Abstract
Catalytic asymmetric hydroboration of unsaturated bonds has been recognized as the most straightforward method for the construction of chiral organoboron compounds. Although catalytic asymmetric hydroboration of alkenes has been well-developed, enantioselective hydroboration of allenes still remains rare probably due to the challenges in controlling the enantio-, stereo-, and regioselectivity. Additionally, the hydroboration products might go through over-borohydride, making the catalytic asymmetric dihydroboration of allenes challenging. Here, we report a cobalt-catalyzed asymmetric dihydroboration of allenes using a ligand relay strategy with two simple ligands. This protocol shows excellent enantio-, stereo-, and regioselectivity with positive functional group compatibilities in the construction of chiral 1,4-diboronate products. The applications of this reaction are demonstrated by various product derivatizations, gram-scale reactions, and the preparation of artigenin analogues. Mechanistic studies indicate that the achiral ligand controls the first hydroboration of allenes, and the chiral oxazoline iminopyridine ligand is responsible for the subsequent isomerization and asymmetric hydroboration.
Similar content being viewed by others
Introduction
Chiral organoboron compounds are acknowledged as exceptionally versatile building blocks in chemical synthesis, owing to their diverse reactivity profile1,2,3,4,5,6, and exhibit extensive applications in pharmaceuticals, materials science, and agrochemistry7,8,9,10,11,12,13. The quest for effective and selective protocols to prepare these valuable molecules has been a longstanding and challenging task in chemical synthesis. In this context, transition metal-catalyzed asymmetric hydroboration of unsaturated compounds, such as alkenes and alkynes, has emerged as a potent tool for constructing chiral organoboron compounds14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. Despite significant advances achieved in the asymmetric hydroboration of alkenes, the selective hydroboration of allenes with boranes to access chiral organoboron compounds poses a formidable challenge34,35,36,37. This is due to the potential generation of a series of organoboron products, resulting from diverse regio- and stereoselectivity issues in this reaction38,39,40. Consequently, the development of effective and selective protocols for the precise enantioselective synthesis of chiral organoboron compounds from readily accessible allenes and commercially available base metal catalysts remains significant and highly desirable.
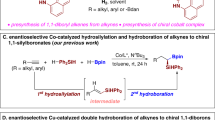
The utility of allenes in the preparation of a diverse range of compounds has been widely explored41,42,43,44,45,46,47,48,49,50,51,52,53,54, highlighting exceptional versatility in chemical synthesis accentuated by the heightened reactivity of their two orthogonal cumulative π-systems. However, the intrinsic instability and elevated activity of allenes present challenges in the hydroboration process for constructing organoboron compounds, leading to intricate regional and stereoselectivity. This complexity contrasts with the hydroboration reactions of alkenes and alkynes, introducing the potential for over-borohydride during the reaction38,39. Over the last two decades, transition metal-catalyzed hydroboration of allenes with pinacolborane (HBpin) has been well developed by researchers, including Ma55,56,57,58, Ge59,60,61, Huang58, Tsuji62, Miyaura40, and Zhan63, providing various allylic boronate and alkenyl boronate derivatives with excellent regio- and stereoselectivities (Fig. 1a)40,55,56,57,58,59,60,61,62,63. Despite significant progress in the hydroboration of allenes, the enantioselective hydroboration of allenes is rarely developed. The pioneering work includes the hydroboration of allenes developed by Rush using stoichiometric chiral borane reagents to generate chiral allylic boranes, which then undergo allylboration with aldehydes to furnish homoallylic alcohols in high enantioselectivities (Fig. 1b)34,35,36,37. Moreover, the Hoveyda group disclosed a Cu-catalyzed asymmetric protoboration of allenes to provide chiral alkenylboron products in high stereoselectivity using chiral NHC–Cu complexes, B2(pin)2 as the boron source, and tBuOH as the proton source (Fig. 1c)64. However, the catalytic asymmetric direct hydroboration of allenes with borones is yet to be reported to our knowledge, emphasizing the urgent need for a more atom-economical and efficient method in the development of asymmetric hydroboration of allenes for obtaining chiral organoboron compounds.
The alkenyl boronate and allylic boronate derivatives obtained from the hydroboration of allenes through transition metal catalysis can potentially undergo the second hydroboration due to the presence of an unsaturated bond23,31,65,66,67,68. Intriguingly, the sequential dihydroboration of allenes by transition metal catalysis has not been reported to our knowledge (Fig. 1a), but it is highly anticipated, as the resulting dihydroboration products and their derivatives could serve as versatile precursors to various high-value chemical products23,69,70,71,72,73,74. We envision that the asymmetric dihydroboration of allenes represents an efficient method for producing chiral 1,n-diboronate products with high atomic and step economies. However, the challenge lies in designing a reaction system that can promote all steps of dihydroboration of allenes with simultaneous control of regio-, stereo-, and even enantioselectivity. Inspired by the ligand relay catalysis strategy in asymmetric multi-step reactions75,76,77,78,79,80,81,82,83,84,85,86,87,88, we hypothesized that the asymmetric dihydroboration of allenes could be realized with two simple ligands in a relay catalysis fashion. Starting with 1,1-disubstituted allenes, we expect one ligand to control the first hydroboration to selectively form achiral allylic boronate intermediates, and the other ligand to produce the desired chiral diboronates via the second asymmetric hydroboration (Fig. 1d). However, several challenges need to be contemplated, such as the need for well-controlled regioselectivity in the first hydroboration step and excellent stereoselectivity in the second asymmetric hydroboration step. Additionally, each ligand has to recognize two similar hydroboration processes to avoid excessive reactions and cross-side reactions.
Here we have successfully developed a regio-, stereo-, and enantioselective sequential hydroboration/ isomerization/asymmetric hydroboration of 1,1-disubstituted allenes using a ligand relay catalysis strategy, resulting in diverse chiral 1,4-diboronate products with high yields and enantiomeric excess values (Fig. 1d).
Results
We started our investigation with buta-2,3-dien-2-ylbenzene 1a as the model substrate and HBpin as the boron source to explore the asymmetric dihydroboration through the ligand relay catalysis strategy. Based on our previous research on the cobalt-catalyzed enantioselective hydroboration and isomerization/hydroboration of alkenes89,90,91, we selected cobalt as the catalyst. As shown in Table 1, the desired 1,4-diol product 2a was obtained in 40% yield with 82% ee when using an achiral N,N,N-tridentate ligand terpyridine (tpy, for the first hydroboration) and a chiral oxazoline iminopyridine ligand L*1 (for the second hydroboration) with Co(acac)2 in MTBE at 30 °C for 30 h (entry 1). Encouraged by this promising result, various chiral oxazoline iminopyridine ligands with different substituted oxazolines were initially examined, revealing that the steric hindrance of the substituent R’ significantly influences the yield and the enantiomeric excess. Gratifyingly, the product 2a was isolated in 79% yield with 95% ee using the sterically hindered chiral oxazoline iminopyridine ligand L*4 (entry 4), and extending the reaction time slightly increased the yield to 82% with 94% ee (entry 5). The less bulky oxazoline iminopyridine ligands L*1–L*3, compared to L*4, resulted in both lower yields and enantiomeric excesses (entries 1–3). Unfortunately, the chiral ligand L*5 was ineffective in this catalytic system (entry 6). Evaluation of other achiral tridentate nitrogen ligands showed that the phenyl substituent with an electron-donating group (L1) gave the product 2a in 42% yield with 94% ee, and the one with an electron-withdrawing group (L2) provided 2a in 11% yield with 70% ee (entries 7-8). As expected, no desired product 2a was observed when bipyridine was applied as the ligand (entry 9). The above experimental results demonstrated that both achiral and chiral ligands are necessary to control the enantio-, stereo-, and regioselectivities of the asymmetric dihydroboration of allenes. Other cobalt catalysts such as Co(OAc)2 and Co(acac)3 were also investigated, leading to lower efficiency (entries 10–11). Finally, solvents were optimized. When Et2O and THF were used as the solvent, the diol 2a was obtained in 76% yield with 88% ee and 27% yield with 79% ee, respectively (entries 12–13). The nonpolar solvent hexane was also screened, providing product 2a in 20% yield with 29% ee (entry 14).
With the optimal reaction conditions in hand, we next explored the substrate scope of allenes in this regio-, stereo-, and enantioselective dihydroboration reaction. As shown in Fig. 2, a wide range of allenes reacted smoothly to afford the corresponding products in good yields and excellent enantioselectivities. A broad range of substituents at the para- position including electron-donating and electron-withdrawing groups, such as -Me, -iBu, -OMe, -OPh, -OBn, -OBz, -OTBS, -CH2OH, -SMe, morpholinyl, -TMS and -F were well compatible, providing the diol products 2b-m in good yields and excellent enantioselectivities. We were pleased to find that the mild reaction conditions tolerated the free hydroxyl group (2i) and the saturated nitrogen heterocycle morpholine (2k), which afforded the desired products in 76% yield with 93% ee and 68% yield with 96% ee, respectively. Additionally, good reactivities were observed with a variety of substituents at meta-position such as -F, -CF3, -Ph, Me, -OMe, -OBn, and acetal, delivering the corresponding 1,4-diols 2n–t in 58–85% yields with 91–95% ee. Furthermore, the allenes with a di- and tri-substituted phenyl group were competent substrates, affording the products 2u and 2v in 67% yield with 95% ee and 82% yield with 90% ee, respectively. This reaction was also applicable to benzo rings such as benzo-1,4-dioxane, leading to the formation of the diol product 2w in moderate yield and high enantioselectivity. In addition to phenyl rings, polycyclic aromatic and heterocyclic motifs such as fluorene (2x), xanthene (2y), and naphthalene (2z) can be readily incorporated into the products 50–76% yields with 72–95% ee. A single recrystallization and removal of the mother liquor by filtration gave the white crystal products 2x and 2z in 90% ee and 88% ee, respectively. We envisioned that the lower optical purity of products 2x and 2z is attributed to the participation of the achiral ligand tpy in the subsequent isomerization/hydroboration after the first hydroboration step to produce a partial racemization products 2x and 2z. Likewise, a series of heteroaromatic substrates, such as those containing furan (2aa, 2ac), thiophene (2ab), indole (2ad), pyridine (2ae'), and pyrrole (2af) were proved to be compatible, delivering the corresponding products in 25–77% yield with 80–96% ee. Of particular note is that the substrates derived from natural products, such as(−)-menthol (2ag) and estrone (2ah), were shown to be well tolerated up to 63% yield with excellent diastereoselectivities with great potential in organic synthesis. Unfortunately, the ethylated, propylated, silylated, and dialkyl allenes failed to provide the desired products (for more details in Supplementary Table 2).
aUnless otherwise noted the reactions were carried out with 1 (0.3 mmol), Co(acac)2 (10 mol%), tpy (5 mol%), L*4 (5 mol%), HBpin (3.5 equiv), MTBE (1.5 mL), 30 oC, 39 h, then oxidized by H2O2 (30%, 0.6 mL), NaOH (3 M, 0.6 mL) in THF (2 ml); Isolated yields. Ee was determined by HPLC on a chiral stationary phase. bWithout oxidation. cNaBO3•4H2O was used as an oxidant. dHBpin (4.5 equiv). eThe reaction was conducted at 20 °C. fEnantiomeric excess after recrystallization in the parentheses.
The synthetic utilities of this method were then investigated. First, a gram-scale reaction of 1a was carried out to give the 1,4-diboronate product 2a’ in 1.38 g and 72% yield with 94% ee, which was used for further transformations (Fig. 3a). Initially, product 2a’ was reacted with vinyl magnesuim bromide in the presence of I2 to afford 4-vinyl borylalkane 3 in 52% yield and 93% ee92, which can go further both carbon–carbon double bond functionalization and C–B bond transformation to give more complex chiral fragments. 1,6-Diene 4 can also be formed in 57% yield with 94% ee by increasing the equivalents of vinyl magnesuim bromide and I292. The coupling of furan with 2a’ led to the formation of 5 in 64% yield with 94% ee93. The chiral diamine 6 was obtained in 52% yield and 94% ee via a Cu-catalyzed cross-coupling reaction94. In addition, Pd-catalyzed Suzuki-Miyaura coupling of 2a’ with 6-bromo-1-benzothiophene allowed the synthesis of product 7 in good yield and excellent enantioselectivity95, which can be used for downstream diversification. The synthetic utility of this method was further highlighted by the synthesis of a bioactive molecule. Starting from allene 1d, the desired chiral 1,4-diol (S)-2d was isolated in 58% yield with 95% ee under the standard reaction conditions with (R)-L*4 as the ligand. The Ru-catalyzed oxidation of (S)-2d provided the chiral lactone 996. Subsequent treatment of 9 with LDA followed by trapping with the electrophile m-OMeC6H4CH2Br gave the artigenin analog 10 in 48% yield and 95% ee (Fig. 3b)97.
To gain more insight into the reaction mechanism, we carried out a series of control experiments (Fig. 4a). Initially, we explored the first hydroboration step of allene 1a in the presence of tpy, which gave E-allylic boronate 11 in 87% yield after 1.5 h (1.5 equiv HBpin, Fig. 4a, i), and extending the reaction time to 39 h resulted in the formation of dihydroboration product 2a in 44% yield (3.5 equiv HBpin, Fig. 4a, ii). Standing in sharp contrast with tpy, when L*4 was alternatively used as the sole ligand, a trace amount of product 11 was detected and 85% of 1a was recovered in 1.5 h (1.5 equiv HBpin, Fig. 4a, iii). The extension of reaction time (39 h) did not improve the efficiency, furnishing the 2a in 17% yield with 94% ee (3.5 equiv HBpin, Fig. 4a, iv). These results indicate that the tpy is an effective ligand for both the first and second hydroboration steps (Fig. 4a, i-ii), and is essential for the high regio- and stereoselectivity in the first hydroboration step. Moreover, compared with the tpy, the L*4 showed much lower reactivity for the first hydroboration step (Fig. 4a, i vs iii). Based on the above results, we hypothesized that E-allylic boronate 11 might be the key intermediate in this asymmetric dihydroboration reaction. The E-allylic boronate 11 was then synthesized and subjected to the standard conditions (2.0 equiv HBpin), which provided the desired diol product 2a in 68% yield with 61% ee (Fig. 4a, v). In contrast, Z-allylic boranate 12 failed to give the 2a’ under the standard conditions (Fig. 4a, vi). These results suggested that the E-allylic boronate 11 was the intermediate and responsible for the second hydroboration. Moreover, we investigated the reactivities of L*4 and tpy in the second isomerization/hydroboration step. Not surprisingly, it was found that the addition of L*4 as a ligand delivered the 2a in 85% yield with 95% ee (Fig. 4a, vii), whereas only 42% yield of 2a’ was obtained with the use of tpy instead (Fig. 4a, viii). Time course studies of the isomerization/hydroboration of compound 11 with ligand L*4 and tpy prove that the reaction rate of L*4 was much faster than that of tpy (for more details in Supplementary Fig. 2). The proposed intermediate E was also synthesized and subjected to the standard conditions with 2.0 equiv HBpin (Fig. 4a, ix), which afforded the product 2a′ in 67% yield and 33% ee along with allylic boronate 11 in 14% yield. When L*4 was alternatively used as the sole ligand, the reaction gave 2a′ in 99% yield and 95% ee. However, the use of terpyridyl ligand delivered the 1,4-diboronate 2a′ and allylic boronate 11 in 51% and 23% yields, respectively. Time course studies showed that substrate 1a was consumed in approximately one minute along with the formation of E-allylic boronate 11 intermediate (Fig. 4b). These results indicated that 1) the ligands tpy and L*4 are responsible for the first and second hydroboration respectively, 2) although the formation of D via the addition of Co to the more hindered position is thermodynamically unfavored, the subsequent β-H elimination and the hydroboration could provide the driving force necessary to compensate for the endergonic insertion of the intermediate 11 into the Co-H (Fig. 5).
Deuterium-labeling experiments were conducted as well (Fig. 4c). When 1a was treated with DBpin (1.5 equiv) for 1.5 h, the corresponding product 13-d1 was afforded in 92% yield (E/Z = 13:1) with 37% D atom incorporations at the C1 position. Previous studies suggest that the hydroboration reaction of allenes proceeds through a Co–H intermediate58, which is consistent with this deuterium-labeling experiment. In addition, we carried out the reaction of 1a with DBpin (3.5 equiv) under the standard conditions, and the corresponding product 2a-d1 was gained in 86% yield with 38%, 26%, 42% D atom incorporations observed at C1–C3 positions. Similarly, D atom incorporations observed at the positions of C1–C3 were 29%, 13%, and 33%, respectively when deuterated substrate 1a-d1 was treated with the standard conditions. These results are consistent with an alkene isomerization mechanism98,99,100. Furthermore, we performed a crossover experiment using a mixture of deuterated 1a-d1 and 1v and found that the H/D scrambled products could be isolated with 86% (2a-d3) and 81% (2v-d) yields, respectively (Fig. 4d), suggesting that a dissociative mechanism was involved in the isomerization.
Based on the above mechanistic experiments and previous literature40,55,56,57,58,59,60,61,62,63,98,99,100,101,102,103, a possible catalytic cycle is proposed as shown in Fig. 5. The cobalt hydride species A can be obtained from the reaction of Co(acac)2 with tpy and HBpin. The allene 1a coordinates with species A, followed by the insertion of 1a into the cobalt hydride to deliver π-allyl-cobalt species C, which then reacts with HBpin via σ-bond metathesis or oxidative addition/reductive elimination to give E-allylic boronate intermediate 11 and regenerating the cobalt hydride species A. The intermediate 11 next goes through the migratory insertion into the cobalt hydride species A’ to afford intermediate D. The following β-H elimination and subsequent migratory insertion generate the alkyl Co species F, which reacts with HBpin to release the final product 2a’ and regeneratethe cobalt hydride species A’.
Discussion
In summary, we have developed a sequential hydroboration/isomerization/asymmetric hydroboration of allenes by ligand relay catalysis, which provides a robust and reliable method for the synthesis of chiral 1,4-diboronate products in good yields and excellent enantioselectivities. This protocol overcame the challenges of stereoselectivity, regioselectivity, and enantioselectivity. The robustness and practicability of this reaction were demonstrated by gram-scale reactions, diverse product derivatizations, and asymmetric syntheses of artigenin analogs. Preliminary mechanistic studies revealed that the achiral ligand was used for the first hydroboration of allenes to deliver E-allylic boranate intermediates with excellent regio- and stereoselectivities, and the subsequent isomerization/asymmetric hydroboration was catalyzed by the Co/chiral oxazoline iminopyridine ligand complex, providing the valuable diboronate products in high enantioselectivities. Additionally, the reaction involves a dissociative alkene isomerization and a cobalt-catalyzed asymmetric hydroboration. This strategy increased the utilization of unsaturated bonds of allenes and enriched the chemistry of allenes.
Methods
In a glove box, to an oven-dried 10-mL vial were added Co(acac)2 (7.8 mg, 0.03 mmol), tpy (3.5 mg, 0.015 mmol), L*4 (6.1 mg, 0.015 mmol) and anhydrous MTBE (1.5 mL). The resulting suspension was stirred for 30 min at room temperature, at which time HBpin (135 mg, 1.05 mmol) was added. After the resulting mixture was stirred for an additional 5 min, allene 1 (0.3 mmol) was then added. The reaction was stirred for 39 h at 30 °C. The resulting solution was quenched with DCM and concentrated. The residue was dissolved in THF (2.0 mL) and cooled to 0 °C, and NaOH (0.6 mL, 3.0 M) and H2O2 (0.6 mL, 30%) were then added. The resulting mixture was stirred for 30 min, then extracted with EtOAc (three times), dried with Na2SO4, filtered and concentrated. The residue was purified by silica gel chromatography to afford the desired products (R)-diol 2.
Data availability
The authors declare that the data Supplementary the findings of this study are available within the paper and its Supplementary Information file. The experimental procedures and characterization of all new compounds are provided in the Supplementary Information. All data are available from the corresponding authors upon request.
References
Brown, H. C. & Singaram, B. The development of a simple general procedure for synthesis of pure enantiomers via chiral organoboranes. Acc. Chem. Res. 21, 287–293 (1988).
Scott, H. K. & Aggarwal, V. K. Highly enantioselective synthesis of tertiary boronic esters and their stereospecific conversion to other functional groups and quaternary stereocentres. Chem. Eur. J. 17, 13124–13132 (2011).
Leonori, D. & Aggarwal, V. K. Lithiation–borylation methodology and its application in synthesis. Acc. Chem. Res. 47, 3174–3183 (2014).
Leonori, D. & Aggarwal, V. K. Stereospecific couplings of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 54, 1082–1096 (2015).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Fyfe, J. W. B. & Watson, A. J. B. Recent developments in organoboron chemistry: old dogs, new tricks. Chemistry 3, 31–55 (2017).
Bank, S. Organic syntheses via boranes (Brown, Herbert C.). J. Chem. Educ. 53, A274 (1976).
Entwistle, C. D. & Marder, T. B. Boron chemistry lights the way: optical properties of molecular and polymeric systems. Angew. Chem. Int. Ed. 41, 2927–2931 (2002).
Hall, D.G. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. (Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, 2011).
Smoum, R., Rubinstein, A., Dembitsky, V. M. & Srebnik, M. Boron containing compounds as protease inhibitors. Chem. Rev. 112, 4156–4220 (2012).
Mellerup, S. K. & Wang, S. Boron-based stimuli responsive materials. Chem. Soc. Rev. 48, 3537–3549 (2019).
Fernández, E. Advances in Organoboron Chemistry towards Organic Synthesis. (Georg Thieme Verlag, Stuttgart, 2020).
Yu, I. F., Wilson, J. W. & Hartwig, J. F. Transition-metal-catalyzed silylation and borylation of C–H bonds for the synthesis and functionalization of complex molecules. Chem. Rev. 123, 11619–11663 (2023).
Li, Y., Lu, X. & Fu, Y. Recent advances in cobalt-catalyzed regio- or stereoselective hydrofunctionalization of alkenes and alkynes. CCS Chem. Just published. https://doi.org/10.31635/ccschem.024.202303678 (2024).
Beletskaya, I. & Pelter, A. Hydroborations catalysed by transition metal complexes. Tetrahedron 53, 4957–5026 (1997).
Crudden, Cathleen, M. & Edwards, D. Catalytic asymmetric hydroboration: recent advances and applications in carbon−carbon bond-forming reactions. Eur. J. Org. Chem. 2003, 4695–4712 (2003).
Carroll, A.-M., O’Sullivan, T. P. & Guiry, P. J. The development of enantioselective rhodium-catalysed hydroboration of olefins. Adv. Synth. Catal. 347, 609–631 (2005).
Collins, B. S. L., Wilson, C. M., Myers, E. L. & Aggarwal, V. K. Asymmetric synthesis of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 56, 11700–11733 (2017).
Fan, W., Li, L. & Zhang, G. Branched-selective alkene hydroboration catalyzed by earth-abundant metals. J. Org. Chem. 84, 5987–5996 (2019).
Yang, X. & Ge, S. Recent progress in cobalt-catalyzed enantioselective hydrogenation and hydroboration reactions of alkenes. Curr. Opin. Green. Sustain. Chem. 31, 100542 (2021).
Kanti Das, K., Manna, S. & Panda, S. Transition metal catalyzed asymmetric multicomponent reactions of unsaturated compounds using organoboron reagents. Chem. Commun. 57, 441–459 (2021).
Hu, J., Ferger, M., Shi, Z. & Marder, T. B. Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 50, 13129–13188 (2021).
Paul, S., Das, K. K., Aich, D., Manna, S. & Panda, S. Recent developments in the asymmetric synthesis and functionalization of symmetrical and unsymmetrical gem-diborylalkanes. Org. Chem. Fron. 9, 838–852 (2022).
Lee, Y., Jang, H. & Hoveyda, A. H. Vicinal diboronates in high enantiomeric purity through tandem site-selective NHC−Cu-catalyzed boron−copper additions to terminal alkynes. J. Am. Chem. Soc. 131, 18234–18235 (2009).
Guo, J., Cheng, B., Shen, X. & Lu, Z. Cobalt-catalyzed asymmetric sequential hydroboration/hydrogenation of internal alkynes. J. Am. Chem. Soc. 139, 15316–15319 (2017).
Yu, S., Wu, C. & Ge, S. Cobalt-catalyzed asymmetric hydroboration/cyclization of 1,6-enynes with pinacolborane. J. Am. Chem. Soc. 139, 6526–6529 (2017).
Huang, Y., del Pozo, J., Torker, S. & Hoveyda, A. H. Enantioselective synthesis of trisubstituted allenyl–B(pin) compounds by phosphine–Cu-catalyzed 1,3-enyne hydroboration. Insights regarding stereochemical integrity of Cu–allenyl intermediates. J. Am. Chem. Soc. 140, 2643–2655 (2018).
Gao, D. W. et al. Cascade CuH-catalysed conversion of alkynesinto enantioenriched 1,1-disubstituted products. Nat. Catal. 3, 23–29 (2020).
Jin, S., Liu, K., Wang, S. & Song, Q. Enantioselective cobalt-catalyzed cascade hydrosilylation and hydroboration of alkynes to access enantioenriched 1,1-silylboryl alkanes. J. Am. Chem. Soc. 143, 13124–13134 (2021).
You, Y. E. & Ge, S. Cobalt-catalyzed one-pot asymmetric difunctionalization of alkynes to access chiral gem-(borylsilyl)alkanes. Angew. Chem. Int. Ed. 60, 20684–20688 (2021).
Jin, S. et al. Enantioselective Cu-catalyzed double hydroboration of alkynes to access chiral gem-diborylalkanes. Nat. Commun. 13, 3524 (2022).
Tan, B. B., Hu, M. & Ge, S. Cobalt-catalyzed regiodivergent ring-opening dihydroboration of arylidenecyclopropanes to access skipped diboronates. Angew. Chem. Int. Ed. 62, e202307176 (2023).
Parsutkar, M. M. et al. Ligand control in Co-catalyzed regio- and enantioselective hydroboration: homoallyl secondary boronates via uncommon 4,3-hydroboration of 1,3-dienes. J. Am. Chem. Soc. 145, 7462–7481 (2023).
Chen, M., Handa, M. & Roush, W. R. Enantioselective synthesis of 2-methyl-1,2-syn- and 2-methyl-1,2-anti-3-butenediols via allene hydroboration−aldehyde allylboration reaction sequences. J. Am. Chem. Soc. 131, 14602–14603 (2009).
Ess, D. H., Kister, J., Chen, M. & Roush, W. R. Origin of thermodynamic versus kinetic control of allene hydroboration with 9-borabicyclo[3.3.1]nonane and 10(R)-trimethylsilyl-9-borabicyclo[3.3.2]decane. Org. Lett. 11, 5538–5541 (2009).
Kister, J., DeBaillie, A. C., Lira, R. & Roush, W. R. Stereoselective synthesis of γ-substituted (Z)-allylic boranes via kinetically controlled hydroboration of allenes with 10-TMS-9-borabicyclo[3.3.2]decane. J. Am. Chem. Soc. 131, 14174–14175 (2009).
Chen, M. & Roush, W. R. Enantioconvergent hydroboration of a racemic allene: enantioselective synthesis of (E)-δ-stannyl-anti-homoallylic alcohols via aldehyde crotylboration. J. Am. Chem. Soc. 133, 5744–5747 (2011).
Brown, H. C., Liotta, R. & Kramer, G. W. Hydroboration. 51. Hydroboration of representative allenes with 9-borabicyclo[3.3.1]nonane. An exceptional directive effect providing a direct synthesis of B-allyl-9-borabicyclo[3.3.1]nonane derivatives. J. Am. Chem. Soc. 101, 2966–2970 (1979).
Knörzer, G., Seyffer, H. & Siebert, W. Synthese von diboraheterocyclen mit einer BC3B-gruppierung / synthesis of diboraheterocycles with a BC3B unit. Z. Naturforsch. 45, 1136–1138 (1990).
Yamamoto, Y., Fujikawa, R., Yamada, A. & Miyaura, N. A A regio- and stereoselective platinum(0)-catalyzed hydroboration of allenes controlled by phosphine ligands. Chem. Lett. 28, 1069–1070 (2003).
Ma, S. Transition metal-catalyzed/mediated reaction of allenes with a nucleophilic functionality connected to the α-carbon atom. Acc. Chem. Res. 36, 701–712 (2003).
Ma, S. Pd-catalyzed coupling reactions involving propargylic/allenylic species. Eur. J. Org. Chem. 2004, 1175–1183 (2004).
Ma, S. Some typical advances in the synthetic applications of allenes. Chem. Rev. 105, 2829–2872 (2005).
Ma, S. Transition-metal-catalyzed reactions of allenes. Pure Appl. Chem. 78, 197–208 (2006).
Ma, S. Control of regio- and stereoselectivity in electrophilic addition reactions of allenes. Pure Appl. Chem. 79, 261–267 (2007).
Bai, T., Ma, S. & Jia, G. Insertion reactions of allenes with transition metal complexes. Coord. Chem. Rev. 253, 423–448 (2009).
Ma, S. Electrophilic addition and cyclization reactions of allenes. Acc. Chem. Res. 42, 1679–1688 (2009).
Yu, S. & Ma, S. How easy are the syntheses of allenes? Chem. Commun. 47, 5384–5418 (2011).
Soriano, E. & Fernández, I. Allenes and computational chemistry: from bonding situations to reaction mechanisms. Chem. Soc. Rev. 43, 3041–3105 (2014).
Ye, J. & Ma, S. Palladium-catalyzed cyclization reactions of allenes in the presence of unsaturated carbon–carbon bonds. Acc. Chem. Res. 47, 989–1000 (2014).
Zhang, X. & Ma, S. Transition metal-catalyzed benzannulation towards naturally occurring carbazole alkaloids. Isr. J. Chem. 58, 608–621 (2018).
Huang, X. & Ma, S. Allenation of terminal alkynes with aldehydes and ketones. Acc. Chem. Res. 52, 1301–1312 (2019).
Blieck, R., Taillefer, M. & Monnier, F. Metal-catalyzed intermolecular hydrofunctionalization of allenes: easy access to allylic structures via the selective formation of C–N, C–C, and C–O bonds. Chem. Rev. 120, 13545–13598 (2020).
Campeau, D., León Rayo, D. F., Mansour, A., Muratov, K. & Gagosz, F. Gold-catalyzed reactions of specially activated alkynes, allenes, and alkenes. Chem. Rev. 121, 8756–8867 (2021).
Yuan, W. & Ma, S. Ligand controlled highly selective copper-catalyzed borylcuprations of allenes with bis(pinacolato)diboron. Adv. Synth. Catal. 354, 1867–1872 (2012).
Yuan, W., Zhang, X., Yu, Y. & Ma, S. Amide-controlled highly selective catalytic borylcupration of allenes. Chem. Eur. J. 19, 7193–7202 (2013).
Yuan, W., Song, L. & Ma, S. Copper-catalyzed borylcupration of allenylsilanes. Angew. Chem. Int. Ed. 55, 3140–3143 (2016).
Li, C. et al. Cobalt-catalyzed regio- and stereoselective hydroboration of allenes. Angew. Chem. Int. Ed. 59, 6278–6283 (2020).
Wu, C. & Ge, S. Ligand-controlled cobalt-catalyzed regiodivergent hydroboration of aryl,alkyl-disubstituted internal allenes. Chem. Sci. 11, 2783–2789 (2020).
Zhao, Y. & Ge, S. Chromium-catalyzed selective dimerization/hydroboration of allenes to access boryl-functionalized skipped (E,Z)-dienes. Angew. Chem. Int. Ed. 60, 2149–2154 (2021).
Yang, X., Yuan, C. & Ge, S. Ligand-enabled stereodivergence in nickel-catalyzed regioselective hydroboration of internal allenes. Chem 9, 198–215 (2023).
Semba, K., Shinomiya, M., Fujihara, T., Terao, J. & Tsuji, Y. Highly selective copper-catalyzed hydroboration of allenes and 1,3-dienes. Chem. Eur. J. 19, 7125–7132 (2013).
Yang, Y., Zeng, J.-H. & Zhan, Z.-P. Regio-divergent hydroboration of terminal allenes controlled by nickel and cobalt catalysts. Org. Chem. Front. 8, 2537–2542 (2021).
Jang, H., Jung, B. & Hoveyda, A. H. Catalytic enantioselective protoboration of disubstituted allenes. Access to alkenylboron compounds in high enantiomeric purity. Org. Lett. 16, 4658–4661 (2014).
Geier, S. J., Vogels, C. M., Melanson, J. A. & Westcott, S. A. The transition metal-catalysed hydroboration reaction. Chem. Soc. Rev. 51, 8877–8922 (2022).
Zhao, Y. & Ge, S. Synergistic hydrocobaltation and borylcobaltation enable regioselective migratory triborylation of unactivated alkenes. Angew. Chem. Int. Ed. 61, e202116133 (2022).
Wang, Y., Li, Y., Wang, L., Ding, S., Song, L., Zhang, X., Wu, Y.-D. & Sun, J. Ir-catalyzed regioselective dihydroboration of thioalkynes toward gem-diboryl thioethers. J. Am. Chem. Soc. 145, 2305–2314 (2023).
Zhang, M., Liu, Z. & Zhao, W. Rhodium-catalyzed remote borylation of alkynes and vinylboronates. Angew. Chem. Int. Ed. 62, e202215455 (2023).
Miralles, N., Maza, R. J. & Fernández, E. Synthesis and reactivity of 1,1-diborylalkanes towards C–C bond formation and related mechanisms. Adv. Synth. Catal. 360, 1306–1327 (2018).
Nallagonda, R., Padala, K. & Masarwa, A. gem-diborylalkanes: recent advances in their preparation, transformation and application. Org. Biomol. Chem. 16, 1050–1064 (2018).
Wu, C. & Wang, J. Geminal bis(boron) compounds: their preparation and synthetic applications. Tetrahedron Lett. 59, 2128–2140 (2018).
Jo, W., Lee, J. H. & Cho, S. H. Advances in transition metal-free deborylative transformations of gem-diborylalkanes. Chem. Commun. 57, 4346–4353 (2021).
Jiang, X.-M. et al. 1,2-Boryl migration enables efficient access to versatile functionalized boronates. Eur. J. Org. Chem. 2022, e202101463 (2022).
Aiken, S. G. et al. Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A. Nat. Chem. 15, 248–256 (2023).
Reetz, M. T. Combinatorial transition-metal catalysis: mixing monodentate ligands to control enantio-, diastereo-, and regioselectivity. Angew. Chem. Int. Ed. 47, 2556–2588 (2008).
Wang, P.-S., Chen, D.-F. & Gong, L.-Z. Recent progress in asymmetric relay catalysis of metal complex with chiral phosphoric acid. Top. Curr. Chem. 378, 9 (2019).
Martínez, S., Veth, L., Lainer, B. & Dydio, P. Challenges and opportunities in multicatalysis. ACS Catal. 11, 3891–3915 (2021).
Sun, Y., Wang, B. & Lu, Z. Ligand relay catalysis: a newly emerged synthetic strategy. Org. Chem. Front. 10, 4146–4160 (2023).
Fors, B. P. & Buchwald, S. L. A multiligand based pd catalyst for C−N cross-coupling reactions. J. Am. Chem. Soc. 132, 15914–15917 (2010).
Chen, C., Peters, J. C. & Fu, G. C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 596, 250–256 (2021).
Kim-Lee, S.-H., Mauleón, P., Gómez Arrayás, R. & Carretero, J. C. Dynamic multiligand catalysis: a polar to radical crossover strategy expands alkyne carboboration to unactivated secondary alkyl halides. Chemistry 7, 2212–2226 (2021).
Zhang, Y. et al. A relay catalysis strategy for enantioselective nickel-catalyzed migratory hydroarylation forming chiral α-aryl alkylboronates. Chem 7, 3171–3188 (2021).
He, Y. et al. Regio- and enantioselective remote hydroarylation using a ligand-relay strategy. Nat. Commun. 13, 2471 (2022).
Ji, X. et al. Asymmetric Asymmetric double hydroxycarbonylation of alkynes to chiral succinic acids enabled by palladium relay catalysis. Angew. Chem. Int. Ed. 61, e202204156 (2022).
Jiang, X., Sheng, F.-T., Zhang, Y., Deng, G. & Zhu, S. Ligand relay catalysis enables asymmetric migratory reductive acylation of olefins or alkyl halides. J. Am. Chem. Soc. 144, 21448–21456 (2022).
Sun, Y., Guo, J., Shen, X. & Lu, Z. Ligand relay catalysis for cobalt-catalyzed sequential hydrosilylation and hydrohydrazidation of terminal alkynes. Nat. Commun. 13, 650 (2022).
Wang, B., Sun, Y. & Lu, Z. Cobalt-catalyzed difunctionalization of styrenes via ligand relay catalysis. Chin. J. Chem. 41, 3633–3638 (2023).
Wang, T.-Z., Guan, Y.-Q., Zhang, T.-Y. & Liang, Y.-F. Ligand relay for nickel-catalyzed decarbonylative alkylation of aroyl chlorides. Adv. Sci. n/a, 2306923 (2023).
Lei, Y., Huang, J. & Zhao, W. Cobalt-catalyzed remote hydroboration of alkenyl amines. Org. Lett. 23, 7797–7802 (2021).
Dong, W., Ye, Z. & Zhao, W. Enantioselective cobalt-catalyzed hydroboration of ketone-derived silyl enol ethers. Angew. Chem. Int. Ed. 61, e202117413 (2022).
Zhao, P. et al. Ligand-controlled cobalt-catalyzed remote hydroboration and alkene isomerization of allylic siloxanes. Chem. Commun. 58, 302–305 (2022).
Sonawane, R. P. et al. Enantioselective construction of quaternary stereogenic centers from tertiary boronic esters: methodology and applications. Angew. Chem. Int. Ed. 50, 3760–3763 (2011).
Bonet, A., Odachowski, M., Leonori, D., Essafi, S. & Aggarwal, V. K. Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters. Nat. Chem. 6, 584–589 (2014).
Sueki, S. & Kuninobu, Y. Copper-catalyzed N- and O-alkylation of amines and phenols using alkylborane reagents. Org. Lett. 15, 1544–1547 (2013).
Mlynarski, S. N., Schuster, C. H. & Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014).
Ishii, Y., Osakada, K., Ikariya, T., Saburi, M. & Yoshikawa, S. Ruthenium complex catalyzed regioselective dehydrogenation of unsymmetrical.Alpha.,.Omega.-diols. J. Org. Chem. 51, 2034–2039 (1986).
Rečnik, L.-M. et al. Synthesis and pharmacological characterisation of arctigenin analogues as antagonists of ampa and kainate receptors. Org. Biomol. Chem. 19, 9154–9162 (2021).
Larionov, E., Li, H. & Mazet, C. Well-defined transition metal hydrides in catalytic isomerizations. Chem. Commun. 50, 9816–9826 (2014).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Obligacion, J. V. & Chirik, P. J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat. Rev. Chem. 2, 15–34 (2018).
Chen, C. et al. Cobalt-catalyzed asymmetric sequential hydroboration/isomerization/hydroboration of 2-aryl vinylcyclopropanes. Angew. Chem. Int. Ed. 61, e202205619 (2022).
Liu, W. et al. Desymmetrizing isomerization of alkene via thiazolinyl iminoquinoline cobalt catalysis. Org. Lett. 24, 1158–1163 (2022).
Acknowledgements
We thank the National Natural Science Foundation of China (Grant Nos. 22271086, 21971059, and 22471218), the National Program for Thousand Young Talents of China, the Fundamental Research Funds for the Central Universities and the Science and Technology Innovation Program of Hunan Province (2021RC2052) for financial support, Shaanxi Fundamental Science Research Project for Chemistry & Biology (22JHQ002), the Program for Young Talents of Shaanxi Province (5113190023), and Natural Science Basic Research Program of Shaanxi (2024JC-ZDXM-08) are greatly appreciated.
Author information
Authors and Affiliations
Contributions
W.Z. and Z-.Q.R. conceived the project. W.Z., Z-.Q.R., and Y.L. wrote the paper. Y.L. and Y.K. performed the experiments and prepared the Supplementary Information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zheng Huang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lei, Y., Kong, Y., Rong, ZQ. et al. Asymmetric dihydroboration of allenes enabled by ligand relay catalysis. Nat Commun 15, 8186 (2024). https://doi.org/10.1038/s41467-024-51774-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51774-z
This article is cited by
-
Transition metal-catalyzed selective hydroboration of allenes
Science China Chemistry (2025)