Abstract
Asymmetric binary catalysis, particularly combining chiral Brønsted acids with Lewis acids, is an emerging strategy in synthetic chemistry. Although a few catalyst combinations exist for stereoselective transformations, their scope is generally limited to pre-organized, activated substrates. Here, we report binary catalysis combining an organosuperacid with bismuth, where the counteranion of chiral N-triflyl phosphoramide acts as a flexible-coordinating ligand. This system demonstrates exceptional reactivity and enantioselectivity in the asymmetric allylation of α-keto thioesters, forming enantio-enriched α-hydroxy thioesters with a tetra-substituted stereogenic carbon center (up to > 99% yield and 97% ee). The success is attributed to bismuth’s flexibility during activation, enhancing also interactions with the thioester-tethered substrate. Integrated experimental, analytical, and computational studies highlight the unique assembly enabled by the chiral Brønsted acid and bismuth salt system.
Similar content being viewed by others
Introduction
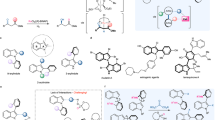
Chiral α-hydroxy carbonyls hold significant importance as essential building blocks for fine chemicals and, more critically, for the synthesis of pharmaceuticals, where their stereochemical integrity is often crucial for biological activity (Fig. 1a). Therefore, developing efficient chemical processes for synthesizing such chiral structures has become crucial in asymmetric catalysis. Asymmetric binary catalysis can be a highly promising tool for achieving these challenging stereoselective reactions. Over the past decades, the combination of transition metals (e.g., Ti, Pd, Rh, Ag, Au, and Fe) or rare-earth metals (e.g., Yb) with chiral Brønsted acid organocatalysts has emerged as a useful approach, enabling highly efficient asymmetric transformations (Fig. 1b)1,2,3,4,5,6,7,8. A conventional approach to asymmetric Lewis acid catalysis comprehends utilizing chiral amine- or alcohol-modified Lewis basic ligands coordinated with metal centers, facilitated by structurally fixed, redox-neutral chiral complexes9. Contrary to this, binary strategy leverages the distinct activation modes of each component efficiently and synergistically: the metal functions as a redox1 or Lewis acidic10 center, while the organic acid serves as a precursor for counteranion or mono-/bidentate ligand after deprotonation. Chiral phosphoric acids11,12 (CPAs: pKa = 12.7 (in MeCN, measured)13, when (R = Ph)) have exhibited wide applicability as organic counterparts in a plethora of catalytic reactions14,15,16. Considerable catalytic activities and selectivities have been achieved by utilizing the unique metal–CPA binary platforms based on their strong coordinating abilities, which offer benefits that a sole catalyst cannot realize17,18,19,20. Recent studies have expanded their usage in conjunction with main group elements (e.g., Li, Al, Ca, and Mg)10,21,22,23.
a Representative bioactive compounds bearing chiral α-hydroxy carbonyls with stereogenic carbon center(s). b Previous works: [CPAs] X [Lewis acids]. c Underdeveloped binary acid catalysis: [NTPA] X [Bi]. d This work: Thioester-directed asymmetric allylation via organic-bismuth binary acid catalysis. Ac acetyl, Bn benzyl, Boc tert-butyloxycarbonyl, Bz benzoyl, Cy cyclohexyl, DFT density functional theory, DMSO dimethyl sulfoxide, ee enantiomeric excess, Et ethyl, iBu isobutyl, iPr isopropyl, Ln ligand, Me methyl, OAc acetate, Ph phenyl, Tf trifluoromethanesulfonyl (triflyl).
Bismuth (Bi) is a post-transition metal that offers a sustainable alternative to conventional metals for various applications24,25,26,27. Unlike other heavy metals, bismuth is cost-effective and significantly stable in aqueous environments. Additionally, it has exceptionally low toxicity—lower than that of common table salt—making it safe for use in oral medications (e.g., bismuth subcitrate: for the treatment of Helicobacter pylori infections28). Despite these advantages, only a limited number of asymmetric bismuth-based binary catalytic approaches have been disclosed in recent years. Cheng, Li, and coworkers showed the synergistic effect of Bi(III) salt and CPAs in diverse asymmetric transformations, such as 1,2-allylation reactions, kinetic resolution, and Mukaiyama–Mannich reaction29,30,31,32,33,34,35. Although these Bi–CPA binary systems have demonstrated remarkable success, their applications have been mainly limited to electrophiles with reactive, cyclic (rigid) imine or carbonyl backbones, such as isatin-derived N-protected ketimines, cyclic oxocarbenium ions, N-protected isatins, dibenzo[b,f][1,4]oxazepines, β,γ-unsaturated α-keto esters, and racemic 2H-azirines. Catalytic reactions utilizing functionalized acyclic substrates are deemed challenging, likely due to the weaker binding of substrate to the binary active site in these systems. Therefore, we hypothesized that a chiral strong Brønsted acid might be an efficient precursor for the counteranion of the elaborate system because it contains multiple transient coordinating functional atoms. This flexible coordination may promote a harmonic synergy between the complex’s stability and its catalytic activity by leveraging the coordination–decoordination equilibrium36,37. While one part of the ligand remains firmly anchored, a weakly binding unit of the potential multidentate ligand can reversibly dissociate from the reaction center, creating an active site for substrate interaction. In this context, we envisioned that a bismuth-counteranion of chiral organosuperacid38,39,40 as a ligand could pave the way for a new approach in asymmetric binary catalysis. This strategy may offer broad compatibility towards challenging substrates and achieve high levels of regio- and stereoselectivity (Fig. 1c).
Herein, we report a binary catalytic system that combines a chiral organosuperacid with a Bi(III) salt (Fig. 1d). We found that the bismuth complexes of counteranions derived from chiral N-triflyl phosphoramides38,40 (NTPAs: pKa = 6.4 (in MeCN, measured)13 and pKa = −3.36 (in dimethyl sulfoxide (DMSO), calculated)41, respectively, when (R = Ph)), efficiently catalyze the enantioselective allylation reaction of α-keto thioesters. This discovery is significant because (i) the use of asymmetric binary acid catalysis employing chiral organosuperacids as flexible-coordinating ligand precursors represents an underexplored approach (with only a few chiral NTPAs documented as counteranions42,43,44,45 or as compatible Brønsted acids46,47,48,49,50 in metal catalysts), and (ii) the underlying mechanism of chiral induction in post-transition metals such as bismuth has been scarcely investigated owing to their complex coordination behaviors. By utilizing the distinctive binding properties of this catalytic approach, we achieved (iii) excellent enantioselectivities in challenging allylation reactions51,52,53,54,55 under mild conditions, enabling the formation of thioester-tethered tetra-substituted stereogenic carbon center in a single protocol. Thioesters are highly reactive yet sufficiently stable intermediates and play a pivotal role in biology and organic synthesis, enabling key metabolic processes and further versatile chemical transformations56. Experimental, analytical, and computational mechanistic studies corroborated the cooperative covalent and non-covalent interactions between the Bi(III)–NTPA binary acid framework and the coordinated substrates.
Results and discussion
Catalyst evaluation
For the model reaction, we selected S-isopropyl α-keto thioester 1a as the acceptor to study catalytic efficiency for acyclic substrates, underdeveloped in previous catalytic systems. Initially, we investigated the reaction between 1a and allylboronic acid pinacol ester (allyl–Bpin, 2), using 3 mol% of Bi(OAc)3 and 1,1′-bi-2-naphthol (BINOL)-derived chiral acid organocatalyst, in CH2Cl2 at ambient temperature for 24 h (Table 1). By exploiting CPA-1 (CPA featuring 3,3′-bis(2,4,6-triisopropylphenyl)-substituents), we obtained the desired chiral S-isopropyl α-hydroxy thioester 3a in 56% conv., whereas with low enantioselectivity (27% ee, Entry 1). Other attempted CPAs did not render satisfactory results (Entries 2–6), driving us to explore alternative core structures, such as chiral thiophosphoric acids. While some promising results were obtained, the efficacy of the CTA scaffold proved to be limited (Entries 7–10).
For stronger acids like chiral NTPAs, an improvement in catalytic performance in terms of reactivity and enantioselectivity was observed (Entries 11–17). Notably, the reaction using the catalyst NTPA-7 (chiral NTPA bearing 3,3′-bis(2,4,6-triisopropylphenyl)-substituents), brought significantly enhanced outcome compared to its CPA analog CPA-1 (NTPA-7: > 99% conv. and 47% ee (Entry 17) vs CPA-1: 56% conv. and 27% ee (Entry 1)). The most dramatic enantioselectivity increment was obtained in toluene (PhMe) among the examined solvents (up to 75% ee, Entries 18–21). Finally, at a lowered temperature (−20 °C) for an extended reaction time (48 h), a superior result was furnished (92% yield and 91% ee, Entry 22). Other factors did not demonstrate a significant positive impact (see Supplementary Information for details). Although CTA-4 and NTPA-4 were promising candidates in the early stages, their catalytic efficacies were less consistent under various reaction conditions (see Supplementary Information for details).
Experimental and analytical mechanistic investigations
To gain insights into the reaction mechanism, we performed experimental and analytical studies, emphasizing the significance of the combined binary acid catalytic system. As shown in Fig. 2a, control reactions conducted with or without the catalytic components, Bi(OAc)3 and NTPA-7, indicate that both are crucial for achieving the desired reactivity and enantioselectivity. Noticeably, the model reaction was inactive when either Bi(OAc)3 or NTPA-7 was absent (< 2 conv.). Using CPA-1 instead of NTPA-7 under the standard reaction condition resulted in considerably declined reactivity and enantioselectivity (74% yield and 12% ee). These facts indicate that the triflyl group (−Tf = −SO2CF3) at NTPA-7 interacts with Bi(OAc)3, and such synergistic action plays a critical role in efficient stereoselective catalysis.
a Control reactions for catalyst evaluation. b 1H NMR (500 MHz) study for in situ-generated catalytic assembly. c Employing pre-prepared intermediate I as catalyst. d Examination of various Lewis acid catalysts and others. e Reaction using α-keto oxoester as starting material. aYield (%) was determined by 1H NMR integration. bEnantiomeric excess (ee) value was determined by chiral HPLC analysis. ent = opposite enantiomer as major form. Ac acetyl, Bpin boronic acid pinacol ester, iPr isopropyl, Me methyl, OAc acetate, OMe methoxy, OTf triflate, Ph phenyl, Tf trifluoromethanesulfonyl (triflyl).
We next carried out a series of 1H NMR measurements to detect the in situ binary acid catalytic assembly (Fig. 2b). When NTPA-7 and Bi(OAc)3 were mixed, the Brønsted acid peak of NTPA-7 immediately disappeared (δ = 2.23 ppm in MeCN-d3) and the generation of acetic acid was simultaneously observed (δ = 1.59 ppm in PhMe-d8), presumably through anion exchange. Furthermore, a new 31P NMR signal (in PhMe-d8) appeared at δ = −35.72 ppm (condition (b)) shifting from δ = 2.68 ppm (condition (a)), and we assigned the resulting species as a potential intermediate I (see Supplementary Figs. 269 and 270). When allyl–Bpin (2) was added to the pre-formed putative intermediate I, the formation of a potential intermediate II—through transmetalation-like step—was witnessed by the observation of a typical 11B NMR signature of the concomitant byproduct, AcO–Bpin (δ = 22.64 ppm in PhMe-d8, see Supplementary Figs. 269 and 270). These stoichiometric reactivities were consistent with computational studies (vide infra). Moreover, removing acetic acid from the reaction mixture and using the pre-prepared intermediate I as the catalyst substantially decreased both reactivity and enantioselectivity (Fig. 2c). This fact indicates that the generated acetic acid is essential for achieving a successful catalytic outcome.
As shown in Fig. 2d, alternative Bi(III) and Bi(V) salts, used in place of Bi(OAc)3, proved ineffective under the optimized reaction condition, obtaining the product 5a with low reactivities and poor enantioselectivities (up to <67% conv. and 9% ee, Entries B–F). When Bi(OAc)3 was replaced by other acetates such as HOAc, NaOAc, and KOAc, no catalytic activity was observed (Entries G–I). Other main group and transition metal Lewis acids resulted in fruitless outcomes (Entries J–O). The importance of the thioester group was further evaluated by synthesizing an oxygen analog (Fig. 2e). When subjected to the standard reaction condition, α-keto oxoester, instead of 4a, exhibited poor reactivity across a broad temperature range. This finding emphasizes the critical role of the thioester group in achieving a successful catalytic outcome (see Supplementary Fig. 268).
Mechanistic investigations by density functional theory (DFT)-based computation
To examine kinetically viable reaction mechanism, we performed computational studies based on DFT calculations as the SMD(toluene) M06-2X/{6-311 + G**/def2-TZVPP for Bi}//{6-31 G**/SDD} level of theory57. At the outset, we investigated the complexation between Bi(OAc)3 and NTPA-7 (Fig. 3). Our initial consideration included various possible coordination modes upon anion exchange. The results indicated that the formation of a six-membered bidentate complex I is highly exergonic with a driving force of 21.9 kcal/mol, and the spontaneous deprotonation that releases acetic acid as a byproduct was consistent with analytical observations by 1H NMR monitoring in Fig. 2b. Other monodentate conformations (Ia and Ib) were less stable than complex I, with energies of 17.2 and 17.0 kcal/mol, respectively. Meanwhile, intermediate Ic, which exhibited weaker binding by the triflate portion, converged to the complex I during the geometry optimization process.
Having understood the stability of the six-membered-like chelated complex, we next evaluated the subsequent allylation reactivity on the substrates (Fig. 4). Initially, the pre-formed complex I can undergo either transmetalation of allyl–Bpin (2) or binding of α-keto thioester 4a. Our computational investigations on both pathways suggested that transmetalation is kinetically favored under the optimal reaction condition (see Supplementary Fig. 291). Hence, the complex I reacts with allyl–Bpin (2) through a transmetalation process, forming an allyl-bismuth complex II with a kinetic barrier of 17.8 kcal/mol (I → I-TS). Then, α-keto thioester 4a binds to complex II, affording a catalyst–substrates complex III. This complex has the potential to traverse four different enantio-determining carbon–carbon (C–C) coupling transition states (see Supplementary Fig. 296), structures and energies of two representatives (III-TS and III′-TS) are displayed in Fig. 4. The transition states III-TS and III′-TS lead to the products (S)-5a and (R)-5a, respectively, where III-TS exhibited an activation energy that is 3.0 kcal/mol lower than that of III′-TS. This computational kinetic barrier for the C–C coupling aligned with our spectroscopic observation confirming the (S) absolute configuration. Finally, the product (S)-5a can be released through facile protonation of the resulting catalyst–adduct complex IV by exogenous acetic acid, facilitating the regeneration of the complex I to close and turn over the catalytic cycle. Attempts to explicitly locate the transition states for monotonous ligand dissociation and association were unsuccessful on electronic energy surfaces because the entropic contribution plays a critical role during these processes58 (see Supplementary Fig. 293).
*Transition states that were not located on their electronic energy surfaces are marked with asterisks. DFT calculations were performed at SMD(toluene) M06-2X/{6-311 + G**/def2-TZVPP for Bi}//{6-31 G**/SDD} level of theory. Ac acetyl, Bpin boronic acid pinacol ester, ee enantiomeric excess, OAc acetate, Ph phenyl, PMB p-methoxybenzyl, TS transition state.
The fundamental origin of exceptional enantioselectivity was further examined by utilizing complexes IV and IV′. Electronic energy components suggested that the energy differences between III-TS and III′-TS were well-reflected in the structures of their respective resultants, IV and IV′ (see Supplementary Fig. 296). As enumerated in Fig. 5a, analyzed by the non-covalent interactions (NCI) plot59, an attractive interaction between the aryl moiety of the adduct and the ligand backbone in IV was revealed, showing a hydrogen–hydrogen (H–H) distance of 3.200 Å (d(H–H) = 3.200 Å) and an O–Bi–O angle of 91.1° (∠(O–Bi–O) = 91.1°). Whereas a similar interaction was observed in IV′, the nature of non-covalent interaction was repulsive, due to the shorter distances between the adduct and the ligand (d(H–H) = 2.508 and 3.019 Å). In essence, the shorter oxygen–oxygen (O–O) distance between the adduct and the ligand in IV′ (d(O–O) = 2.777 Å), along with the smaller O–Bi–O angle (∠(O–Bi–O) = 71.8°), induced a strong repulsive interaction. We concluded that such interaction is substantiated in the bidentate coordination mode enabled by NTPA-7.
Energy decomposition analysis60 was further conducted to quantify the unique interactions (Fig. 5b). By dividing IV and IV′ into two fragments (the catalyst and the adduct, respectively), we were able to deconvolute the steric- and orbital-interaction terms using the Amsterdam Density Functional quantum chemical package61. The total bonding energy in IV is 2.4 kcal/mol lower, leading to stabilization of the complex. The steric interaction, on the other hand, contributes significantly to the energy difference of 4.2 kcal/mol, while the orbital interaction is marginal. These results underscore the influence of the bidentate ligand and non-covalent interactions, where the additional oxygen atom in IV plays a crucial role in differentiating the energy, and thereby affecting enantioselectivity.
Based on the mechanistic information, we propose a plausible catalytic cycle (Fig. 6). The chiral organosuperacid NTPA-7 is readily activated in situ by anion exchange with Bi(OAc)3, generating the active catalyst I. Subsequently, transmetalation of allyl–Bpin (2) forms the allyl-bismuth complex II, and the α-keto thioester (1 or 4) binds to create the adduct species III. After enantio-determining C–C coupling takes place to afford a tetra-substituted stereogenic carbon center, further protonation releases the chiral product (3 or 5) and completes the catalytic cycle.
Substrate scope
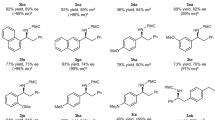
To probe the generality of the developed catalytic method, a variety of α-keto thioesters (1 or 4) was subjected to the optimized reaction condition (Figs. 7 and 8). Intensive modulation of thioester moiety was initially performed by varying S-substituents on α-keto thioesters 1 (Fig. 7). It was found that α-keto thioesters consisting of S-secondary alkyl substituents (1a–1d) and S-tertiary alkyl substituents (1e and 1f) smoothly underwent the reaction, offering the corresponding chiral products (3a–3f) with high levels of reactivity (84–94% yields) and enantioselectivity (71–90% ee). The reaction utilizing α-keto thioesters consisting of S-aryl substituents with electron-donating or -withdrawing groups (1g–1j) also conducted well, attaining the corresponding chiral products (3g–3j) with moderate to good reactivities (59–84% yields) and enantioselectivities (65–89% ee). Encouragingly, α-keto thioesters being made up of S-benzyl substituents (1k–1o) were well fitted in the reaction, exhibiting the corresponding chiral products (3k–3o) with good to excellent reactivities (74–93% yields) and enantioselectivities (73–91% ee).
aReaction conditions: α-Keto thioesters (1, 0.3 mmol), allyl–Bpin (2, 1.0 equiv., 0.3 mmol), Bi(OAc)3 (3 mol%, 0.009 mmol), NTPA-7 (3 mol%, 0.009 mmol), PhMe (0.1 M, 3.0 mL), −20 °C, 48 h. bYield (%) was determined after column chromatographic purification. cEnantiomeric excess (ee) value was determined by chiral HPLC analysis. dReaction was performed at 25 °C. eDiastereomeric ratio (d.r.) value was determined using 1H NMR analysis. Bpin boronic acid pinacol ester, Me methyl, OAc acetate, OMe methoxy, Ph phenyl.
aReaction conditions: α-Keto thioesters (4, 0.3 mmol), allyl–Bpin (2, 1.0 equiv., 0.3 mmol), Bi(OAc)3 (3 mol%, 0.009 mmol), NTPA-7 (3 mol%, 0.009 mmol), PhMe (0.1 M, 3.0 mL), −20 °C, 48 h. bYield (%) was determined after column chromatographic purification. cEnantiomeric excess (ee) value was determined by chiral HPLC analysis. dReaction was performed at 25 °C. eReaction was performed in 0.2 mmol scale. Me methyl, OMe methoxy.
Notably, S-(p-methoxybenzyl) (S-PMB) α-keto thioester 4a was identified as the optimal substrate for achieving the corresponding chiral S-PMB α-hydroxy thioester 5a with notable reactivity (96% yield) and promising enantioselectivity (93% ee). A wide range of S-PMB α-keto thioesters 4 was attempted through a similar process (Fig. 8). Gratifyingly, S-PMB α-keto thioesters comprising of phenyl and naphthyl substituents (4a–4c), aryl substituents with electron-donating groups (4d–4i), and fused cyclic substituents (4j–4m) nicely underwent the reaction, furnishing the corresponding chiral products (5a–5m) with good to excellent reactivities (65–96% yields) and enantioselectivities (85–97% ee). Additionally, S-PMB α-keto thioesters comprising of aryl substituents with electron-withdrawing groups (4n–4s) and heteroaryl substituents (4t–4v) were also smoothly converted to the corresponding chiral products (5n–5v) with strikingly high levels of reactivity (75– > 99% yields) and enantioselectivity (90–95% ee). To our delight, it was found that the S-PMB α-keto thioesters comprising of alkyl substituents, such as benzyl and phenethyl groups (4w and 4x, respectively), can be readily adapted to the reaction at ambient temperature, thereby creating the desired products with preeminent results ((5w; 87% yield and 72% ee) and (5x; 92% yield and 62% ee)). It should be noted that the enantioselective utilization of this type of substrate has been a challenging issue. The absolute configuration of the obtained chiral products was determined by the single crystal X-ray structure analysis. Based on the X-ray analysis of 5c, its absolute configuration was assigned as (S). The absolute configuration of the other chiral products was assigned by analogy.
Synthetic applications
The feasibility of our protocol was disclosed through a gram-scale reaction of 4a, using a reduced loading of the optimal catalytic combination (2 mol%), resulting the product 5a without erosion of reactivity and enantioselectivity (> 99% yield and 93% ee, Fig. 9a). Moreover, suggested functional group derivatizations of model chiral S-PMB α-hydroxy thioester 5a, while maintaining enantioselectivity, highlighted the versatility of our catalytic method (Fig. 9b). The consecutive hydroboration–oxidation of 5a by utilizing 9-borabicyclo[3.3.1]nonane (9-BBN) was conducted to obtain the corresponding product 6 in 47% yield. The subsequent Ley–Griffith oxidation of pre-isolated 6 by utilizing 4-methylmorpholine N-oxide and tetrapropylammonium perruthenate gave rise to the production of a γ-lactone 7, which constitutes a tetra-substituted stereogenic carbon center on its γ-position, in 60% yield. The olefin metathesis between 5a and ethyl acrylate gave the corresponding product 8 in 68% yield. The transesterification of 5a rendered the desired α-hydroxy oxoester 9 in 85% yield. In addition, the positive specific rotation of 9, which is in accordance with (S) when compared with the reported values62,63, confirmed the (S) absolute configuration of its precursor 5a (see Supplementary Information for details). The coupling reagent-free direct amidation of 5a with p-anisidine and 4-methoxybenzylamine gave the corresponding α-hydroxy amides 10 and 11, respectively in 75% and 71% yield.
a Scale-up synthesis. b Synthetic applications. 9-BBN 9-boracyclo[3.3.1]nonane, Bpin boronic acid pinacol ester, Cy cyclohexyl, ee enantiomeric excess, Et ethyl, Me methyl, Mes 2,4,6-trimethylphenyl (mesityl), NMMO 4-methylmorpholine N-oxide, OAc acetate, OMe methoxy/methoxide, Ph phenyl, THF tetrahydrofuran, TPAP tetrapropylammonium perruthenate, r.t. room temperature.
In summary, we developed the asymmetric organosuperacid-bismuth binary catalysis by exploiting the counteranion of chiral NTPA as a flexible-coordinating ligand. Based on the chiral redox-neutral bismuth catalytic system, we successfully achieved the enantioselective allylation reaction of α-keto thioesters to access enantio-enriched α-hydroxy thioesters containing a tetra-substituted stereogenic carbon center with excellent reactivities and enantioselectivities (up to >99% yield and 97% ee). The unique features of this unexplored system, including the flexible-coordinating chiral NTPA counteranion ligand, thioester-directed catalysis, and cooperative non-covalent interactions, were highlighted through an in-depth examination of the underlying reaction mechanism. Our future works will aim to develop a broader range of bismuth-catalyzed sustainable reactions that utilize various organic acid catalysts, challenging systems accelerated by water as a reaction medium64. We hope this organic-bismuth binary acid system will establish a pioneering approach in the asymmetric catalysis community, unlocking new reactivity and selectivity that have not been explored earlier.
Methods
General procedure for the catalytic enantioselective allylation reaction
In a flame-dried capped test tube, equipped with a magnetic stirring bar and filled with Ar gas, Bi(OAc)3 (3 mol%, 0.009 (or 0.006) mmol), NTPA-7 (3 mol%, 0.009 (or 0.006) mmol), and PhMe (anhydrous, 0.2 M, 1.5 (or 1) mL) were added. The reaction mixture then sealed to stir to −20 °C (@ constant temperature bath) for 0.5 h. Subsequently, allyl–Bpin (2, 1 equiv., 0.3 (or 0.2) mmol) was added to the reaction mixture (dropwise), then sealed to stir at −20 °C for 5 min. To the crude mixture at −20 °C, S-PMB α-keto thioester (1 or 4, 0.3 (or 0.2) mmol, in PhMe (anhydrous, 0.2 M, 1.5 (or 1) mL)) was added (dropwise), then sealed to stir at −20 °C (@ constant temperature bath (or rt)) for 48 h. The resulting mixture was concentrated in vacuo, and the residue was purified by column chromatography on silica gel (EtOAc:hexanes = 1:50 to 1:3 v/v) to afford corresponding chiral S-PMB α-hydroxy thioester (3 or 5). The NMR spectra and the mass data were obtained using the Bruker Ascend™ 500 spectrometer and the Xevo G2-XS QTof mass spectrometer (combined in supercritical fluid chromatography (SFC; quadrupole TOF analyzer; Waters, Milford, MA, USA)), respectively, at the Chiral Material Core Facility Center of Sungkyunkwan University.
Data availability
All relevant data are presented in the main article and/or the Supplementary Information. CCDC 2302605 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033. Source data are provided with the manuscript. Other data are available from corresponding author upon request. Source data are provided with this paper.
References
Mayer, S. & List, B. Asymmetric counteranion-directed catalysis. Angew. Chem. Int. Ed. 45, 4193–4195 (2006).
Shao, Z. & Zhang, H. Combining transition metal catalysis and organocatalysis: a broad new concept for catalysis. Chem. Soc. Rev. 38, 2745–2755 (2009).
Du, Z. & Shao, Z. Combining transition metal catalysis and organocatalysis – an update. Chem. Soc. Rev. 42, 1337–1378 (2013).
Chen, D.-F., Han, Z.-Y., Zhou, X.-L. & Gong, L.-Z. Asymmetric organocatalysis combined with metal catalysis: concept, proof of concept, and beyond. Acc. Chem. Res. 47, 2365–2377 (2014).
Yang, L., Melot, R., Neuburger, M. & Baudoin, O. Palladium(0)-catalyzed asymmetric C(sp3)–H arylation using a chiral binol-derived phosphate and an achiral ligand. Chem. Sci. 8, 1344–1349 (2017).
Luo, W. et al. Asymmetric ring-opening of donor–acceptor cyclopropanes with primary arylamines catalyzed by a chiral heterobimetallic catalyst. ACS Catal. 9, 8285–8293 (2019).
Teator, A. J. & Leibfarth, F. A. Catalyst-controlled stereoselective cationic polymerization of vinyl ethers. Science 363, 1439–1443 (2019).
Yang, L.-L. et al. Enantioselective diarylcarbene Insertion into Si–H bonds induced by electronic properties of the carbenes. J. Am. Chem. Soc. 142, 12394–12399 (2020).
Lacour, J. & Linder, D. A counterion strategy. Science 317, 462–463 (2007).
Hatano, M., Moriyama, K., Maki, T. & Ishihara, K. Which is the actual catalyst: chiral phosphoric acid or chiral calcium phosphate? Angew. Chem. Int. Ed. 49, 3823–3826 (2010).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Kaupmees, K., Tolstoluzhsky, N., Raja, S., Rueping, M. & Leito, I. On the acidity and reactivity of highly effective chiral brønsted acid catalysts: establishment of an acidity scale. Angew. Chem. Int. Ed. 52, 11569–111572 (2013).
Akiyama, T. Stronger brønsted acids. Chem. Rev. 107, 5744–5758 (2007).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived brønsted acid and metal catalysis: history and classification by mode of activation; brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Akiyama, T. & Mori, K. Stronger brønsted acids: recent progress. Chem. Rev. 115, 9277–9306 (2015).
Rueping, M., Koenigs, R. M. & Atodiresei, I. Unifying metal and brønsted acid catalysis—concepts, mechanisms, and classifications. Chem. Eur. J. 16, 9350–9365 (2010).
Fang, G.-C., Cheng, Y.-F., Yu, Z.-L., Li, Z.-L. & Liu, X.-Y. Recent advances in first-row transition metal/chiral phosphoric acid combined catalysis. Top. Curr. Chem. 377, 23 (2019).
Brodt, N. & Niemeyer, J. Chiral organophosphates as ligands in asymmetric metal catalysis. Org. Chem. Front. 10, 3080–3109 (2023).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Ed. 52, 518–533 (2013).
Hatano, M., Ikeno, T., Matsumura, T., Torii, S. & Ishihara, K. Chiral lithium salts of phosphoric acids as Lewis acid–base conjugate catalysts for the enantioselective cyanosilylation of ketones. Adv. Synth. Catal. 350, 1776–1780 (2008).
Yue, T., Wang, M.-X., Wang, D.-X., Masso, G. & Zhu, J. Catalytic asymmetric passerini-type reaction: chiral aluminum–organophosphate-catalyzed enantioselective α-addition of isocyanides to aldehydes. J. Org. Chem. 74, 8396–8399 (2009).
Lv, J., Li, X., Zhong, L., Luo, S. & Cheng, J.-P. Asymmetric binary-acid catalysis with chiral phosphoric acid and MgF2: catalytic enantioselective Friedel–Crafts reactions of β,γ-unsaturated α-ketoesters. Org. Lett. 12, 1096–1099 (2010).
Ollevier, T. New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 11, 2740–2755 (2013).
Moon, H. W. & Cornella, J. Bismuth redox catalysis: an emerging main-group platform for organic synthesis. ACS Catal. 12, 1382–1393 (2022).
Mato, M. & Cornella, J. Bismuth in radical chemistry and catalysis. Angew. Chem. Int. Ed. 63, e202315046 (2024).
Pramanik, M., Guerzoni, M. G., Richards, E. & Melen, R. L. Recent advances in asymmetric catalysis using p-block elements. Angew. Chem. Int. Ed. 63, e202316461 (2024).
Lambert, J. R. & Midolo, P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 11, 27–33 (1997).
Wang, J. et al. Bi(III)-catalyzed enantioselective allylation reactions of ketimines. iScience 16, 511–523 (2019).
Pan, Y.-L. et al. Enantioselective allylation of oxocarbenium ions catalyzed by Bi(OAc)3/chiral phosphoric acid. ACS Catal. 10, 8069–8076 (2020).
Wang, J. et al. Bi(OAc)3/chiral phosphoric acid catalyzed enantioselective allylation of isatins. Chem. Commun. 56, 261–264 (2020).
Cai, L., Pan, Y.-L., Chen, L., Cheng, J.-P. & Li, X. Bi(OAc)3/chiral phosphoric acid catalyzed enantioselective allylation of seven-membered cyclic imines, dibenzo[b,f][1,4]oxazepines. Chem. Commun. 56, 12383–12386 (2020).
Pan, Y.-L. et al. Kinetic resolution of 2H-azirines by asymmetric allylation reactions. ACS Catal. 11, 13752–13760 (2021).
Liu, X.-S., Li, Y. & Li, X. Bi(OAc)3/chiral phosphoric acid-catalyzed enantioselective 1,2- and formal 1,4-allylation reaction of β,γ-unsaturated α-ketoesters. Org. Lett. 23, 9128–9133 (2021).
Pan, Y.-L. et al. Asymmetric difluorocarbonylation reactions of non-active imines catalyzed by Bi(OAc)3/chiral phosphoric acid. Org. Chem. Front. 9, 3990–3997 (2022).
Bassetti, M. Kinetic evaluation of ligand hemilability in transition metal complexes. Eur. J. Inorg. Chem. 2006, 4473–4482 (2006).
Elsby, M. R. & Baker, R. T. Strategies and mechanisms of metal–ligand cooperativity in first-row transition metal complex catalysts. Chem. Soc. Rev. 49, 8933–8987 (2020).
Nakashima, D. & Yamamoto, H. Design of chiral N-Triflyl phosphoramide as a strong chiral brønsted acid and its application to asymmetric Diels–Alder reaction. J. Am. Chem. Soc. 128, 9626–9627 (2006).
Cheon, C. H. & Yamamoto, H. Super brønsted acid catalysis. Chem. Commun. 47, 3043–3056 (2011).
Caballero-García, G. & Goodman, J. M. N-Triflylphosphoramides: highly acidic catalysts for asymmetric transformations. Org. Biomol. Chem. 19, 9565–9618 (2021).
Yang, C., Xue, X.-S., Li, X. & Cheng, J.-P. Computational study on the acidic constants of chiral brønsted acids in dimethyl sulfoxide. J. Org. Chem. 79, 4340–4351 (2014).
Rueping, M. & Koenigs, R. M. Brønsted acid differentiated metal catalysis by kinetic discrimination. Chem. Commun. 47, 304–306 (2011).
Knipe, P. C. & Smith, M. D. Enantioselective one-pot synthesis of dihydroquinolones via BINOL-derived Lewis acid catalysis. Org. Biomol. Chem. 12, 5094–5097 (2014).
Bai, J.-F., Sasagawa, H., Yurino, T., Kano, T. & Maruoka, K. In situ generation of N-Boc-protected alkenyl imines: controlling the E/Z geometry of alkenyl moieties in the Mukaiyama–Mannich reaction. Chem. Commun. 53, 8203–8206 (2017).
Franchino, A., Martí, À. & Echavarren, A. M. H-bonded counterion-directed enantioselective Au(I) Catalysis. J. Am. Chem. Soc. 144, 3497–3509 (2022).
Han, Z.-Y. et al. Hybrid metal/organo relay catalysis enables enynes to be latent dienes for asymmetric Diels–Alder reaction. J. Am. Chem. Soc. 134, 6532–6535 (2012).
Wang, P.-S. et al. Enantioselective relay catalytic cascade intramolecular hydrosiloxylation and Mukaiyama aldol reaction. Chem. Eur. J. 19, 6234–6238 (2013).
Wu, X., Li, M.-L. & Wang, P. Hybrid gold/chiral brønsted acid relay catalysis allows an enantioselective synthesis of (−)-5-epi-Eupomatilone-6. J. Org. Chem. 79, 419–425 (2014).
Li, N., Chen, D.-F., Wang, P.-S., Han, Z.-Y. & Gong, L.-Z. Relay catalytic cascade hydrosiloxylation and asymmetric hetero-Diels – Alder reaction. Synthesis 46, 1355–1361 (2014).
Zhao, P. et al. Asymmetric intramolecular hydroalkoxylation of unactivated alkenes catalyzed by chiral N-triflyl phosphoramide and TiCl4. Chin. J. Chem. 38, 565–569 (2020).
Kennedy, J. W. J. & Hall, D. G. Recent advances in the activation of boron and silicon reagents for stereocontrolled allylation reactions. Angew. Chem. Int. Ed. 42, 4732–4739 (2003).
Denmark, S. E. & Fu, J. Catalytic enantioselective addition of allylic organometallic reagents to aldehydes and ketones. Chem. Rev. 103, 2763–2793 (2003).
Marek, I. & Sklute, G. Creation of quaternary stereocenters in carbonyl allylation reactions. Chem. Commun. 1683–1691 https://doi.org/10.1039/B615042J (2007).
Yus, M., González-Gómez, J. C. & Foubelo, F. Diastereoselective allylation of carbonyl compounds and imines: application to the synthesis of natural products. Chem. Rev. 111, 5595–5698 (2013).
Huo, H.-X., Duvall, J. R., Huang, M.-Y. & Hong, R. Catalytic asymmetric allylation of carbonyl compounds and imines with allylic boronates. Org. Chem. Front. 1, 303–320 (2014).
Schnepel, C. et al. Thioester-mediated biocatalytic amide bond synthesis with in situ thiol recycling. Nat. Catal. 6, 89–99 (2023).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetic, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Ryu, H. et al. Pitfalls in computational modeling of chemical reactions and how to avoid them. Organometallics 37, 3228–3239 (2018).
Contreras-García, J. et al. NCIPLOT: a program for plotting noncovalent interaction regions. J. Chem. Theory Comput. 7, 625–632 (2011).
Kitaura, K. & Morokuma, K. A new energy decomposition scheme for molecular interactions within the Hartree-Fock approximation. Int. J. Quantum Chem. 10, 325–340 (1976).
Te Velde, G. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Ooi, T., Fukumoto, K. & Maruoka, K. Construction of enantiomerically enriched tertiary α-hydroxycarboxylic acid derivatives by phase-transfer-catalyzed asymmetric alkylation of diaryloxazolidin-2,4-diones. Angew. Chem. Int. Ed. 45, 3839–3842 (2006).
Zheng, K., Qin, B., Liu, X. & Feng, X. Highly enantioselective allylation of α-ketoesters catalyzed by N,N′-dioxide–In(III) complexes. J. Org. Chem. 72, 8478–8483 (2007).
Kitanosono, T., Masuda, K., Xu, P. & Kobayashi, S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 118, 679–746 (2018).
Acknowledgements
Dedicated to Prof. Dr. Choong Eui Song for his 70th birthday. The generous supports of the Ministry of Science, ICT and Future Planning of Korea (MSIT, RS-2023-00259659 and RS-2023-00219859), the Ministry of Education (MOE, National Research Facilities and Equipment Center: 2022R1A6C101A751 and 2022R1A6C102A913), and Korea Toray Fellowship are gratefully acknowledged for H.Y.B. (SKKU). This work was funded by the MSIT (2022R1F1A106268, RS-2023-00213491, RS-2024-00405261, and RS-2025-00642970); the Creative Research Program and the KAIST Cross-Generation Collaborative Lab Project at KAIST to Y.P. We thank the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science in Korea for allowing the use of computational resources. This work was supported by the Sungkyunkwan University and the BK21 FOUR (Graduate School Innovation) funded by the MOE, and National Research Foundation of Korea.
Author information
Authors and Affiliations
Contributions
Y.P. and H.Y.B. administrated the project. J.H.P. and M.H.S. conducted the conceptualization, experiments, and analytical investigations. S.Y.Y. and S.J. conducted the computational studies and data processing. All authors involved in discussions and writing–editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Lili Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, J.H., Yoo, S.Y., Shin, M.H. et al. Superacid counteranion as flexible-coordinating ligand for asymmetric organo-bismuth catalysis. Nat Commun 16, 6090 (2025). https://doi.org/10.1038/s41467-025-61265-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61265-4