-induced nitric oxide production in RAW264.7 cells are mediated by heme oxygenase-1 and its product carbon monoxide. Inflamm Res 2005;54:508–13.
Qin S, Du R, Yin S, Liu X, Xu G, Cao W. Nrf2 is essential for the anti-inflammatory effect of carbon monoxide in LPS-induced inflammation. Inflamm Res 2015;64:537–48.
Pae HO, Lee CY, Chung HT. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm 2008;2:159–65.
Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, et al. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci USA 2006;103:5191–6.
Li M, Li Q, Zhao Q, Zhang J, Lin J. Luteolin improves the impaired nerve functions in diabetic neuropathy: behavioral and biochemical evidences. Int J Clin Exp Pathol 2015;8:10112–20.
Khan A, Wang F, Shal B, Khan AU, Zahra SS, Haq IU, et al. Anti-neuropathic pain activity of ajugarin-i via activation of Nrf2 signaling and inhibition of TRPV1/TRPM8 nociceptors in STZ-induced diabetic neuropathy. Pharm Res 2022;183:106392.
Zhao P, Wei Y, Sun G, Xu L, Wang T, Tian Y, et al. Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway. J Neuroinflammation Amm 2022;19:269.
Kopeina GS, Zhivotovsky B. Programmed cell death: past, present and future. Biochem Biophys Res Commun 2022;633:55–8.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516.
Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol 2017;17:151–64.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 2017;42:245–54.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015;526:660–5.
Zhang Z, Tang M, Liu W, Song Y, Gao M, Ni P, et al. Dapagliflozin prevents kidney podocytes pyroptosis via miR-155-5p/HO-1/NLRP3 axis modulation. Int Immunopharmacol 2024;131:111785.
Zuo Y, Chen L, He X, Ye Z, Li L, Liu Z, et al. Atorvastatin regulates MALAT1/miR-200c/NRF2 activity to protect against podocyte pyroptosis induced by high glucose. Diab Metab Syndr Obes 2021;14:1631–45.
Lv C, Cheng T, Zhang B, Sun K, Lu K. Triptolide protects against podocyte injury in diabetic nephropathy by activating the Nrf2/HO-1 pathway and inhibiting the NLRP3 inflammasome pathway. Ren Fail 2023;45:2165103.
Li G, Liu C, Yang L, Feng L, Zhang S, An J, et al. Syringaresinol protects against diabetic nephropathy by inhibiting pyroptosis via NRF2-mediated antioxidant pathway. Cell Biol Toxicol. 2023;39:621–39.
Wei Z, pinfang K, jing Z, zhuoya Y, Shaohuan Q, Chao S. Curcumin improves diabetic cardiomyopathy by Inhibiting pyroptosis through AKT/Nrf2/ARE pathway. Mediat Inflamm 2023;2023:3906043.
Li J, Cao F, Yin H-l, Huang Z-j, Lin Z-t, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis 2020;11:88.
Li Y, Zeng X, Lu D, Yin M, Shan M, Gao Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum Reprod 2021;36:951–64.
Zhu W, Liu D, Lu Y, Sun J, Zhu J, Xing Y, et al. PHKG2 regulates RSL3-induced ferroptosis in Helicobacter pylori related gastric cancer. Arch Biochem Biophys 2023;740:109560.
Feng Q, Yang Y, Qiao Y, Zheng Y, Yu X, Liu F, et al. Quercetin ameliorates diabetic kidney injury by inhibiting ferroptosis via activating Nrf2/HO-1 signaling pathway. Am J Chin Med 2023;51:997–1018.
Feng X, Wang S, Sun Z, Dong H, Yu H, Huang M, et al. Ferroptosis enhanced diabetic renal tubular injury via HIF-1α/HO-1 pathway in db/db mice. Front Endocrinol 2021;12:626390.
Xu P, Lin B, Deng X, Huang K, Zhang Y, Wang N. VDR activation attenuates osteoblastic ferroptosis and senescence by stimulating the Nrf2/GPX4 pathway in age-related osteoporosis. Free Radic Bio Med 2022;193:720–35.
Hu Z, Zhang H, Yi B, Yang S, Liu J, Hu J, et al. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis 2020;11:73.
Li L, Li WJ, Zheng XR, Liu QL, Du Q, Lai YJ, et al. Eriodictyol ameliorates cognitive dysfunction in APP/PS1 mice by inhibiting ferroptosis via vitamin D receptor-mediated Nrf2 activation. Mol Med 2022;28:11.
Wang H, Yu X, Liu D, Qiao Y, Huo J, Pan S, et al. VDR activation attenuates renal tubular epithelial cell ferroptosis by regulating Nrf2/HO-1 signaling pathway in diabetic nephropathy. Adv Sci 2024;11:2305563.
Gordon DM, Adeosun SO, Ngwudike SI, Anderson CD, Hall JE, Hinds TD, et al. CRISPR cas9-mediated deletion of biliverdin reductase A (BVRA) in mouse liver cells induces oxidative stress and lipid accumulation. Arch Biochem Biophys. 2019;672:108072.
Almourani R, Chinnakotla B, Patel R, Kurukulasuriya LR, Sowers J. Diabetes and cardiovascular disease: an update. Curr Diab Rep 2019;19:161.
Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21:21.
Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharm Sin 2019;40:1–8.
Gu J, Wang S, Guo H, Tan Y, Liang Y, Feng A, et al. Inhibition of p53 prevents diabetic cardiomyopathy by preventing early-stage apoptosis and cell senescence, reduced glycolysis, and impaired angiogenesis. Cell Death Dis 2018;9:82.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018;61:21–8.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy. Circ Res 2018;122:624–38.
Khan S, Ahmad SS, Kamal AM. Diabetic cardiomyopathy: from mechanism to management in a nutshell. Endocr Metab Immune 2021;21:268–81.
Maamoun H, Zachariah M, McVey JH, Green FR, Agouni A. Heme oxygenase (HO)-1 induction prevents endoplasmic reticulum stress-mediated endothelial cell death and impaired angiogenic capacity. Biochem Pharmacol 2017;127:46–59.
Maamoun H, Benameur T, Pintus G, Munusamy S, Agouni A. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Front Physiol 2019;10:70.
Liu J, Wang L, Tian XY, Liu L, Wong WT, Zhang Y, et al. Unconjugated bilirubin mediates heme oxygenase-1–induced vascular benefits in diabetic mice. Diabetes 2014;64:1564–75.
Zhong ZY, Tang Y. Upregulation of periostin prevents high glucose-induced mitochondrial apoptosis in human umbilical vein endothelial cells via activation of Nrf2/HO-1 signaling. Cell Physiol Biochem 2016;39:71–80.
Luo Y, Lu S, Dong X, Xu L, Sun G, Sun X. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis 2017;22:1013–24.
Collino M, Pini A, Mugelli N, Mastroianni R, Bani D, Fantozzi R, et al. Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Dis Model Mech 2013;6:1012–20.
Liu X, Wei J, Peng DH, Layne MD, Yet S-F. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes 2005;54:778–84.
Wang H, Siren J, Perttunen S, Immonen K, Chen Y-C, Narumanchi S, et al. Deficiency of heme oxygenase 1a causes detrimental effects on cardiac function. J Cell Mol Med 2024;28:e18243.
Kovtunovych G, Ghosh MC, Ollivierre W, Weitzel RP, Eckhaus MA, Tisdale JF, et al. Wild-type macrophages reverse disease in heme oxygenase 1-deficient mice. Blood 2014;124:1522–30.
Yachie A. Heme oxygenase-1 deficiency and oxidative stress: a review of 9 independent human cases and animal models. Int J Mol Sci 2021;22:1514.
Evans JM, Navarro S, Doki T, Stewart JM, Mitsuhashi N, Kearns-Jonker M. Gene transfer of heme oxygenase-1 using an adeno-associated virus serotype 6 vector prolongs cardiac allograft survival. J Transplant 2012;2012:740653.
Liu X, Simpson JA, Brunt KR, Ward CA, Hall SRR, Kinobe RT, et al. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol Heart C 2007;293:H48–H59.
Bellner L, Lebovics NB, Rubinstein R, Buchen YD, Sinatra E, Sinatra G, et al. Heme oxygenase-1 upregulation: a novel approach in the treatment of cardiovascular disease. Antioxid Redox Signal 2019;32:1045–60.
Ndisang JF, Jadhav A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. Am J Physiol Endocrinol M 2009;296:E829–E41.
Xu G, Ma Y, Jin J, Wang X. Activation of AMPK/p38/Nrf2 is involved in resveratrol alleviating myocardial ischemia-reperfusion injury in diabetic rats as an endogenous antioxidant stress feedback. Ann Transl Med 2022;10:890.
Zhao C, Zhang Y, Liu H, Li P, Zhang H, Cheng G. Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem Biophys Res Commun 2017;490:552–9.
Ge X, Wang L, Fei A, Ye S, Zhang Q. Research progress on the relationship between autophagy and chronic complications of diabetes. Front Physiol 2022;13:956344.
Zhao Y, Zhang L, Qiao Y, Zhou X, Wu G, Wang L, et al. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. Plos One 2013;8:e75927.
Ni J, Li Y, Xu Y, Guo R. Salidroside protects against cardiomyocyte apoptosis and ventricular remodeling by AKT/HO-1 signaling pathways in a diabetic cardiomyopathy mouse model. Phytomedicine 2021;82:153406.
Liao HH, Zhu JX, Feng H, Ni J, Zhang N, Chen S, et al. Myricetin possesses potential protective effects on diabetic cardiomyopathy through Inhibiting IκBα/NFκB and enhancing Nrf2/HO-1. Oxid Med Cell Longev 2017;2017:8370593.
Wei Z, Shaohuan Q, Pinfang K, Chao S. Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovasc Ther 2022;2022:3159717.
Wu X, Zhou X, Lai S, Liu J, Qi J. Curcumin activates Nrf2/HO-1 signaling to relieve diabetic cardiomyopathy injury by reducing ROS in vitro and in vivo. FASEB J 2022;36:e22505.
Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, et al. Diabetic foot ulcer classifications: a critical review. Diabetes Metab Res Rev 2020;36:e3272.
Dixon D, Edmonds M. Managing diabetic foot ulcers: pharmacotherapy for wound healing. Drugs 2021;81:29–56.
Grochot-Przeczek A, Dulak J, Jozkowicz A. Heme oxygenase-1 in neovascularisation: a diabetic perspective. Thromb Haemost 2010;104:424–31.
Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 2014;31:817–36.
Chen VY, Siegfried LG, Tomic-Canic M, Stone RC, Pastar I. Cutaneous changes in diabetic patients: primed for aberrant healing? Wound Repair Regen 2023;31:700–12.
Chen QY, Wang GG, Li W, Jiang YX, Lu XH, Zhou PP. Heme oxygenase-1 promotes delayed wound healing in diabetic rats. J Diab Res 2016;2016:9726503.
Fan J, Liu H, Wang J, Zeng J, Tan Y, Wang Y, et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J Cell Mol Med 2021;25:652–65.
Ram M, Singh V, Kumar D, Kumawat S, Gopalakrishnan A, Lingaraju MC, et al. Antioxidant potential of bilirubin-accelerated wound healing in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 2014;387:955–61.
Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 2010;9:728–43.
Rodella FL, Vanella L, Peterson JS, Drummond G, Rezzani R, Falck RJ, et al. Heme oxygenase-derived carbon monoxide restores vascular function in type 1 diabetes. Drug Metab Lett 2008;2:290–300.
Tu C, Lu H, Zhou T, Zhang W, Deng L, Cao W, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022;286:121597.
Krzyszczyk P, Kang HJ, Kumar S, Meng Y, O’Reggio MD, Patel K, et al. Anti-inflammatory effects of haptoglobin on LPS-stimulated macrophages: role of HMGB1 signaling and implications in chronic wound healing. Wound Repair Regen 2020;28:493–505.
Cano Sanchez M, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants 2018;7:98.
Leal EC, Carvalho E. Heme oxygenase-1 as therapeutic target for diabetic foot ulcers. Int J Mol Sci 2022;23:12043.
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 2018;9:419.
Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu Z, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv 2022;29:214–28.
Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M, et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv 2021;7:eabj0153.
Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, et al. Stromal cell–derived factor 1 promotes angiogenesis via a heme oxygenase 1–dependent mechanism. J Exp Med 2007;204:605–18.
Badr, El-Hossary G, Lasheen FM, FE-dM, Negm NZ, Khalaf M, et al. Cold atmospheric plasma induces the curing mechanism of diabetic wounds by regulating the oxidative stress mediators iNOS and NO, the pyroptotic mediators NLRP-3, caspase-1 and IL-1β and the angiogenesis mediators VEGF and Ang-1. Biomed Pharmacother 2023;169:115934.
Kuo CS, Chen CY, Huang HL, Tsai HY, Chou RH, Wei JH, et al. Melatonin improves ischemia-induced circulation recovery impairment in mice with streptozotocin-induced diabetes by improving the endothelial progenitor cells functioning. Int J Mol Sci 2022;23:9839.
Xu C, Hu L, Zeng J, Wu A, Deng S, Zhao Z, et al. Gynura divaricata (L.) DC. promotes diabetic wound healing by activating Nrf2 signaling in diabetic rats. J Ethnopharmacol 2024;323:117638.
Huang H, Wang L, Qian F, Chen X, Zhu H, Yang M, et al. Liraglutide via activation of AMP-activated protein kinase-hypoxia inducible factor-1α-heme oxygenase-1 signaling promotes wound healing by preventing endothelial dysfunction in diabetic mice. Front Physiol 2021;12:660263.
Hasan Maleki M, Siri M, Jafarabadi A, Rajabi M, Amirhossein Mazhari S, Noori Z, et al. Boosting wound healing in diabetic rats: the role of nicotinamide riboside and resveratrol in UPR modulation and pyroptosis inhibition. Int Immunopharmacol 2024;132:112013.
Zhou D, Liang Q, Ge X, Xu J. Allogeneic platelet-rich plasma inhibits ferroptosis in promoting wound repair of type 2 diabetic ulcers. Free Radic Bio Med 2024;215:37–47.
Lu Y, Zhao D, Cao G, Yin S, Liu C, Song R, et al. Research progress on and molecular mechanism of vacuum sealing drainage in the treatment of diabetic foot ulcers. Front Surg 2024;11:1265360.
Lee HB, Ha H, Kim SI, Ziyadeh FN. Diabetic kidney disease research: where do we stand at the turn of the century? Kidney Int 2000;58:S1–S2.
Pradhan D, Sahu PK, Purohit S, Ranajit SK, Acharya B, Sangam S, et al. Therapeutic interventions for diabetes mellitus-associated complications. Curr Diab Rev 2024;20:1–21.
Kuang Y, Yang J, Sun M, Rui T, Yang Z, Shi M. Depression of LncRNA DANCR alleviates tubular injury in diabetic nephropathy by regulating KLF5 through sponge miR-214-5p. BMC Nephrol 2024;25:130.
Matoba K, Takeda Y, Nagai Y, Sekiguchi K, Yokota T, Utsunomiya K, et al. The physiology, pathology, and therapeutic interventions for ROCK isoforms in diabetic kidney disease. Front Pharmacol 2020;11:585633.
Ptilovanciv EON, Fernandes GS, Teixeira LC, Reis LA, Pessoa EA, Convento MB, et al. Heme oxygenase 1 improves glucoses metabolism and kidney histological alterations in diabetic rats. Diabetol Metab Synd 2013;5:3.
Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative Stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med 2021;171:179–89.
Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diab Metab Synd 2019;13:1165–72.
Ma L, Wu F, Shao Q, Chen G, Xu L, Lu FA-O. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Dev Ther 2021;15:3207–21.
Zhang B, Zhang X, Zhang C, Shen Q, Sun G, Sun X. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-Mediated HO-1 expression. Molecules 2019;24:247.
Pham TK, Nguyen THT, Yun HR, Vasileva EA, Mishchenko NP, Fedoreyev SA, et al. Echinochrome A prevents diabetic nephropathy by inhibiting the PKC-iota pathway and enhancing renal mitochondrial function in db/db mice. Mar Drugs 2023;21:222.
Chen ZQ, Sun XH, Li XJ, Xu ZC, Yang Y, Lin ZY, et al. Polydatin attenuates renal fibrosis in diabetic mice through regulating the Cx32-Nox4 signaling pathway. Acta Pharm Sin 2020;41:1587–96.
Chen Q, Tao J, Xie X. Astaxanthin promotes Nrf2/ARE signaling to inhibit HG-induced renal fibrosis in GMCs. Mar Drugs 2018;16:117.
Chen Q, Tao J, Li G, Zheng D, Tan Y, Li R, et al. Astaxanthin ameliorates experimental diabetes-induced renal oxidative stress and fibronectin by upregulating connexin43 in glomerular mesangial cells and diabetic mice. Eur J Pharmacol 2018;840:33–43.
Yu WC, Huang RY, Chou TC. Oligo-Fucoidan improves diabetes-induced renal fibrosis via activation of Sirt-1, GLP-1R, and Nrf2/HO-1: an in vitro and in vivo study. Nutrients 2020;12:3068.
Wei X, Ma Y, Li Y, Zhang W, Zhong Y, Yu Y, et al. Anti-apoptosis of podocytes and pro-apoptosis of mesangial cells for telmisartan in alleviating diabetic kidney injury. Front Pharmacol 2022;13:876469.
Antar SA, Abdo W, Taha RS, Farage AE, El-Moselhy LE, Amer ME, et al. Telmisartan attenuates diabetic nephropathy by mitigating oxidative stress and inflammation, and upregulating Nrf2/HO-1 signaling in diabetic rats. Life Sci 2022;291:120260.
Alaofi AL. Sinapic acid ameliorates the progression of streptozotocin (STZ)-induced diabetic nephropathy in rats via NRF2/HO-1 mediated pathways. Front Pharmacol 2020;11:1119.
Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C, et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol 2020;888:173574.
Jin T, Chen C. Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem Toxicol 2022;163:112892.
Wu Y, Zhao Y, Yang H-z, Wang Y-j, Chen Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep. 2021;41:BSR20202924.
Kour V, Swain J, Singh J, Singh H, Kour H. A review on diabetic retinopathy. Curr Diab Rev 2024;20:74–88.
Cano-Cano F, Alcalde-Estévez E, Gómez-Jaramillo L, Iturregui M, Sánchez-Fernández EM, García Fernández JM, et al. Anti-inflammatory (M2) response is induced by a sp2-iminosugar glycolipid sulfoxide in diabetic retinopathy. Front Immunol 2021;12:632132.
Sun Z, Wang Y, Xu R, Zhang S, Yang H, Song J, et al. Hydroxysafflor yellow a improved retinopathy via Nrf2/HO-1 pathway in rats. Open Life Sci 2022;17:284–92.
Li S, Lu S, Wang L, Liu S, Zhang L, Du J, et al. Effects of amygdalin on ferroptosis and oxidative stress in diabetic retinopathy progression via the NRF2/ARE signaling pathway. Exp Eye Res 2023;234:109569.
Ke B, Zhang T, An T, Lu R. Soy isoflavones ameliorate the cognitive dysfunction of Goto-Kakizaki rats by activating the Nrf2-HO-1 signalling pathway. Aging 2020;12:21344–54.
Wang F, Shang Y, Zhang R, Gao X, Zeng Q. A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and anti‑inflammatory mechanisms. Mol Med Rep 2019;19:1040–8.
Liang E, Ma M, Wang L, Liu X, Xu J, Zhang M, et al. The BET/BRD inhibitor JQ1 attenuates diabetes-induced cognitive impairment in rats by targeting Nox4-Nrf2 redox imbalance. Biochem Biophys Res Commun 2018;495:204–11.
Deng C, Meng Z, Chen H, Meng S. Tetramethylpyrazine ameliorates systemic streptozotocin-induced Alzheimer-like pathology. J Chem Neuroanat 2023;127:102207.
Xiaojun M, Min S, Yushan Y, Gaofei R, Jingwen H, Guijun Q. et al. Albiflorin alleviates cognitive dysfunction in STZ-induced rats. Aging. 2021;13:18287–97.
Sun H, Lu S, Qu G, Li J, Song B. Mesenchymal stem cells-derived exosomes ameliorate high glucose and lipopolysaccharide-induced HPMECs injury through the Nrf2/HO-1 pathway. Autoimmunity 2023;56:2290357.
Song JX, An JR, Chen Q, Yang XY, Jia CL, Xu S, et al. Liraglutide attenuates hepatic iron levels and ferroptosis in db/db mice. Bioengineered 2022;13:8334–48.
Cheng X, Huang J, Li H, Zhao D, Liu Z, Zhu L, et al. Quercetin: a promising therapy for diabetic encephalopathy through inhibition of hippocampal ferroptosis. Phytomedicine 2024;126:154887.
Yao Q, Sun R, Bao S, Chen R, Kou L. Bilirubin protects transplanted islets by targeting ferroptosis. Front Pharmacol 2020;11:907.
Gao Z, Zhang Z, Gu D, Li Y, Zhang K, Dong X, et al. Hemin mitigates contrast-induced nephropathy by inhibiting ferroptosis via HO-1/Nrf2/GPX4 pathway. Clin Exp Pharm P 2022;49:858–70.
Stancic A, Velickovic K, Markelic M, Grigorov I, Saksida T, Savic N, et al. Involvement of ferroptosis in diabetes-induced liver pathology. Int J Mol Sci 2022;23:9309.
Wu J, Zhao Y, Fan Z, Chen Q, Chen J, Sun Y, et al. Soluble epoxide hydrolase inhibitor protects against blood-brain barrier dysfunction in a mouse model of type 2 diabetes via the AMPK/HO-1 pathway. Biochem Biophys Res Commun 2020;524:354–9.
Wu S, Zhu J, Wu G, Hu Z, Ying P, Bao Z, et al. 6-Gingerol alleviates ferroptosis and Inflammation of diabetic cardiomyopathy via the Nrf2/HO-1 pathway. Oxi Med Cell Longev 2022;2022:3027514.
Puhari SSM, Yuvaraj S, Vasudevan V, Ramprasath T, Arunkumar K, Amutha C, et al. Fucoidan from sargassum wightii reduces oxidative stress through upregulating Nrf2/HO-1 signaling pathway in alloxan-induced diabetic cardiomyopathy rats. Mol Biol Rep 2023;50:8855–66.
Uddandrao VVS, Parim B, Singaravel S, Ponnusamy P, Ponnusamy C, Sasikumar V, et al. Polyherbal formulation ameliorates diabetic cardiomyopathy through attenuation of cardiac inflammation and oxidative stress via NF-κB/Nrf-2/HO-1 pathway in diabetic rats. J Cardiovasc Pharm 2022;79:e75–e86.
Li H, Shi Y, Wang X, Li P, Zhang S, Wu T, et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem Biol Interact 2019;310:108754.
Umar U, Ahmed S, Iftikhar A, Iftikhar M, Majeed W, Liaqat A, et al. Phenolics extracted from Jasminum sambac mitigates diabetic cardiomyopathy by modulating oxidative stress, apoptotic mediators and the Nfr-2/HO-1 pathway in alloxan-induced diabetic rats. Molecules 2023;28:5453.
Li X, Wu D, Tian Y. Fibroblast growth factor 19 protects the heart from oxidative stress-induced diabetic cardiomyopathy via activation of AMPK/Nrf2/HO-1 pathway. Biochem Biophys Res Commun 2018;502:62–68.
Wei Z, Jing Z, Pinfang K, Chao S, Shaohuan Q. Quercetin inhibits pyroptosis in diabetic cardiomyopathy through the Nrf2 pathway. J Diabetes Res 2022;2022:9723632.
Chen J, Guo P, Han M, Chen K, Qin J, Yang F. Cognitive protection of sinomenine in type 2 diabetes mellitus through regulating the EGF/Nrf2/HO-1 signaling, the microbiota-gut-brain axis, and hippocampal neuron ferroptosis. Phytother Res 2023;37:3323–41.
Wang B, Zhu S, Guo M, Ma R-D, Tang Y-L, Nie Y-X, et al. Artemisinin ameliorates cognitive decline by inhibiting hippocampal neuronal ferroptosis via Nrf2 activation in T2DM mice. Mol Med 2024;30:35.
Zou W, Yuan J, Tang ZJ, Wei HJ, Zhu WW, Zhang P, et al. Hydrogen sulfide ameliorates cognitive dysfunction in streptozotocin-induced diabetic rats: involving suppression in hippocampal endoplasmic reticulum stress. Oncotarget 2017;8:64203–16.
Xianchu L, Kang L, Beiwan D, Huan P, Ming L. Apocynin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Bratisl Lek Listy 2021;122:78–84.
Ma C, Long H. Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. NeuroToxicology 2016;57:104–11.
Yuan S, Liang X, He W, Liang M, Jin J, He QA-O. ATF4-dependent heme-oxygenase-1 attenuates diabetic nephropathy by inducing autophagy and inhibiting apoptosis in podocyte. Ren Fail 2021;43:968–79.
Jiang N, Zhao H, Han Y, Li L, Xiong S, Zeng L, et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO–1–mediated control of mitochondrial dynamics. Cell Prolif 2020;53:e12909.
Raish M, Ahmad A, Bin Jardan YA, Shahid M, Alkharfy KM, Ahad A, et al. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed Pharmacother 2022;145:112412.
Yan Y, Yuan N, Chen Y, Ma Y, Chen A, Wang F, et al. SKP alleviates the ferroptosis in diabetic kidney disease through suppression of HIF-1α/HO-1 pathway based on network pharmacology analysis and experimental validation. Chin Med 2024;19:31.
Wang G, Zhang X, Lu X, Liu J, Zhang Z, Wei Z, et al. Fish oil supplementation attenuates cognitive impairment by inhibiting neuroinflammation in STZ-induced diabetic rats. Aging 2020;12:15281–9.
Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J, et al. Melatonin suppresses ferroptosis induced by high glucose via activation of the Nrf2/HO-1 signaling pathway in type 2 diabetic osteoporosis. Oxid Med Cell Longev 2020;2020:9067610.
Li Y, Liu J, Ma X, Bai X. Maresin-1 inhibits high glucose induced ferroptosis in ARPE-19 cells by activating the Nrf2/HO-1/GPX4 pathway. BMC Ophthalmol 2023;23:368.
Huang YC, Chen BC, Chang KF, Hsieh MC, Sheu GT, Hsiao CY, et al. The alleviation effects of n-butylidenephthalide on apoptosis, senescence, and tight junction impairment of retinal pigment epithelium by activating Nrf-2/HO-1 signaling pathway in early diabetic retinopathy. Life Sci 2023;327:121815.
Xu Z, Li S, Li K, Wang X, Li X, An M, et al. Urolithin a ameliorates diabetic retinopathy via activation of the Nrf2/HO-1 pathway. Endocr J 2022;69:971–82.
Zhang XX, Ji YL, Zhu LP, Wang ZH, Fang CQ, Jiang CH, et al. Arjunolic acid from cyclocarya paliurus ameliorates diabetic retinopathy through AMPK/mTOR/HO-1 regulated autophagy pathway. J Ethnopharmacol 2022;284:114772.
Song Y, Lv P, Yu J. Platycodin d inhibits diabetic retinopathy via suppressing TLR4/MyD88/NF-κB signaling pathway and activating Nrf2/HO-1 signaling pathway. Chem Biol Drug Des 2024;103:e14419.
Fang J, Bai W, Yang L. Astaxanthin inhibits oxidative stress and apoptosis in diabetic retinopathy. Acta Histochem 2023;125:152069.
Yang X, Li D. Tricin attenuates diabetic retinopathy by inhibiting oxidative stress and angiogenesis through regulating Sestrin2/Nrf2 signaling. Hum Exp Toxicol 2023;42:09603271231171642.
D’Agata V, D’Amico AG, Maugeri G, Bucolo C, Rossi S, Giunta S. Carnosol attenuates high glucose damage in human retinal endothelial cells through regulation of ERK/Nrf2/HO-1 pathway. J Asian Nat Prod Res 2023;25:783–95.
Shi Q, Cheng Y, Dong X, Zhang M, Pei C, Zhang M. Effects of rhaponticin on retinal oxidative stress and inflammation in diabetes through NRF2/HO-1/NF-κB signalling. J Biochem Mol Toxic 2020;34:e22568.
Pu Q, Guo XX, Hu JJ, Li AL, Li GG, Li XY. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed Pharmacother 2022;147:112659.
Wang P, Fan S, Hu X, Luo L, Ying J, Li J. MG132, attenuates the retinal vascular injury through the upregulation of Nrf2 expression. J Ocul Pharm Ther 2023;39:661–71.
Yang J, Hua Z, Zheng Z, Ma X, Zhu L, Li Y. Acteoside inhibits high glucose-induced oxidative stress injury in RPE cells and the outer retina through the Keap1/Nrf2/ARE pathway. Exp Eye Res 2023;232:109496.
Lin HD, Lee YC, Chiang CY, Lin YJ, Shih CY, Tsai RK, et al. Protective effects of Scoparia dulcis L. extract on high glucose-induced injury in human retinal pigment epithelial cells. Front Nutr 2023;10:1085248.
Hao Y, Gao X. Diosgenin protects retinal pigment epithelial cells from inflammatory damage and oxidative stress induced by high glucose by activating AMPK/Nrf2/HO-1 pathway. Immun Inflamm Dis 2022;10:e698.
Zhu J, Sun H, Kang X, Zhu H, Yan X. Acidic polysaccharides from Buddleja officinalis inhibit angiogenesis via the Nrf2/ARE pathway to attenuate diabetic retinopathy. Food Funct 2022;13:9021–31.
Xu Z, Liu Y, Ma R, Chen J, Qiu J, Du S, et al. Thermosensitive hydrogel incorporating Prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl Mater Interfaces 2022;14:14059–71.
Vendidandala NR, Yin TP, Nelli G, Pasupuleti VR, Nyamathulla S, Mokhtar SI. Gallocatechin‑silver nanoparticle impregnated cotton gauze patches enhance wound healing in diabetic rats by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Life Sci 2021;286:120019.
Zhang Z, Zheng Y, Chen N, Xu C, Deng J, Feng X, et al. San Huang Xiao Yan recipe modulates the HMGB1-mediated abnormal inflammatory microenvironment and ameliorates diabetic foot by activating the AMPK/Nrf2 signalling pathway. Phytomedicine 2023;118:154931.
Wang S, Shi M, Zhou J, Wang W, Zhang Y, Li Y Circulating exosomal mir-181b-5p promoted cell senescence and inhibited angiogenesis to impair diabetic foot ulcer via the nuclear factor erythroid 2-related factor 2/Heme oxygenase-1 pathway. Front Cardiovasc Med. 2022;9844047.
Lin S, Zhang Q, Li S, Zhang T, Wang L, Qin X, et al. Antioxidative and angiogenesis-promoting effects of tetrahedral framework nucleic acids in diabetic wound healing with activation of the Akt/Nrf2/HO-1 Pathway. ACS Appl Mater Interfaces 2020;12:11397–408.
Yin X, Fan X, Zhou Z, Li Q. Encapsulation of berberine decorated ZnO nano-colloids into injectable hydrogel using for diabetic wound healing. Front Chem 2022;10:964662.
Pan-Yue Q, Ya-Jing X, Xiang-Duo Z, Jun-Hua D, Bin Q, Xue-Fang L, et al. Effect and mechanisms of Polygonatum kingianum (polygonati rhizome) on wound healing in diabetic rats. J Ethnopharmacol 2022;298:115612.
Wang G, Li W, Chen Q, Jiang Y, Lu X, Zhao X. Hydrogen sulfide accelerates wound healing in diabetic rats. Int J Clin Exp Pathol 2015;8:5097–104.
Li M, Yu H, Pan H, Zhou X, Ruan Q, Kong D, et al. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front Pharmacol 2019;10:1099.
Acknowledgements
We apologize to all contributors to this field of research whose work is not discussed here owing to space restrictions. We thank Servier Medical Art for providing the drawing materials.
Funding
This present study was supported by the Foundation of Hubei Educational Committee (grant no. BXLBX0809/Q20202803) and the Transverse Project on of Hubei University of Science and Technology (grant nos. 2022HX152) and the Doctoral Scientific Research Foundation of Hubei University of Science and Technology (grant no. BK202010).
Author information
Authors and Affiliations
Contributions
Bao-qing Zhao and Xi-ying Guo conducted topic selection, outline, figures, and tables. Jing-jing Zhang and Ping Ni drafted the original manuscript. Yi Song and Man-Jun Gao revised the draft critically. Bao-qing Zhao writing, review and editing, supervision, and funding acquisition. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Jj., Ni, P., Song, Y. et al. Effective protective mechanisms of HO-1 in diabetic complications: a narrative review. Cell Death Discov. 10, 433 (2024). https://doi.org/10.1038/s41420-024-02205-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-024-02205-x
This article is cited by
-
Unraveling the oxidative stress landscape in diabetic foot ulcers: insights from bulk RNA and single-cell RNA sequencing data
Biology Direct (2025)
-
Structural characterization and cardioprotective activities of natural polysaccharides from Apocynum venetum L
Chemical and Biological Technologies in Agriculture (2025)
-
Ferroptosis-related proteins ALOX15 and HMOX1 are upregulated and associated with inflammation in patients with diabetic nephropathy: a prospective cross-sectional study
International Urology and Nephrology (2025)