Abstract
The World Health Organization describes brucellosis as one of the world’s leading zoonotic diseases, with the Middle East a global hotspot. Brucella melitensis is endemic among livestock populations in the region, with zoonotic transmission occurring via consumption of raw milk, amongst other routes. Control is largely via vaccination of small ruminant and cattle populations. Due to sociocultural and religious influences camel milk (camelus dromedarius) is widely consumed raw, while milk from other livestock species is largely boiled. To investigate the potential public health impact of Brucella in camels we conduct a cross-sectional study in southern Jordan including 227 herds and 202 livestock-owning households. Here we show daily consumption of raw camel milk is associated with Brucella seropositive status among the study population, ORadj 2.19 (95%CI 1.23–3.94) on multivariable analysis, highlighting the need for socioculturally appropriate control measures; targeted interventions among the camel reservoir being crucial for effective control.
Similar content being viewed by others
Introduction
With over two million human cases estimated to occur annually worldwide, the World Health Organization (WHO) has described brucellosis as one of the world’s leading zoonotic diseases. Most cases occur within lower-and-middle-income countries (LMIC) with limited resources for prevention and control, prompting WHO to designate brucellosis a global Neglected Disease1,2,3. Human infection causes a variety of symptoms including recurrent fevers, headaches, arthralgia and chronic malaise, while reproductive symptoms also occur, including miscarriages, infertility and orchitis4,5. In addition, infection can also progress to cause osteomyelitis, endocarditis, meningoencephalitis, and in some cases death4. Human infections occur largely via consumption of infected raw milk and dairy products and through physical contact with infected livestock, via contaminated birthing products and reproductive discharges6. As such, brucellosis also represents an important production disease in livestock, resulting in potentially severe economic losses through abortions, still-births, metritis, orchitis and infertility6.
The Middle East represents an important global hotspot for brucellosis, with more than half a million human cases estimated to occur annually7,8,9. B. melitensis is the dominant causative agent (alongside, to a lesser extent, B. abortus), with reported livestock seroprevalences across the region being among the highest in the world7,10,11. The majority of human cases in the region occur among sub-populations working closely with livestock, with consumption of unpasteurized dairy products and occupational exposure to breeding ruminants being the most frequently identified risk factors for infection8,12,13. Within Jordan, brucellosis has been identified as one of the most important zoonotic diseases of public health impact, with government-led control strategies largely focussed on livestock vaccination schemes14,15,16,17,18. However, despite several decades of sustained vaccination initiatives, seroprevalences among small ruminant and cattle populations remain high19,20,21,22. While population level seroprevalences in Jordan remain largely unexamined, previous studies have reported seroprevalences among sub-populations including children, refugees, individuals working with livestock, those consuming livestock products and among hospitalised pyrexic patients17,23,24,25,26.
Due to sociocultural and religious perceptions, camel milk is widely consumed raw across the region, while milk from other livestock species is usually boiled27,28. As a result, camels (camelus dromedarius) pose a unique public health threat from milk-borne pathogens, including Brucella29,30. However, while the public health threat associated with brucellosis among other livestock species in the region is widely recognized10,31,32,33, the potential zoonotic impact of dromedary camels remains largely unexamined – despite previous reports of small-scale outbreaks in the region traced to the consumption of raw milk from infected camels28,34,35,36. In addition, the lack of a commercially available Brucella vaccine licensed for use in camels means that vaccination is limited to off-label use only, and while practiced in some countries, is not widespread across the region (including Jordan where government-led Brucella livestock vaccination programmes do not include camels, and access by the private sector is restricted)37.
This study therefore represents the first large-scale population-based study examining the potential zoonotic threat posed by Brucella in camels among a high-risk population in the Middle East10. To address key knowledge gaps, we conducted a cross-sectional study among livestock-owning communities and their camels in southern Jordan (Aqaba and Ma’an governorates). The aims of this study were to, i) estimate the seroprevalence of Brucella among the camel population, ii) estimate the seroprevalence of Brucella among members of livestock-owning households (camel owning and non-camel owning), iii) identify risk factors for Brucella seropositive status among camel populations, iv) identify risk factors for Brucella seropositive status among livestock-owning household members, and v) identify socioculturally appropriate control strategies. In this work we show daily consumption of raw camel milk as being associated with Brucella seropositive status on multivariable analysis, highlighting the need for socioculturally appropriate control measures, with targeted interventions among the camel reservoir being crucial for effective control.
Results
Camels
We collected blood samples from 902 camels, of which 26 samples were of insufficient volume for testing, with 884 samples remaining representing 227 herds. Of these 227 herds, 131 herds (455 camels) were owned by households included in a parallel human study, where household members were also tested for evidence of Brucella seropositive status. Median herd size was 10 [interquartile range (IQR) 5–20], with a mean of 3.9 camels sampled per herd (Fig. 1). The median age of camels sampled was 5 years (interquartile range [IQR] 2-8 years). There were 33/884 (3.7%) camels positive on Rose Bengal Plate test (RBPT), of which 8/884 (0.9%) were positive on Compliment Fixation Test (CFT), 9/884 (1.0%) were positive on indirect ELISA; 10/884 (1.1%) being positive on either CFT or indirect ELISA.
In Ma’an and Aqaba respectively, there were 7/340 (2.1%) and 1/544 (0.2%) positive camels (positive on RBPT and confirmed by CFT, as per OIE guidelines)37, with true seroprevalence (adjusted for test performance values) being 2.4% (95% CI 1.1–4.8) and 0.1% (95% CI 0.0–1.1) respectively; true seroprevalence being 1.0% (95% CI 0.5–2.1) across both regions. There were 7/227 (3.1%) herds with at least one seropositive camel sampled, of which 6/81 (7.4%) were in Ma’an and 1/146 (0.7%) were in Aqaba. Adjusted herd-level seroprevalence (adjusted for test performance values and herd sampling proportion) was 30.4% (95% CI 22.2–39.5) in Ma’an and 4.8% (95% CI 2.1–8.2) in Aqaba; adjusted herd level seroprevalence (also weighted for region) across both regions being 18.7% (95% CI 14.3–23.3) (Table 1).
Due to the low seroprevalence of positives on RBPT and CFT (8/884, 0.9%), and the singularity of subsequent multivariable logistic regression models created, samples positive on RBPT and CFT or ELISA (10/884, 1.1%) were considered positive for evidence of Brucella exposure for purposes of logistic regression analyses to determine potential risk factors for infection. On univariable analysis, the following variables were found to be associated (p < 0⋅20) with seropositive status, region, purchasing camels (in the last year), closed herd management practices and one or more goats also being owned (Table 2). On multivariable analysis, positive status was associated with region (Ma’an) ORadj 6.23 (95%CI 1.53–41.81) p = 0.0093) and purchasing camels (ORadj 3.84 (95%CI 1.04–18.09) p = 0.043); with evidence to suggest closed herd management practices are protective (OR 0.00, p = 0.072) in a singular model (singularity being due to the absence of any positive camels sampled from among closed herds) (Table 3).
Humans
We collected blood samples from 879 members of livestock-owning households of which 12 samples were of insufficient volume for diagnostic testing, with 867 samples remaining representing 203 households (Table 4). Median household size was 10 [IQR 7–13]), with a mean of 4.2 members sampled per household. The median age of individuals sampled was 27 (IQR 15–44). Of the 203 households sampled, 172 were camel-owning households (COH) (medium household size 10 [IQR 7–14]) including 667 household members (median age 27 years [IQR 16–44]), with a mean of 3.8 members sampled per household. The remaining 31 households were non-camel owning households (NCOH) (medium household size 11 [IQR 9–13]), including 200 household members (median age 26 years [IQR 15–44], with a mean of 6.5 individuals sampled per household (Fig. 1). Among COH, there were 131/172 (76.2%) households from which one or more camels from the herd were also tested for Brucella serological status. Jordanian Ministry of Agriculture (MoA) records show 3089 livestock owning households in Ma’an, of which 317/3089 (10.3%) own camels, and 1359 livestock-owning households in Aqaba, of which 265/1389 (19.1%) own camels, with camel ownership being 582/4448 (13.1%) overall. No households included in the study reported owning cattle (with cattle almost entirely absent from the south of Jordan, due to the arid, desert environment).
There were 68/867 (7.8%) individuals seropositive on RBPT and 62/867 (7.2%) individuals seropositive on IgG ELISA, with 91/867 (10.5%) seropositive to either test. A Kappa coefficient of 0.57 (95% CI 0.47–0.68) indicated moderate agreement between test results, individuals being considered positive if positive to either test38. The estimated intracluster correlation coefficient (ICC) for within household seropositivity to either test was 0.46 (95% CI 0.33–0.90). Adjusted population prevalence among members of livestock-owning households (adjusted for combined test performance values and weighted for region and camel ownership status) was 8.7% (95% CI 6.9–10.7). In Ma’an and Aqaba regions, 75/478 (15.7%) and 16/389 (4.1%) individuals were seropositive respectively, adjusted seroprevalences being 10.0% (95% CI 7.5–12.9) and 5.9% (95% CI 3.9–8.6) respectively (adjusted for combined test performance values and camel ownership status) (Table 5).
Among COH, 79/667 (11.8%) members were seropositive, adjusted seroprevalence (adjusted for combined test performance values and weighted for region) being 12.7% (95% CI 10.3–15.4). In Ma’an and Aqaba regions respectively, there were 68/379 (17.9%) and 11/288 (3.8%) seropositive individuals in COH; adjusted seroprevalences being 19.5% (95% CI 15.7–23.6) and 4.8% (95% CI 2.7–7.7) respectively. Among NCOH, 12/200 (6.0%) members were seropositive, with adjusted seroprevalence being 8.2% (95% CI 4.9–12.5); there were 7/99 (7.1%) and 5/101 (5.0%) positive individuals in Ma'an and Aqaba respectively, adjusted seroprevalences being 9.0% (95% CI 4.4–15.7) and 6.2% (95% CI 2.6–12.0) respectively (Table 5).
The following variables, with <10% missing values, were found to be associated (p <0.20) with seropositivity on RBPT or ELISA on univariable analysis: region, sub-region, region with camel owning status, sex, history of brucellosis, household dwelling type (tent), handwashing (>5 times/day), household owns sheep, drinking raw camel milk, drinking raw small ruminant milk, consuming raw small ruminant dairy products, birthing camels, birthing small ruminants, disposing of camel afterbirth, disposing of small ruminant afterbirth, slaughtering camels, slaughtering small ruminants, frequent interaction with livestock, any livestock engagement and hand hygiene when working with livestock (Table 6).
Pairwise correlations (R ≥0.4) were identified between birthing camels, disposing of camel afterbirth and slaughtering camels, with birthing of camels selected for inclusion in multivariable models. Pairwise correlations were also identified between frequently (≥weekly) working with livestock, frequently working with small ruminants, frequently working with camels and any history of livestock engagement, with frequently working with livestock selected for inclusion. Region, sub-region and region with camel owning status were also pairwise correlated, with region selected for inclusion. In addition, correlation was also identified between birthing small ruminants and disposing of small ruminant afterbirth, with birthing small ruminants selected for inclusion in multivariable models. Drinking raw milk from small ruminants and consuming raw small ruminant dairy products were correlated, with each variable tested in a separate multivariable model, adjusted for the same covariates. The composite variable hand hygiene when frequently working with livestock, was correlated with constituent variables frequently working with livestock and handwashing and therefore tested in a separate model adjusted for the same covariates.
On multivariable analysis, evidence of association was found between seropositivity and region (Ma’an) (ORadj 3.27 [1.86–6.07], p = <0.0001), drinking raw camel milk daily (categorical, p = 0⋅016), birthing small ruminants (≥weekly, in season) (ORadj 1.87 [1.02–3.39], p = 0.044), and poor hand hygiene (handwashing <5 times/day) when working with livestock (≥weekly) (categorical, p = 0.047) (Table 7).
Among individuals drinking raw camel milk daily, 27.6% (43/156) were seropositive in Ma’an and 4.9% (3/61) were seropositive in Aqaba (OR 9.42 [2.36–62.70], p = 0.0047), with daily consumption of raw camel milk associated with seropositive status in Ma’an, (ORadj 6.48 [3.22–13.74]) though not in Aqaba (ORadj 1.04 [0.23–3.47]) in a post-hoc logistic regression model, with Aqaba (not drinking camel milk daily) as a baseline and household as a random effect, adjusted for the same covariates (age, sex, region, household dwelling type, owning sheep, drinking camel milk, consuming small ruminant dairy products, birthing small ruminants and birthing camels). Similarly, region with camel owning status was associated with seropositivity (Ma’an COH (ORadj 4.24 [2.20–8.88]) with Aqaba COH as a baseline), when adjusted for the same covariates. Among COH where at least one camel was tested for Brucella seropositive status, seroprevalence among individuals drinking raw milk from a herd with a positive camel tested was 50.0% (2/4), compared to 15.7% (44/281) among households where camels tested in the herd were negative (OR 24.31 [0.69–851.92], p = 0.079). In a follow-up survey conducted among 69/172 (40.1%) of COH including 503 household members, 308/503 (61.2%) reported history of consuming camel milk in the past 6 months, of whom 285/308 (92.5%) reported always consuming this raw. Among 320/503 (63.6%) who reported history of consuming small ruminant milk in the past 6 months, 278/320 (86.9%) reported they never or rarely consumed this raw.
Among 577 individuals working frequently (≥weekly) with livestock, hand washing >5 times/day was found to be protective (ORadj 0.52 [0.30–0.92], p = 0.023), in a logistic regression model with household as a random effect adjusted for the same covariates (age, sex, region, household dwelling type, owning sheep, drinking camel milk, consuming small ruminant dairy products, birthing small ruminants and birthing camels). Use of soap (yesterday) when handwashing >5 times/day was protective (ORadj 0.52 [0.29–0.91]).
Among households owning small ruminants and reporting Brucella vaccination status with Rev 1 vaccine administered by the MoA (at least once during the previous 5 years), vaccine uptake was 65.4% (44/55) among flocks in Ma’an and 70.5% (31/44) among flocks in Aqaba, and 75.8% (75/99) overall. When weighted for camel owning status, estimated vaccine uptake among the study population was 89.0% in Ma’an and 87.4% in Aqaba, and 88.5% overall (vaccine uptake among COH flocks being 72.2% (57/79) compared to 90.0% (18/20) among NCOH flocks in the sample population, OR 0.29 [0.43–1.21]) p = 0.097). Vaccination of small ruminant flocks was found to be protective among household members owning vaccinated flocks; 17.8% (19/107) of individuals being seropositive among households owning unvaccinated flocks, compared to 8.8% (33/373) of individuals among households owning vaccinated flocks (ORmin.adj 0.36 [0.14–0.91], p = 0.030), adjusted for a priori variables (age and sex) and region.
Among children of school age (≤15 yrs), seropositivity among those not attending school was 18.6% (8/43), compared to 6.3% (11/175) among those attending school; not attending school being associated with seropositive status (ORmin.adj 3.23 [1.10–9.61], p = 0.033), when adjusted for priori variables (age and sex) and region. Among adults (>15 yrs), secondary education was found to be protective (ORmin.adj 0.37 [0.18–0.79], p = 0.010), when adjusted for the same covariates. Among Brucella seropositive individuals included in the study, 74.7% (68/91) had no known history of a brucellosis.
Discussion
Historically, dromedary camels have provided the only means of survival, transport, and sustained existence in the harsh desert environments of the Middle East and North Africa39,40. Consequently, they occupy a unique status within Arab Islamic culture41. The Qur’an (surra 88) entreats unbelievers to consider four things that God has made: the earth, the heavens, the mountains, and the camel – with camels perceived as being an example of God’s power; a miracle of creation holding an entirely different position to all other animals41. The Islamic Hadith (the life and teachings of the prophet, transcribed by his followers), describes the healing powers of drinking camels’ milk and urine41,42,43. As a result, camels are perceived as a uniquely clean livestock species among Islamic communities in the Middle East and beyond44. Study participants expressed the perception that owning camels was something of great honor – camels being seen as uniquely ‘righteous’ and ‘clean’ animals, compared to other livestock species27. A perception frequently expressed among the study population was that drinking camel milk imparted vital medicinal benefits, including increasing male libido, controlling blood sugar levels, healing gastrointestinal problems, and treating of cancer, amongst other medicinal properties27,45. Among the study population children drink camel’s milk raw from an early age, with participants explaining that milk comes from the camel ‘pure and ready to drink’ – and should never be boiled, for fear of damaging its healing properties26,46. This perception, combined with an unfavourable change in taste after boiling, means that camel milk is almost universally consumed raw among camel-owning communties across the region, while milk from small ruminants (and cattle where present) is usually boiled due to a perception of risk from zoonotic diseases, such as alhumaa almalitia (brucellosis)30,34,47.
It is within this context that our study findings identified daily consumption of raw camel milk as being associated with Brucella seropositive status among the study population ORadj 2.19 (95%CI 1.23–3.94), following multivariable analysis to adjust for potential confounders. When stratified by region, daily consumption of raw camel milk was found to be associated with seropositive status in Ma’an, though not in Aqaba. This reflects the high herd-level seroprevalences identified in Ma’an, where almost a third of herds were estimated to be positive (containing one or more seropositive camels), compared to Aqaba, where less than one in twenty herds were estimated to be positive. Likewise, half of all households members owning infected camels and drinking raw camel milk were found to be seropositive, compared to 15% among those drinking camel milk from herds where all sampled camels were seronegative. Even where individual camel seroprevalences may be relatively low (2.4% among camel populations in Ma’an, for example), widespread consumption of camel milk raw means that exposure to an infected animal becomes increasingly likely over time, particularly when drinking daily34. As such, boiling camel milk prior to consumption would have an important protective effect, particularly in areas where camel seroprevalences are higher (such as Ma’an)46,48. However, due to the strong sociocultural influences described (and an unfavourable change in taste after boiling) efforts to promote such profound behavioural change are likely to be challenging46,48,49.
For this reason, control of Brucella among the camel reservoir (alongside other measures) is of paramount importance50,51. Following multivariable analysis, purchasing of camels (in the past year) was found to be associated with seropositive status among animals in the receiving herds (ORadj 3.84; 95%CI: 1.04–18.09). Due to their high economic value camels that abort are often sold at market, rather than slaughtered, with potential purchasers then unaware of their reproductive history and potential Brucella positive status (as reported by study participants)22. Conversely, study findings suggested evidence that closed herd management practices are protective against Brucella entering the herd (p = 0.072), in a singular model (all camels sampled in closed herds testing negative)48. To reduce potential within herd transmission, breeding camels should be separated from the main herd during parturition, as previously described52,53. To reduce potential transmission between livestock species, camel herds should be managed away from small ruminants where possible, particularly during lambing/kidding periods, as previously described12,36,54. Small ruminant seroprevalences in the region are known to be high22, with Rev 1 (B. melitensis) vaccination of flocks offering a potentially protective effect, where camel herds and small ruminant flocks are managed together50,51.
Camel herds in the study population had not been vaccinated against Brucella. Government-led vaccination programmes in Jordan do not include camels and private access to the Rev 1 vaccine is heavily restricted, with those herd owners surveyed reporting absence of vaccination in their herds. In addition, importing of camels from neighbouring countries, including Saudi Arabia (where Rev 1 vaccination may potentially have occurred), is prohibited unless for direct slaughter, suggesting the Brucella seroconversion identified in the study population is likely attributable to natural infection44,50. While off-label use of the live-attenuated Rev 1 vaccine in camels should be considered where appropriate (see FAO guidelines), challenges with efficacy and the risk of contaminated milk mean that protective herd management strategies likely offer the most accessible means of control for many herd owners (until licensed vaccines become available)51. Testing and slaughter schemes, using serum PCR testing where possible, including breeding males shared between herds, also offer a potential route toward reducing the risk of Brucella infection from raw camel milk in Jordan and the wider region (though such schemes require sufficient financial compensation be provided to succeed)55.
Rev 1, being a live-attenuated vaccine, incurs risk of infection for veterinarians when administering the vaccine, and risk of abortion among pregnant livestock if accidently inoculated – both factors creating challenges in vaccine deployment across the region56,57. Reported Rev 1 vaccine uptake (at least once during the past 5 years) among small ruminant flocks was 89.0% in Ma’an and 87.4% among flocks in Aqaba, and 88.5% overall (weighted for camel-owning status), suggesting the observed difference in seroprevalence between regions is not attributable to differences in vaccine uptake. Vaccination of small ruminant flocks was found to offer a significantly protective effect among household members, with an apparent seroprevalence of 17.8% (19/107) among individuals owning unvaccinated flocks, compared to 8.8% (33/373) among those owning vaccinated flocks, (ORmin.adj 0.36 [0.14–0.91]), when adjusted for a priori variables (age and sex) and region. These findings highlight the importance of small ruminant vaccination in protecting high-risk communities. Vaccine uptake was lower among COH (72.2%) compared to NCOH (90.0%), potentially explained by increased challenges in geographic accessibility to COH, compared to NCOH, and suggesting targeted interventions aimed at increasing Rev 1 uptake among small ruminant flocks owned by COH would have beneficial effects in reducing human rates of infection among such communities22.
Birthing of small ruminants (≥weekly in season) was associated with seropositive status on multivariable analysis, ORadj 1.86 [1.00–3.38], consistent with previous findings, with weak evidence that drinking raw small ruminant milk (daily) also presents risk of infection, ORadj 1.96 [0.95–3.93]. These findings highlight the need for educational messages that reaffirm the importance of boiling small ruminant milk and dairy products before consumption22,58, (due to its colloidal structure and protein composition, camel milk is unsuitable for making dairy products), and the use of gloves when birthing, where possible59.
Infected livestock shed high volumes of Brucella in aborted materials and uterine discharges, with subsequent zoonotic transmission occurring via several potential routes, including ingestion of contaminated material on dirty hands and exposure of skin wounds (particularly on the hands and arms) or mucus membranes (eyes, nose and mouth) to contaminated materials6. Within this context, poor hand hygiene was found to be associated with Brucella seropositive status among individuals frequently working with livestock, with good hygiene (handwashing >5 times/day with soap) found to be protective (ORadj 0.52 [0.29–0.91]). Hand hygiene initiatives, such as WASH, therefore offer a potentially important control strategy for brucellosis among high risk communities in Jordan and the wider region60. (Due to frequency of handwashing being closely connected to concepts of prayer and ritual washing (‘wudu’) among Islamic communities, baseline handwashing frequency was set at ≤5 /day)60.
As a WHO neglected disease, brucellosis presents a disease of poverty, with secondary education found to be protective among the study population (ORmin.adj 0.37 [0.18–0.79]), while children not in school were at greater risk of infection, compared to contemporaries in school (ORmin.adj 3.23 [1.10–9.61])26,46. Diagnosis rates among the study population were low, with just a quarter of seropositive individuals reporting a known history of brucellosis, suggesting a need for promotion of awareness regarding symptoms, diagnosis and treatment among rural communities in Jordan (and potentially the wider region), together with a need for increased capacity in diagnostic and medical services among such communities58.
Among members of livestock-owning households in southern Jordan, Brucella seroprevalence was estimated to be 8.7% (95% CI 7.0-10.7) overall (adjusted for test performance values and weighted for region and camel ownership status), with adjusted seroprevalences in Ma’an and Aqaba being 10.0% (95% CI 7.5–12.9) and 5.9% (95% CI 3.9–8.6) respectively. Among COH, adjusted seroprevalences were 19.5% (95% CI 15.7–23.6) and 4.8% (95% CI 2.7–7.7) in Ma’an and Aqaba respectively, reflecting the high camel-herd level seroprevalences identified in Ma’an, compared to Aqaba (30.4% (22.2–39.5) and 4.8% (2.1–8.2) respectively). The ICC among included households was 0.46 (95% CI 0.33–0.90), suggesting moderate clustering; likely reflecting households owning infected herds (or flocks) with subsequent zoonotic transmission via consumption of infected milk (or milk products), alongside other potential routes.
This study has some limitations, with non-probabilistic and probabilistic sampling methods being used among camel herds during two distinct periods (2014–15 and 2017–18 respectively), however, we do not believe this has had an important impact on seroprevalence estimates or risk factor analysis (with seroprevalence among randomly selected camel herds being similar to those among non-probabilistically sampled herds, across the study period).
In conclusion, Brucellosis represents a serious public health threat among livestock-owning communities in southern Jordan and the wider region, with camels presenting an important source of zoonotic transmission via consumption of raw milk (with milk from other livestock species being largely consumed boiled). Due to deep sociocultural perceptions, efforts to encourage boiling of camel milk prior to consumption are likely to be challenging, (though should be attempted nonetheless, in a culturally appropriate context). For this reason, reducing Brucella infection rates among camel populations in Jordan, and the wider region, is of crucial importance in protecting human populations associated with such herds from infection via consumption of raw camel milk. This is potentially best achieved through a combination of targeted management practices (such as closed herd management, with purchasing only where necessary from trusted (preferably tested) sources), alongside possible government-led test and slaughter schemes and off-label use of the B. melitensis Rev 1 vaccine, where appropriate. Small ruminant vaccination had a significantly protective effect on associated household members, highlighting the importance of government-led vaccination programmes and the need for increased capacity, so as to improve vaccine uptake where possible (a quarter of flocks in sample population had not being vaccinated during the previous 5 years). Among individuals working frequently with livestock (≥weekly) handwashing with soap >5 times/day was found to be significantly protective, highlighting the importance of initiatives that promote hand hygiene, such as WASH, among high-risk rural communities in the region. Education had a significantly protective effect (including through being in school), highlighting the importance of education promotion strategies and increased capacity (including accessibility) among high-risk rural communities in the region (with challenges to this for desert dwelling, semi-nomadic Bedouin communities, such as the study population). Levels of diagnosis were low, suggesting the need for improved awareness strategies and increased capacity for diagnostic testing and treatment among high-risk rural communities in the region.
Methods
Ethical approval
The present study was done with institutional ethical review board approvals from The Royal Veterinary College and London School of Hygiene & Tropical Medicine (both London, UK) and Jordan University of Science and Technology (JUST) (Irbid, Jordan).
Camels
Study design and participants
Camel sampling was conducted in Aqaba and Ma’an governorates of southern Jordan across two distinct periods during 2014–2015 and 2017–2018 (Fig. 2). Due to the absence of an adequate sampling frame between February 9th 2014 and December 30th 2015, we conducted non-probabilistic sampling of herds belonging to camel-owing clients of a centrally located private veterinary practice (Al Quwayrah, Aqaba governorate) and camel-owing clients of local government veterinarians working in the study area. Between September 27th, 2017, and October 11th, 2018, we conducted a cross-sectional study using a sampling frame provided by the MoA comprising a list of all households owning one or more camels within the four sub-regions (Aqaba East, Aqaba West, Ma’an East, and Ma’an West), with herds being randomly selected by a co-author (SN) using computer-generated randomisation lists (Stata, version 15.1); (these camel-owning households being also eligible for inclusion in the parallel human study).
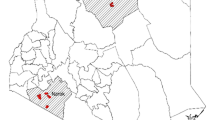
Location of 227 camel herds sampled for Brucella in southern Jordan February 2014 to October 2018 (due to local grazing movements there were three herds selected from the MoA list for Ma’an (west) that were sampled in the neighbouring region, Tafilah. Results from these herds were attributed to Ma’an.).
Institutional and national guidelines for the care of animals were followed at all times. Informed consent was obtained from all herd owners at the time of sampling, with a veterinary surgeon clinically examining all camels included in the study before sampling.
Sample collection and laboratory procedures
Selected herds were visited by members of the sampling team, including a veterinarian and community facilitator, who provided study information, assessed eligibility, and obtained consent. Based on expected owner compliance, in herds >12 camels we sampled 12 camels per herd and in herds of ≤12, we sampled all camels, subject to accessibility and owner permissions. Blood samples were collected from selected camels alongside administration of a structured questionnaire regarding potential camel-level and herd-level risk factors for Brucella exposure during the previous year, including Brucella vaccination. Questionnaires were administered in the local dialect on paper (2014–2015) and on android tablets (2017–2018) using the application Open Data Kit (version 1.10). Blood samples were collected in 8 mL plain serum vacutainer tubes and centrifuged centrally in Aqaba, at 2000 revolutions per minute (961×g) for 10 min, followed by serum collection and storage at –20 °C. All samples were stored centrally in Aqaba before transport to the diagnostic laboratory at the Veterinary Health Centre, JUST, Irbid for laboratory testing.
Serum samples were tested using the Rose-Bengal test (RBPT) for the detection of antibodies against Brucella antigens supplied by Jovac, Amman, Jordan. Briefly, a sample of serum (0.05 ml) was mixed with 0.05 ml of antigen on a microscope slide, producing a zone approximately 2 cm in diameter. This mixture was then gently agitated at room temperature for four minutes and observed for agglutination, the sample being considered positive where a visible reaction was observed. Sensitivity and specificity were assumed to be 86.7% and 99.1% respectively61. Serum samples positive on RBPT were also tested using Compliment Fixation Test (CFT) using standardized Brucella antigens supplied by Jovac, Amman, Jordan, as described by the OIE37. CFT plates were left to stand for one hour to allow unlysed cells to settle before results were read, with positive and negative controls being run for test validation. Results ≥20 IU/ml were considered positive, based on an absence of haemolysis. Sensitivity and specificity were estimated to be 99.0% and 98.4% respectively62. (Combined performance values of RBPT confirmed on CFT being 85.8% and >99.9% respectively).
Serum samples positive on RBPT were also tested using a commercially available multispecies Brucella IgG indirect ELISA supplied by Idvet (with these results being used for purposes of risk factor analysis, together with those samples positive on CFT)63 Tests were performed according to the instructions of the manufacturer, with optical densities measured at 450 nm. For each sample, the %OD was obtained as: %OD = 100 × (S−N)/(P−N), where S is the sample OD, N is the OD of the negative control and P the OD of the positive control. Samples with a % OD ≥ 120% were considered positive. Sensitivity and specificity were estimated as being 74.4% and 96.3% respectively64,65,66.
Outcomes
The primary outcome was evidence of Brucella seropositive status (seropositive on RBPT, confirmed seropositive on CFT)37. Secondary outcomes were potential associations between possible risk factors and evidence of Brucella exposure (seropositive on RBPT, confirmed by seropositivity on either CFT or ELISA).
Statistical analysis
A sample size estimate of 903 camels was used, with a total camel population size of 7750 (MoA figures for Aqaba and Ma’an governorates), an expected seroprevalence of 12.1% (Al Majali et al.)36, a ± 2% precision, 95% confidence interval and design effect of 1.0. Seroprevalence estimates and their 95% CIs were calculated at the camel and herd levels, with herd-level prevalence defined according to herds with at least one seropositive individual.
To adjust for herd sampling proportion, herd-level seroprevalence was estimated accounting for the uncertainty arising from sampling only a proportion of each herd (the proportion being different in each herd), based on the method described by Beauvais et al.67. Due to low prevalence, a binomial prior distribution for the number of positives in each herd was used, using a true prevalence distribution calculated for each region. This was used in a Bayesian computation with herd sample results, giving a discrete probability distribution for positives within a herd. Each herd was then simulated as being positive or negative using a random sample from a binomial distribution. This was repeated 1,000 times to create an uncertainty distribution, where the 2.5th and 97.5th percentiles gave a 95% credible interval and the 50th percentile gave the most likely herd-level prevalence.
Univariable associations between hypothetical risk factors and serological status were assessed using logistic regression, with serological status as a binary outcome, without weighting for region. Potential risk factors, including age, sex, racing status and herd management practices during the previous year, were analysed as categorical variables at the individual level (including the herd-level management practice variables).
Variables with a p value <0·20 in univariable analysis were considered for inclusion in the multivariable models48. Collinearities between variables were examined with use of the Pearson’s coefficient, with a threshold of 0·4 or greater to define direct collinearity and –0·4 for inverse collinearity. Any collinear variables were to be excluded from the same multivariable model and tested in separate models, except for a-priori variables (age and sex), which were included alongside any collinear variables in the same model. Models that contained a singular variable were rebuilt with the singular variable excluded, with the singular variable then adjusted in a separate model using the same covariates.
Multivariable models were constructed using logistic regression, with herd as a random effect. Models were first constructed with a backwards stepwise method; the variable to be removed at each step was identified by comparing models with each of the non-a-priori variables removed and selecting the model with the lowest Akaike Information Criterion score, provided that removing the variable did not change the log odds ratio of any non-a-priori variables by more than 10%48. Models were then constructed with a forward stepwise method to ensure the same model was constructed by either method. Regression analysis confidence intervals were generated with the profile likelihood technique.
All statistical analyses were done in R (version 4.0.3).
Humans
Study design and participants
We conducted a cross-sectional study between September 27th, 2017, and October 11th, 2018, among livestock-owning households in four sub-regions of southern Jordan (Aqaba East, Aqaba West, Ma’an East, and Ma’an West) (Fig. 3). Livestock-owning households were randomly selected by a co-author (SN) using computer-generated randomisation lists (Stata, version 15.1) from a sampling frame provided by the MoA, comprising a list of all households owning one or more camels, and a list of those owning sheep or goats, within the four sub-regions. Both house and tent dwellings were eligible for inclusion.
All household members present at the time of sampling were invited to participate, except children younger than 5 years or individuals without the capacity to provide informed consent (as judged by a medical clinician and household head; the household head being the listed herd or flock owner in MoA lists). Household membership was defined as either: 1) self-identifying as a household member and sleeping in the household the previous night; or 2) being a household employee.
Written informed consent was obtained from all participating individuals at the time of sampling, along with parent or guardian consent for children aged 5–15 years. Institutional and national guidelines for the care of participating individuals were followed at all times.
Sample collection and laboratory procedures
Selected households were visited by members of the sampling team, including a medical practitioner and community facilitator, who provided study information, assessed eligibility, and obtained consent. Blood samples were collected from participating individuals together with administration of a structured questionnaire regarding potential risk factors for Brucella exposure during the previous 6 months, including questions regarding spatial data, personal information (including age and sex (observed), household information, consumption of livestock products, livestock engagement activities and hand hygiene (with a follow-up questionnaire administered among COH regarding milk boiling practices). An additional questionnaire was administered to the household head regarding household-level risk factors, including age and sex of any unsampled members. Questionnaires were administered in the local dialect on android tablets using the application Open Data Kit (version 1.10). Due to cultural sensitivity, information regarding participant sex was obtained from interviewer observation. Information regarding nationality was self-reported, according to national identity card or passport details.
Blood samples were collected in 8 mL plain serum vacutainer tubes and centrifuged centrally in Aqaba, at 2000 revolutions per minute (961×g) for 10 min, followed by serum collection and storage at –20 °C. All samples were stored centrally in Aqaba before transport to the diagnostic laboratory at the Veterinary Health Centre, JUST, Irbid for laboratory testing.
Serum samples were tested for antibodies against Brucella using the commercially available Brucella IgG ELISA classic, supplied by Institut Virion/Serion, GmbH, Würzburg, Germany68. The technique was performed according to the manufacturer’s instructions, with optical densities measured at 450 nm. Samples with a titre above 25 IU/ml were considered positive for IgG. Sensitivity and specificity provided by the manufacturer were >99% and 99.3% respectively69.
Serum samples were also tested using the Rose-Bengal test (RBPT) for the detection of antibodies against Brucella antigen supplied by Jovac, Amman, Jordan. Briefly, a sample of serum (0.05 ml) was mixed with 0.05 ml of antigen on a microscope slide to produce a zone approximately 2 cm in diameter. The mixture was agitated gently for four minutes at room temperature and observed for agglutination, the sample being considered positive where a visible reaction was observed. Sensitivity and specificity were assumed to be 87.5% and 100% respectively70. (Combined performance values, positive on either RBPT or ELISA being calculated as 90% and 99.3% respectively).
Outcomes
The primary outcome was Brucella seropositive status on either RBPT or ELISA. Secondary outcomes were potential associations between possible risk factors and seropositive status.
Statistical analysis
Sample size calculations were based on an expected seroprevalence of 4% among livestock-owning household members, a 4:1 ratio of COH members to NCOH, 80% power, with 90% confidence level, and a design effect of 1·25. The target sample size was calculated as 946 individuals: 757 COH members and 189 NCOH members. 160 COH (40 per region) and 40 NCOH (10 per region) were randomly selected for potential inclusion, based on an estimated mean household sample size of 4·7, with additional households randomly selected (80 COH, 20 per region; and 20 NCOH, five per region) as required.
Seroprevalence estimates and their 95% CIs were calculated at the individual level for COHs and NCOHs, weighted by the number of COHs and NCOHs in each administrative region. The intraclass correlation coefficient was calculated to assess the extent to which seropositive individuals clustered within households. Cohen’s κ coefficient was calculated as a measure of agreement between ELISA and RBPT results.
Univariable associations between hypothetical risk factors and serological status were assessed by means of mixed-effects logistic regression, with household as a random effect and serological status as a binary outcome, without weighting for the region. Potential risk factors including temporospatial data, personal information, health and hygiene data and history of livestock contact during the previous 6 months, were analysed as categorical variables at the individual level (including the household-level variables of household size, dwelling type, water source, any home air-cooling system, number of livestock herds/flocks nearby, and all herd/flock-specific variables).
Variables with a p value of less than 0·20 in univariable analysis were considered for inclusion in the multivariable models, except for any variables missing more than 10% of values, which were examined separately48. Collinearities between variables were examined with use of the Pearson’s coefficient, with a threshold of 0·4 or greater to define direct collinearity and –0·4 for inverse collinearity. Collinear variables were excluded from the same multivariable model and tested in separate models, except for a-priori variables (age and sex), which were included alongside any collinear variables in the same model.
Multivariable models were constructed using mixed-effects regression, with household as a random effect. Models were first constructed with a backwards stepwise method; the variable to be removed at each step was identified by comparing models with each of the non-a-priori variables removed and selecting the model with the lowest Akaike Information Criterion score, provided that removing the variable did not change the log odds ratio of any non-a-priori variables by more than 10%48. Models were then constructed with a forward stepwise method to ensure the same model was constructed by either method. Regression analysis confidence intervals were generated with the profile likelihood technique.
All statistical analyses were done in R (version 4.0.3) with mixed-effects models generated with use of the glmer function of the package lme4 (version 1.1–26).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All authors had full access to all data in the study. The data supporting the findings of this study is available upon reasonable request to the corresponding author, subject to approval by the relevant ethics committee, along with study questionnaires.
References
World Health Organization. Brucellosis Fact Sheet, WHO, [Available from: https://www.who.int/news-room/fact-sheets/detail/brucellosis (2020).
World Health Organization. Estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. WHO Geneva, (2015).
Franc, K. A., Krecek, R. C., Häsler, B. N. & Arenas-Gamboa, A. M. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health 18, e125 (2018).
Dean, A. S. et al. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 6, e1929 (2012).
Abo-shehada, M. N. & Abu-Halaweh, M. Seroprevalence of Brucella species among women with miscarriage in Jordan. East Mediterr. Health J. 17, 871–874 (2011).
Radostits O. M., GAY, C., Hinchcliff, K. W. & Constable, P. D. A textbook of the diseases of cattle, horses, sheep, pigs and goats. Veterinary Medicine 10th edition London: Saunders. 1548–1551 (2007).
Laine, C. G., Johnson, V. E., Scott, H. M. & Arenas-Gamboa, A. M. Global Estimate of Human Brucellosis Incidence. Emerg. Infect. Dis. 29, 1789–1797 (2023).
Dadar, M. et al. Human brucellosis and associated risk factors in the Middle East region: A comprehensive systematic review, meta-analysis, and meta-regression. Heliyon 10, e34324 (2024).
Alhussain, H. et al. Seroprevalence of camel brucellosis in Qatar. Trop. Anim. Health Prod. 54, e351 (2022).
Musallam, I. I., Abo-Shehada, M. N., Hegazy, Y. M., Holt, H. R. & Guitian, F. J. Systematic review of brucellosis in the Middle East: disease frequency in ruminants and humans and risk factors for human infection. Epidemiol. Infect. 144, 671–685 (2016).
Bagheri Nejad, R., Krecek, R. C., Khalaf, O. H., Hailat, N. & Arenas-Gamboa, A. M. Brucellosis in the Middle East: Current situation and a pathway forward. PLoS Negl. Trop. Dis. 14, e0008071 (2020).
Al-Ani, F. et al. Human and animal brucellosis in the Sultanate of Oman: an epidemiological study. J. Infect. Dev. Ctries 17, 52–58 (2023).
Al-Marzooqi, W. et al. Seroprevalence and Risk Factors of Brucellosis in Ruminants in Dhofar Province in Southern Oman. Vet. Med Int 2022, e3176147 (2022).
Shaqra QM, Abu Epidemiological aspects of brucellosis in Jordan. Eur. J. Epidemiol. 16, 581–584 (2000).
Abutarbush, S. M. et al. Implementation of One Health approach in Jordan: Review and mapping of ministerial mechanisms of zoonotic disease reporting and control, and inter-sectoral collaboration. One Health 15, e100406 (2022).
McAlester, J. & Kanazawa, Y. Situating zoonotic diseases in peacebuilding and development theories: Prioritizing zoonoses in Jordan. PLoS One 17, e0265508 (2022).
Abo-Shehada, M. N., Odeh, J. S., Abu-Essud, M. & Abuharfeil, N. Seroprevalence of brucellosis among high risk people in northern Jordan. Int J. Epidemiol. 25, 450–454 (1996).
Dajani, Y. F., Masoud, A. A. & Barakat, H. F. Epidemiology and diagnosis of human brucellosis in Jordan. J. Trop. Med Hyg. 92, 209–214 (1989).
Samadi, A., Ababneh, M. M., Giadinis, N. D. & Lafi, S. Q. Ovine and Caprine Brucellosis (Brucella melitensis) in Aborted Animals in Jordanian Sheep and Goat Flocks. Vet. Med Int 2010, e458695 (2010).
Al-Talafhah, A. H., Lafi, S. Q. & Al-Tarazi, Y. Epidemiology of ovine brucellosis in Awassi sheep in Northern Jordan. Prev. Vet. Med 60, 297–306 (2003).
Aldomy, F. M., Jahans, K. L. & Altarazi, Y. H. Isolation of Brucella melitensis from aborting ruminants in Jordan. J. Comp. Pathol. 107, 239–42 (1992).
Musallam, I. I., Abo-Shehada, M., Omar, M. & Guitian, J. Cross-sectional study of brucellosis in Jordan: Prevalence, risk factors and spatial distribution in small ruminants and cattle. Prev. Vet. Med 118, 387–96 (2015).
Abo-Shehada, M. N., Rabi, A. Z. & Abuharfeil, N. The prevalence of brucellosis among veterinarians in Jordan. Ann. Saudi Med 11, 356–357 (1991).
Al-Amr, M. et al. Epidemiology of human brucellosis in military hospitals in Jordan: A five-year study. J. Infect. Dev. Ctries 16, 1870–1876 (2022).
Abo-Shehada, M. N. & Abu-Halaweh, M. Risk factors for human brucellosis in northern Jordan. East Mediterr. Health J. 19, 135–140 (2013).
Issa, H. & Jamal, M. Brucellosis in children in south Jordan. East Mediterr. Health J. 5, 895–902 (1999).
Galali, Y. & Al-Dmoor, H. M. Miraculous properties of camel milk and perspective of modern science. J. Fam. Med. Dis. Prev. 5, e095 (2019).
Shimol, S. B. et al. Human brucellosis outbreak acquired through camel milk ingestion in southern Israel. Isr. Med Assoc. J. 14, 475–478 (2012).
Zhu, S., Zimmerman, D. & Deem, S. L. A review of zoonotic pathogens of dromedary camels. Ecohealth 16, 356–377 (2019).
Sprague, L. D., Al-Dahouk, S. & Neubauer, H. A review on camel brucellosis: a zoonosis sustained by ignorance and indifference. Pathog. Glob. Health 106, 144–149 (2012).
Almuzaini, A. M. An epidemiological study of brucellosis in different animal species from the al-qassim region, Saudi Arabia. Vaccines (Basel). 11, e694 (2023).
Dahl, M. O. Brucellosis in food-producing animals in Mosul, Iraq: A systematic review and meta-analysis. PLoS One 15, e0235862 (2020).
Hegazy, Y. M., Moawad, A., Osman, S., Ridler, A. & Guitian, J. Ruminant brucellosis in the Kafr El Sheikh Governorate of the Nile Delta, Egypt: prevalence of a neglected zoonosis. PLoS Negl. Trop. Dis. 5, e944 (2011).
Bardenstein, S., Gibbs, R. E., Yagel, Y., Motro, Y. & Moran-Gilad, J. Brucellosis Outbreak Traced to Commercially Sold Camel Milk through Whole-Genome Sequencing, Israel. Emerg. Infect. Dis. 27, 1728–1731 (2021).
Garcell, H. G. et al. Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. J. Infect. Public Health 9, 523–527 (2016).
Al-Majali, A. M., Al-Qudah, K. M., Al-Tarazi, Y. H. & Al-Rawashdeh, O. F. Risk factors associated with camel brucellosis in Jordan. Trop. Anim. Health Prod. 40, 193–200 (2008).
World Organizarion of Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals Ch 3.1.4 (WOAH, Paris, 2022).
Mangtani, P. et al. The prevalence and risk factors for human Brucella species infection in a cross-sectional survey of a rural population in Punjab, India. Trans. R. Soc. Trop. Med Hyg. 114, 255–263 (2020).
Alnaeem, A. et al. Some pathological observations on the naturally infected dromedary camels (Camelus dromedarius) with the Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia 2018-2019. Vet. Q 40, 190–197 (2020).
Chatty D. From Camel to Truck: The Bedouin in the Modern World. (White Horse Press, Cambridge, 2013).
Qur’an, Al-Ghashiyah (Surra 88), verse 17.
Al Zahrani, A. et al. Use of camel urine is of no benefit to cancer patients: observational study and literature review. East Mediterr. Health J. 29, 657–663 (2023).
Sahih Al Bukhari, The Book of Medicine (Book 76), Hadith 9.
Abutarbush, A. S., Hijazeen, Z., Doodeen, R. & Hawaosha, M. Analysis, description and mapping of camel value chain in jordan ministry of agriculture. Glob. Veterinaria 20, 144–152 (2019).
Abuelgasim, K. A. et al. The use of complementary and alternative medicine by patients with cancer: a cross-sectional survey in Saudi Arabia. BMC Complement Alter. Med 18, e88 (2018).
Shaalan, M. A. et al. Brucellosis in children: clinical observations in 115 cases. Int J. Infect. Dis. 6, 182–186 (2002).
Musallam, I. I., Abo-Shehada, M. N. & Guitian, J. Knowledge, attitudes, and practices associated with brucellosis in livestock owners in jordan. Am. J. Trop. Med Hyg. 93, 1148–1155 (2015).
Holloway, P. et al. Risk factors for middle east respiratory syndrome coronavirus infection among camel populations, Southern Jordan, 2014-2018. Emerg. Infect. Dis. 27, 2301–2311 (2021).
Dadar, M., Tiwari, R., Sharun, K. & Dhama, K. Importance of brucellosis control programs of livestock on the improvement of one health. Vet. Q 41, 137–151 (2021).
Radwan, A. I. et al. Control of Brucella melitensis infection in a large camel herd in Saudi Arabia using antibiotherapy and vaccination with Rev. 1 vaccine. Rev. Sci. Tech. 14, 719–732 (1995).
Food and Agriculture Organisation, Animal Production and Health Paper 156 Ch. 9 (FAO, Rome, 2003)
Holloway, P. et al. A cross-sectional study of Q fever in Camels: Risk factors for infection, the role of small ruminants and public health implications for desert-dwelling pastoral communities. Zoonoses Public Health 70, 238–247 (2023).
Ahad, A. A., Megersa, B. & Edao, B. M. Brucellosis in camel, small ruminants, and Somali pastoralists in Eastern Ethiopia: a One Health approach. Front Vet. Sci. 11, e1276275 (2024).
Mohammed, A. et al. Seroprevalence and risk factors of brucellosis in dromedary camels (Camelus dromedarius) in Sudan from 1980 to 2020: a systematic review and meta-analysis. Vet. Q 43, 1–15 (2023).
Dadar, M. & Alamian, S. Isolation of Brucella melitensis from seronegative camel: potential implications in brucellosis control. Prev. Vet. Med 185, e105194 (2020).
Blasco, J. M. A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats. Prev. Vet. Med 31, 275–283 (1997).
Vives-Sotoa, M. P.-G. A., Pereirab, J., Rodríguez-Sánchez, J. & Solerac, E. J. What risk do Brucella vaccines pose to humans? A systematic review of the scientific literature on occupational exposure. PLoS Negl. Trop. Dis. 18, e0011889 (2024).
Ducrotoy, M. J. et al. Integrated health messaging for multiple neglected zoonoses: Approaches, challenges and opportunities in Morocco. Acta Trop. 152, 17–25 (2015).
Mbye, M., Ayyash, M., Abu-Jdayil, B. & Kamal-Eldin, A. The texture of camel milk cheese: effects of milk composition, coagulants, and processing conditions. Front Nutr. 9, e868320 (2022).
Al-Zoubi, M. et al. WASH in Islam. Guide on Water, Sanitation and Hygiene (WASH) from an Islamic Perspective. (Sanitation for Millions, Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH, Germany 2020).
Elsohaby, I. et al. Bayesian Evaluation of Three Serological Tests for Diagnosis of Brucella infections in Dromedary Camels Using Latent Class Models. Prev. Vet. Med 208, e105771 (2022).
Khan, F. K. R. et al. Comparative performance study of four different serological tests for the diagnosis of dromedary brucellosis. J. Camel Pract. Res. 23, 213–217 (2016).
IDvet. ID Screen® Brucellosis Serum Indirect Multi-species, (https://www.innovative-diagnostics.com/produit/id-screen-brucellosis-serum-indirect-multi-species/) (2023).
Gwida, M. M. et al. Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res Notes 4, e525 (2011).
Getachew, T., Getachew, G., Sintayehu, G., Getenet, M. & Fasil, A. Bayesian Estimation of Sensitivity and Specificity of Rose Bengal, Complement Fixation, and Indirect ELISA Tests for the Diagnosis of Bovine Brucellosis in Ethiopia. Vet. Med Int 2016, e8032753 (2016).
Wernery, U. Camelid brucellosis: a review. Rev. Sci. Tech. 33, 839–857 (2014).
Beauvais, W., Orynbayev, M. & Guitian, J. Empirical Bayes estimation of farm prevalence adjusting for multistage sampling and uncertainty in test performance: a Brucella cross-sectional serostudy in southern Kazakhstan. Epidemiol. Infect. 144, 3531–3539 (2016).
Serion ELISA classic Brucella spp. IgG. https://www.serion-diagnostics.de/en/products/serion-elisa-classic-antigen/brucella/ (2023).
Al Dahouk, S., Tomaso, H., Nöckler, K., Neubauer, H. & Frangoulidis, D. Laboratory-based diagnosis of brucellosis–a review of the literature. Part II: serological tests for brucellosis. Clin. Lab 49, 577–589 (2003).
Ekiri, A. B. et al. Utility of the rose bengal test as a point-of-care test for human brucellosis in endemic african settings: a systematic review. J. Trop. Med 2020, e6586182 (2020).
Acknowledgements
We sincerely thank the Ministry of Health and Ministry of Agriculture in Jordan. Also Fares A. Altakhainah, Ghassab H. Hasanat, Hassan H. Alhusainat, Abdalmajeed M. Alajlouni, Suk Woo Lee, Yeong Kyoung Kang, and Hitesh Patel. This research was supported by a Medical Research Council Global Challenges Research Fund Foundation award (2017–2018, URN 2017 1735-3, JG), a Foreign and Commonwealth Office Bilateral Programme Budget Fund award (2013–2014, JG) from the British Embassy in Amman and the UK International Biosecurity Programme (within the context of a WOAH twinning initiative between the RVC and JUST).
Author information
Authors and Affiliations
Contributions
P.H., J.G., P.M., and J.C. designed the study, A.A., E.A., and W.H. facilitated fieldwork in Jordan, and T.H., P.H., and M.G. coordinated and carried out the field work. B.A. performed laboratory testing. M.G. managed the data, performed the statistical analysis, and generated figures, P.H., J.G., and S.N. participated in the statistical analysis. P.H. wrote the manuscript, M.G., J.G., P.M., J.C., I.M., I.P., T.H., and B.C. participated in writing the manuscript. All authors critically reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.A. was project coordinator for the project “Reducing the threat of MERS-CoV and avian influenza in Jordan and strengthening regional disease surveillance capacity”, supported by the US Department of Defence Threat Reduction Agency, Biological Threat Reduction Program. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Abdelmalik Ibrahim Khalafalla and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holloway, P., Gibson, M., Holloway, T. et al. Camel milk is a neglected source of brucellosis among rural Arab communities. Nat Commun 16, 861 (2025). https://doi.org/10.1038/s41467-024-55737-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55737-2
This article is cited by
-
Camel brucellosis: a narrative review of epidemiology and control strategies
Veterinary Research Communications (2026)
-
Evaluation of the genetic profiles of Brucella with different biotypes using MLVA and MLST techniques
Animal Diseases (2025)