Abstract
Although chiral substituents have been incorporated into ansa chains to stabilize the conformations of cyclophanes and modulate the biological activities of pharmaceuticals, the asymmetric syntheses of these atropisomers relies on substrate-induced diastereoselective macrocyclization. To the best of our knowledge, enantio-, atrop-, and diastereoselective macrocyclizations are yet to be reported. Herein, we describe an N-heterocyclic carbene (NHC) and chiral phosphoric acid (CPA) dual-catalytic process for the desymmetrization of 1,3-diols, to achieve macrocyclization and stereoselective control over two chiral elements. It is deduced that the hydrogen bonding of CPA with the 1,3-diols enhances the diastereoselectivity of the process. As a result, various planar-chiral cyclophanes bearing chiral ansa chains are synthesized. Thermodynamic experiments reveal that the presence of an all-carbon quaternary carbon center on the ansa chain significantly increases the rotational barriers of the cyclophanes. Moreover, density functional theory calculations suggest that the chiral substituent shrinks the ansa chain by compressing the bond angle, thereby rendering the conformational rotation reaction more challenging.
Similar content being viewed by others
Introduction
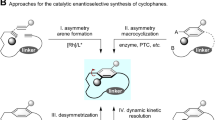
Three-dimensional (3D) conformations of molecules play a significant role in determining its properties, which is of particular importance in the area of drug discovery1. Over the past few decades, drug discovery has shifted from linear and disk-shaped molecules to spherical frameworks2,3, such as macrocycles that are rich in sp3-hybridiced carbon atoms. Due to their tunable conformations, flexible functional group organization, and a certain degree of constraint imparted by the macrocycles, these structures have been used to modulate challenging biotargets4,5,6,7. For example, cross-linked cyclophanes are a subset of macrocyclic molecules that possess unique 3D conformations8,9. In recent years, cyclophanes containing conformationally locked benzene rings have become increasingly common in the areas of asymmetric catalysis10,11, functional materials12,13, and drug development14,15. To produce a conformationally stable cyclophane, bulky substituents are required on the benzene ring to prevent rotation around the macrocycle (Fig. 1A, type I)8,9,16,17,18. In addition, short ansa chains also cause rotational obstruction of the benzene ring, resulting in type II planar-chiral cyclophanes (Fig. 1A, type II)8,9,16,17,18. Furthermore, the installation of a chiral substituent on the ansa chain increases the rotational barrier of the benzene ring, generating type III cyclophanes (Fig. 1A, type III). Notably, in type III atropisomers, both the chiral ansa chain and substituted benzene ring co-stabilize the conformation.
A Approaches to stabilize the conformation of planar-chiral cyclophanes. B Selected example of natural products and drugs containing a type III planar-chiral cyclophane skeleton. C Enantio- and atroposelective macrolactonization for the synthesis of planar-chiral cyclophanes. D Dual catalysis process to form cyclophanes bearing a chiral ansa chain. E Design of a dual catalysis protocol for enantio-, atrop-, and diastereoselective macrocyclization. CALB = Candida antarctical lipase B.
Conformationally defined type III atropisomers form the cores of many bioactive natural products, including Fijiolide A19 (an NFκB inhibitor) and Cihunamide B20,21, exhibit antibacterial activity against Gram-positive pathogens (Fig. 1B). Therefore, unlocking the conformation–biological activity relationships of cyclophanes is crucial in the context of drug discovery. Previously, chiral substituents were introduced on the ansa chain to stabilize the cyclophane conformation and tune the biological activities of pharmaceuticals14,15,22,23. In addition, the presence of a methyl substituent on the ansa chain of Lorlatinib, a United States Food and Drug Administration (US FDA)-approved drug for the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer, prevents rotation of the benzene ring, resulting in a significantly improved activity compared to its conformationally free derivative (Fig. 1B)14,23. Despite considerable efforts being devoted to the enantio- and atroposelective syntheses of planar-chiral cyclophanes, the majority of the works have focused on type I and II atropisomers24,25,26,27,28,29,30,31,32,33,34,35,36,37,38. In contrast, the asymmetric synthesis of type III cyclophanes relies on a chiral pool and substrate-induced diastereoselective macrocyclization39,40,41,42. To date, the enantioselective synthesis of planar-chiral cyclophanes bearing a central chiral ansa chain has been less explored.
To access planar-chiral cyclophanes, atroposelective macrocyclization has been considered as a straightforward approach. For example, Collins pioneered the biocatalytic macrolactonization protocol for the enantioselective and atroposelective syntheses of cyclophanes (Fig. 1C)43. Subsequently, Wang44, Chi45, and Zhao46 described N-heterocyclic carbene-catalyzed macrocyclizations to construct indolophane, naphthalenophane, and cyclophane skeletons, respectively. More recently, Collins extended the macrolactonization process to access cyclophanes bearing a chiral ansa chain by merging transition metal (TM) catalysis with biocatalysis (Fig. 1D)47. Although this strategy does not achieve planar-chiral control, the dual-catalysis protocol offers a potential solution to address the assembly of planar and central chiralities in a single step.
Inspired by Collins’ dual-catalysis model47 and Wang’s desymmetrization approach for the synthesis of medium-sized ring48, it was envisioned that the desymmetrization of 1,3-diols by an intramolecular N-heterocyclic carbene (NHC)-bound acyl azolium would achieve macrocyclization and stereoselective control over the two chiral elements49,50,51,52,53. Furthermore, although the hydrogen bonding of a chiral phosphoric acid (CPA) catalyst with 1,3-diols offers enhanced diastereoselectivities (Fig. 1E)48, this approach is challenging due to several difficulties. More specifically, no enantio-, atrop-, and diastereoselective macrocyclization protocols are known, and the thermodynamic stabilities of type III planar-chiral cyclophanes have yet to be explored in detail. Furthermore, the desymmetrization protocol has not been reported within the context of macrocyclization. Given our group’s interest in the asymmetric synthesis of cyclophanes46,54,55,56,57,58,59, we herein report a dual-catalytic process for the formation of planar-chiral cyclophanes. This method combines NHC and CPA catalysis to afford type III cyclophanes. The stereoselectivity of this process is evaluated, and preliminary conformational stability studies are performed to compare the rotational barriers of the type III planar-chiral [15]cyclophanes with those of derivatives bearing no ansa chain substituents.
Results and discussion
Optimization of reaction conditions
This investigation began with investigating the macrocyclization of linear substrate 1 bearing a prochiral alkyl chain. As shown in Table 1, using 4 Å molecular sieves (4 Å MS) as an additive, 3,3’,5,5’-tetratert-butyldiphenoquinone (DQ) as an oxidant, K2CO3 as a base, and tetrahydrofuran (THF) as the solvent, a series of carbene pre-catalysts was tested for the desymmetrization process. The N substituent of the indanol-derived NHC was found to have a clear impact on the diastereoselectivity of the reaction (Table 1, entries 1–6), with N-C6F5 II and N-2,4,6-(iPr)3C6H2 IV yielding product 2 with high enantiomeric ratio (er) values and low diastereomeric ratios (dr; Table 1, entries 2 and 4). The diastereoselectivity increased when the N-2,6-(Et)2C6H3 precatalyst VI was used (Table 1, entry 6; 14:1 dr). In addition, the pyrrolotriazolium- and amino alcohol-derived pre-catalysts VII and VIII afforded the desired product in moderate dr and er values (Table 1, entries 7 and 8). Previous reports have suggested that chiral Brønsted acids can take part in hydrogen-bonding interactions with 1,3-diols and NHC-bound acyl azolium moieties, thereby enhancing the enantioselectivity in the desymmetrization of 1,3-diols for the synthesis of medium-sized rings48. Thus, as expected, the addition of spirophosphoric acid produced macrocycles with enhanced diastereoselectivities (Table 1, entries 9 and 10). Furthermore, the configurations of the NHC and CPA catalysts do not affect stereo control in the reaction48. However, in the current system, the incorporation of IX (enantiomer of VI) into the CPA catalyst further improved the dr (Table 1, entry 11). Variations in the base and solvent had no significant effect on the reaction outcome (Table 1, entries 12–19), with the optimal conditions being those described in Table 1: entry 11.
Scope of the substrates
With the optimized reaction conditions in hand, the substrate scope of the macrolactonization reaction was investigated using various substituted 1,3-diols (Fig. 2). It was found that a methyl substituent was well tolerated, affording the desired cyclophane (3) in a 60% yield with excellent er and dr values. Alkyl chain non-substituted substrate afforded a slightly reduced diastereoselectivity (4, 10:1 dr). Substrates bearing benzyl derivatives exhibited good reactivities and stereoselectivities (5–9). Naphthalen-2-ylmethyl (10), and cinnamyl (11), and propargyl (12) groups were also compatible, indicating broad functional group tolerance.
The substrate scope was further investigated in terms of the effect of the benzene ring. More specifically, substrates bearing phenyl, 4-methoxyl-phenyl, 4-tbutyl-phenyl, 4-pyridyl, 2-naphthalenyl, and benzofuranyl groups at the C-5 position were tolerated, delivering the desired products (13–15, 17–19) with excellent er and dr values. Although the substrate containing a 4-phenyl group afforded high er, a poor dr was observed (16, 3:1 dr). In addition, substrates bearing various 2,5-dialkoxyl groups smoothly underwent macrocyclization (20–21). Subsequently, the absolute configuration of 14 was determined by single-crystal X-ray crystallography (CCDC 2383321), and the other structures were assigned by analogy. The analysis for 14 revealed that the benzene ring plane is bent with dihedral angle of α1 = 1.58° and α2 = 1.49° (where α1 represents the angle between the m − m’−p’ and m − m’−o’−o planes and α2 represents the angle between the o − o’−p and m − m’−o’−o planes). Moreover, bonds adjacent to the benzene ring (e.g., C1–C2 and C3–C4) are twisted out of the aryl ring plane with the angles of β1 = 3.70° and β2 = 3.54° (where β1 represents the angle between the C1 and C2 bond and m − m’−p’ plane, β2 represents the angle between the C3 and C4 bond and o − o’−p plane)59.
The effects of ansa chain length and substituents on the macrocyclization reaction were also evaluated. It was found that reducing the ansa chain length to 11 and 12 atoms resulted in a decrease in the yield and stereoselectivity, probably due to the conformational stain present in the [11]- and [12]cyclophane systems (22–23)44. It was also found that 14- and 15-membered ansa chains containing ether groups were tolerated (24–25), and some 15-membered substrates delivered conformationally stable atropisomers (26–30). However, a further increase in the ansa chain length yielded a product with low diastereoselectivity (31, 2:1 dr), and an inseparable broad peak was observed using chiral HPLC. The rotational transition state of 31 was determined to be TSrot(31), which possessed a computed barrier of 21.9 kcal/mol and half-life of 11.0 min for the rotational reaction. These results suggested that bond flip of benzene ring around the macrocycle might been fast. In addition, 17- and 18-membered ansa chains led to the loss of planar chirality (32–33).
Synthetic transformations
The applicability of this synthetic transformation was subsequently illustrated by performing a Dess–Martin oxidation of the primary alcohol moiety in cyclophane 2, yielding the corresponding aldehyde (34) without a loss of stereoselectivity (Fig. 3A). In addition, functional group manipulation of 2, including mesylation, azidation, Staudinger reduction, and thioureation of the amine, afforded a type III cyclophane-derived thiourea catalyst 35. Although 35 exhibited a low catalytic activity in the eight-membered ring formation reaction60, it offers a potential solution for improved catalyst design.
A Synthetic transformations the potential applicability. B Ring cleavage of 30 and 39. C Ring distortion of 31. Reaction conditions: (i) Dess–Martin oxidant, CH2Cl2, 0 °C, 95% yield. (ii) MsCl, Et3N, CH2Cl2, 0 °C, 93% yield. (iii) NaN3, DMSO, 100 °C, 56% yield. (iv) Ph3P, THF/H2O, 25 °C. (v) Phenyl isothiocyanate, THF, rt, 65% yield. (vi) MOMCl, DIPEA, CH2Cl2. (vii) NaOH, MeOH.
To investigate the thermodynamic epimerization for type III cyclophanes, 30 was heated in toluene at 110 °C for 12 h, resulting decreased dr and er values (39; 2:1 dr, 95.5:4.5 er for the major isomer and 85.5:14.5 er for the minor isomer) (Fig. 3B). Ring cleavage of compounds 30 and 39 yielded the same product 40 in a 95.5:4.5 er, revealing that the epimerization of 30 occurred due to the loss of planar chirality during the heating process (for details, see the Supplementary Fig. S5). Furthermore, the high degree of ring distortion in 31 allowed the preparation of the acyclic product 41 in a 93:7 er, indicating that central chirality control leads to efficient macrocyclization during the formation of 31 (Fig. 3C).
Configurational stability studies
Previous reports have suggested that the introduction of a chiral substituent onto the ansa chain stabilizes the conformations of planar-chiral cyclophanes15,22,61. However, very few detailed configurational stability studies have been performed. Thus, to clarify the effects of ansa chain substitution on the rotational barrier, thermodynamic experiments were carried out (Fig. 4A; for details, see the Supplementary Information Section 8 and Supplementary Data 1). For the ansa chain of non-substituted [14]cyclophane 42, a rotational barrier of 37.6 kcal/mol was identified, along with a half-life of 323.1 h for the rotational reaction at 150 °C. However, no racemization was observed for the type III [14]cyclophane 2 after heating at the same temperature for 120 h. A similar stabilizing effect was observed for [15]cyclophanes (43 vs. 26)46. Additionally, changing the methyl substituent (26) to a benzyl (27) or 3,5-di-tert-butylbenzyl (30) group resulted in an increased rotational barrier, indicating a clear correlation between the ansa chain chiral substituent and conformational stability.
Subsequently, computational studies were performed to explore the origin of the configurational stability caused by chiral substituents on the ansa chain. For this purpose, the rotational transition states 43, 26, 27, and 30 were optimized and designated as TSrot(43), TSrot(26), TSrot(27), and TSrot(30), respectively. The calculated results indicate that the energy barriers for these transition states increase in the order TSrot(43) <TSrot(26) <TSrot(27) <TSrot(30), which is in good agreement with the experimental results (Fig. 4B). Geometry analyses of these transition states suggest that the presence of the chiral substituent on ansa chain should reduce the C1 − C2 − C3 bond angle, with larger substituents exhibiting a greater degree of reduction (119.02° <115.64° <115.40° <115.14°) (Fig. 4C). This bond angle reduction shrinks the ansa chain and inhibits the rotational process, as reflected by distortion of the methoxy group and benzene ring in each transition state. Notably, distortion of the C7 − O − C8 bond angle and the increase in the C4 − C5 − C6 − C7/C4 − C10 − C9 − C7 dihedral angle occurred to approximately the same extent. In addition, steric hindrance between the chiral substituent and ester group may also cause the higher energy barriers observed for TSrot(27) and TSrot(30). Finally, the key steric repulsive interactions were demonstrated using noncovalent interactions, as detailed in the Supplementary Information.
In summary, this work demonstrates an enantio-, atrop-, and diastereoselective macrolactonization reaction based on a combination of NHC and CPA catalysis to prepare type III cyclophanes. It was found that the NHC-bound acyl azolium promoted the desymmetrization of 1,3-diols within the context of macrocyclization and stereoselective control over the two chiral elements. In addition, hydrogen-bonding between CPA, the 1,3-diols, and the acyl azolium moieties further stabilized the transition state, enhancing the diastereoselectivity of the reaction. Consequently, this approach afforded a variety of planar-chiral cyclophanes bearing chiral ansa chains. The potential applications of the obtained type III cyclophane was illustrated by its transformation into a thiourea catalyst. Conformational stability studies indicated that the incorporation of an all-carbon quaternary carbon center on the ansa chain significantly increased the rotational barrier of the benzene ring around the macrocycle. Furthermore, computational studies revealed that the presence of a chiral substituent shrinks the ansa chain by compressing the bond angle, thereby hindering the rotational process. Notably, previous approaches for the syntheses of type III cyclophanes relied on a substrate-induced diastereoselective macrocyclization process. Thus, the dual catalytic protocol described herein offers a potential and efficient route to these atropisomers. While the dual-catalytic protocol shows promise, further work is needed to optimize yields across a broader substrate range and to evaluate its scalability for asymmetric catalysis and library inclusion. These studies are under investigation in our laboratory, and the results will be presented in due course.
Methods
General procedure for the synthesis of cyclophanes
To a 15 mL Schlenk tube equipped with a magnetic stirring bar was added substrate (0.10 mmol, 1.0 equiv.), NHC IX (4.3 mg, 0.01 mmol, 0.1 equiv.), CPA (3.2 mg, 0.01 mmol, 0.1 equiv.), K2CO3 (2.76 mg, 0.02 mmol, 0.2 equiv.), DQ (49.0 mg, 0.12 mmol, 1.2 equiv.) and 4 Å MS (50 mg). The tube was closed with a septum, evacuated, and refilled with nitrogen (3 cycles). After that, dry THF (10.0 mL) was added to the reaction mixture at 25 oC and stirred for 24 h. Upon the reaction completion (monitored by TLC), the solvent was evaporated and the residue was purified by silica gel column chromatography affording products.
Data availability
Experimental details, characterization of compounds and copies of NMR data are available with the submitted manuscript. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2383321 (for 14). This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Data supporting the findings of this manuscript are also available from the corresponding author upon request.
References
Diaz, D. B. et al. Illuminating the dark conformational space of macrocycles using dominant rotors. Nat. Chem. 13, 218–225 (2021).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Ciardiello, J. J., Stewart, H. L., Sore, H. F., Galloway, W. R. J. D. & Spring, D. R. A novel complexity-to-diversity strategy for the diversity-oriented synthesis of structurally diverse and complex macrocycles from quinine. Bioorg. Med. Chem. 25, 2825–2843 (2017).
Isidro-Llobet, A. et al. Diversity-oriented synthesis of macrocyclic peptidomimetics. Proc. Natl. Acad. Sci. USA. 108, 6793–6798 (2011).
O’Connell, K. M. G. et al. A two-directional strategy for the diversity-oriented synthesis of macrocyclic scaffolds. Org. Biomol. Chem. 10, 7545–7551 (2012).
Lau, Y. H. et al. Double strain-promoted macrocyclization for the rapid selection of cell-active stapled peptides. Angew. Chem. Int. Ed. 54, 15410–15413 (2015).
Cram, D. J. & Cram, J. M. Cyclophane chemistry: bent and battered benzene rings. Acc. Chem. Res. 4, 204–213 (1971).
Tobe, Y. & Sonoda, M. In Modern Cyclophane Chemistry. (Wiley-VCH, 2004).
Hassan, Z., Spuling, E., Knoll, D. M., Lahann, J. & Bräse, S. Planar chiral [2.2]paracyclophanes: from synthetic curiosity to applications in asymmetric synthesis and materials. Chem. Soc. Rev. 47, 6947–6963 (2018).
Yoshida, Y., Kanashima, Y., Mino, T. & Sakamoto, M. Asymmetric syntheses and applications of planar chiral hypervalent iodine(V) reagents with crown ether backbones. Tetrahedron 75, 3840–3849 (2019).
Roy, I., David, A. H. G., Das, P. J., Pe, D. J. & Stoddart, J. F. Fluorescent cyclophanes and their applications. Chem. Soc. Rev. 51, 5557–5605 (2022).
Li, J. et al. Chirality of perylene diimides: design strategies and applications. Angew. Chem. Int. Ed. 61, e202202532 (2022).
Syed, Y. Y. Lorlatinib: first global approval. Drugs 79, 93–98 (2019).
Glunz, P. W. et al. Atropisomer control in macrocyclic factor VIIa inhibitors. J. Med. Chem. 59, 4007–4018 (2016).
Liu, Z., Nalluri, S. K. M. & Stoddart, J. F. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 46, 2459–2478 (2017).
Tanaka, K. Catalytic enantioselective synthesis of planar chiral cyclophanes. Bull. Chem. Soc. Jpn. 91, 187–194 (2018).
Kotha, S., Shirbhate, M. E. & Waghule, G. T. Selected synthetic strategies to cyclophanes. Beilstein J. Org. Chem. 11, 1274–1331 (2015).
Nam, S.-J. et al. Fijiolides A and B, inhibitors of TNF-α-induced NFκB activation, from a marine-derived sediment bacterium of the genus Nocardiopsis. J. Nat. Prod. 73, 1080–1086 (2010).
An, J. S. et al. Discovery and biosynthesis of Cihunamides, macrocyclic antibacterial RiPPs with a unique C−N linkage formed by CYP450 catalysis. Angew. Chem. Int. Ed. 62, e202300998 (2023).
Yu, L., Nagata, Y. & Nakamura, H. Atroposelective total synthesis of Cihunamide B. J. Am. Chem. Soc. 146, 2549–2555 (2024).
Elleraas, J. et al. Conformational studies and atropisomerism kinetics of the ALK clinical candidate Lorlatinib (PF-06463922) and desmethyl congeners. Angew. Chem. Int. Ed. 55, 3590–3595 (2016).
Wang, Z. et al. Recent progress toward developing axial chirality bioactive compounds. Eur. J. Med. Chem. 243, 114700–114706 (2022).
Wang, Y. & Joullié, M. M. Approaches to cyclophane-types of cyclopeptide alkaloids. Chem. Rec. 21, 906–923 (2021).
López, R. & Palomo, C. Planar chirality: a mine for catalysis and structure discovery. Angew. Chem. Int. Ed. 61, e202113504 (2022).
Li, J., Huang, D., Fang, Q. & Zhao, C. Progress on synthesis of optically pure mechanical planar chiral rotaxanes and planar chiral macrocycle molecules. Chin. Sci. Bull. 67, 2383–2392 (2022).
Yang, G. & Wang, J. Recent advances on catalytic atroposelective synthesis of planar-chiral macrocycles. Angew. Chem. Int. Ed. 63, e202412805 (2024).
Dong, Z., Li, J. & Zhao, C. Catalytic enantioselective macrocyclization for the synthesis of planar-chiral cyclophanes: recent updates. Eur. J. Org. Chem. 27, e202400841 (2024).
Zhao, Y.-H., Zhu, D. & Chen, Z.-M. Enantioselective construction of planar-chiral molecules by catalytic asymmetric late-stage functionalizations. ChemCatChem. 16, e202401312 (2024).
Wang, D., Shao, Y.-B., Chen, Y., Xue, X.-S. & Yang, X. Enantioselective synthesis of planar-chiral macrocycles through asymmetric electrophilic aromatic amination. Angew. Chem. Int. Ed. 61, e202201064 (2022).
Yu, S., Shen, G., He, F. & Yang, X. Asymmetric synthesis of planar-chiral macrocycles via organocatalyzed enantioselective macrocyclization. Chem. Commun. 58, 7293–7296 (2022).
Zhu, D. et al. Enantioselective synthesis of planar-chiral sulfur-containing cyclophanes by chiral sulfide catalyzed electrophilic sulfenylation of arenes. Angew. Chem. Int. Ed. 63, e202318625 (2024).
Nogami, J. et al. Enantioselective synthesis of planar chiral zigzag-type cyclophenylene belts by rhodium-catalyzed alkyne cyclotrimerization. J. Am. Chem. Soc. 142, 9834–9842 (2020).
Akagawa, K., Higuchi, J., Yoshikawa, I. & Kudo, K. Kinetic resolution of ansa cyclophanes by peptide-catalyzed Aldol/Retro-Aldol reactions. Eur. J. Org. Chem. 2018, 5278–5281 (2018).
Ding, Q., Wang, Q., He, H. & Cai, Q. Asymmetric synthesis of (−)-Pterocarine and (−)-Galeon via chiral phase transfer-catalyzed atropselective formation of diarylether cyclophane skeleton. Org. Lett. 19, 1804–1807 (2017).
Wei, S., Chen, L.-Y. & Li, J. Enantioselective synthesis of planar chiral macrocyclic metacyclophanes by Pd-catalyzed C–O cross-coupling. ACS Catal 13, 7450–7456 (2023).
Tan, L., Sun, M., Wang, H., Kim, J. & Lee, M. Enantiocontrolled macrocyclization by encapsulation of substrates in chiral capsules. Nat. Synth. 2, 1222–1231 (2023).
Yang, G., Liu, S., Ji, S., Wu, X. & Wang, J. Pd/NHC sequentially catalyzed atroposelective synthesis of planar-chiral macrocycles. Chem. Sci. 15, 19599–19603 (2024).
Jia, Y., Bois-Choussy, M. & Zhu, J. Synthesis of diastereomers of Complestatin and Chloropeptin I: substrate-dependent atropstereoselectivity of the intramolecular Suzuki-Miyaura reaction. Angew. Chem. Int. Ed. 47, 4167–4172 (2008).
Jia, Y., Bois-Choussy, M. & Zhu, J. Synthesis of DEFG ring of Complestatin and Chloropeptin I: highly atropdiastereoselective macrocyclization by intramolecular Suzuki-Miyaura reaction. Org. Lett. 9, 2401–2404 (2007).
Wang, Z., Bois-Choussy, M., Jia, Y. & Zhu, J. Total synthesis of Complestatin (Chloropeptin II). Angew. Chem. Int. Ed. 49, 2018–2022 (2010).
Hu, W. et al. Stereocontrolled and efficient total synthesis of (−)-Stephanotic acid methyl ester and (−)-Celogentin C. Org. Lett. 12, 956–959 (2010).
Gagnon, C. et al. Biocatalytic synthesis of planar chiral macrocycles. Science 367, 917–921 (2020).
Yang, G., He, Y., Wang, T., Li, Z. & Wang, J. Atroposelective synthesis of planar-chiral indoles via carbene catalyzed macrocyclization. Angew. Chem. Int. Ed. 63, e202316739 (2024).
Lv, X. et al. Carbene organic catalytic planar enantioselective macrolactonization. Nat. Commun. 15, 958 (2024).
Wang, J. et al. N-heterocyclic carbene-catalyzed highly enantioselective macrolactonization to access planar-chiral macrocycles. Org. Lett. 26, 1040–1045 (2024).
Guerrero-Morales, J., Scaglia, M., Fauran, E., Lepage, G. & Collins, S. K. Chemoenzymatic synthesis of macrocycles via dynamic kinetic resolution of secondary alcohols. Nat. Synth. 3, 1275–1282 (2024).
Wu, Z. & Wang, J. Enantioselective medium-ring lactone synthesis through an NHC-catalyzed intramolecular desymmetrization of prochiral 1,3-diols. ACS Catal. 7, 7647–7652 (2017).
Li, B.-S., Wang, Y., Proctor, R. S. J., Jin, Z. & Chi, Y. R. Carbene-catalyzed desymmetrization of 1,3-diols: access to optically enriched tertiary alkyl chlorides. Chem. Commun. 52, 8313–8316 (2016).
Zhang, Y., Cai, H., Gan, X. & Jin, Z. N-heterocyclic carbene-catalyzed enantioselective (dynamic) kinetic resolutions and desymmetrizations. Sci. China Chem. 67, 482–511 (2024).
Zhang, M., Yang, X., Peng, X., Li, X. & Jin, Z. Asymmetric construction of axial and planar chirality with N-heterocyclic carbene (NHC) organocatalysis. Sci. China Chem. 68, 815–825 (2025).
Lv, X. et al. Access to planar chiral ferrocenes via N-heterocyclic carbene-catalyzed enantioselective desymmetrization reactions. ACS Catal. 12, 2706–2713 (2022).
Lv, Y. et al. Asymmetric synthesis of planar chiral carbonitriles and amines via carbene-catalyzed kinetic resolution. Org. Lett. 26, 1584–1588 (2024).
Li, J. & Zhao, C. Highly enantioselective synthesis of planar-chiral cyclophanes through a Brønsted acid-catalyzed asymmetric transfer hydrogenation. ACS Catal. 13, 14155–14162 (2023).
Dong, Z., Li, J., Yao, T. & Zhao, C. Palladium-catalyzed enantioselective C−H olefination to access planar-chiral cyclophanes by dynamic kinetic resolution. Angew. Chem. Int. Ed. 62, e202315603 (2023).
Li, J. et al. N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution. Nat. Commun. 15, 2338 (2024).
Li, J., Dong, Z., Zhai, H., Wu, J. & Zhao, C. An approach for highly enantioselective synthesis of meta-disubstituted [n]paracyclophanes. J. Org. Chem. 89, 15374–15379 (2024).
Zhai, H. et al. Rhodium(III)-catalyzed atroposelective indolization to access planar-chiral macrocycles. J. Am. Chem. Soc. 146, 29214–29223 (2024).
Li, J., Dong, Z., Liu, Y., Liu, X. & Zhao, C. Catalytic enantioselective synthesis of monosubstituted [n]paracyclophane using a desymmetrization/kinetic resolution sequence. Chem. Eur. J. 31, e202404610 (2025).
Wang, J., Li, J. & Zhao, C. A Lewis acid-promoted Michael addition and ring-expansion cascade for the construction of nitrogen-containing medium-sized rings. Molecules 28, 1650 (2023).
Glunz, P. W. Recent encounters with atropisomerism in drug discovery. Bioorg. Med. Chem. Lett. 28, 53–60 (2018).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 22171027 to C.Z.), Fundamental Research Funds for the Central Universities (No. 2233300007 to C.Z.), Natural Science Foundation of Shandong Province (No. ZR2022QB063 to K.L.), and Research Fund of Jining University (2022ZYRC9 to K.L.).
Author information
Authors and Affiliations
Contributions
C.Z. conceived and designed the experiments. J.W. and Y.W. performed the experiments. K.L. and T.L. carried out the DFT calculations. C.Z. and K.L. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xingxing Wu, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Lv, K., Wen, Y. et al. Enantio-, atrop-, and diastereoselective macrolactonization to access type III cyclophanes. Nat Commun 16, 3170 (2025). https://doi.org/10.1038/s41467-025-58241-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58241-3