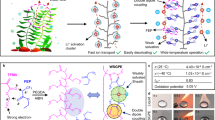
Abstract
Li-rich layered oxides exhibit promising potential applications in high-energy-density solid-state lithium metal batteries. Nevertheless, the strong oxidative oxygen species generate at high voltage, which poses great challenges to positive electrode-side interface stability. Herein, a robust in-situ polymerization gel polymer electrolyte with bifunctional additives is designed for interface modification. These additives, include lithium difluoro(oxalate) borate and LiPO2F2, regulate the Li+ chemical environment in gel polymer electrolyte to enhance crosslink density without residual oligomer, which reduce gas generation and suppress contact loss, thus avoiding interfacial impedance divergence. Concurrently, the designed gel polymer electrolyte enables a wide electrochemical stability window (up to 4.7 V) and a high Li+ transference number (0.82). Additionally, the additives induced F- and B-rich inorganic cathode-electrolyte interphase inhibits side reactions and oxygen/transition metal loss effectively, stabilizing the chemical interface. The as-constructed Li-rich layered oxides-based ceramic solid-state lithium metal batteries with gel polymer electrolyte interface modification exert a high discharge capacity of 276.5 mAh g-1 at 30 °C without external pressure, delivering a retention of 81.7% after 100 cycles at 25 mA g-1 during 2.0-4.7 V. This work provides a guideline for developing high-voltage solid-state lithium metal batteries via interfacial design.
Similar content being viewed by others
Introduction
The emerging diversified application scenarios call for modern lithium batteries to develop towards high energy density1,2,3. Currently, the most liquid electrolyte (LE)-based batteries have difficulty to break through the bottleneck of 350 Wh kg−1 in energy density, even with the combination of an advanced nickel-rich layered positive electrode and silicon/carbon composite negative electrode4,5. Solid-state lithium metal batteries (SSLBs) have the advantages of high safety and high energy density, particularly when employing high-voltage positive electrodes. Li-rich layered oxide (LLO) positive electrodes [x Li2MnO3·(1-x) LiTMO2 (0 < x ≤ 1, transition metal (TM) = Ni, Co, Mn, etc.)] can deliver high discharge specific capacity (> 300 mAh g_1) and energy density (> 1000 Wh kg−1) due to that extra anions (lattice oxygen) participate in the redox reactions at high voltage (> 4.5 V, vs. Li/Li+) for charge compensation6,7. When the LLO positive electrode combines with the lithium metal negative electrode (3860 mAh g−1, − 3.04 V vs. H2/H+), a significant elevation of energy density will be achieved for SSLBs. However, currently the LLOs-based SSLBs are still in their infancy, which is required to overcome tough challenges on the electrode-electrolyte solid-solid interface, especially on the positive electrode side. These issues can be viewed through two perspectives: physical and chemical interface.
For physical interface, SSLBs experience difficulties in maintaining optimal electrode-electrolyte contact continually, like LE-based batteries. Even a minor volume change of the active materials during Li insertion/extraction would lead to a significant local stress8. This is further exacerbated by irreversible lattice oxygen release and asymmetric changes in lattice constants of LLOs, finally resulting in the contact loss and microstructural cracking, and driving the failures in SSLBs9,10,11. Therefore, it is essential to maintain a high stack pressure to ensure the operation of SSLBs. However, advanced insight into SSLBs requires a minimum stack pressure for practical applications8,12. In addition, LLOs materials exhibit poor electron and Li+ transport13,14. It is a critical problem to maintain long-term stability and low-resistance interfaces between the active materials and the solid-state electrolyte under a low stack pressure. For chemical interface, the degradation of solid-state electrolytes remains a great challenge, particularly at high voltage over 4.5 V, resulting in the formation of a high impedance interphase due to continuous side reactions15,16,17. This is a major factor in performance fading over time. In addition, the structural instability of LLOs positive electrode during cycling, as well as its O-derivatives, further aggravate the challenges on chemical/electrochemical stability18. It is of paramount importance to passivate the positive electrode-electrolyte interface to inhibit side reactions and suppress potential TM and O loss from lattice19,20,21. Gel polymer electrolyte (GPE) as an interface modifier offers a promising strategy to establish a flexible interface for physical contact22. The chemical/electrochemical stability can be further enhanced by introducing functional additives. For example, B-containing salts (e.g., LiDFOB) promote the formation of cathode electrolyte interphase (CEI) with a B-O bond that benefits lattice oxygen stabilization23, while F-rich additives (e.g., LiPO2F2) facilitate the formation of HF-resistant CEI24, thereby enabling a stable positive electrode-electrolyte interface. Up to now, the physically and chemically stable interface on the positive electrode side is still a great challenge for high-voltage LLOs-based SSLBs.
Herein, an in situ polymerization GPE is exquisitely designed for interface modification by introducing lithium difluoro(oxalate) borate (LDFB) and LiPO2F2 (LPF) additives simultaneously (labeled as LDFB + LPF GPE). The additives not only promote to form a beneficial CEI layer rich in inorganic components on the positive electrode surface, but also regulate the Li+ chemical environment to enhance the crosslink density and immobilize anions in GPE, leading to elevated Li+ migration and oxidation stability. As a result, the LDFB + LPF GPE exhibits a higher Li+ transference number of 0.82 and a wide electrochemical window up to 4.7 V. Furthermore, GPE featuring high polymerization density has lower residual oligomer and reduced gas generation, benefiting to maintain the physical interface contact and suppress the increase and divergence in interfacial impedance. In addition, the generated F- and B-rich inorganic CEI layer can effectively suppress potential O and TM loss, finally reinforcing chemical interface stability in LLOs. As a result, the ceramic SSLBs based on LLOs positive electrode achieve an initial capacity of 276.5 mAh g−1 at 25 mA g−1 with a capacity retention of 81.7% after 100 cycles under ambient conditions.
Results
Improvement mechanisms of additives modified gel polymer electrolyte
The GPE with a crosslinked network is well-suited for high-voltage batteries due to its high oxidation window25. The based gel polymer electrolyte (Base GPE), LDFB GPE (with LiDFOB additive) and LDFB + LPF GPE (with LiDFOB and LiPO2F2 additives) was synthesized by means of a crosslink reaction between butyl acrylate (BA) and triethylene glycol diacrylate (TEGDA) (Experimental Section). The Fourier transform infrared (FTIR) spectra (Supplementary Fig. 1a, b) reveal that the absorption peaks of C = C and = C-H stretching vibrations at ~ 1630 cm−1 and 2830 cm−1 almost disappear in the GPEs, indicating that polymerization of the monomer and crosslinker has been completed25,26,27. After polymerization, Base GPE, LDFB GPE, and LDFB + LPF GPE exhibit monomer conversion rates of 90.2%, 97.0%, and 97.5%, respectively (Supplementary Fig. 1c, d), with sequentially fewer mobile oligomer or monomer residues on the bottle wall (Supplementary Fig. 2, right), indicating that LDFB + LPF GPE has the lowest residual monomer content and improved crosslinking feature. The Young’s modulus of LDFB + LPF GPE, detected through atomic force microscopy (AFM, Supplementary Fig. 3), presents the highest mechanical strength of 4.1 MPa (Fig. 1c) compared to that of Base GPE (1.4 MPa, Fig. 1a) and LDFB GPE (2 MPa, Fig. 1b), which is beneficial for the formation of a self-supporting LDFB + LPF GPE film (Supplementary Fig. 4). The fitted results (Fig. 1d and Supplementary Fig. 5) from small angel X-ray scattering (SAXS, Supplementary Fig. 6) also reveal that the increase in surface fractal dimension (Ds) is mainly due to the introduction of LiPO2F2. The values of LDFB + LPF GPE (2.23) and LPF GPE (2.11) are higher than that of Base GPE (1.61) and LDFB GPE (1.98), respectively, meaning a reinforced physical entanglement (which represents internal molecular interaction occurring around the polymer chains)28. The GPE with enhanced mechanical strength is also beneficial to suppress the growth of Li dendrites. The radius of gyration (Rg) values derived from Guinier plots (Supplementary Fig. 7) increase progressively from 19.0 nm (Base GPE) to 19.4 nm (LDFB GPE) and 19.6 nm (LDFB + LPF GPE). M (polymer molecular weight) is positively correlated with Rg, confirming that the LDFB + LPF GPE achieves higher crosslinking efficiency (reduced residual oligomers) and elevated M value than Base GPE and LDFB GPE.
a–c The corresponding Young’s Modulus images for Base GPE (a), LDFB GPE (b), and LDFB + LPF GPE (c). d SAXS data of Base GPE, LDFB GPE, and LDFB + LPF GPE and the corresponding fitted curves (solid lines) by Power Law. e, f 7Li (e) and 31P (f) NMR spectra of the Base GPE, LDFB GPE, and LDFB + LPF GPE. g The chronoamperometry curves and EIS spectra before and after polarization of Li symmetric cells with LDFB + LPF GPE. h The Li+ conductivity and Nyquist plots (inset) of different GPEs with stainless steel symmetric cells at 30 °C. Error bar: standard deviation of 3 measurements. i LSV of pristine LE and different GPEs. j The HOMO/LUMO energy levels and molecular orbital models of various GPE components (molecular orbital models of TEGDA and TEGDA + BA (4 Units) are presented in Supplementary Fig. 10).
The introduction of additives regulates Li+ chemical environment, the 7Li peaks in the nuclear magnetic resonance (NMR) spectra of Base GPE, LDFB GPE, and LDFB + LPF GPE (Fig. 1e) exhibit a downfield shift, suggesting a decreased electronegativity and thus promoting Li+ migration. Meanwhile, the higher dissociation and concentration of Li+ can serve as a cation template to promote polymerization, resulting in stronger crosslinking density29. Consequently, the anions of macromolecules are immobilized by enhanced polymer networks, a clear upfield shift is observed in the 31P NMR spectra of LDFB + LPF GPE (Fig. 1f), indicating an enhanced interaction between GPE and P-containing derivatives (Detailed discussions attached to Supplementary Fig. 8). This presents that additives can also improve polymerization density, which differs from the traditional additives that mainly facilitate the formation of CEI. Hence, the Li+ transference number of LDFB + LPF GPE is as high as 0.82 (Fig. 1g, Supplementary Fig. 9, and Supplementary Table 1) owing to the strengthened immobilization for anions, which is better than that of Base GPE (0.68) and LDFB GPE (0.76). The Li+ conductivity of the different GPEs were calculated through the electrochemical impedance spectra (EIS, Fig. 1h). The introduction of different additives has no obvious effect on the conductivity of GPEs, whose Li+ conductivity all reaches the order of 10−3 S cm−1 (Supplementary Table 2).
However, the incorporation of additives has a significant influence on the electrochemical window of GPEs from the linear sweep voltammetry (LSV) results in Fig. 1i. The carbonate-based electrolyte (Pristine LE), used as a plasticizer in GPEs, undergoes oxidation at 4.0 V. The electrochemical window of Base GPE (3.4 V) is even lower than Pristine LE, attributed to the oxidation of its oligomer or monomer residues. This is also confirmed by the calculated results that the HOMO energy level of BA (− 7.77 eV) and TEGDA (− 7.54 eV) is higher than that of EC (− 8.47 eV) and DMC (− 8.22 eV), indicating the inferior oxidation stability of Base GPE (Fig. 1i). Although the HOMO energy level of crosslinked TEGDA + BA (4 Units) is also only − 7.54 eV (Fig. 1j), the LDFB GPE and LDFB + LPF GPE still exhibit enhanced oxidation voltages of 4.3 V and 4.7 V, respectively. This is attributed to increased crosslinking density achieved by introducing LDFB and LPF additives, which decrease monomer residues. More importantly, the decomposition of LiPO2F2, occurring within the voltage range of 3.5 to 4.0 V (Fig. 1i), can produce Li3PO4 and LiF and form an electronically inert interface30. Thus, the LDFB + LPF GPE ultimately exhibits the strongest electrochemical stability.
The effect of micro contact loss on physical interface
The cobalt-free Li-rich oxide positive electrode, Li1.2Ni0.2Mn0.6O2 (CFLLO), is selected to assemble Li|GPE | CFLLO coin cells using commercial polypropylene as a separator to conveniently study the positive electrode|electrolyte physical interface. Firstly, the morphology and element distribution of scanning electron microscopy (SEM) (Fig. 2a and Supplementary Figs. 11–13) all indicate that GPE (LDFB + LPF GPE as a representative example) has been tightly filled into the inner pores between positive electrode particles, which ensures rapid Li+ transport within the composite positive electrode and solves the issue of poor kinetics caused by solid-solid interface contact in SSLBs.
a The cross-sectional SEM image and corresponding elements distribution of the CFLLO composite positive electrode filled with LDFB + LPF GPE. b The first charge/discharge curves of the Li|GPE | CFLLO cells in 2.0–4.8 V at 25 mA g−1 and 30 °C. The marked capacities with triangles represent the lattice oxygen plateau. c, d The charging voltage profiles in 2.0–4.8 V and corresponding in situ DEMS results of mass signals of m/z 44 (CO2, CH3CHO) for the Li|Base GPE | CFLLO (c) and Li|LDFB + LPF GPE | CFLLO (d) batteries. e, f SEM images of composite positive electrode with Base GPE (e) and LDFB + LPF GPE (f) after cycling. g The impedance parameters of bulk (Rb), CEI (RCEI), and interface (Ri) of the corresponding batteries from fitting results.
To fully activate the lattice oxygen redox activity in the CFLLO positive electrode, the Li|GPE | CFLLO batteries were cycled in 2.0–4.8 V in the first cycle at 25 mA g−1 (Fig. 2b). The fluctuation in the initial charge-discharge curves mainly originates from the decomposition of residual monomers, and disappears in the following several cycles (Supplementary Fig. 14). The cell with Base GPE exhibits an irreversible decomposition plateau of residual oligomers or monomers at ~ 3.4 V, which may result in a lot of gaseous by-products. In addition, due to a lack of stable CEI protection and the poor crosslinking density, Base GPE decomposes above 4.5 V. Both of them would damage the interface contact and Li+ conduction, finally leading to a low discharge capacity of only 215.3 mAh g−1 with a coulombic efficiency of only 33.6% due to the incompletely activated lattice oxygen activity of CFLLO. Similarly, LDFB GPE also contains some residual oligomer that undergo slight decomposition between 3.6 and 4.5 V, resulting in an initial discharge capacity of 233.5 mAh g−1 with a low coulombic efficiency of 49.2%. Differently, LDFB + LPF GPE with high crosslinking density has no residue decomposition plateau below 3.5 V. It should be pointed out that the decomposition products of LiDFOB and LiPO2F2 guarantee an effective CEI protection (as discussed in Fig. 3), thus LDFB + LPF GPE-based cells exhibit the highest discharge capacity of 270.7 mAh g-1 with fully activated lattice oxygen and the highest initial coulombic efficiency of 82.4%. The improved electrochemical performance (Supplementary Figs. 15–17) can be attributed to the synergistic effects of LiDFOB and LiPO2F2 additives (Detailed discussions attached to Supplementary Figs. 16 and 17).
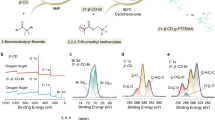
a–d TOF-SIMS 2D mappings of NiF- (a, c) and BF2O- (b, d) in CFLLO electrodes matched with Base GPE and LDFB + LPF GPE after 90 cycles at 25 mA g–1 during 2.0–4.6 V. The color ribbons represent signal intensity in arbitrary units. e–j XPS spectra of C 1 s (e, h), F 1 s (f, i), and P 2p (g, j) for the cycled CFLLO electrodes matched with Base GPE and LDFB + LPF GPE after 0 and 30 s etching. k The adsorption energy between CEI components and lattice oxygen on the (003) surface of the Li2MnO3 phase. l, m XANES spectra (l) and EXAFS (m) of Mn K-edge of CFLLO electrodes with Base GPE, LDFB GPE, and LDFB + LPF GPE after 10 cycles.
In situ DEMS experiments were carried out to characterize the gas release (m/z 44, CO2, CH3CHO; m/z 32, O2) from Li|GPE | CFLLO batteries in the voltage range of 2.0–4.8 V (Fig. 2c, d and Supplementary Figs. 18 and 19). The decomposition plateau below 3.5 V is not detected due to the volatilization of the residual oligomers/monomers during the heating and purging process. Owing to the high voltage condition and the strong anionic oxidation of the positive electrode, the Base GPE with poor crosslinking feature experiences the most severe interfacial side reactions, resulting in a gas production of 0.00375 μmol μL−1 (Fig. 2c). The crosslinking density and CEI protection effect of LDFB GPE and LDFB + LPF GPE gradually improve, both of them (Supplementary Fig. 18 and Fig. 2d) show a decrease in gas production amount, which are 0.00275 μmol μL−1 and 0.00175 μmol μL−1, respectively. The concentration of gas generated from the Base GPE-based cell increases continuously throughout the charging process, while that from the LDFB GPE and the LDFB + LPF GPE-based cells increases until 4.6 V and then decreases. This difference is attributed to the presence of more oligomer residues in Base GPE and the degradation of Base GPE itself under high voltage exceeding 4.6 V. More gas production results in more pores and severe contact loss in the composite positive electrode with Base GPE (Fig. 2e) after cycling, which are not observed in the composite positive electrode with LDFB + LPF GPE (Fig. 2f). Therefore, the greatly reduced gas production of LDFB + LPF GPE benefits to maintain the electrode-electrolyte interface stability.
The EIS tests are conducted for different GPE-based cells before and after 50 cycles at 75 mA g−1, Fig. 2g shows the fitting results derived from Supplementary Fig. 20a–d. Notably, the interface impedance changes (Ri) in Base GPE and LDFB GPE-based cells range from 96.3 to 996.4 Ω and 109.6 to 911.9 Ω, respectively, which are significantly higher than that of LDFB + LPF GPE counterpart (from 149.8 to 302.8 Ω). In addition, the Ri of cells based on Base GPE and LDFB GPE is composed of two parts: Riʹ and Riʹʹ. The interfacial impedance divergence is caused by the formed void of cracked material and micro contact loss of the composite positive electrode31. The CFLLO material matched with Base GPE and LDFB GPE experiences more severe structural decay (Supplementary Figs. 21b, c) and particle breakage (Supplementary Figs. 22b, c) with larger gas generation, which inevitably disrupts the mechanical contact between GPE and CFLLO positive electrode. Due to the protection of additive-modified CEI, CFLLO matched with LDFB + LPF GPE maintains a stable structure without cracks and voids (Supplementary Figs. 21 d and 22 d). The divergence of Ri is absent in LDFB + LPF GPE-based cells, so it exhibits the lowest interface impedance of 302.8 Ω after cycling. Overall, LDFB + LPF GPE-based cells exhibit significantly enhanced material/interface mechanical stability.
The effect of CEI on stabilizing chemical interface
To further investigate the mechanism of CEI, which is formed by LiDFOB and LiPO2F2 in stabilizing the chemical interface, time-of-flight secondary ion mass spectrometry (TOF-SIMS) and X-ray photoelectron spectroscopy (XPS) measurements were carried out for the CFLLO electrode after 90 cycles. As shown in Fig. 3a–d and Supplementary Figs. 23–25, the CH2- fragments in Supplementary Fig. 24a mainly originate from unstable organic components such as ROCO2Li or polycarbonate in CEI, while CO3- fragments in Supplementary Fig. 24b mainly come from harmful phase of Li2CO3 that can produce CO2 by its decomposition32. The difference indicates that the introduction of LiDFOB and LiPO2F2 significantly reduces the proportion of organic and harmful components. The reason is the reduced side reactions of GPE with additives, which are caused by three factors: (1) The additives, as discussed previously, enhance the antioxidant ability of GPE; (2) The decomposition product BF2OH of LiDFOB can immobilize O anion with strong oxidizing feature23, which is consistent with the changes of O2- and BF2O- signal (Fig. 3b, d and Supplementary Figs. 23b and 24c). Similarly, Fig. 3k and Supplementary Fig. 26 indicate that the B-rich CEI can form a strong bond with the lattice oxygen. The adsorption energy of these B-containing CEI components (− 1.75 eV, − 1.89 eV, and − 2.36 eV for BF2OH, LiBO2, and BF2OBF2, respectively) is stronger than that of CH3COOLi (− 0.73 eV) on (003) surface of Li2MnO3 phase; (3) The CEI layer is rich in inorganic components, including LiBO2 (Supplementary Figs. 25a and 28c from LiDFOB), LiF, and Li3PO4 (Fig. 3g, j and Supplementary Figs. 27c and 29a, from LiPO2F2), which can effectively passivate positive electrode|electrolyte interface to inhibit side reaction.
The lower HOMO energy level represents a stronger antioxidant ability. The higher value of CH3COOLi (− 7.15 eV) than LiF (− 7.7 eV) and BF2OH (− 10.58 eV) indicates the formed inorganic components-rich CEI layer is more stable at high voltage (Supplementary Fig. 29). Although LiDFOB improves the stability of lattice oxygen, it still causes damage to the structure of CFLLO (Supplementary Fig. 21). The signal results of NiF- and NiO- in Fig. 3a, c and Supplementary Figs. 23a and 25b indicate that single LiDFOB cannot inhibit potential TM dissolution effectively. The X-ray absorption near edge structure (XANES) of Mn K-edge in Fig. 3l displays a significantly increased pre-edge peak intensity for cycled CFLLO with Base GPE compared to that with LDFB GPE and LDFB + LPF GPE, implying the enhancement of local structure distortion33,34. The inferior structure stability is assigned to O and TM loss of CFLLO matched with Base GPE, as shown in the results of weakening coordination of Mn-O and Mn-TM in extended X-ray absorption fine structure (EXAFS) in Fig. 3m. Supplementary Fig. 30 reveals gradual increase lattice oxygen content in the subsurface region of the cycled CFLLO from Base GPE (60.6%) to LDFB GPE (71.4%) and LDFB + LPF GPE (74.6%). Clearly, CFLLO without LiDFOB additive exhibits the most severe lattice oxygen loss in Base GPE-based battery, suggesting the introduction of B-containing additive benefits to improve lattice oxygen stability in CFLLO. The electron paramagnetic resonance spectra of the cycled CFLLO exhibit gradually decreased oxygen vacancy signal at g value of about 2.003 from Base GPE to LDFB GPE and LDFB + LPF GPE-based batteries (Supplementary Fig. 31), further showing better oxygen stability of CFLLO in LDFB + LPF GPE-based battery, which is consistent with above O 1 s XPS results (Supplementary Fig. 30). Therefore, LiDFOB combined with LiPO2F2 can synergistically optimize CEI to simultaneously solve the issues of potential TM and O loss in CFLLO.
Figure 3e–j and Supplementary Figs. 27 and 28 show the XPS spectra of cycled composite positive electrodes with Base GPE, LDFB GPE, and LDFB + LPF GPE. The peak positions are summarized in Supplementary Table 4 and 5. In the F 1 s XPS spectra (Fig. 3f, i and Supplementary Fig. 27b), LiPxFyOz/LiPxFy (686-686.8 eV) in Base GPE arise from the decomposition of PF635, while the peak completely disappears in LDFB + LPF GPE, implying that LDFOB and LiPO2F2 can effectively inhibit the decomposition of electrolytes. In addition, the CEI with rich LiF (F 1 s, 685.0 eV, Fig. 3i), Li3PO4 (P 2p, 133.5/134.3 eV, Fig. 3j), and LiBO2 (B 1 s, 191.2 eV, Supplementary Fig. 28c) inorganic components are formed on the CFLLO surface with LDFB + LPF GPE. The LiF-rich CEI obtained from the decomposition of LiPO2F2 protects the positive electrode from HF attack (Detailed discussions attached to Supplementary Fig. 27), which ensures good stability of positive electrode structure and TM chemical states (Supplementary Fig. 32). Therefore, the CFLLO electrode matched with LDFB + LPF GPE does not detect signal peak of TM in C 1 s, P 2p and O 1 s spectra (C-Ni of 283.4 eV, TM-P of 130/131.1 eV, TM-O of 529.4 eV), which are present in XPS spectra of Base GPE and LDFB GPE. This proves that LiPO2F2 combined with LiDFOB can inhibit the potential TM dissolution of CFLLO, and the TM element content on the Li surface exhibits a decrease trend (Supplementary Figs. 33 and 34). All above results sufficiently suggest that LDFB + LPF GPE benefits to form a stable chemical interface between positive electrode and electrolyte.
LLOs-based ceramic SSLBs design under ambient conditions
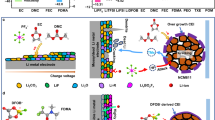
Clearly, LDFB + LPF GPE exhibits satisfactory capability in regulating physical/chemical interface and the LLOs-based ceramic SSLBs have been devised (Fig. 4a). The Li1.2Ni0.13Co0.13Mn0.54O2 with higher specific capacity is selected as positive electrode to examine the interfacial regulating ability of LDFB + LPF GPE, because the catalytic activity of Co may lead to a more severe interfacial side reactions36,37. The Li1.4Al0.4Ti1.6(PO4)3 (LATP) electrolyte with mechanical stiffness means worse interface contact. The ceramic SSLBs were achieved with only trace amount GPE serving as an interfacial modification layer at 30 °C and ambient pressure. The sintered LATP has a typical topography (Supplementary Fig. 35), crystal structure and Li+ conductivity (Supplementary Fig. 36). LDFB + LPF GPE can provide intimate contact between composite positive electrode and LATP (Supplementary Fig. 37). The LATP with a thickness of 300 μm is modified with a 10 μm of ultrathin GPE on the negative electrode side (Supplementary Fig. 38).
a The design principle of crosslinking GPE with enhanced physical and chemical interface for ceramic solid-state lithium batteries: (i) The additives change the Li chemical environment in the GPE, where the augmented crosslink density of GPE immobilizes the anion, thereby enhancing its oxidation stability and Li+ transference. (ii) The decomposition of additives forms F- and B-rich inorganic CEI layer, which effectively suppress interfacial side reactions, stabilize O, and prevent HF attack. (iii) The inorganic CEI and the GPE featuring high crosslink density alleviates GPE decomposition and gas generation, reducing physical contact loss (R represents alkyl group). b Voltage profiles of the Li|GPE-LATP-GPE|Li symmetric cells at 0.1 mA cm−2 and 0.05 mAh cm−2 (partial magnification shown in the inset). c Cycle performance of the Li|GPE-LATP-GPE | LLO in 2.0–4.7 V at 25 mA g−1 and 30 °C (the voltage range of first cycle is 2.0–4.8 V). d Rate performance and subsequent cycle performance at 50 mA g−1 of the Li|GPE-LATP-GPE | LLO batteries in 2.0–4.7 V. The above GPE all represents LDFB + LPF GPE. e Comparison of the electrochemical performance for the constructed LLOs-based SSLBs with halide, sulfide, and ceramic electrolytes. The source of the literature data shown in this figure can be found in Supplementary Table 6.
As shown in Supplementary Fig. 39, the Li|LATP|Li symmetric cell can hardly work, and its impedance increases rapidly after 60 h (Supplementary Fig. 40a), indicating severe reduction of LATP by Li (as shown in the inset). Differently, the Li|GPE-LATP-GPE|Li symmetric cell displays a decreased impedance from 450 Ω to 320 Ω after 60 h cycling at 0.1 mA cm−2, proving that LDFB + LPF GPE effectively improves the negative electrode-side interface stability and prevents LATP from Li reduction (Detailed discussions attached to Supplementary Fig. 40b)38,39. Besides, the crosslinked polymerized network can not only regulate the uniform growth of solid electrolyte interphase (SEI), but also benefit to induce a relatively homogeneous Li+ flux25,40. The formed LiF, as a critical SEI component, through LiPO2F2 decomposition improves the interface stability of Li negative electrode, thereby decreasing battery polarization (Supplementary Fig. 41). Consequently, the assembled Li|LDFB + LPF GPE|Li cell displays a low voltage hysteresis of 15 mV during 500 h cycling when tested at 0.1 mA cm−2 for 0.05 mAh cm−2 (Supplementary Fig. 42a). The interface impedance decreases to approximate 107 Ω after cycling that is attributed to the formation of stable Li|SEI | GPE interface (Supplementary Fig. 42b). Unfortunately, the voltage hysteresis experiences slight drops at 105 and 163 h, respectively, corresponding to the internal slight short circuit caused by Li dendrite growth. There is still a risk of short circuits in the GPE system. Differently, as shown in Fig. 4b, the constructed Li|GPE-LATP-GPE|Li symmetric cell can cycle stably for 1000 h at 0.1 mA cm−2 and 0.05 mAh cm−2, accompanied by a steady voltage polarization of 50 mV without significant oscillation, signifying the stable negative electrode-electrolyte interface. In Supplementary Fig. 43, the R1 of fresh symmetric batteries in high-frequency region is indicative of the materials impedance of composite solid-state electrolytes, which exhibits an increase from 119 Ω to 146 Ω after cycling. This can be attributed to the formation of SEI and the emergence of impedance in the secondary high-frequency region. The interfacial impedance (Ri) decreases from 349 Ω to 82 Ω after cycling, indicating the enhanced Li+ transport across the negative electrode-electrolyte interface. Consequently, the rigid LATP matrix and stable SEI interface synergistically inhibit the uncontrollable growth of lithium dendrites.
As demonstrated in Fig. 4c, the constructed Li|GPE-LATP-GPE | LLO cell (where GPE is LDFB + LPF GPE) demonstrates an initial discharge capacity of 276.5 mAh g−1 in the voltage range of 2.0–4.7 V at 25 mA g−1 with a capacity retention of 81.7% after 100 cycles. The good voltage retention (95.8%) after 100 cycles reflects the highly reversible structure of the positive electrode (Supplementary Fig. 44). Similarly, the impedance of batteries remains stable before and after long cycling, even without external pressure (Supplementary Fig. 45), showing benign interface contact. The greatly enhanced electrochemical performance indicates that LDFB + LPF GPE plays a dual role of electrochemical and mechanical modification on both positive electrode and negative electrode sides of SSLBs, as expected. Figure 4d shows the rate capability of the designed ceramic SSLBs at 25, 50, 125, and 250 mA g−1. At current densities of 125 and 250 mA g−1, the LLOs-based ceramic SSLBs exhibit specific capacities of 199.8 and 152.1 mAh g−1, respectively. The following cycling performance of batteries can reach 182.6 mAh g−1 after 200 cycles at 50 mA g−1 (with a capacity retention of 76.1%), evidencing that LDFB + LPF GPE successfully achieves stable physical/chemical interface at both positive electrode and negative electrode side in ceramic solid-state batteries. Clearly, the LLOs-based ceramic SSLBs without liquid electrolyte or external stack pressure show favorable electrochemical properties among the previously reported LLOs-based inorganic SSLBs (Fig. 4e and Supplementary Table 6).
Discussion
The combination of LiDFOB and LiPO2F2 additives can optimize the properties of GPE, resulting in a high-crosslinking density. The elaborately designed LDFB + LPF GPE has an electrochemical window up to 4.7 V and a high Li+ transference number of 0.82. The mechanism for enhanced interface stability is investigated and revealed. It is found that the high crosslinking density of LDFB + LPF GPE with less residual oligomer can ensure a more stable GPE|LLOs interface without divergence and significant growth of interface impedance. In-situ polymerization effectively constructs continuous Li+ transfer pathways and good interface contact. The LiF and BF2OH in inorganic component-enrich CEI layer can effectively mitigate the potential TM and O loss by resisting HF and immobilizing O, thus maintaining the structure integrity of the LLOs material during cycling. As a result, the assembled LATP-based solid-state batteries and symmetric batteries can achieve good long-term cycling performance under ambient conditions. This research provides guideline for the practical application of high-energy density SSLBs.
Methods
Preparation of the GPEs
To synthesize the GPE41, TEGDA (triethylene glycol diacrylate, C12H18O6, Weng Jiang Reagent, AR) and BA (butyl acrylate, C7H12O2, ALFA, 99%) were collected as the monomer mixtures in a weight ratio of 4: 2. The 2,2’-Azobis-(2,4-dimethylvaleronitrile) (ABVN, C14H24N4, Aladdin, 98%), accounting for 1 wt% of the monomer mixtures, was added as thermal initiator. The liquid electrolyte (Pristine LE) is 1 M LiPF6 in EC/DMC/EMC (1: 1: 1, v/v/v) (DoDoChem Co., Ltd.), which is added together with Al2O3 (30 nm, Aladdin, 99.9%) to form a precursor solution after stirring for 4 h. The composition weight ratio of precursor solution of liquid electrolyte/monomer mixtures/Al2O3 is 94: 6: 5.3. The procedures were carried out in the glove box under argon atmosphere (O2 < 1 ppm, H2O < 1 ppm). The Base GPE was formed by heating the precursor solution at 80 °C for 30 min via in situ thermal polymerization. The LDFB GPE, LPF GPE, and LDFB + LPF GPE have a similar preparation process as Base GPE except that the precursor solution requires the addition of 0.7 wt% LiDFOB, 0.7 wt% LiPO2F2, and 1.4 wt% LiDFOB/LiPO2F2 (1: 1, w/w), respectively (Al2O3/LiDFOB or LiPO2F2 = 5.3: 0.7).
Preparation of LATP electrolyte
The Li1.4Al0.4Ti1.6(PO4)3 powder materials were purchased from BTR Co., Ltd. 400 mg of powders were isostatically pressed into a 16 mm green pellets at 250 MPa for 10 minutes. The green pellets were sintered at 900 °C for 6 h in air atmosphere. The LATP electrolyte was obtained and further polished to 300 μm in thickness.
Preparation of positive electrode materials
The cobalt-free Li1.2Ni0.2Mn0.6O2 and Li1.2Ni0.13Co0.13Mn0.54O2 were prepared through co-precipitation42. Firstly, a certain concentration of chelating agent aqueous solution was obtained in a continuously stirred tank reactor (CSTR). Then, the metal salts of CoSO4·7H2O (Aladdin, 99%), MnSO4·H2O (Aladdin, 99%), and NiSO4·6H2O (Aladdin, 99%) were added to 10 L deionized water in a certain ratio (Mn2+/Ni2+/Co2+ = 3: 1: 0 or Mn2+/Ni2+/Co2+ = 4: 1: 1) to obtain 1.0 M solution A. Next, solution A and 10 L of 1.0 M Na2CO3 (Aladdin, 99%) aqueous solution were simultaneously pumped in CSTR with 800 rpm at 60 °C under Ar protection and aged for 5 h. After that, the cleaned and dried carbonate precursor was pre-calcined at 500 °C for 2 h. Finally, the carbonate precursor was mixed with LiOH·H2O and calcined at 800 °C in air with a ramping rate of 2 °C min−1 for 10 h.
Preparation of composite positive electrode and batteries
To prepare the LLO composite positive electrode, the active materials powder (3 g, 90 wt%), Super P (0.167 g, 5 wt%), and polyvinylidene fluoride (PVDF, 1.67 g, 5 wt%, Aladdin, 99.9%) were uniformly mixed in N-methyl-2-pyrrolidone (NMP, 7.8 g, Aladdin, 99.9%) through stirring in serum bottle for 24 h. Then, the obtained slurry was coated on carbon-coated Al foil using a 300 μm doctor blade, and further dried for 12 h in a vacuum oven at 85 °C to remove the solvent. The composite electrode was punched into discs with a diameter of 12 mm using a punching machine (MSK-T10), and the mass loading of active materials is about 2.4–2.6 mg cm−2. All batteries, for electrochemical testing, were assembled by CR2025-type coin cells in an Ar-filled glovebox (O2 < 1 ppm, H2O < 1 ppm). Li metal batteries were assembled using CFLLO and fresh Li metal without pretreatment (China Energy Lithium Co., Ltd., 99.9%, 16 mm diameter × 1.5 mm thickness) as positive electrode/negative electrode, respectively. Celgard 2500 was punched into discs with a diameter of 19 mm as the separator, different GPEs were used as the electrolyte for gel lithium batteries. The quasi ceramic SSLBs used LLO as the positive electrode and the LATP ceramic pellet as the solid-state electrolyte with LDFB + LPF GPE modified layer (30 μL mg−1 for each positive electrode|negative electrode side) at the interface. The Li | |Li symmetric batteries utilized the same electrolyte except for the Li|LATP|Li cell without GPE. All cells with GPEs need to age at 30 °C for 2 h to ensure that the precursor solution has wetted the electrodes sufficiently. Subsequently, the cells were heated at 80 °C for 30 min in an oven to trigger in situ polymerization.
Material characterization
The FTIR spectra was recorded using an infrared instrument (Thermo Fisher Nicolet iS 10) over the wavenumbers range from 4000 to 500 cm−1. 7Li NMR spectra were obtained on a Bruker AVANCE NEO 600 Solid-State Nuclear Magnetic Resonance spectrometer. The morphologies were examined using SEM (FEI TECNAI F30) equipped with an energy dispersive spectrometer, and the microstructures were investigated using TEM (Talos F200). The crystal structure of the samples was characterized by an X-ray diffractometer (XRD, Bruker-D8-A25) with a Cu-Kα radiation in the 2θ range of 10–80°. The CEI composition of the long-cycled electrodes was characterized using XPS (Thermo Scientific ESCALAB Xi + ) with an etching time interval of 30 s. TOF-SIMS (IONTOF M6) was carried out to characterize the interface chemistry. The topography and Young’s modulus of the GPEs were tested via AFM (Bruker Dimension ICON PT). All nuclear magnetic resonance (NMR) spectra were collected using AVANCE NEO 600 MHz. The microstructure change of GPEs was characterized using SAXS (Xenocs Xeuss 3.0 HR). X-ray absorption spectroscopy (XAS) measurements were collected at 12-BM-B at the Advanced Photon Source (APS) in Argonne National Laboratory. The radiation was monochromatized by a Si (111) double-crystal monochromator. Electron paramagnetic resonance (EPR) of the cycled CFLLO positive electrodes was recorded on a Bruker EMX plus-9.5/12/P/L.
Electrochemical measurements
The GPE membranes were sandwiched between two stainless steel (SS, 15.8 mm × 0.8 mm thickness) electrodes (SS | GPE | SS), and the Pt was sputtered on both sides of the LATP pellet (14 mm diameter × 1 mm thickness) to fabricate the symmetric cell (Pt|LATP|Pt). The SS and Pt were the Li+ blocking electrode. And the coin cells were assembled to determine the ionic conductivity values. Potentiostatic EIS was conducted using a PARSTAT 3000A-DX electrochemical workstation (AMETEK Instrument Co., Ltd.) with a 10 mV voltage amplitude. The measurement range from 1 MHz to 0.1 Hz at an open-circuit voltage after a 2 s equilibration time, with 10 points per decade at 30 °C. The ionic conductivity values were obtained by the following equation:
where L (cm) is the thickness of the electrolyte, R (Ω) is the bulk resistance of the electrolyte obtained from EIS, and S (cm2) denotes the contact area of the electrode and electrolyte. The Nyquist plots of Li|GPE | CFLLO were fitted by the ZView software. The electrochemical stability window of the GPEs was determined through LSV tests from 0 to 5 V at a scan rate of 1 mV s−1 using Li|GPE | SS.
Li+ transference number (tLi+) was measured by chronoamperometry and EIS using an electrochemical working station. The test cell was Li|GPE|Li, and the step voltages ΔV was 10 mV. The tLi+ was calculated with the following equation:
Among them, I0 and ISS represent the initial and steady-state current values, respectively. Simultaneously, R0 and RSS are the initial and steady-state values of bulk resistances, and Ri0 and RiSS are interfacial resistances before and after the polarization.
The galvanostatic charge/discharge measurements were conducted using the batteries testing system (CT-4008, Neware Electronics Co., Ltd). Li symmetric cells were cycled at 0.1 mA cm−2 with charge/discharge for 0.5 h. All the electrochemical tests were carried out at 30 °C. Three cells were tested for a single electrochemical experiment.
DEMS (QAS100-Li, LingLu Co., Ltd) analysis was carried out to detect the gases generated during the initial charge process. During testing, the cell was replenished with Ar, which would carry the evolved gases from the cell to the mass spectrometer for analyzing. After that, the m/z 44 mass signals, corresponding to the CO2 or CH3CHO, were selected and recorded as a function of time and the cell voltage. The mass loading of positive materials is 8 mg cm−2, the separator and negative are the same as those used in a coin cell.
Theoretical calculations
All density functional theory (DFT) calculations were performed by using the Gaussian 16 software43. Geometric configurations were optimized at the B3LYP level with a 6-311 + + G (d,p) basis set under gas-phase conditions36. The GD3BJ correction was used for all calculations. The results were analyzed with Multiwfn programs44,45.
The theoretical calculations were implemented using the Vienna ab initio simulation package (VASP)46. The local generalized gradient approximation (GGA) of Perdew, Burke, and Ernzerhof (PBE) was used to treat the exchange-correlation energy47. The plane wave cut-off energy was set to be 520 eV, and the convergence criteria were 10−5 eV and 0.04 eV Å−1 for electron energy and ions force, respectively, in all calculations. The k-point sampling of the Monkhorst-Pack scheme was used to sample Brillouin-zone integrations48, which were set 1 × 1 × 1 for Li2MnO3-BF2OH, Li2MnO3-LiBO2, Li2MnO3-BF2OBF2, and Li2MnO3-CH3COOLi. The adhesion can be acquired according to the following equation49:
Where \({W}_{{ad}}\) is the work of adhesion for the interface, \({E}_{{interface}}\), \({E}_{{{Li}}_{2}{Mn}{O}_{3}}\) and \({E}_{X-{slab}}\) (X = BF2OH, LiBO2, BF2OBF2, and CH3COOLi) are referenced to the total energy of interfacial supercell, isolated Li2MnO3 (003) and X surface slab, respectively, and S refers to interfacial area. The optimized structures for calculation are available in Supplementary Data 1 and 2.
Data availability
The data that support the findings of this study are available from the corresponding author on request. Source data are provided in this paper.
References
Kang, K. S., Meng, Y. S., Bréger, J., Grey, C. P. & Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311, 977–980 (2006).
Zhang, C. et al. Regulating Lewis acid-base interaction in poly (ethylene oxide)-based electrolyte to enhance the cycling stability of solid-state lithium metal batteries. Small Struct. 5, 2300301 (2023).
Xia, Y. et al. Van der Waals forces between S and P ions at the CoP-C@MoS2/C heterointerface with enhanced lithium/sodium storage. Adv. Funct. Mater. 33, 2302830 (2023).
Yuan, Y., Nai-Fang, H., Yong-Cheng, J., Jun, M. & Guang-Lei, C. Research advance of lithium-rich cathode materials in all-solid-state lithium batteries. Acta Phys. Sin. 72, 1–10 (2023).
Han, Q. et al. Exploiting iodine redox chemistry for achieving high-capacity and durable PEO-based all-solid-state LiFePO4/Li batteries. Adv. Energy Mater. 13, 2301462 (2023).
He, W. et al. Challenges and recent advances in high capacity Li-rich cathode materials for high energy density lithium-ion batteries. Adv. Mater. 33, 2005937 (2021).
Zuo, W. et al. Li-rich cathodes for rechargeable Li-based batteries: reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 13, 4450–4497 (2020).
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Liu, T. C. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305–312 (2022).
Kalnaus, S., Dudney, N. J., Westover, A. S., Herbert, E. & Hackney, S. Solid-state batteries: The critical role of mechanics. Science 381, eabg5998 (2023).
Krauskopf, T., Richter, F. H., Zeier, W. G. & Janek, J. Physicochemical concepts of the lithium metal anode in solid-state batteries. Chem. Rev. 120, 7745–7794 (2020).
Wan, H., Wang, Z., Zhang, W., He, X. & Wang, C. Interface design for all-solid-state lithium batteries. Nature 623, 739–744 (2023).
Liu, B. et al. Direct observation of Li-ion transport heterogeneity induced by nanoscale phase separation in Li-rich cathodes of solid-state batteries. Angew. Chem. Int. Ed. 61, e202209626 (2022).
Du, W. et al. High-energy and long-cycling all-solid-state lithium-ion batteries with Li- and Mn-rich layered oxide cathodes and sulfide electrolytes. ACS Energy Lett. 7, 3006–3014 (2022).
Auvergniot, J. et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 29, 3883–3890 (2017).
Walther, F. et al. Visualization of the interfacial decomposition of composite cathodes in argyrodite-based all-solid-state batteries using time-of-flight secondary-ion mass spectrometry. Chem. Mater. 31, 3745–3755 (2019).
Minnmann, P. et al. Designing cathodes and cathode active materials for solid-state batteries. Adv. Energy Mater. 12, 2201425 (2022).
Cabanero Martinez, M. A. et al. Are polymer-based electrolytes ready for high-voltage lithium battery applications? An overview of degradation mechanisms and battery performance. Adv. Energy Mater. 12, 2201264 (2022).
Hestenes, J. C. & Marbella, L. E. Beyond composition: surface reactivity and structural arrangement of the cathode-electrolyte interphase. ACS Energy Lett. 8, 4572–4596 (2023).
Xu, J. Critical review on cathode-electrolyte interphase toward high-voltage cathodes for Li-ion batteries. Nano Micro Lett. 14, 2–22 (2022).
Wu, Y. et al. Development of cathode-electrolyte-interphase for safer lithium batteries. Energy Storage Mater. 37, 77–86 (2021).
Han, S. Y. et al. Li-ion exchange-driven interfacial buffer layer for all-solid-state lithium metal batteries. Adv. Funct. Mater. 34, 2405152 (2024).
Yang, X. et al. Understanding of the working mechanism of lithium difluoro(oxalato) borate in Li||NCM85 battery with enhanced cyclic stability. Energy Mater 3, 300029, (2023).
Zhang, A. P. et al. Regulating electrode/electrolyte interfacial chemistry enables 4.6 V ultra-stable fast charging of commercial LiCoO2. Energy Environ. Sci. 17, 3021 (2024).
Zhang, C. et al. Highly oxidation-resistant ether gel electrolytes for 4.7 V high-safety lithium metal batteries. Adv. Energy Mater. 13, 2203870 (2023).
Wu, J. et al. A synergistic exploitation to produce high-voltage quasi-solid-state lithium metal batteries. Nat. Commun. 12, 5746 (2021).
Wang, H. et al. A strongly complexed solid polymer electrolyte enables a stable solid state high-voltage lithium metal battery. Energy Environ. Sci. 15, 5149–5158 (2022).
Wang, H. C. et al. An entanglement association polymer electrolyte for Li-metal batteries. Nat. Commun. 15, 2500 (2024).
Liu, J., Li, J.-J., Luo, Z.-H. & Zhou, Y.-N. Fast room-temperature self-healing vitrimers enabled by accelerated associative exchange kinetics. Chem. Eng. J. 452, 139452 (2023).
Liu, B., Zhou, H., Yin, C., Guan, H. & Li, J. Enhanced electrochemical performance of LiNi0.5Mn1.5O4 cathode by application of LiPF2O2 for lithium difluoro(oxalate)borate electrolyte. Electrochim. Acta 321, 134690 (2019).
McGrogan, F. P., Bishop, S. R., Chiang, Y. M. & Van Vliet, K. J. Connecting particle fracture with electrochemical impedance in LixMn2O4. J. Electrochem. Soc. 164, A3709–A3717 (2017).
Kim, S. et al. Wide-temperature-range operation of lithium-metal batteries using partially and weakly solvating liquid electrolytes. Energy Environ. Sci. 16, 5108–5122 (2023).
Ogley, M. J. W. et al. Metal–ligand redox in layered oxide cathodes for Li-ion batteries. Joule 9, 1–14 (2024).
Oishi, M. et al. Charge compensation mechanisms in Li1.16Ni0.15Co0.19Mn0.50O2 positive electrode material for Li-ion batteries analyzed by a combination of hard and soft X-ray absorption near edge structure. J. Power Sources 222, 45–51 (2013).
Zhao, J. et al. Interphase engineering by electrolyte additives for lithium-rich layered oxides: advances and perspectives. ACS Energy Lett. 6, 2552–2564 (2021).
Nie, K. et al. Increasing poly(ethylene oxide) stability to 4.5 V by surface coating of the cathode. ACS Energy Lett. 5, 826–832 (2020).
Han, Q. et al. Realizing compatibility of high voltage cathode and poly (ethylene oxide) electrolyte in all-solid-state lithium batteries by bilayer electrolyte design. Chem. Eng. J. 454, 140104 (2023).
Kong, W. et al. Stabilizing Li1.3Al0.3Ti1.7(PO4)3|Li metal anode interface in solid-state batteries by kevlar aramid nanofiber-based protective coating. Adv. Funct. Mater. 33, 2306748 (2023).
Didwal, P. N. et al. An advanced solid polymer electrolyte composed of poly(propylene carbonate) and mesoporous silica nanoparticles for use in all-solid-state lithium-ion batteries. Energy Storage Mater. 37, 476–490 (2021).
Zhou, D. et al. Stable conversion chemistry-based lithium metal batteries enabled by hierarchical multifunctional polymer electrolytes with near-single ion conduction. Angew. Chem. Int Ed. 58, 6001–6006 (2019).
Fan, H., Li, H., Fan, L. Z. & Shi, Q. Preparation and electrochemical properties of gel polymer electrolytes using triethylene glycol diacetate-2-propenoic acid butyl ester copolymer for high energy density lithium-ion batteries. J. Power Sources 249, 392–396 (2014).
Guo, W. et al. A universal strategy toward the precise regulation of initial coulombic efficiency of Li-rich Mn-based cathode materials. Adv. Mater. 33, 2103173 (2021).
Frisch, M. J., et al. Gaussian 16 Rev. C.01. (2016).
Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange” - Becke AD (1993) J Chem Phys 98:5648-52. Theor. Chem. Acc. 103, 361–363 (2000).
Lu, T. & Chen, F. W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput Chem. 33, 580–592 (2012).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Liu, Y. & Ning, X. S. Influence of α-Al2O3 (0001) surface reconstruction on wettability of Al/Al2O3 interface: A first-principle study. Comp. Mater. Sci. 85, 193–199 (2014).
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (Grant Nos. 52272240[Q.S.X.], 52431009[D.-L.P.] and U22A20118[D.-L.P.]), Science and Technology Planning Projects of Fujian Province of China (Grant No. 2023H0003[Q.S.X.]), the Fundamental Research Funds for the Central Universities of China (Xiamen University: Nos. 20720220074[Q.S.X.] and 20720240053[Q.S.X.]), and the “Double-First Class” Foundation of Materials Intelligent Manufacturing Discipline of Xiamen University. This work was also supported by the Clean Vehicles, US–China Clean Energy Research Center (CERC-CVC2[K.A.]) under the US DOE EERE Vehicle Technologies Office. This research used resources of the Advanced Photon Source (beamline 12-BM-B), a U.S. DOE Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02- 06CH11357.
Author information
Authors and Affiliations
Contributions
J.T.L., D.-L.P., K.A., and Q.S.X. guide the project; X.C.H. and Q.S.X. designed this research; X.C.H., S.Q.S., M.J.F., S.S.L., Y.G.Z., H.L.W., G.Y.G., Y.Y.L., S.Y.Z., and C.K.Z performed research; J.S.W and B.S.S. carried out the theory calculations; X.C.H., J.T.L., L.S.W., D.-L.P., K.A., and Q.S.X. discussed the data and results; and X.C.H., J.T.L., and Q.S.X. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hong Yuan and the other anonymous reviewer(s) for their contribution to the peer review of this work. [A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, X., Shen, S., Li, J. et al. Modulating physicochemical interfaces enables li-rich oxides based ceramic solid-state li batteries under ambient conditions. Nat Commun 16, 9338 (2025). https://doi.org/10.1038/s41467-025-64396-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64396-w