Abstract
Coating Ni-rich layered oxide positive electrodes is essential to improve their electrochemical performance in sulfide-based all-solid-state Li batteries, but achieving cost-effective, high-performance positive electrodes remains challenging. In this work, we apply a thin (~3 nm) conductive binary Li borate glass coating (0.5Li2O·0.5B2O3) onto single-crystal LiNi0.8Co0.1Mn0.1O2 using a simple dry process and heating. This coated positive electrode delivers 209 mAh g−1 specific capacity at a specific current of 20 mA g−1 with 79.7% initial Coulombic efficiency, retains 87.8% capacity after 1000 cycles at a specific current of 200 mA g−1, and achieves 14.6 mAh cm−2 areal capacity. Pouch cells with this positive electrode reach 383 Wh kg−1 specific energy, and sustain 300 cycles at a specific current of 66.67 mA g−1. Various characterizations reveal that this coating can enhance Li-ion transport, stabilize the positive electrode lattice, and strengthen the interface between positive electrode and sulfide electrolyte. Here we show that conductive glass coatings enable high-voltage positive electrodes with high stability and specific energy in all-solid-state batteries.
Similar content being viewed by others
Introduction
Li-ion batteries (LIBs) have surpassed competitors like lead-acid batteries, dominating markets such as electric vehicles and mobile devices due to their high specific energy, good rate capability, and stable cycling performance1,2. However, meeting the 2030 target of 500 Wh kg−1 remains challenging, as the theoretical specific energy of LIBs is limited to 400 Wh kg−1 3. More critically, the safety risk posed by flammable liquid electrolytes continues to be a major concern. In response, next-generation all-solid-state Li batteries (ASSLBs) offer a solution, not only addressing safety with non-flammable solid electrolytes (SEs) but also increasing specific energy through tighter cell architectures and eliminating certain components4,5,6. SEs are essential for the development of ASSLBs and can be categorized into four types: oxide SEs, polymer SEs, sulfide SEs (SSEs), and halide SEs7. Among SEs, SSEs have gained significant attention due to their favorable elastic modulus (7–30 GPa)8 and high room temperature (25 °C) ionic conductivity (\({\sigma }_{i}\), 10−3 ~ 10−2 S cm−1)9. However, the further development of SSE-based ASSLBs faces challenges, particularly at the positive electrode–electrolyte interface when using high-voltage layered oxide positive electrodes10. This interface suffers from issues like undesirable chemical/electrochemical reactions, space charge layers (SCL), and poor solid–solid contact11.
Stabilizing positive electrode interface through artificial coatings on the positive electrode active material (CAM) is an effective approach. Such coatings must exhibit good electrochemical stability, high \({\sigma }_{i}\) for fast Li+ transport, low electronic conductivity (\({\sigma }_{e}\)) to prevent SSE decomposition12,13,14, and must be thin enough (~5 nm) to avoid increasing cell resistance15. Various coatings have been developed for CAMs in ASSLBs, including LiNbO316, Li3PO417, Li7TaO618, Li2O–ZrO219, CoO–Li2CO3/C20, Li2CoTi3O813, LiZr2(PO4)321, Li3P1+xO4S4x14, TiNb2O722, Li1.4Al0.4Ti1.6(PO4)3–cyclized polyacrylonitrile23, and Li borates. Among these, Li borates such as Li3BO3–Li2CO324, Li3BO325, Li2B4O726, Li3B11O1827, and LiBa(B3O5)328, have attracted interest due to their low-cost precursors, scalable preparation processes, and ability to enhance the electrochemical performance of CAMs. However, their relatively low \({\sigma }_{i}\) remains a limitation, with \({\sigma }_{i}\) for crystalline Li3BO3, LiBO2, and Li2B4O7 are only 10−9 S cm−1 (25 °C)24, 10−8 S cm−1 (300 °C)29, and 10−7 S cm−1 (300 °C)29, respectively. Research has shown that binary Li borate glasses (xLi2O·(1−x)B2O3) are promising Li+ conductors30. For instance, 0.5Li2O·0.5B2O3 (LOBO) exhibits a \({\sigma }_{i}\) of approximately 10−6 S cm−1 at 25 °C31. In addition, the recently developed dry coating technology challenges the conventional notion that coatings must be applied via wet processes15. Compared to traditional wet coating, dry coating is simpler, faster, more environmentally friendly, and cost-effective, making it a highly promising approach for surface modification.
In this work, we developed and applied a LOBO coating (~3 nm, 0.3 wt%) on single-crystal LiNi0.8Co0.1Mn0.1O2 (NCM) CAM via a simple dry process followed by calcination. The resulting NCM@LOBO CAM significantly improves electrochemical performance in Li6PS5Cl (LPSC)-based ASSLBs. Specifically, Li–In|LPSC|NCM@LOBO full cells achieve a specific capacity of 209 mAh g−1 at a specific current of 20 mA g−1, with an initial Coulombic efficiency (CE) of 79.7%, 87.8% capacity retention after 1000 cycles at a specific current of 200 mA g−1, and a high areal capacity of 14.6 mAh cm−2 with stable cycling. Additionally, NCM@LOBO-based ASS pouch cells achieve a specific energy of 383 Wh kg−1 and long-term cycling over 300 cycles. Further analysis shows that the LOBO coating significantly enhances \({\sigma }_{i}\) and reduces \({\sigma }_{e}\) of NCM, improving Li+ diffusion at the interface and reducing cell impedance. The LOBO coating also stabilizes NCM, mitigates SSE decomposition, and promotes the formation of a uniform, dense positive electrode electrolyte interphase (CEI), thereby improving the overall stability of full cells. This study demonstrates that selecting coatings with high \({\sigma }_{i}\) and low \({\sigma }_{e}\) is a key strategy for enhancing the electrochemical performance of CAMs in SSE-based ASSLBs.
Results and discussion
Materials preparation
Figure 1a illustrates the preparation process of NCM@LOBO. First, the coating precursors, Li2O and B2O3, were thoroughly mixed via ball milling. Next, NCM was added to the mixture and subjected to additional ball milling to ensure the precursors were uniformly coated onto the NCM particles. Finally, the target NCM@LOBO was obtained through high-temperature calcination at 600 °C for 10 h in air. The thickness of the LOBO coating can be adjusted by varying the precursor amounts. In this study, 0.3 wt% LOBO-coated NCM and uncoated NCM (b-NCM) were selected for structural characterization and comparison. X-ray diffraction (XRD) was employed to examine the crystal structure of the samples. As shown in Fig. S1, the XRD pattern of NCM@LOBO is consistent with that of pristine b-NCM, with no observable peak shifts and changes in the intensity ratios of specific peaks. This indicates that the coating process did not alter the NCM crystal structure32,33. To confirm the formation of the LOBO coating, X-ray photoelectron spectroscopy (XPS) was performed. The XPS spectra of NCM@LOBO overlap well with those of b-NCM, except for the appearance of a peak at 192.0 eV, attributed to LOBO (Fig. S2)34. This observation further confirms the unchanged structure of the NCM core. Figure 1b, c show the Li 1s and B 1s XPS spectra of NCM@LOBO at different depths. At the surface, these spectra confirm the presence of the LOBO coating. However, at a subsurface depth of 5 nm, LOBO is undetectable, and the Li 1s XPS peak at 54.4 eV is assigned to NCM35. These findings confirm the successful deposition of a thin LOBO layer (<5 nm) on the NCM surface.
a Preparation: (i) Dry mixing of Li2O, B2O3, and NCM; (ii) Calcination at 600 °C for 10 h in air. b Li 1s and c B 1s XPS spectra at surface (0 nm) and subsurface (5 nm) depths. d Cross-sectional SEM image. e HRTEM image. f, g HAADF-STEM images of regions ① and ② in (e), showing the amorphous LOBO layer and intact NCM structure. h Elemental mapping, highlighting the distribution of B element.
The morphologies and internal microstructures of the samples were examined using scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), and high-angle annular dark-field scanning TEM (HAADF-STEM). SEM images (Figs. S3 and S4) reveal that both NCM@LOBO and b-NCM particles exhibit a typical single-crystal morphology. Cross-sectional SEM images (Figs. 1d, S5, and S6)36 show that the internal structures of both pristine and coated NCM particles remain homogeneous and intact. Notably, NCM@LOBO particles are smaller than pristine b-NCM particles, suggesting partial cracking of single-crystal particles during the coating process. This observation is further supported by particle size distribution analysis (Fig. S7). The properties of the LOBO coating were further analyzed using HRTEM and HAADF-STEM. HRTEM images (Figs. 1e and S8) show that the LOBO coating adheres tightly to the NCM particle surfaces, with an average thickness of ~3 nm. HAADF-STEM images of the LOBO (Fig. 1f) and NCM (Fig. 1g) regions reveal that the LOBO layer is amorphous, while the underlying NCM maintains an intact layered structure. In contrast, no coating or impurities were observed on the surface of b-NCM (Fig. S9). Elemental mapping (Fig. 1h) further confirms the uniform distribution of B on the NCM particle surface.
Electrochemical performance test
To evaluate the electrochemical performance of the as-prepared NCM@LOBO CAMs, prototype ASSLBs with a Li–In negative electrode and a home-made LPSC SSE (Fig. S10) were assembled and tested at 35 MPa and 25 °C. Achieving an optimal coating thickness is critical for enhancing NCM performance, as the coating must fully cover the NCM particles without being so thick that it hinders Li+ transport15. Accordingly, NCM@LOBO with three different LOBO contents (0.1, 0.3, and 0.5 wt%) were prepared and tested in full cells. From the initial galvanostatic charge–discharge (GCD) profiles (Fig. 2a), NCM@LOBO with 0.1, 0.3, and 0.5 wt% LOBO coatings deliver specific capacities of 192, 209, and 207 mAh g−1, respectively, with initial CEs of 71.8%, 79.7%, and 76.6% at a specific current of 20 mA g−1. In comparison, the b-NCM shows a lower specific capacity of 175 mAh g−1 and an initial CE of 69.5%. Differential capacity (\({dQ}/{dV}\)) curves (Fig. S11) confirm that all as-prepared CAMs exhibit typical NCM redox characteristics. The NCM@LOBO-0.3% exhibits the smallest polarization value, which correlates with its highest electrochemical performance.
a Voltage profiles at a specific current of 20 mA g−1, b cycling stability, c rate capability, d voltage profiles at a specific current of 200 mA g−1, and e long-term stability. f Maximum areal capacities, and g stability under maximum areal capacity. h Maximum areal capacity comparison, also including other reported CAMs, such as NMC111@LLSTO56, NCM85@ZrO257, NCM811@LiNbO354, NCM712+ionomer58, NCM622@LiNbO359, NCM622@Li3N60, B-coated/doped-NCM9061, and NCM811+2%PTFE62.
Durability testing at a specific current of 20 mA g−1 (Figs. 2b and S12) reveals that after 100 cycles, the capacity retentions for b-NCM, NCM@LOBO-0.1%, NCM@LOBO-0.3%, and NCM@LOBO-0.5% are 51.1%, 57.1%, 94.3%, and 86.9%, respectively. The rate capability of the samples was also evaluated (Fig. 2c), with NCM@LOBO-0.3% demonstrating the relatively improved performance. It achieves specific capacities of 209, 190, 167, 152, and 74 mAh g−1 at specific currents of 20, 40, 100, 200, and 400 mA g−1, respectively. The voltage profiles (Figs. S13 and 2d) and \({dQ}/{dV}\) curves (Figs. S14 and S15) show that NCM@LOBO-0.3% exhibits the smallest polarization among all samples. Long-term stability was assessed through GCD testing at a specific current of 200 mA g−1 for 1000 cycles, following an activation sequence of specific currents of 20 mA g−1 for 2 cycles, 40 mA g−1 for 2 cycles, and 100 mA g−1 for 2 cycles. As shown in Figs. 2e and S16, NCM@LOBO-0.3% achieves a capacity retention of 87.8%, which is larger than those of NCM@LOBO-0.5% (84.3%), NCM@LOBO-0.1% (57.4%), and b-NCM (42.1%). These results highlight that the LOBO coating significantly enhances the specific capacity, cycling stability, and rate capability of NCM, with 0.3 wt% LOBO identified as the optimal content. Additionally, NCM@LOBO not only beats b-NCM in electrochemical performance but also exceeds the performance of CAMs with other reported coatings (Table S1).
Increasing the CAM mass loading, and thereby the areal capacity, is crucial for improving the specific energy of ASSLBs. To determine the maximum areal capacities of the as-prepared CAMs, the mass loading was progressively increased to as high as 89.2 mg cm−2, corresponding to a composite positive electrode thickness of ~430 μm (Fig. S17). As shown in Fig. 2f, the areal capacity of NCM@LOBO-0.3% steadily increases with mass loadings ranging from 17.8 to 80.3 mg cm−2, reaching a maximum of 14.6 mAh cm−2 at a mass loading of 80.3 mg cm−2. However, further increases in mass loading (to 89.2 mg cm−2) do not result in higher areal capacities due to the increased composite positive electrode thickness, which impedes Li+ transport, raises impedance, and reduces specific capacity (Fig. S18). In comparison, the maximum areal capacity for b-NCM is 5.6 mAh cm−2, achieved at a mass loading of 53.5 mg cm−2 (Fig. S19).
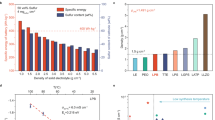
Achieving stable cycling with high mass loading CAMs (above 50 mg cm−2) is a significant challenge, with few reports to date37. Here, we evaluated the cycling stability of the as-prepared CAMs at their maximum areal capacities (Fig. 2g). NCM@LOBO demonstrates more stable cycling, retaining 89.5% of its capacity after 50 cycles at a specific current of 20 mA g−1, compared to 56.7% for b-NCM. It is important to note that the maximum areal capacity of full cells is influenced not only by the CAMs but also by the paired negative electrodes and SSEs. For example, full cells with Si-based negative electrodes38 or modified Li10GeP2S12 (LGPS) SSE39 have achieved higher areal capacities. To put the performance of NCM@LOBO into perspective, we compared its maximum areal capacity with those of reported modified CAMs paired with Li–In negative electrodes and common SSEs (e.g., LPSC, LGPS). As shown in Fig. 2h and Table S2, the maximum areal capacities of previously reported coated CAMs do not exceed 5 mAh cm−2, which is significantly lower than that of NCM@LOBO. This can be attributed to the inferior \({\sigma }_{i}\) of other coating materials.
Electrochemical properties measurement
To understand the differences in electrochemical performance between NCM@LOBO and b-NCM CAMs, their intrinsic electrochemical properties—such as \({\sigma }_{i}\) and \({\sigma }_{e}\), interfacial impedance with the SSE, and Li+ diffusion coefficient (\({D}_{{{Li}}^{+}}\))—were systematically analyzed. The \({\sigma }_{i}\) values of the CAMs were obtained via electrochemical impedance spectroscopy (EIS) by sandwiching CAM pellets between two SSE pellets. As shown in Fig. 3a, the calculated \({\sigma }_{i}\) values for NCM@LOBO and b-NCM are 8.9 and 5.0 mS cm−1, respectively, indicating that the LOBO coating significantly enhances Li+ transport between NCM particles. The \({\sigma }_{e}\) values of the CAMs were measured using the staircase potentiometry method. After fitting and calculations, NCM@LOBO and b-NCM exhibit \({\sigma }_{e}\) values of 5.4 × 10−7 and 5.1 × 10−3 S cm−1, respectively (Figs. 3b and S20). The considerably reduced \({\sigma }_{e}\) in NCM@LOBO compared to b-NCM suggests that LOBO is more effective at suppressing SSE degradation.
Interfacial impedance between the CAMs and LPSC SSE was assessed via EIS of full cells. The Nyquist plots in Fig. S21 display a high-frequency semicircle followed by Warburg impedance, and the interfacial resistance (\({R}_{{Int}}\)) was determined through equivalent circuit fitting (Table S3). The \({R}_{{Int}}\) values for cells with NCM@LOBO and b-NCM are 262.9 and 799.5 Ω, respectively, with NCM@LOBO showing the lower interfacial impedance, attributed to its high \({\sigma }_{i}\) and good compatibility with LPSC. \({D}_{{{Li}}^{+}}\) were measured using the galvanostatic intermittent titration technique (GITT) on the full cells14. The initial transient discharge voltage profiles (Figs. 3c and S22) reveal electrochemical polarizations of 5 and 9 mV for NCM@LOBO and b-NCM, respectively. These correspond to geometric mean \({D}_{{{Li}}^{+}}\) values of 1.14 × 10−8 and 4.37 × 10−9 cm2 s−1, respectively (Fig. 3d), demonstrating a faster Li+ diffusion kinetics in NCM@LOBO. Overall, the Li+-conductive and electron-insulating LOBO layer enhances Li+ transport between the CAM and SSE while suppressing electrolyte decomposition (Fig. 3e), effectively addressing the interface challenges between the electrode and electrolyte (Fig. 3f).
Initial positive electrode interface evolution
Monitoring the evolution of the positive electrode–electrolyte interface during initial cycling is critical for understanding the functioning mechanism of the LOBO coating. To assess the evolution of Li+ kinetics at the interface, in situ EIS measurements were conducted on full cells with NCM@LOBO and b-NCM CAMs during the initial charge and discharge cycle40. The Nyquist plots (Fig. 4a) and corresponding equivalent circuit fitting results (Table S4) for the NCM@LOBO-based cell show that the \({R}_{{Int}}\) increases from 107.9 to 574.5 Ω as the cell charges from 3.1 to 3.7 V, but then gradually decreases to 231.7 Ω during discharge to 2.5 V. This corresponds to an overall impedance increase of 466.6 Ω after experiencing a full charging process. In comparison, the b-NCM-based cell exhibits a similar trend, but with double the impedance increase (915.8 Ω), rising from 214.2 to 1130.0 Ω during the charging process (Fig. S23a and Table S4). These results indicate that NCM@LOBO maintains a more stable and higher Li+ conductive interface with LPSC compared to b-NCM.
a In situ Nyquist plots, b calculated DRT spectra, and c corresponding 2D intensity color map for the NCM@LOBO-based full cell, cycled at a specific current of 20 mA g−1 and 25 °C. d–g Ex situ XPS measurements of the extracted composite positive electrodes at selected states: 0%, 50%, 100% SOC, and 50%, 100% SOD. The full cells were cycled at a specific current of 20 mA g−1 and 25 °C with a CAM loading of 10 mg cm−2.
The distribution of relaxation time (DRT) technique was used to transform the frequency-domain Nyquist plots into time-domain spectra, providing detailed insights into the electrochemical contributions in the EIS41. Fig. 4b, c show the DRT spectra corresponding to the data in Fig. 4a. The peaks at 10−5 s are attributed to the resistance of the solid electrolyte interphase (SEI)/CEI41. Peaks between 10−3 and 10−1 s represent the charge transfer resistance at the positive electrode interface (\({R}_{{ct}}1\))41, while peaks between 100 and 102 s are related to the charge transfer resistance at the negative electrode interface (\({R}_{{ct}}\)2)42. The SEI/CEI resistances exhibit minimal changes during cycling, while \({R}_{{ct}}1\) and \({R}_{{ct}}\)2 increase significantly as the cell charges from 3.5 to 3.7 V, before decreasing during discharge. In contrast, the b-NCM-based cell shows similar SEI/CEI, \({R}_{{ct}}1\), and \({R}_{{ct}}\)2 peaks at the same relaxation times, but with more pronounced increases in \({R}_{{ct}}1\) and \({R}_{{ct}}\)2 during the charge from 3.5 to 3.7 V (Fig. S23b, c). The observed changes in \({R}_{{ct}}\)1 during charging and discharging can be attributed to (1) anisotropic volume changes in CAMs40; (2) the reversible decomposition and formation of LPSC43; and (3) the impact of the SCL44. These factors are effectively mitigated when using LOBO-coated NCM CAM, but not in b-NCM CAM.
To gain deeper insight into the structural and compositional evolution of positive electrode interface during the initial cycling process, we characterized CAMs extracted at various states of charge (SOC: 0%, 50%, and 100%) and states of discharge (SOD: 50% and 100%) using HRTEM, electron energy loss spectroscopy (EELS), and XPS.
As shown in the HRTEM images of the extracted NCM@LOBO (Fig. S24), the formation of the CEI begins immediately upon contact with the LPSC. The resulting CEI blends seamlessly with the LOBO coating, making it difficult to visually distinguish the two layers. As the NCM@LOBO undergoes charging from 0% to 100% SOC and subsequent discharging to 100% SOD, the CEI thickness increases progressively from 6.1 nm to 9.6 nm and then to 13.4 nm. Similarly, in the case of the b-NCM, the CEI layer also thickens from 5.6 nm to 8.6 nm and ultimately to 13.0 nm during the same cycling stages. Notably, even when excluding the thickness of the LOBO layer, the CEI formed on the cycled b-NCM is thicker than that on the cycled NCM@LOBO. This indicates that the LOBO coating improves interfacial stability between NCM and SSE and mitigates SSE decomposition.
To further investigate the interfacial chemical evolution, we employed EELS to examine the valence states of Ni, Co, Mn, Li, and O in CAMs at different SOC and SOD conditions. For the Ni L-edge in the EELS spectra of cycled NCM@LOBO (Fig. S25), a distinct shift to higher energy loss values is observed during charging, which returns to its original position upon discharging—indicating reversible redox behavior of Ni. In contrast, the chemical states of Co and Mn remain largely unchanged, suggesting the structural integrity of NCM@LOBO is maintained throughout cycling. In the case of cycled b-NCM (Fig. S26), both the Ni L-edge and, to a lesser extent, the Co L-edge exhibit similar reversible energy loss shifts, indicating partial redox activity of Co during cycling. The Mn L-edge remains stable, further confirming the preserved structure of b-NCM during initial cycling. These findings regarding the interfacial chemical state evolution of Ni, Co, and Mn are corroborated by XPS results (Fig. S27), providing an independent validation via a complementary surface-sensitive technique. Additionally, analysis of the Li and O K-edges in the EELS spectra reveals the presence of Li2O species within the CEI of both cycled CAMs (Fig. S28), in agreement with previously reported findings45,46.
To quantitatively analyze the positive electrode interfacial evolution during the initial cycling, we conducted ex situ XPS measurements on composite positive electrodes at various SOC and SOD. As shown in the P 2p and S 2p XPS spectra of the cycled composite positive electrodes (Fig. 4d–g), several oxidation products originating from LPSC—such as Li3PO4, P2O5, and polysulfides/sulfur—can be clearly identified. For both b-NCM- and NCM@LOBO-based composite positive electrodes, the content of these oxidation products increases as the SOC progresses from 0% to 100%, but slightly decreases upon further discharge. This suggests that some of the oxidized LPSC species can be partially reduced under discharging conditions. More importantly, under identical cycling conditions, the NCM@LOBO-based composite positive electrode exhibits significantly fewer LPSC oxidation products than the b-NCM-based composite positive electrode. This finding indicates that the LOBO coating effectively suppresses the interfacial oxidation of LPSC by the NCM, thereby enhancing interface stability.
Next, in situ Raman spectroscopy was conducted on composite positive electrodes during the initial charge and discharge cycle using customized full cells to track the evolution of the LPSC component44. Fig. S29a, b present the in situ Raman spectra of the cycled composite NCM@LOBO positive electrode. Five characteristic peaks at 189, 261, 418, 565, and 592 cm−1, corresponding to PS43− in LPSC47, exhibit minimal intensity changes during cycling, indicating a stable interface between LPSC and NCM@LOBO. Additionally, a subtle peak at 463 cm−1 emerges and intensifies during charging, then gradually fades during discharge. This peak corresponds to the S–S bond in polysulfides/sulfur, derived from LPSC oxidation47. Its appearance and disappearance indicate the reversible redox behavior of LPSC, which is consistent with the XPS results. A similar phenomenon was observed in the in situ Raman spectra of the cycled composite b-NCM positive electrode (Fig. S29c).
Gas evolution is a well-known issue in LIBs with liquid electrolytes and high-voltage layered oxide positive electrodes48. However, gas production in sulfide-based ASSLBs has rarely been reported. To address this, in situ differential electrochemical mass spectrometry (DEMS) was employed to monitor gas generation in NCM@LOBO-based full cells during the initial charge and discharge cycle. Eight potential gas species were selected for detection, and their evolution patterns are shown in Fig. S30. The signals for N2 and O2 remain constant throughout the cycling, with a ratio consistent with air composition, suggesting no O2 release from the NCM@LOBO lattice during operation. Other air components, such as H2O and CO2, are also detected, though only trace amounts of gases like H2S and HCl are observed, likely resulting from reactions between LPSC and leaked air, unrelated to the cell’s charging and discharging processes. Thus, gas evolution is minimal in NCM@LOBO-based sulfide ASSLBs, effectively eliminating the risk of cell swelling or explosion.
Long-term positive electrode interface evolution
To understand the enhanced cycling stability of NCM@LOBO compared to b-NCM, full cells cycled for 1000 cycles were disassembled, and the composite NCM@LOBO and b-NCM positive electrodes were extracted for characterization. Before this, the EIS measurements of the cycled full cells were conducted. From the Nyquist plots and corresponding fitting analysis (Fig. S31 and Table S5), the \({R}_{{Int}}\) of the NCM@LOBO-based cell increases by only 100.2 Ω after 1000 cycles, reaching 363.1 Ω, while the b-NCM-based cell shows a much larger increase of 283.7 Ω, indicating that a more conformal and stable interface formed in the NCM@LOBO-based cell.
The microstructures of the cycled composite positive electrodes were examined using SEM and TEM. The surface morphologies (Fig. S32) show that the cycled NCM@LOBO positive electrode remains smooth with no cracks or voids, whereas the cycled b-NCM positive electrode develops an uneven surface with numerous cracks and voids. Cross-sectional SEM images of the cycled NCM@LOBO (Fig. 5a) reveal a flat and intact structure with no internal cracks or voids in the single-crystal particles. In contrast, the uncoated b-NCM particles (Fig. 5b) experience significant breakage after cycling. Cross-sectional TEM images (Figs. 5c, d, S33, and S34) further reveal that a uniform, dense, and continuous CEI layer (~60 nm thick) formed on the NCM@LOBO particles. However, the CEI on the cycled b-NCM surface is rough, loose, and inconsistent in thickness, ranging from tens to hundreds of nanometers, which is unable to fully separate NCM from LPSC. These findings suggest that the LOBO coating plays a critical role in stabilizing the CAM–SSE interface and induces the formation of a favorable CEI layer, contributing to the long-term stability of NCM@LOBO CAM in sulfide-based ASSLBs.
Cross-sectional a, b SEM and c, d TEM images. e, f S 2p and g, h P 2p XPS spectra. i, j Mn k-edge XANES and k, l κ3-weighted FT EXAFS spectra. After cycling the full cells at a specific current of 200 mA g−1 and 25 °C for 1000 cycles with a CAM mass loading of 10 mg cm−2, the cycled composite positive electrodes were extracted under 100% SOD and sampled for the ex situ measurements.
The decomposition degree of the SSE in cycled composite positive electrodes was analyzed via XPS to assess the stability between SSE and CAMs. The S 2p and P 2p XPS spectra of the cycled positive electrodes (Fig. 5e–h) show that LPSC decomposes into S–S (163.2 and 164.4 eV), sulfate (168.7 eV), phosphate (133.6 and 134.5 eV), and P2O5 (135.2 eV) after cycling24,49. The decomposition degree, defined as the ratio of generated species to total species in the XPS spectra, was calculated to be ~50% for the cycled NCM@LOBO positive electrode, compared to ~75% for the cycled b-NCM positive electrode (Table S6), indicating that the LOBO coating effectively suppresses side reactions between LPSC and NCM.
The intrinsic stability of the layered oxide positive electrodes was further evaluated by examining the valence changes of the Mn element, a key indicator of structural stability50. Mn K-edge X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopies were performed on fresh and cycled NCM@LOBO and b-NCM positive electrodes (Fig. 5i–l). The XANES spectra of NCM@LOBO show no significant shifts in edge position or pre-edge features after 1000 cycles, indicating that the Mn oxidation state and NCM@LOBO structure remain stable. In contrast, the Mn in b-NCM is notably reduced after cycling, as evidenced by a shift to lower energy in the spectra50. The EXAFS spectra of fresh and cycled NCM@LOBO show two dominant peaks at 1.48 Å and 2.53 Å, corresponding to Mn–O and Mn–Mn bonds, respectively51. However, the Mn–O bond length changes after cycling in the b-NCM positive electrodes, further demonstrating their structural instability. In conclusion, the formation of a desirable CEI, the suppression of LPSC/NCM side reactions, and the improved internal stability of NCM particles contribute to the high stability of NCM@LOBO CAM in sulfide-based ASSLBs.
Pouch cell application
To assess the practical applicability of the as-prepared NCM@LOBO CAM, a 5 cm × 10 cm single-layer ASS pouch cell was assembled using an NCM@LOBO positive electrode, LPSC SSE, and a Li/μ-Si negative electrode (Fig. 6a). The positive electrode and SSE membranes were fabricated through a dry process with polytetrafluoroethylene as a binder, while the negative electrode membrane was prepared using a coating process with polyisobutene as the binder. The pouch cell was tested under ambient temperature and 50 MPa. As shown in Fig. 6b, at a specific current of 10 mA g−1, the cell delivers a specific capacity of 204 \({{{{\rm{mAh\; g}}}}}_{{{{\rm{CAM}}}}}^{-1}\) with an initial CE of 80.9%, corresponding to a total capacity of 323 mAh and a specific energy of 383 Wh kg−1 (Table S7). The relatively low initial CE observed in this practical pouch cell results from both the formation of the interfacial layer and parasitic side reactions52. At a higher specific current of 66.67 mA g−1, the specific capacity remains at 153 \({{{{\rm{mAh\; g}}}}}_{{{{\rm{CAM}}}}}^{-1}\). Additionally, the cycling stability of the pouch cell was evaluated at a specific current of 66.67 mA g−1. It can run over 300 cycles and exhibits 80% capacity retention after 229 cycles, with an average CE of over 99.5% (Fig. 6c). Notably, the oscillating trend observed in the capacity of the pouch cell is primarily attributed to fluctuations in ambient temperature during testing. This effect can be eliminated by conducting the test under constant 25 °C condition (Fig. S35).
Currently, sulfide-based ASS pouch cells are in the early stages of development, with many reported cells requiring high operating temperatures (around 60 °C)53, delivering low energy densities54, or showing poor cycling stability (Table S8)55. Considering the test conditions, specific energy, and cycling stability, the NCM@LOBO-based ASS pouch cell demonstrates robust electrochemical performance, highlighting the significant practical potential of NCM@LOBO CAM for future applications.
In conclusion, a thin (~3 nm) LOBO-coated NCM CAM was successfully prepared using a dry process followed by calcination, resulting in higher electrochemical performance compared to b-NCM and other reported coated NCM-type CAMs. Specifically, the Li–In|LPSC|NCM@LOBO full cell achieved a specific capacity of 209 mAh g−1 at a specific current of 20 mA g−1, 87.8% capacity retention after 1000 cycles at a specific current of 200 mA g−1, and a high areal capacity of 14.6 mAh cm−2 with stable cycling. Additionally, the Li/μ-Si|LPSC|NCM@LOBO pouch cell demonstrated a high specific energy of 383 Wh kg−1 and an 80% capacity retention after 229 cycles.
The high electrochemical performance of NCM@LOBO CAM is attributed to (1) its high \({\sigma }_{i}\) and low \({\sigma }_{e}\), which enhance Li+ diffusion kinetics while suppressing LPSC decomposition; and (2) its surface LOBO coating, which induces the formation of a favorable CEI, mitigating further LPSC decomposition and stabilizing the NCM structure during cycling. This study provides valuable insights into the design and fabrication of highly conductive and stable coatings for high-voltage, high-capacity CAMs in sulfide-based ASSLBs.
Methods
Preparation of coated Ni-rich positive electrodes
Binary Li borate glass (0.5Li2O·0.5B2O3) coated single-crystal LiNi0.8Co0.1Mn0.1O2 (NCM@LOBO) were synthesized through a dry process followed by calcination. Initially, a stoichiometric mixture of Li2O (99.99%, Aladdin) and B2O3 (99.9%, Macklin) was prepared via ball milling at 300 rpm for 2 h, with the specific amounts of Li2O and B2O3 adjusted to achieve the desired coating ratios (Table S9). Subsequently, NCM powders (99.45%, Canrd Technology Co., Ltd.) were added to the mixture, and ball milling was continued at 300 rpm for an additional 4 h. Finally, the NCM@LOBO material was obtained by heat-treating the mixture at 600 °C for 10 h in air.
Preparation of Li6PS5Cl sulfide solid electrolyte (LPSC SSE)
LPSC SSE was prepared using a solid-state sintering method. A stoichiometric mixture of reagent-grade Li2S (62.5 mol%, 99.9%, Alfa), LiCl (25.0 mol%, 99.9%, Aladdin), and P2S5 (12.5 mol%, 99%, Aladdin) powders was ball-milled at 550 rpm for 17 h. The resulting mixture was then cold-pressed into pellets and placed in a vacuum-sealed quartz tube for sintering. The sintering process involved heating the mixture to 550 °C for 10 h at a rate of 1 °C min−1. All procedures were carried out under an Ar atmosphere in a dry glovebox (O2 < 0.1 ppm, H2O < 0.1 ppm).
Preparation of composite positive electrode powder
The composite positive electrode powder was prepared by dry ball milling a mixture of positive electrode active material (CAM, 70 wt%), LPSC SSE (29 wt%), and vapor-grown carbon fiber (VGCF, 1 wt%; Canrd Technology Co., Ltd.) at 360 rpm for 4 h under an Ar atmosphere. The ball milling procedure for the preparation of CAMs, SSE, and composite positive electrode powder was performed using a planetary ball mill (FP-400H, FOCUCY) in a ZrO2 jar with ZrO2 balls.
Materials characterizations
X-ray diffraction (XRD) patterns were collected on a Bruker D8 Advance diffractometer using Cu Kα radiation (λ = 1.5406 Å). The data were acquired in a 2θ range of 10° to 80° with a step size of 0.05° and a scan rate of 6° min−1. The particle size distribution of the CAMs was investigated by using laser particle analyzer (S3500, Microtrac). Scanning electron microscopy (SEM) and energy dispersive spectroscopy were conducted on super-resolution field emission scanning electron microscope and energy spectrometer (JSM-7800F, JEOL), with beam voltages of 5 kV and 15 kV, respectively. For cross-sectional SEM analysis, the materials were embedded in epoxy resin and sectioned at 25 °C using a three-ion beam cutting instrument (Leica EM TIC 3×). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) were carried out on 200 kV field emission transmission electron microscope (Talos F200X G2, Thermo Scientific). For cross-sectional TEM analysis, the materials were embedded in epoxy resin and sectioned at 25 °C using an ultramicrotome (Leica EM UC7). High-angle annular dark-field scanning TEM (HAADF-STE) and electron energy loss spectroscopy (EELS) were performed on spherical aberration corrected field emission transmission electron microscope (Spectra 300, ThermoFisher Scientific). All samples for microstructural characterizations were fabricated in an Ar atmosphere at 25 °C. To minimize air exposure, each sample was promptly transferred to the microscope chamber immediately prior to analysis. The chemical composition of the materials was characterized by X-ray photoelectron spectroscopy (XPS) (AXIS UltraDLD, Shimadzu) using monochromatic Al Kα radiation (1486.6 eV). Charge correction was applied by referencing the C 1 s peak to 284.8 eV. Depth-profiling XPS analysis of the CAMs was conducted using Ar+ ion etching at an energy of 1000 eV under low-current conditions. The sputtering rate was estimated to be 0.05 nm s−1 based on a Ta2O5 calibration, with an etching time of 100 s. For ex situ XPS analysis, composite positive electrode samples were prepared under an Ar atmosphere at 25 °C and transferred to the instrument using a custom-built airtight transfer chamber to prevent air exposure. The X-ray absorption fine structure (XAS) data were acquired in transmission (or fluorescence) mode utilizing a RapidXAFS 2 M instrument (Anhui Absorption Spectroscopy Analysis Instrument Co., Ltd.), operating at 10 kV and 20 mA. The spectrometer was equipped with a 500 mm radius-of-curvature Si (440) spherically bent crystal analyzer for examining the Mn absorption edge.
Prototype cell assembly and test
All assembly procedures were conducted within an Ar-filled glovebox, ensuring low levels of oxygen (O2 < 0.1 ppm) and moisture (H2O < 0.1 ppm). The prototype full cells were assembled using customized cell molds consisting of two conductive die steel bars and a poly(ether ether ketone) cylinder with an internal diameter of 10 mm. First, 90 mg of LPSC SSE was cold-pressed into a pellet (10 mm diameter, ca. 700 μm thickness) at 80 MPa for 30 s. Next, the composite positive electrode powder (CAM: 10 mg cm−2, ca. 55 μm thickness) were applied to one side of the SSE pellet and pressed at 360 MPa for 30 s. A 9 mm diameter Li–In foil (prepared by pressing 50-μm-thick Li and 100-μm-thick In foils together at 80 MPa for 30 s) was then placed on the opposite side of the SSE pellet. The resulting sandwich structure was inserted into the cell mold, and a stack pressure of 35 MPa was applied using a digital tablet press. The pressure was maintained by securing the cell between two steel plates with bolts at each corner. For full cells with higher CAM loadings (17.8 to 89.2 mg cm−2), the thickness of the Li–In foil increased accordingly.
Galvanostatic charge–discharge (GCD) tests were performed using a Neware Battery cycler (CT-4008Q, China) at constant 25 ± 0.5 °C (in environmental chamber). The potential cutoff was set between 2.5 and 3.7 V (vs. Li+/Li–In). For rate testing, the cells were cycled at specific currents ranging from 20 to 400 mA g−1, while long-term cycling tests were conducted at a specific current of 200 mA g−1. Cells with high positive electrode loading were cycled at a specific current of 20 mA g−1. For each item of electrochemical experiment, at least two prototype cells were tested.
Electrochemical impedance spectroscopy (EIS) tests
The EIS full cells were configured the same as the 10 mg cm−2 CAM loading cell. Ex situ EIS tests were conducted on cells in their fresh state [2 h open-circuit voltage (OCV) applied before testing] and after 1000 cycles [at a specific current of 200 mA g−1 with potential cutoff between 2.5 and 3.7 V (vs. Li+/Li–In)]. In situ EIS was conducted to investigate the cell’s impedance behavior under different voltage conditions during initial cycling [voltage intervals of 0.1 V (from 3.1 to 3.7 V) during charging and 0.2 V during discharging (from 3.7 to 2.5 V) at a specific current of 20 mA g−1]. Potentiostatic EIS measurements were conducted using a BioLogic SP-300 potentiostat at an amplitude of 10 mV, scanning frequencies from 100 kHz to 0.01 Hz with a total of 42 data points.
Li+ diffusion coefficient (\({{{{\boldsymbol{D}}}}}_{{{{{\boldsymbol{Li}}}}}^{+}}\)) tests
The full cells for \({D}_{{{Li}}^{+}}\) testing were fabricated using the same configuration as the baseline cell with a CAM loading of 10 mg cm−2. The specific current for these tests was set at 20 mA g−1 with potential cutoff between 2.5 and 3.7 V (vs. Li+/Li–In). Each applied galvanostatic specific current was held for 1 min 30 s, followed by a rest period of 1 h. The \({D}_{{{Li}}^{+}}\) was calculated using the GITT according to the following Eq. (1):
Where \(S\) represents the interfacial contact area between positive electrodes and SSE in this case; \(\tau\) is pulse duration; \({\Delta E}_{S}\) is the steady-stage voltage change; \({\Delta E}_{\tau }\) is the transient voltage change; \({m}_{B}\) is the mass of CAMs in the composite positive electrode; \({M}_{B}\) is the molecular weight of CAMs; \({V}_{m}\) is the molar volume of CAMs.
Ionic conductivity (\({{{{\boldsymbol{\sigma }}}}}_{{{{\boldsymbol{i}}}}}\)) tests
A CAM pellet (100 mg weight, 520 μm thickness, and 10 mm diameter) was placed between two SSE pellets (100 mg weight, 815 μm thickness, and 10 mm diameter each) and inserted into the cell mold. EIS was performed to measure the total resistance, from which the resistance of the CAMs was calculated (by subtracting the resistance of the SSE). The \({\sigma }_{i}\) of the CAMs was determined using the formula (2):
where \(R\) is the resistance, \(l\) is the thickness, and \(S\) is the area of the CAM pellet.
Electronic conductivity (\({{{{\boldsymbol{\sigma }}}}}_{{{{\boldsymbol{e}}}}}\)) tests
A CAM pellet (15 mg weight, 80 μm thickness, and 10 mm diameter) was placed between two stainless steel (SS) sheets and inserted into the cell mold. The \({\sigma }_{e}\) of the CAMs was measured using the staircase potentiometry method, with potential applied in the range of 0.2 to 1 V relative to the OCV.
In situ measurements
The in situ full cells were identical in configuration to the 10 mg cm−2 CAM loading cell. The testing conditions were set as a specific current of 20 mA g−1 and potential cutoff between 2.5 and 3.7 V (vs. Li+/Li–In). In situ Raman spectroscopy was performed using a confocal Raman spectrometer (gora-Lite, Ideaoptics Co., Ltd.) equipped with a customized spectro-electrochemical cell (SSD-RAMAN, Beijing Scistar Tech. Co., Ltd.). Li–In|LPSC|NCM@LOBO and Li–In|LPSC|b-NCM full cells were assembled for these measurements and subjected to initial charge and discharge. The Raman spectra, focused on the composite positive electrode, were collected in the range of 100–2900 cm−1 using a 532 nm laser (10 mW power) and a 20× objective. For in situ gas evolution analysis during the initial cycling of the Li–In|LPSC|NCM@LOBO cell, differential electrochemical mass spectrometry (DEMS) was performed using the QAS 100Li system (Linglu Instruments Co., Ltd, Shanghai) with a constant argon gas flow of 0.7 mL min−1.
Pouch cell assembly and tests
The electrode/electrolyte membrane preparation and cell assembly procedures were conducted within an Ar-filled glovebox, ensuring low levels of oxygen (O2 < 0.1 ppm) and moisture (H2O < 0.1 ppm). The composite positive electrode (31.7 mg cm−2) and SSE (65 μm) membranes were prepared via a dry process using polytetrafluoroethylene (PTFE, 640XT X, Dupont) as a binder. The composite positive electrode consisted of 70 wt% CAM, 28 wt% SSE, 1 wt% VGCF, and 1 wt% PTFE. The SSE membrane contained 0.5 wt% PTFE binder. The Li/μ-Si negative electrode membrane (3 mg cm−2) was fabricated using a coating process. Specifically, a μ-Si slurry [96 wt% μ-Si (99.95%, Canrd Technology Co., Ltd.) and 4 wt% polyisobutene (Mw: 3050000 g mol−1, RG, Adamas) in ultradry n-hexane (98%, Macklin), the solvent-to-solid is 6:4 in mass ratio] was coated onto the surface of a Li foil (20 μm thickness) by using automatic coating machine (MSK-AFA-HC100, Shenzhen Kejing Star Technology Co., Ltd), followed by vacuum drying to remove the solvent. An electrode punching machine (MSK−180-S, Shenzhen Kejing Star Technology Co., Ltd) was used to cut all electrode membranes to the target dimensions of 5 cm × 10 cm. The N/P ratio was calculated to be 1.7, based on theoretical specific capacities of 3579 mAh g−1 for Si and 200 mAh g−1 for NCM.
The pouch cell was assembled by sequentially stacking an Al foil (99.6%, 12 μm thickness), composite positive electrode membrane, SSE membrane, Li/μ-Si negative electrode membrane, and Cu foil (99.9%, 8 μm thickness). The stack was then sealed in an aluminum plastic pouch. The pouch cell was clamped between two iron plates and tightened with screws under a pressure of 50 MPa for cycling. The pouch cell was tested using a Neware Battery cycler (CT-4008Q, China) at ambient temperature or at constant 25 ± 0.5 °C (in environmental chamber), with a cutoff potential range of 2.5 to 4.3 V (vs. Li+/Li–Si). The cell was cycled at a specific current of 10 mA g−1 for the initial two cycles, and then at a specific current of 66.67 mA g−1 for subsequent cycles.
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Viswanathan, V. et al. The challenges and opportunities of battery-powered flight. Nature 601, 519–525 (2022).
Meng, Y. S., Srinivasan, V. & Xu, K. Designing better electrolytes. Science 378, eabq3750 (2022).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Wu, D., Chen, L., Li, H. & Wu, F. Solid-state lithium batteries-from fundamental research to industrial progress. Prog. Mater. Sci. 139, 101182 (2023).
Yang, C. et al. Copper-coordinated cellulose ion conductors for solid-state batteries. Nature 598, 590–596 (2021).
Yu, S. et al. Design of a trigonal halide superionic conductor by regulating cation order-disorder. Science 382, 573–579 (2023).
Jia, L. et al. Li–solid electrolyte interfaces/interphases in all-solid-state Li batteries. Electrochem. Energy Rev. 7, 12 (2024).
Kato, A. et al. Mechanical properties of sulfide glasses in all-solid-state batteries. J. Ceram. Soc. Jpn. 126, 719–727 (2018).
Yu, C., Zhao, F., Luo, J., Zhang, L. & Sun, X. Recent development of lithium argyrodite solid-state electrolytes for solid-state batteries: synthesis, structure, stability and dynamics. Nano Energy 83, 105858 (2021).
Li, N., Luo, J., Zhu, J. & Zhuang, X. Cathodic interface in sulfide-based all-solid-state lithium batteries. Energy Storage Mater. 63, 103034 (2023).
Wu, Z. et al. Interfaces in sulfide solid electrolyte-based all-solid-state lithium batteries: characterization, mechanism and strategy. Electrochem. Energy Rev. 6, 10 (2023).
Minnmann, P. et al. Designing cathodes and cathode active materials for solid-state batteries. Adv. Energy Mater. 12, 2201425 (2022).
Wang, C.-W. et al. Engineering the interface between LiCoO2 and Li10GeP2S12 solid electrolytes with an ultrathin Li2CoTi3O8 interlayer to boost the performance of all-solid-state batteries. Energy Environ. Sci. 14, 437–450 (2021).
Liang, J. et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun. 14, 146 (2023).
Wu, E. A. et al. A facile, dry-processed lithium borate-based cathode coating for improved all-solid-state battery performance. J. Electrochem. Soc. 167, 130516 (2020).
Chen, Y. et al. Elucidating and minimizing the space-charge layer effect between NCM cathode and Li6PS5Cl for sulfide-based solid-state lithium batteries. Adv. Energy Mater. 14, 2304443 (2024).
Deng, S. et al. Dual-functional interfaces for highly stable Ni-rich layered cathodes in sulfide all-solid-state batteries. Energy Storage Mater. 27, 117–123 (2020).
Shi, J. et al. Coupling novel Li7TaO6 surface buffering with bulk Ta-doping to achieve long-life sulfide-based all-solid-state lithium batteries. J. Mater. Chem. A 10, 21336–21348 (2022).
Lee, Y.-G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020).
Zhang, M. et al. Self-sacrificing reductive interphase for robust and high-performance sulfide-based all-solid-state lithium batteries. Adv. Energy Mater. 14, 2303647 (2024).
Wang, L. et al. Bidirectionally compatible buffering layer enables highly stable and conductive interface for 4.5 V sulfide-based all-solid-state lithium batteries. Adv. Energy Mater. 11, 2100881 (2021).
Sun, N. et al. Surface-to-bulk synergistic modification of single crystal cathode enables stable cycling of sulfide-based all-solid-state batteries at 4.4 V. Adv. Energy Mater. 12, 2200682 (2022).
Liang, Y. et al. Heuristic design of cathode hybrid coating for power-limited sulfide-based all-solid-state lithium batteries. Adv. Energy Mater. 12, 2201555 (2022).
Jung, S. H. et al. Li3BO3–Li2CO3: rationally designed buffering phase for sulfide all-solid-state Li-ion batteries. Chem. Mater. 30, 8190–8200 (2018).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Huo, H. et al. Chemo-mechanical failure mechanisms of the silicon anode in solid-state batteries. Nat. Mater. 23, 543–551 (2024).
Zhang, Y. Q. et al. Direct visualization of the interfacial degradation of cathode coatings in solid state batteries: a combined experimental and computational study. Adv. Energy Mater. 10, 1903778 (2020).
Wang, C.-W. et al. Mechanochemical reactions between polyanionic borate and residue Li2CO3 on LiCoO2 to stabilize cathode/electrolyte interface in sulfide-based all-solid-state batteries. Nano Energy 108, 108192 (2023).
Button, D. P., Mason, L. S., Tuller, H. L. & Uhlmann, D. R. Structural disorder and enhanced ion transport in amorphous conductors. Solid State Ion. 9-10, 585–592 (1983).
Tuller, H. L., Button, D. P. & Uhlmann, D. R. Fast ion transport in oxide glasses. J. Non Cryst. Solids 40, 93–118 (1980).
Levasseur, A., Kbala, M., Hagenmuller, P., Couturier, G. & Danto, Y. Elaboration and characterization of lithium conducting thin film glasses. Solid State Ion. 9-10, 1439–1444 (1983).
Ko, G. et al. Doping strategies for enhancing the performance of lithium nickel manganese cobalt oxide cathode materials in lithium-ion batteries. Energy Storage Mater. 60, 102840 (2023).
Yu, H. et al. Reversible configurations of 3-coordinate and 4-coordinate boron stabilize ultrahigh-Ni cathodes with superior cycling stability for practical Li-ion batteries. Adv. Mater. 37, 2412360 (2025).
Chowdari, B. V. R. & Rong, Z. Study of the fluorinated lithium borate glasses. Solid State Ion. 78, 133–142 (1995).
Balasubramaniam, R. et al. Composite solid electrolyte for high voltage solid-state Li-metal battery. ChemElectroChem 9, e202200317 (2022).
Tian, R. et al. High-voltage stability of small-size single crystal Ni-rich layered cathode for sulfide-based all-solid-state lithium battery at 4.5 V. Adv. Energy Mater. 13, 2300850 (2023).
Zhu, J. et al. A porous Li-Al alloy anode toward high-performance sulfide-based all-solid-state lithium batteries. Adv. Mater. 36, 2407128 (2024).
Yan, W. et al. Hard-carbon-stabilized Li–Si anodes for high-performance all-solid-state Li-ion batteries. Nat. Energy 8, 800–813 (2023).
Li, Y. et al. A lithium superionic conductor for millimeter-thick battery electrode. Science 381, 50–53 (2023).
Zhang, Z. et al. An ultraconformal chemo-mechanical stable cathode interface for high-performance all-solid-state batteries at wide temperatures. Energy Environ. Sci. 16, 4453–4463 (2023).
Zhao, W. et al. Maintaining interfacial transports for sulfide-based all-solid-state batteries operating at low external pressure. ACS Energy Lett. 8, 5050–5060 (2023).
Lu, Y. et al. The carrier transition from Li atoms to Li vacancies in solid-state lithium alloy anodes. Sci. Adv. 7, eabi5520 (2021).
Tan, D. H. S. et al. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte. ACS Energy Lett. 4, 2418–2427 (2019).
Zhang, J. et al. Unraveling the intra and intercycle interfacial evolution of Li6PS5Cl-based all-solid-state lithium batteries. Adv. Energy Mater. 10, 1903311 (2020).
Xu, P. et al. Proton-exchange induced reactivity in layered oxides for lithium-ion batteries. Nat. Commun. 15, 9842 (2024).
Wang, F. et al. Chemical distribution and bonding of lithium in intercalated graphite: identification with optimized electron energy loss spectroscopy. ACS Nano 5, 1190–1197 (2011).
Taklu, B. W. et al. Air-stable iodized-oxychloride argyrodite sulfide and anionic swap on the practical potential window for all-solid-state lithium-metal batteries. Nano Energy 112, 108471 (2023).
Xiang, Y. et al. Gas induced formation of inactive Li in rechargeable lithium metal batteries. Nat. Commun. 14, 177 (2023).
Wang, C. et al. Enhanced air stability and Li metal compatibility of Li-argyrodite electrolytes triggered by In2O3 co-doping for all-solid-state Li metal batteries. Adv. Funct. Mater. 34, 2313308 (2024).
Björklund, E., Brandell, D., Hahlin, M., Edström, K. & Younesi, R. How the negative electrode influences interfacial and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathodes in Li-ion batteries. J. Electrochem. Soc. 164, A3054–A3059 (2017).
Zhang, L. et al. Stabilizing P3-type oxides as cathodes for high-rate and long-life sodium ion batteries by disordered distribution of transition metals. Small Methods 4, 2000422 (2020).
Ham, S.-Y. et al. Overcoming low initial coulombic efficiencies of Si anodes through prelithiation in all-solid-state batteries. Nat. Commun. 15, 2991 (2024).
Ye, L., Lu, Y., Wang, Y., Li, J. & Li, X. Fast cycling of lithium metal in solid-state batteries by constriction-susceptible anode materials. Nat. Mater. 23, 244–251 (2024).
Liu, S. et al. Super long-cycling all-solid-state battery with thin Li6PS5Cl-based electrolyte. Adv. Energy Mater. 12, 2200660 (2022).
Xu, X. Q. et al. Integrating prelithiation and interface protection to achieve high-energy all-solid-state batteries. Angew. Chem. Int. Ed. 63, e202415891 (2024).
Cao, D. et al. Stable thiophosphate-based all-solid-state lithium batteries through conformally interfacial nanocoating. Nano Lett. 20, 1483–1490 (2020).
Ma, Y. et al. Advanced nanoparticle coatings for stabilizing layered Ni-rich oxide cathodes in solid-state batteries. Adv. Funct. Mater. 32, 2111829 (2022).
Hong, S.-B. et al. All-solid-state lithium batteries: Li+-conducting ionomer binder for dry-processed composite cathodes. ACS Energy Lett. 7, 1092–1100 (2022).
Luo, S. et al. Growth of lithium-indium dendrites in all-solid-state lithium-based batteries with sulfide electrolytes. Nat. Commun. 12, 6968 (2021).
Park, S. W. et al. Stable cycling of all-solid-state batteries with sacrificial cathode and lithium-free indium layer. Adv. Funct. Mater. 32, 2108203 (2022).
Kim, U.-H. et al. Microstructure- and interface-modified Ni-rich cathode for high-energy-density all-solid-state lithium batteries. ACS Energy Lett. 8, 809–817 (2023).
Liang, Z. et al. Enabling stable and high areal capacity solid state battery with Ni-rich cathode via failure mechanism study. Energy Storage Mater. 63, 102987 (2023).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52173205 to X. Zhuang). We thank the support from the Instrumental Analysis Center of Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Contributions
The work was conceived and designed by J.Z. and X.Zhuang. J.L., N.L., and Y.F. fabricated the samples. J.L. and L.J. assembled and measured the prototype cells. B.G. and X.Zhang assembled and tested the pouch cell. Q.H. and J.W. conducted the electrochemical properties tests. J.Z., J.L. and J.H. participated in the characterizations. The manuscript was drafted by J.L. and revised by J.Z. and X.Zhuang. All authors participated in the data analysis and discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Andrea Paolella and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, J., Guo, B., Li, N. et al. Conductive binary Li borate glass coating for improved Ni-rich positive electrode in sulfide-based all-solid-state Li batteries. Nat Commun 16, 9462 (2025). https://doi.org/10.1038/s41467-025-64532-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64532-6