Abstract
Bridged-ring scaffolds are prevalent in numerous natural products and pharmaceuticals. However, achieving enantioselective construction of these architectures presents a significant challenge, due to the considerable structural strain of the products and the presence of multiple stereocenters. Herein, we report a Pd/Cu co-catalyzed substrate-dependent enantiodivergent tandem Heck/Sonogashira reaction for the synthesis of alkyne-tethered (R,S,S)-bicyclo[3.2.1]octenes and (S,R,R)-benzo-bicyclo[3.2.1]octanes, each featuring one quaternary and two adjacent tertiary stereocenters. Intramolecular cyclization of the resultant bridged-ring skeletons facilitates the stereospecific synthesis of rigid chiral tricyclodecanes and tetracyclotetradecanes. The alkene, alkyne and ester motifs within bicyclo[3.2.1]octenes and benzo-bicyclo[3.2.1]octanes enables a diverse of transformations, providing access to versatile bridged-ring compounds. Preliminary activity assays demonstrate that two of chiral bridged-ring compounds display good inhibitory effects on the cGAS-STING signaling pathway. Mechanistic studies and DFT calculations indicate that reductive elimination is the rate-determining step in tandem Heck/Sonogashira reaction. Furthermore, key factors responsible for the substrate-dependent enantiodivergence are elucidated through DFT calculations.
Similar content being viewed by others
Introduction
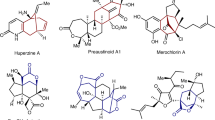
Bridged ring scaffolds are fundamental structural elements found in a wide variety of natural products and pharmaceutical molecules1,2. These rigid three-dimensional motifs are often conducive to increasing molecular stability and improving drug properties, such as solubility and target binding affinity3,4,5. However, the enantioselective construction of bridged ring compounds, remains a formidable challenge, due to the enhanced angular and torsional strains in the reaction transition states and the presence of multiple stereocenters in the products6,7,8,9,10,11,12,13,14,15,16. Recently, the synthesis and transformations of bicyclo[1.1.1]pentanes (BCP)17,18,19,20,21,22, bicyclo[2.1.1]hexanes23,24,25,26,27,28, bicyclo[2.2.1]heptanes29,30,31,32, and bicyclo[3.1.1]heptanes33,34,35,36,37,38 have garnered significant and growing attention from the chemical community, leading to rapid advancements in the field of bridged ring chemistry (Fig. 1a, left). Relatively, the synthesis of chiral bicyclo[3.2.1]octenes and their analogues is still in an underdeveloped stage of research39,40,41,42,43. Furthermore, the asymmetric synthesis of highly rigid and intricate tricyclodecanes, tetracyclotetradecanes and their analogs remains an unaccomplished challenge (Fig. 1a, right).
Alkynes are one of the most important functional groups in organic synthetic chemistry44,45, enabling a variety of transformation reactions, such as cycloisomerization46,47, hydrofunctionalization48,49, hydration50, click reaction51,52, Larock indole cyclization53, and so on. In addition, alkynes are also important structural units in drug molecules and functional materials54,55. Thus, the construction of chiral compounds containing alkyne segments is also one of the hot research fields in chemistry56,57,58,59,60,61,62,63.
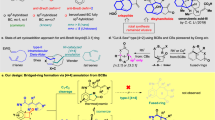
Considering the significance of bridged ring skeletons and the abundant chemical functionality of alkyne groups, we are exploring the feasibility of incorporating alkyne fragments while constructing chiral bridged ring skeletons (Fig. 1b). This endeavor will offer opportunities and potential for obtaining a diverse range of chiral bridged ring compounds, including some previously unattainable scaffolds. However, the development of such protocols may encounter several challenges: 1) Efficiently constructing high strain bridge ring skeletons; 2) Achieving the realization of multiple chiral centers; 3) Addressing and mitigating competitive side reactions involving alkynes; 4) Providing a plausible explanation of the reaction mechanism.
Herein, we describe a palladium and copper co-catalyzed tandem Heck/Sonogashira coupling reaction of 3,3-disubstituted cyclopentenes and terminal alkynes to access alkyne-tethered chiral bridged ring compounds. Interestingly, our strategy involves an unusual substrate-dependent enantiodivergent process. The (E)−3-bromo-2-phenylallylcyclopent-3-enes yielded the products bicyclo[3.2.1]octenes with (R,S,S) configuration with one quaternary and two adjacent tertiary stereocenters, whereas 2-iodobenzylcyclopent-3-enes afforded (S,R,R)-benzo-bicyclo[3.2.1]octanes (Fig. 1c). The alkene, alkyne and ester motifs embedded within these two products demonstrated diverse transformations, providing a good platform for achieving multifunctional chiral bridged compounds. Moreover, the highly rigid chiral tricyclo[5.2.1.04,8]decadienes and benzo-tricyclo[6.2.1.04,9]undecenes could be conveniently synthesized through stereospecific transformations of alkyne-tethered bicyclo[3.2.1]octenes and benzo-bicyclo[3.2.1]octanes. Preliminary activity tests indicated that two of the synthesized chiral bridged ring compounds exhibited inhibitory activity against the cGAS-STING pathway through targeting STING, suggesting the potential as promising lead compounds. Mechanism studies coupled with DFT calculations detailedly described the reaction process of tandem Heck/Sonogashira coupling reaction, and unveiled that reductive elimination is the rate-determining step. Furthermore, DFT calculations also elucidated the key factors responsible for the substrate-dependent enantiodivergence of this reaction.
Results
Reaction development
We initiated our study with (E)−3-bromo-2-phenylallylcyclopent-3-ene 1a and phenylacetylene 2a as the model substrates. After a systematic exploration of the reaction parameters, we determined that the combination of [(π-allyl)PdCl]2 (5.0 mol%), CuI (12.0 mol%), (S)-DTBM-Segphos (L1) (20.0 mol%) and Na2CO3 (3.0 equiv) in DMSO (2.0 mL) at 80 °C for 20 h was the optimal condition, which afforded 3aa in 88% isolated yield with 95% ee and > 20:1 dr (Table 1, entry 1). Replacing L1 with (S)-Segphos (L2), (R)-Binap (L3) and (R)-Difluorphos (L4) led to decreased yield and enantioselectivity (Table 1, entries 2−4). The reaction with (S,S)-BDPP (L5) as the ligand formed product 3aa in 52% yield with 11% ee (Table 1, entry 5). Only trace product could be detected when (R,R)-DIOP (L6) and Josiphos ligand L7 were employed (Table 1, entries 6 and 7). The phosphoramidite ligand L8 performed the reaction in 12% yield (Table 1, entry 8). Other palladium catalysts, such as Pd2(dba)3 and Pd(OAc)2 offered 3aa in 20% and 58% yields, respectively (Table 1, entries 9 and 10). Utilizing DIPEA as the base, 3aa was obtained in 12% yield with 74% ee (Table 1, entry 11), while the use of NaOtBu resulted in only trace amounts of the product (Table 1, entry 12). The test on reaction solvents revealed that THF and DCM were not compatible with this system (Table 1, entry 13). Reducing the amount of L1 to 10.0 mol% impacted the yield and enantioselectivity (Table 1, entry 14). A control experiment demonstrated that the addition of copper salts was crucial, and no product was detected in its absence (Table 1, entry 15).
Synthesis of chiral bicyclo[3.2.1]octenes
Having determined the optimized condition, we then explored the generality of this reaction. The substrate scope of alkynes was explored (Fig. 2a). The introduction of diverse functional groups such as methoxyl (2b), halides (2c, 2k−m), trifluoromethyl (2d), methoxycarbonyl (2e), formyl (2f), cyano (2g), amino (2h), ethenyl (2i) and methyl (2j) moieties on the ortho-, meta- or para-position of the phenyl ring, and disubstituted pepper ring (2n), have no obvious effect on the reaction outcomes, furnishing products 3ab−an in 62−94% yields with 91−95% ee and > 20:1 dr. In addition, the alkynes containing heterocycles, like thiophene (2o), pyridine (2p), pyrimidine (2q), pyrazolo[1,5-a]pyrimidine (2r) and 1-tosyl-1H-indole (2s) were also suitable for this reaction, delivering products 3ao−as in 73–89% yields with 93–95% ee. When enynes were subjected to the reaction, 3at and 3au were obtained in 63% and 87% yields with 91% and 94% ee, respectively. Replacing aromatic rings with alkyl groups such as benzyl (2v), cyclopropyl (2w), hydroxyethyl (2x) and phthalimidylethyl (2y), the reaction worked smoothly as well, yielding the products 3av–ay in 66−79% yields. Moreover, the alkyne bearing a tert-butyldimethylsilyl group (2z) could also be readily utilized to afford product 3az in 80% yield with 94% ee, providing a valuable handle for further transformations.
a Substrate scope of alkynes. b Substrate scope of cyclopentenes. Reaction conditions: 1 (0.10 mmol), 2 (0.15 mmol), [(π-allyl)PdCl]2 (5.0 mol%), CuI (12.0 mol%), L1 (20.0 mol%), Na2CO3 (3.0 equiv) in 2.0 mL DMSO at 80 °C under Ar atmosphere, 20 h. Isolated yields were given. The ee values and dr values were determined by chiral HPLC analysis. b70 °C, 36 h. c24 h. d48 h. e36 h.
To further showcase the generality of this reaction, various cyclopentenes were investigated. As shown in Fig. 2b, switching the methoxycarbonyl group to the N-phenyl amide group (1b), product 3ba was obtained in 62% yield with 94% ee. When N-methoxy-N-methylacetamide (1c), benzoyl (1d) and acetyl (1e) substituted cyclopentenes were subjected to the reaction, products 3ca−ea were obtained in 83–91% yields with 86−94% ee. Methoxymethyl-substituted cyclopentene (1f) led to the formation of product 3fa in 88% yield with 93% ee. Next, the effect of the substituents attached to the alkene moiety was screened. Electron-donating groups (Me, OMe) and electron-withdrawing groups (Cl, CF3, F) on the phenyl ring were all well compatible, offering products 3ga−ka in 77–85% yields with 91–94% ee. Changing the phenyl group to a naphthyl or cyclohexyl group, products 3la and 3ma were isolated in 78% and 79% yields with 92% and 97% ee. The product 3ea was determined to (R,S,S) absolute configuration by single crystal X-ray analysis (see the Supplementary Table 9 for more details), and that of all other products was assigned accordingly.
Synthesis of chiral tricyclo[5.2.1.04,8]decadienes
Having successfully obtained chiral alkyne-tethered bicyclo[3.2.1]octenes, we aimed to achieve the synthesis of previously unrealized and more rigid chiral tricyclo[5.2.1.04,8]decadienes. Treating 3aa with AuPPh3Cl and AgSbF6 resulted in the formation of the desired cycloisomerization product 4aa in 87% yield with 93% ee and >20:1 dr (Fig. 3a). Encouraged by this result, we carried out a primary substrate scope investigation of this protocol, and the representative products 4ab, 4ac, 4ao, 4av, 4fa, 4 ha and 4ia were obtained in 50–91% yields. The absolute configuration of product 4aa was determined by single crystal X-ray analysis (see the Supplementary Table 10 for more details).
a Au-catalyzed intramolecular alkene-alkyne coupling reactions. b Cycloisomerizations of 3aa and further transformations. aAuPPh3Cl, AgSbF6, THF. b24 h. cMeMgBr, THF. dNIS, DCM. e4-MeOC6H4B(OH)2, Pd(OAc)2, S-Phos, K3PO4, Toluene. fEthyl acrylate, Pd(OAc)2, PPh3, Et3N, DMF. gPhenylacetylene, Pd(PPh3)2Cl2, CuI, Et3N/Toluene = 1/3. hCO, Pd(OAc)2, DPPF, DIPEA, MeOH. iDCO2Na, Pd(PPh3)2Cl2, Et3N, DMF. jPhSeSePh, NFSI, THF.
Subsequently, treatment of 4aa with methylmagnesium bromide via a Grignard reaction afforded tertiary alcohol-substituted tricyclo[5.2.1.04,8]decadiene 5 in 88% yield (Fig. 3b). Furthermore, cycloisomerization of 3aa with N-iodosuccinimide (NIS) provided product 6 in 85% yield. The alkenyl iodide motif of compound 6 offers a powerful handle for further manipulations. We employed palladium-catalyzed Suzuki reaction (7), Heck reaction (8), Sonogashira reaction (9) and carbonylation reaction (10) to install a variety of functional groups into the framework, enabling the construction of versatile chiral tricyclo[5.2.1.04,8]decadienes. Additionally, Pd-catalyzed reductive deiodination of 6 with DCO2Na as the deuterium source produced compound 11 in 93% yield with >99% D incorporation. Moreover, we synthesized chiral tricyclo[5.2.1.04,8]decadiene 12 with a phenylselanyl group in 61% yield.
Synthesis of chiral benzo-bicyclo[3.2.1]octanes and chiral benzo-tricyclo[6.2.1.04,9]undecenes
To further explore the generality of the reaction, we then extended this method to the construction of chial benzo-bicyclo[3.2.1]octanes and benzo-tricyclo[6.2.1.04,9]undecenes. Systematic evaluation of the reaction conditions was conducted with 2-iodobenzylcyclopent-3-ene 13a and phenylacetylene 2a as the starting materials. The product 14aa could be isolated in 85% yield with 95% ee and >20:1 dr using 5.0 mol% [(π-allyl)PdCl]2, 12.0 mol% CuI, 20.0 mol% (S)-Segphos (L2) and 3.0 equiv K2CO3 in acetone at 70 °C for 20 h. The substrate scope of alkynes was subsequently evaluated, demonstrating a good tolerance for diverse functional groups, and affording the products 14ab–aab in good yields with excellent enantioselectivities and diastereoselectivities (Fig. 4a). Next, the scope of iodobenzene-attached cyclopentenes was investigated. Replacing the methoxycarbonyl group with N-phenyl amide (13b), N-methoxy-N-methylacetamide (13c), or acetyl groups (13d) yielded products 14ba–da in 64–95% yields with 96–99% ee. The introduction of methyl (13e), methoxyl (13f), and chlorine (13h–j) groups onto the phenyl ring was also examined, offering 14ea, 14fa, and 14ha–ja in 64–83% yields with 94–98% ee and >20:1 dr. However, the p-trifluoromethyl phenyl-substituted substrate 13g produced the product 14ga in 35% yield accompanied by a byproduct formed via direct aryl-alkynyl coupling reaction. The product 14ba was determined to (S,R,R) absolute configuration by single crystal X-ray analysis (see the Supplementary Table 11 for more details).
a Synthesis of chiral benzo-bicyclo[3.2.1]octanes. b Synthesis of chiral benzo-tricyclo[6.2.1.04,9]undecenes. aReaction conditions: 13 (0.10 mmol), 2 (0.15 mmol), [(π-allyl)PdCl]2 (5.0 mol%), CuI (12.0 mol%), L2 (20.0 mol%), K2CO3 (3.0 equiv) in 1.0 mL acetone at 70 °C under Ar atmosphere, 20 h. Isolated yields were given. bReaction conditions: 14 (0.10 mmol), Fe(OTf)3 (10.0 mol%) in 1.0 mL DCE at 80 °C under Ar atmosphere, 2 h. c14 (0.10 mmol) in 0.5 mL TFA and 0.5 mL MeCN at 80 °C under Ar atmosphere, 16 h. d3 h. eFe(OTf)3 (20.0 mol%). f14aa (0.10 mmol), ICl (2.0 equiv), NaHCO3 (2.0 equiv) in 1.0 mL MeCN at room temperature under Ar atmosphere, 20 min.
Chiral benzo-tricyclo[6.2.1.04,9]undecenes were then synthesized via intramolecular cyclization of benzo-bicyclo[3.2.1]octanes (Fig. 4b). Treating 14aa with Fe(OTf)3 offered the product 15aa in 95% yield with 94% ee and >20:1 dr. The adaptability of the reaction was then explored, and the representative products 15ab, 15ac, 15ao, 15da, 15fa, 15ha, 15ia and 15ja were obtained in 57−97% yields. Treatment of 14ca with TFA offered product 15ca in 62% yield. Furthermore, subjecting 14aa to ICl under basic conditions delivered the iodine-induced cyclization product 16 in 89% yield. The absolute configuration of product 15ia was determined by single crystal X-ray analysis (see the Supplementary Table 12 for more details).
Synthetic applications
To demonstrate the reliability of our strategy, we scaled up the reactions of 1a and 13a with 2a to 2.5 mmol, yielding the products 3aa and 14aa in satisfactory quantities, without compromising on efficiency and enantioselectivity, even the loadings of [(π-allyl)PdCl]2 and CuI has been decreased to 2.5 mol% and 6.0 mol% (Fig. 5a). The embedded alkene, alkyne, and ester motifs in products 3 and 14 provided good opportunities for versatile manipulations, and the results were illustrated in Fig. 5b–d. Dihydroxylation reaction of 3aa with OsO4 gave diol 17 in 60% yield (the absolute configuration of 17 was assigned by nOe experiments of the corresponding etherification product, see the Supplementary Fig. 94 for more details). Epoxidation of 3aa with m-CPBA delivered product 18 in 59% yield. When 3aa was treated with Pd/C under a hydrogen atmosphere, both alkenyl and alkynyl groups were reduced, and bicyclo[3.2.1]octane 19 was isolated in 83% yield. The absolute configuration of 18 and 19 were confirmed by X-ray diffraction (see the Supplementary Tables 13 and 14 for more details). Hydrolysis of the ester group in 3aa with LiOH generated a carboxylic acid group, which could be further converted to amino-substituted product 20 in 69% yield via Curtius rearrangement reaction. In addition, treatment of 3az with TBAF resulted in the removal of the tert-butyldimethylsilyl group, affording terminal alkyne 21 in 81% yield. Copper(I)-catalyzed azide-alkyne cycloadditions (CuAAC) of compound 21 with benzyl, glucose-derived and zidovudine azides provided bicyclo[3.2.1]octenes with triazole groups 22a–c in 74–90% yields. Gold-catalyzed hydration reaction of 14aa provided product 23 in 89% yield. TBAF treatment of 14aab removed the trimethylsilyl group, yielding terminal alkyne 24 in 98% yield. Compound 24 could be further converted to amino-substituted product 25 via hydrolysis followed by Curtius rearrangement reaction. The terminal alkyne motif of 24 could participate in several transformations, such as palladium catalyzed Larock indole cyclization and platinum catalyzed hydrosilylation, which delivered products 26 and 27 in 70% yield and 91% yield.
a Scale-up reactions. b Transformations of 3aa. c Desilylation of 3az and click reactions. d Transformations of 14aa and 14aab. Reaction conditions: aOsO4, NMO, Acetone/H2O = 4:1, rt; bm-CPBA, DCM, 0 °C–rt; cPd/C, H2, MeOH, rt; d(i) LiOH, EtOH/H2O = 2:1, 80 °C; (ii) Et3N, DPPA, BnOH, Toluene, 85 °C; eTBAF, THF, 0 °C; fBnN3, CuTc, Toluene, rt; gR1N3, CuBr, bpy, Et3N, Toluene, rt; hR2N3, CuI, DIPEA, AcOH, DCM, rt; iAuPPh3Cl, AgSbF6, THF/H2O = 10:1, 80 °C; jN-tosyl-2-iodoaniline, Pd(PPh3)2Cl2, CuI, Et3N, DMF, 40 °C; kPhMe2SiH, Pt(dba)3, SIPr, THF, rt. SIPr: 1,3-Bis(2,6-di-i-propylphenyl)−4,5-dihydroimidazol-2-ylidine.
Activity tests
As a pivotal component of the innate immune system, the cGAS-STING pathway is related to the pathogenesis of autoimmune inflammatory disorders, and the development of small-molecule inhibitors against this pathway represents a promising therapeutic strategy for autoimmune diseases64. Given that the bridged-ring compound Curcumol has been demonstrated to possess potent inhibitory activity against the cGAS-STING pathway65, we performed a cell-based phenotypic screening of the resulting chiral bridged ring compounds library on THP1-Dual cells, and found that 15 compounds exhibited preliminary inhibitory activity against the cGAS-STING pathway (Fig. 6a, see the Supplementary Table 1 for more details). Compounds 3af, 3ao, 3ar, 4fa, 5 and 25 showed no significant cytotoxicity toward THP1-Dual cells at 10 μM (Fig. 6b). These compounds were advanced to IC50 determination, which 3ao and 5 demonstrated superior inhibitory activity with IC50 values of 2.05 μM and 2.78 μM, respectively (Fig. 6c, d). In SR717-induced THP-1 cells, both compound 3ao and compound 5 downregulated the mRNA levels of IFNB, CXCL10, and ISG15 in a concentration-dependent manner (see the Supplementary Fig. 105 for more details). Further data indicated that, compared to 3ao, compound 5 exhibited more potent inhibitory activity against the cGAS-STING pathway in THP1 cells, human monocytes, and MEF cells stimulated by cGAMP (Fig. 6e). In STING-transfected HEK293T cells, 3ao and 5 also inhibited IFNB transcription (Fig. 6f).
a Cell-based phenotypic screening of compounds in THP1-Dual cells (10 μM), with 100% inhibition rate as the selection criterion (n = 2 biological replicates). b Cytotoxicity of compounds toward THP1-Dual cells (10 μM, 24 h), with 90% cell survival rate as the selection criterion (n = 2 biological replicates). c Concentration-dependent inhibitory curves of compounds 3af, 3ao, 3ar, 4fa, 5, and 25 in THP1-Dual cells measured by luciferase assay (n = 3 biological replicates). d Structures of 3ao and 5. e mRNA levels of IFNB in THP1, human monocytes and MEF cells stimulated by cGAMP (2 μM) with or without 3ao/5 co-treatment (n = 3 biological replicates). Significance vs DMSO+cGAMP/HT-DNA group: ns: no significant; *P < 0.05; **P < 0.01; ***P < 0.001. f mRNA levels of IFNB in HEK293T transfected hSTING plasmid with or without 3ao/5 treatment (n = 3 biological replicates). Significance vs DMSO group: **P < 0.01; ***P < 0.001. g Effects of 3ao and 5 on the enzymatic activity of human cGAS (h-cGAS) assessed by luminescence assay (n = 2 technical replicates). h Binding of 3ao and 5 to human STING (hSTING) assessed by thermal shift assays (TSA) (n = 2 technical replicates); i Binding affinity (Kd) of 3ao and 5 with hSTING assessed by surface plasmon resonance (SPR) analysis. j Predicted binding modes of 3ao and 5 with hSTING (PDB ID: 4F5Y). Data in (a, b, g and h) are shown as mean, while data in (c, e, f, and i) are shown as mean ± S.D.
To identify the potential targets of compounds 3ao and 5, the effects of the two compounds on the enzymatic activity of human cGAS (h-cGAS) were examined. Compounds 3ao and 5 did not exhibit any inhibitory effect on the enzymatic activity at a concentration of 30 μM (Fig. 6g). Thermal shift assays (TSA) showed that both compounds changed the melting temperature (Tm) of hSTING, indicating that 3ao and 5 bound to hSTING (Fig. 6h, see the Supplementary Fig. 107 for more details). Further SPR analysis indicated that 3ao and 5 bound to hSTING with an affinity (Kd) of 17.34 and 5.56 μM, respectively (Fig. 6i). To understand the binding mode of these compounds to STING protein, we subsequently conducted molecular docking and found that both two compounds bound to the cyclic dinucleotide (CDN)-binding pocket of the STING protein (Fig. 6j). The 1-methoxycarbonyl-3-phenyl-bicyclo[3.2.1]octene moiety of 3ao occupies the interior of the pocket and the ester group formed hydrogen bonds with R238, while the thiophene fragment connected by an alkyne extends into the solvent-accessible interface and engaged pi-pi interaction with Y167. Furthermore, 5 adopted an inverted “V” conformation within the pocket, forming hydrogen bonds with V239 and van der Waals interactions with Y167/P264. In conclusion, these data indicate that 3ao and 5 inhibited the cGAS-STING pathway through targeting STING, revealing them as promising lead compounds for drug development.
Further studies
To gain deeper insight into the reaction mechanism, a Hammett analysis was performed. The electronic effects of substituents on the phenyl ring of substrates 1 were first evaluated, yielding a Hammett plot (fitted with Hammett σp substituent constants66) with a reaction constant ρ of −0.14 (Fig. 7a, top). This low ρ value indicates that the electrical properties of substituents on the phenyl ring have a minimal impact on the reaction activity. The reactions involving various substituents on the phenyl ring of substrates 13 resulted in a ρ value of −1.18 (Fig. 7a, bottom), suggesting a more pronounced influence of electronic properties on the reaction rate, with electron-donating substituents facilitating the reaction. These findings clarified that the strong electron-withdrawing group (e.g., CF3) significantly affect the yield of 14ga in Fig. 4, while have a little impact on 3ja in Fig. 2. Additional experiments were conducted with various aryl acetylenes 2, the results revealed Hammett ρ values of −0.86 with substrate 1a (Fig. 7b, top) and −0.38 with substrate 13a (Fig. 7b, bottom). The uniformly negative ρ values indicate that the reductive elimination step might be the rate-determining step in this tandem Heck/Sonogashira coupling reaction. Besides, a linear relationship between the enantiomeric purity of ligand and product indicated that one chiral ligand coordinated with one palladium atom, forming the active catalyst (Fig. 7c).
a The Hammett correlation plots of substrates 1 or 13a with 2a. b The Hammett correlation plots of substrates 2 with 1a or 13a. c Nonlinear effect studies for the reaction between 1a or 13a and 2a. d Gibbs free energy profile for the reaction of 1a and 2a. e Gibbs free energy profile for the reaction of 13a and 2a. The DFT model established at the SMD(DMSO for 1a or Acetone for 13a)//PWPB95-D4/def2-TZVPP//C-PCM(DMSO for 1a or Acetone for 13a)/PBE0-D4/def2-TZVP(for Pd)/def2-SVP (for other atoms) level.
To further elucidate the reaction mechanism, density functional theory (DFT) calculations were performed on the reaction between substrate 1a or 13a and phenylacetylene 2a, as depicted in Fig. 7d and 7e (with L2 as the ligand, see the Supplementary Figs. 129–133 for more details). The active catalyst complex (Pd0L2) was selected as the initial species. The reaction commences with the oxidative addition of 1a to the Pd0L2, passing through the transition state EneTSOA, with an energy barrier of 1.77 kcal/mol, leading to the intermediate EneInt1, which is exergonic by 34.10 kcal/mol. The subsequent departure of the bromide ion from EneInt1, facilitated by sodium ions, forms the exergonic intermediate EneInt2, with an exergonicity of 7.32 kcal/mol. Without sodium ions, this step becomes endergonic by 14.16 kcal/mol. Next, the olefin insertion transition state EneTSInsert is encountered, with a barrier of 9.94 kcal/mol, generating intermediate EneInt4. The conversion from EneInt2 to EneInt4 is exergonic by 20.15 kcal/mol. Coordination and transmetalation between EneInt4 and I−-coordinated copper-acetylide lead to the formation of EneInt6 (using the I−-coordinated species as an example; Cl− and Br−-coordinated copper-acetylides were also considered, see the Supplementary Fig. 131 for more details)67. The subsequent departure of CuI from EneInt6, facilitated by \({{\rm{NaCO}}_{3}^{-}}\), results in intermediate EneInt7, with an exergonicity of 17.32 kcal/mol. In the absence of \({{\rm{NaCO}}_{3}^{-}}\), this step requires an energy barrier of 27.66 kcal/mol. Finally, EneInt7 undergoes reductive elimination via the transition state EneTSRE, with a free energy barrier of 23.32 kcal/mol, yielding the final compound 3aa and Pd0L2. This analysis confirms that reductive elimination is the rate-determining step, with an overall activation energy of 23.32 kcal/mol. The similar findings of the reaction between substrate 13a and phenylacetylene 2a was depicted in Fig. 7e. These findings indicate: 1) the enantioselectivity is determined during the olefin insertion step; 2) the energy surface during transmetalation is flat, and the alkylpalladium(II) cation intermediates (EneInt4 to EneInt6, ArInt4 to ArInt6) undergo rapid and reversible transmetalation processes; 3) reductive elimination is the rate-determining step. DFT calculations for the reaction between 1a and 2a using ligand L1 were also performed, and the energy profile employing L1 resembles that with L2, see the Supplementary Fig. 134 for more details. These mechanistic studies, combined with previous findings68,69, suggest that, compared to the solvent or the halide coordination on palladium in classical Sonogashira reactions, weak olefin/arene coordination in the intermediate (EneInt4 or ArInt4) prior to transmetalation may facilitate the association of alkyne to palladium center. Further kinetic studies uncovered that the reaction between 1a and 2a exhibited first-order dependence in [1a], a negative fractional-order dependence in [2a], fractional order in [Pd]2 and negative first order in [CuI] (Fig. 8a). To validate the aforementioned facts, we deduced the corresponding initial rate equation (see Supplementary Fig. 128 for more details). The rate law was consistent with all of the kinetic data and corroborated the conclusions drawn from DFT calculations.
Based on these experimental and computational results, we propose a catalytic cycle for the reaction using 1a and 2a as the model substrates (Fig. 8b). The cycle initiates with the reduction of the Pd(II) precursor to the active Pd(0) species. Subsequent oxidative addition of 1a to Pd(0) generates the vinylpalladium intermediate I. A stereospecific syn-olefin insert process affords the alkylpalladium intermediate II. Concomitantly, copper-mediated deprotonation of the terminal alkyne 2a in the presence of base yields the halide-coordinated copper acetylide III. Transmetallization between intermediate II and intermediate III produces the species IV, which undergoes reductive elimination to deliver the target product 3aa while regenerating the Pd(0) catalyst.
Interestingly, products 3aa and 14aa exhibited opposite absolute configurations (R,S,S vs S,R,R) of this reaction. Control experiments with L1 and L2 were conducted, as depicted in Fig. 9a. To elucidate the origins of enantioselectivity and rationalize the differing absolute configuration outcomes of the products 3 and 14, DFT calculations were performed on the enantiodetermining step (Fig. 9b–f, with L2 as the ligand, see the Supplementary Figs. 129 and 130 for more details). The calculated \({\Delta \Delta G}_{{{\rm{disf}}}-{{\rm{fav}}}}^{{\ddagger} }\) values are 1.35 kcal/mol for substrate 1a (Fig. 9b) and 2.07 kcal/mol for substrate 13a (Fig. 9c). These calculated enantiomeric ratios (ercalc) are in good agreement with the experimental results (ercalc = 87:13 vs erexp = 85:15 for substrate 1a, and ercalc = 95.5:4.5 vs erexp = 96.5:3.5 for substrate 13a). To further understand the opposite enantioselective outcomes with the same ligand, energy decomposition analysis was conducted. Various contributions to electronic energy difference between two diastereomeric transition states \(\Delta \Delta {E}_{{{\rm{disf}}}-{{\rm{fav}}}}^{{\ddagger} }\) were evaluated (Fig. 9d), including: (i) distortion of ligand, (ii) distortion of substrate, (iii) distortion of Pd coordination sphere, (iv) P-Pd bonds strength and C-Pd bonds strength, (v) noncovalent interactions (NCI). Some of these contributions are interrelated and considered together as the literature70, and the results of energy decomposition analysis are summarized in Fig. 9e (the energy decomposition analysis was also performed for the reaction of 1a using ligand L1, see the Supplementary Fig. 139 for more details). The data shows that the relative ligand distortion term \(\Delta {\Delta }_{{{\rm{Lig}}}}{E}_{{{\rm{disf}}}-{{\rm{fav}}}}^{{\ddagger} }\) is significant for the \(\Delta \Delta {E}_{{{\rm{disf}}}-{{\rm{fav}}}}^{{\ddagger} }\) of EneTSInsert, while the relative Pd coordination sphere distortions term \(\Delta {\Delta }_{{{\rm{Pd}}}}{E}_{{{\rm{disf}}}-{{\rm{fav}}}}^{{\ddagger} }\) is crucial for ArTSInsert. It is also noteworthy that DisfEneTSInsert exhibits a more favorable \({\Delta }_{{{\rm{NCI}}}}{E}_{{{\rm{disf}}}-{{\rm{fav}}}}^{{\ddagger} }\) compared to FavEneTSInsert, suggesting the presence of more favorable noncovalent interactions in DisfEneTSInsert. Top views of these transition states are illustrated in Fig. 9f to clarify the energy decomposition results.
a Control experiments. b The Gibbs free-energy profile of insertion via alkenylpalladium(II) intermediates. c The Gibbs free-enegy profile of insertion via arylpalladium(II) intermediates. d Contributions to electronic energies difference ∆∆E‡. e The results of energy decomposition analysis. f Top views of transition states of olefin insertion. The DFT model established at the SMD(DMSO for 1a, Acetone for 13a)//PWPB95-D4/def2-TZVPP//C-PCM(DMSO for 1a, Acetone for 13a)/PBE0-D4/def2-TZVP(for Pd)/def2-SVP (for other atoms) level.
In summary, we have developed a palladium/copper co-catalyzed substrate-dependent enantiodivergent tandem Heck/Sonogashira coupling reaction of 3,3-disubstituted cyclopentenes with terminal alkynes. This approach enables the efficient synthesis of chiral alkyne-tethered (R,S,S)-bicyclo[3.2.1]octenes and (S,R,R)-benzo-bicyclo[3.2.1]octanes with one quaternary carbon stereocenter and two adjacent tertiary carbon stereocenters, exhibiting high enantio- and diastereoselectivity. The intramolecular cyclizations of the resulting bridged ring frameworks led to the convenient synthesis of versatile chiral tricyclodecanes and tetracyclotetradecanes, which have not been successfully synthesized prior to this achievement. The alkene, alkyne, and ester moieties incorporated within chiral bicyclo[3.2.1]octenes and benzo-bicyclo[3.2.1]octanes facilitate a multitude of transformations, thereby expanding both the structural diversity and functional group compatibility of bridged ring compounds. Furthermore, preliminary biological evaluation demonstrated that the synthesized chiral bridged-ring compounds 3ao and 5 exhibited significant inhibitory activity against the cGAS-STING pathway through targeting STING, highlighting their potential as promising lead compounds for drug development. Moreover, we systematically described the reaction process of tandem Heck/Sonogashira coupling reaction through mechanism studies and DFT calculations, and revealed that reductive elimination is the rate-determining step of the reaction. DFT analysis provided further insight into the pivotal factors influencing substrate-dependent enantiodivergence in the reaction.
Methods
General procedure for the synthesis of alkyne-tethered bicyclo[3.2.1]octenes
An oven-dried 10 mL Schlenk tube was charged with 1 (0.10 mmol, 1.0 equiv), 2 (0.15 mmol, 1.5 equiv), [(π-allyl)PdCl]2 (1.8 mg, 0.005 mmol, 5.0 mol%), CuI (2.3 mg, 0.012 mmol, 12 mol%), L1 (23.6 mg, 0.02 mmol, 20 mol%), Na2CO3 (31.8 mg, 0.3 mmol, 3.0 equiv), and DMSO (2.0 mL) under argon. The reaction mixture was vigorously stirred at 80 °C (oil temperature) for 20 h. After cooling to room temperature, the reaction mixture was added H2O and then extracted with EA for three times. The combined organic extracts were dried over anhydrous Na2SO4, and concentrated under vacuum to give dark residue, which was purified by column chromatography on silica gel with PE/EA to afford products 3.
General procedure for the synthesis of tricyclo[5.2.1.04,8]decadienes through Au-catalyzed cycloisomerization
To a solution of AuPPh3Cl (5.0 mg, 0.01 mmol, 10.0 mol%) in anhydrous THF (0.50 mL) was added AgSbF6 (3.4 mg, 0.01 mmol, 10.0 mol%) under Ar atmosphere and the reaction mixture was stirred at room temperature for 10 min. Then a solution of 3 (0.10 mmol, 1.0 equiv) in anhydrous THF (0.50 mL) was added and the reaction mixture was stirred at 80 °C for 12 h. After cooling to room temperature, the reaction mixture was added H2O and then extracted with EA for three times. The combined organic phases were dried over anhydrous Na2SO4, the solution was concentrated under vacuum, and purified by column chromatography on silica gel with PE/EA to afford products 4.
General procedure for the synthesis of alkyne-tethered benzo-bicyclo[3.2.1]octanes
An oven-dried 10 mL Schlenk tube was charged with 13 (0.1 mmol, 1.0 equiv), 2 (0.15 mmol, 1.5 equiv), [(π-allyl)PdCl]2 (1.8 mg, 0.005 mmol, 5.0 mol%), CuI (2.3 mg, 0.012 mmol, 12 mol%), L2 (12.3 mg, 0.02 mmol, 20 mol%), K2CO3 (41.5 mg, 0.3 mmol, 3.0 equiv), and acetone (1.0 mL) under argon. The reaction mixture was vigorously stirred at 70 °C (oil temperature) for 20 h. After cooling to room temperature, solvent was removed under vacuum to give dark residue, which was purified by column chromatography on silica gel with PE/EA to afford products 14.
General procedure for the synthesis of benzo-tricyclo[6.2.1.04,9]undecenes through Fe-catalyzed intramolecular cyclization
An oven-dried 10 mL Schlenk tube was charged with 14 (0.1 mmol, 1.0 equiv), Fe(OTf)3 (2.1 mg, 0.01 mmol, 10.0 mol%) and DCE (1.0 mL) under argon. The reaction mixture was stirred at 80 °C (oil temperature) for 2 h. After cooling to room temperature, solvent was removed under vacuum to give dark residue, which was purified by column chromatography on silica gel with PE/EA to afford products 15.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6. Data are represented with mean (n = 2) or mean ± Standard Deviation (S.D.) (n = 3). Unpaired Two-tailed Student’s t-tests were used to compare differences between two groups. IC50 values were calculated by using 4-parameter non-linear regression model. Statistical significance was defined as ns: no significant, *P < 0.05, **P < 0.01, and ***P < 0.001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data supporting the findings of this study are available within the article, Supplementary Information file and Source Data file, and are available from the corresponding author upon request. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center, under deposition numbers CCDC 2249271 (3ea), CCDC 2281863 (4aa), CCDC 2281862 (14ba), CCDC 2372251 (15ia), CCDC 2295643 (18) and CCDC 2303095 (19). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Mak, J. Y. W., Pouwer, R. H. & Williams, C. M. Natural products with anti-Bredt and bridgehead double bonds. Angew. Chem. Int. Ed. 53, 13664–13688 (2014).
Xu, Z., Li, X., Rose, J. A. & Herzon, S. B. Finding activity through rigidity: syntheses of natural products containing tricyclic bridgehead carbon centers. Nat. Prod. Rep. 40, 1393–1431 (2023).
Stockdale, T. P. & Williams, C. M. Pharmaceuticals that contain polycyclic hydrocarbon scaffolds. Chem. Soc. Rev. 44, 7737–7763 (2015).
Degorce, S. L., Bodnarchuk, M. S., Cumming, I. A. & Scott, J. S. Lowering lipophilicity by adding carbon: one-carbon bridges of morpholines and piperazines. J. Med. Chem. 61, 8934–8943 (2018).
Tsien, J., Hu, C., Merchant, R. R. & Qin, T. Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. Nat. Rev. Chem. 8, 605–627 (2024).
Zhao, W. Novel syntheses of bridge-containing organic compounds. Chem. Rev. 110, 1706–1745 (2010).
Ruiz, M., López-Alvarado, P., Giorgi, G. & Menéndez, J. C. Domino reactions for the synthesis of bridged bicyclic frameworks: fast access to bicyclo[n.3.1]alkanes. Chem. Soc. Rev. 40, 3445–3454 (2011).
Yang, L.-C. et al. Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions. Nat. Chem. 12, 860–868 (2020).
Teng, H.-L., Yao, L. & Wang, C.-J. Cu(I)-catalyzed regio- and stereoselective [6 + 3] cycloaddition of azomethine ylides with tropone: an efficient asymmetric access to bridged azabicyclo[4.3.1]decadienes. J. Am. Chem. Soc. 136, 4075–4080 (2014).
Zhu, C., Wang, D., Zhao, Y., Sun, W.-Y. & Shi, Z. Enantioselective palladium-catalyzed intramolecular α-arylative desymmetrization of 1,3-diketones. J. Am. Chem. Soc. 139, 16486–16489 (2017).
Hou, S.-H. et al. Enantioselective Type II cycloaddition of alkynes via C–C activation of cyclobutanones: rapid and asymmetric construction of [3.3.1] bridged bicycles. J. Am. Chem. Soc. 142, 13180–13189 (2020).
Xu, Y. et al. Organocatalytic enantioselective Conia-Ene-type carbocyclization of ynamide cyclohexanones: regiodivergent synthesis of morphans and normorphans. Angew. Chem. Int. Ed. 58, 16252–16259 (2019).
Mu, X. et al. Construction of various bridged polycyclic skeletons by palladium-catalyzed dearomatization. Angew. Chem. Int. Ed. 59, 8143–8147 (2020).
Chen, X. M., Zhu, L. & Gong, L.-Z. Chiral indoline-2-carboxylic acid enables highly enantioselective catellani-type annulation with 4-(bromomethyl)cyclohexanone. Angew. Chem. Int. Ed. 60, 24844–24848 (2021).
Chen, J., Wang, Y., Ding, Z. & Kong, W. Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones. Nat. Commun. 11, 1882 (2020).
Tan, J.-P. et al. Asymmetric synthesis of N-bridged [3.3.1] ring systems by phosphonium salt/Lewis acid relay catalysis. Nat. Commun. 13, 357 (2022).
Levin, M. D., Kaszynski, P. & Michl, J. Bicyclo[1.1.1]pentanes, [n]staffanes, [1.1.1]propellanes, and tricyclo[2.1.0.02,5]pentanes. Chem. Rev. 100, 169–234 (2000).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Garlets, Z. J. et al. Enantioselective C–H functionalization of bicyclo[1.1.1]pentanes. Nat. Catal. 3, 351–357 (2020).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Yang, Y. et al. Programmable late-stage functionalization of bridge-substituted bicyclo[1.1.1]pentane bis-boronates. Nat. Chem. 16, 285–293 (2024).
Che, J.-T. et al. Enantioselective synthesis of 2-substituted bicyclo[1.1.1]pentanes via sequential asymmetric imine addition of bicyclo[1.1.0]butanes and skeletal editing. Nat. Chem. 17, 393–402 (2025).
Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem. 15, 535–541 (2023).
Qin, T., He, M. & Zi, W. Palladium-catalysed [2σ + 2π] cycloaddition reactions of bicyclo[1.1.0]butanes with aldehydes. Nat. Synth. 4, 124–133 (2025).
Guo, R. et al. Strain-release [2π + 2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes initiated by energy transfer. J. Am. Chem. Soc. 144, 7988–7994 (2022).
Fu, Q. et al. Enantioselective [2π + 2σ] cycloadditions of bicyclo[1.1.0]butanes with vinylazaarenes through asymmetric photoredox catalysis. J. Am. Chem. Soc. 146, 8372–8380 (2024).
Wu, F. et al. Zinc-catalyzed enantioselective formal (3 + 2) cycloadditions of bicyclobutanes with imines: catalytic asymmetric synthesis of azabicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed. 63, e202406548 (2024).
Hu, S., Pan, Y., Ni, D. & Deng, L. Facile access to bicyclo[2.1.1]hexanes by Lewis acid-catalyzed formal cycloaddition between silyl enol ethers and bicyclo[1.1.0]butanes. Nat. Commun. 15, 6128 (2024).
Bailey, W. F., Khanolkar, A. D. & Gavaskar, K. V. Highly stereoselective tandem cyclizations of 5-hexenyllithiums: preparation of endo-2-substituted bicyclo[2.2.1]heptanes and 3-substituted trans-bicyclo[3.3.0]octanes. J. Am. Chem. Soc. 114, 8053–8060 (1992).
Hong, F.-L. et al. Copper-catalyzed asymmetric reaction of alkenyl diynes with styrenes by formal [3 + 2] cycloaddition via Cu-containing all-carbon 1,3-dipoles: access to chiral pyrrole-fused bridged [2.2.1] skeletons. J. Am. Chem. Soc. 142, 7618–7626 (2020).
Yu, X., Zheng, C. & You, S.-L. Chiral brønsted acid-catalyzed intramolecular asymmetric dearomatization reaction of indoles with cyclobutanones via cascade friedel–crafts/semipinacol rearrangement. J. Am. Chem. Soc. 146, 25878–25887 (2024).
Shen, Z.-A., Guo, J. & Lu, Y.-X. Facile enantioselective synthesis of multi-substituted norbornanes/norbornenes using a latent synthon strategy. Nat. Commun. 15, 8351 (2024).
Zheng, Y. et al. Photochemical intermolecular [3σ + 2σ]-cycloaddition for the construction of aminobicyclo[3.1.1]heptanes. J. Am. Chem. Soc. 144, 23685–23690 (2022).
Nguyen, T. V. T. et al. Photocatalyzed [2σ + 2σ] and [2σ + 2π] cycloadditions for the synthesis of bicyclo[3.1.1]heptanes and 5- or 6‑membered carbocycles. J. Am. Chem. Soc. 145, 25411–25421 (2023).
Yu, T. et al. Selective [2σ + 2σ] cycloaddition enabled by boronyl radical catalysis: synthesis of highly substituted bicyclo[3.1.1]heptanes. J. Am. Chem. Soc. 145, 4304–4310 (2023).
Zhang, X.-G., Zhou, Z.-Y., Li, J.-X., Chen, J.-J. & Zhou, Q.-L. Copper-catalyzed enantioselective [4π + 2σ] cycloaddition of bicyclobutanes with nitrones. J. Am. Chem. Soc. 146, 27274–27281 (2024).
Wang, X., Gao, R. & Li, X. Catalytic asymmetric construction of chiral polysubstituted 3‑azabicyclo[3.1.1]heptanes by copper-catalyzed stereoselective formal [4π + 2σ] cycloaddition. J. Am. Chem. Soc. 146, 21069–21077 (2024).
Dibchak, D. General synthesis of 3-azabicyclo[3.1.1]heptanes and evaluation of their properties as saturated isosteres. Angew. Chem. Int. Ed. 62, e202304246 (2023).
Filippini, M.-H. & Rodriguez, J. Synthesis of functionalized bicyclo[3.2.1]octanes and their multiple uses in organic chemistry. Chem. Rev. 99, 27–76 (1999).
Presset, M., Coquerel, Y. & Rodriguez, J. Syntheses and applications of functionalized bicyclo[3.2.1]octanes: thirteen years of progress. Chem. Rev. 113, 525–595 (2013).
Yuan, Z. et al. Palladium-catalyzed asymmetric intramolecular reductive Heck desymmetrization of cyclopentenes: access to chiral bicyclo[3.2.1]octanes. Angew. Chem., Int. Ed. 58, 2884–2888 (2019).
Li, Q. et al. Enantioselective synthesis of bicyclo[3.2.1]octadienes via palladium-catalyzed intramolecular alkene-alkyne coupling reaction. Angew. Chem. Int. Ed. 62, e202313404 (2023).
Yuan, Z., Zeng, Y., Feng, Z., Lin, A. & Yao, H. Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes. Nat. Commun. 11, 2544 (2020).
Trost, B. M. & Li, C.-J. Modern alkyne chemistry (Wiley-VCH, 2015).
Chinchilla, R. & Nájera, C. Chemicals from alkynes with palladium catalysts. Chem. Rev. 114, 1783–1826 (2014).
Jiménez-Núñez, E. & Echavarren, A. M. Gold-catalyzed cycloisomerizations of enynes: a mechanistic perspective. Chem. Rev. 108, 3326–3350 (2008).
Marinetti, A., Jullien, H. & Voituriez, A. Enantioselective, transition metal catalyzed cycloisomerizations. Chem. Soc. Rev. 41, 4884–4908 (2012).
Kennemur, J. L., Maji, R., Scharf, M. J. & List, B. Catalytic asymmetric hydroalkoxylation of C–C multiple bonds. Chem. Rev. 121, 14649–14681 (2021).
Chen, J., Wei, W.-T., Lia, Z. & Lu, Z. Metal-catalyzed Markovnikov-type selective hydrofunctionalization of terminal alkynes. Chem. Soc. Rev. 53, 7566–7589 (2024).
Hintermann, L. & Labonne, A. Catalytic hydration of alkynes and its application in synthesis. Synthesis 8, 1121–1150 (2007).
Tiwari, V. K. et al. Cu-catalyzed click reaction in carbohydrate chemistry. Chem. Rev. 116, 3086–3240 (2016).
Devaraj, N. K. & Finn, M. G. Introduction: click chemistry. Chem. Rev. 121, 6697–6698 (2021).
Zeni, G. & Larock, R. C. Synthesis of heterocycles via palladium-catalyzed oxidative addition. Chem. Rev. 106, 4644–4680 (2006).
Zhu, X., Liu, J. & Zhang, W. De novo biosynthesis of terminal alkyne-labeled natural products. Nat. Chem. Biol. 11, 115–120 (2015).
Brewitz, L. et al. Alkyne derivatives of SARS-CoV-2 main protease inhibitors including nirmatrelvir inhibit by reacting covalently with the nucleophilic cysteine. J. Med. Chem. 66, 2663–2680 (2023).
Wang, F.-L. et al. Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes. Nat. Chem. 14, 949–957 (2022).
Fu, L., Zhang, Z.-H., Chen, P.-H., Lin, Z.-Y. & Liu, G.-S. Enantioselective copper-catalyzed alkynylation of benzylic C-H bonds via radical relay. J. Am. Chem. Soc. 142, 12493–12500 (2020).
Zhang, S.-L., Zhang, W.-W. & Li, B.-J. Ir-catalyzed regio- and enantioselective hydroalkynylation of trisubstituted alkene to access all-carbon quaternary stereocenters. J. Am. Chem. Soc. 143, 9639–9647 (2021).
Xie, J. et al. Unlocking diverse π‑bond enrichment frameworks by the synthesis and conversion of boronated phenyldiethynylethylenes. J. Am. Chem. Soc. 146, 10167–10176 (2024).
Liu, R.-R. et al. Enantioselective dearomative difunctionalization of indoles by palladium-catalyzed Heck/Sonogashira sequence. Angew. Chem. Int. Ed. 56, 7475–7478 (2017).
Bai, X., Wu, C., Ge, S. & Lu, Y.-X. Pd/Cu-catalyzed enantioselective sequential Heck/Sonogashira coupling: asymmetric synthesis of oxindoles containing trifluoromethylated quaternary stereogenic centers. Angew. Chem. Int. Ed. 59, 2764–2768 (2020).
Zhou, L. et al. Enantioselective difunctionalization of alkenes by a palladium-catalyzed Heck/Sonogashira sequence. Angew. Chem. Int. Ed. 59, 2769–2775 (2020).
Li, Y. et al. Nickel-catalyzed chemodivergent 1,1-difunctionalization of unactivated α-olefins with alkynyl electrophiles and B2pin2. ACS Catal. 10, 4888–4894 (2020).
Zhang, Z. & Zhang, C. Regulation of cGAS–STING signalling and its diversity of cellular outcomes. Nat. Rev. Immunol. 25, 425–444 (2025).
Yang, N. et al. Direct inhibition of macrophage sting signaling by curcumol protects against myocardial infarction via attenuating the inflammatory response. Phytomedicine 138, 156403 (2025).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Wang, X., Song, Y., Qu, J. & Luo, Y. Mechanistic insights into the copper-cocatalyzed Sonogashira cross-coupling reaction: key role of an anion. Organometallics 36, 1042–1048 (2017).
He, C., Ke, J., Xu, H. & Lei, A. Synergistic catalysis in the Sonogashira coupling reaction: quantitative kinetic investigation of transmetalation. Angew. Chem. Int. Ed. 52, 1527–1530 (2013).
Oeschger, R. J., Ringger, D. H. & Chen, P. Gas-phase investigations on the transmetalation step in Sonogashira reactions. Organometallics 34, 3888–3892 (2015).
Orlandi, M. & Licini, G. Computational analysis of enantioselective Pd-catalyzed α-arylation of ketones. J. Org. Chem. 85, 11511–11518 (2020).
Acknowledgements
We acknowledge generous financial support from the National Natural Science Foundation of China (NSFC22071267 and 22371299, H.Y.), Natural Science Foundation of Jiangsu Province (BK20242068, A.L.), the Project Program of State Key Laboratory of Natural Medicines (SKLNMZZ202211, H.Y.) and the Open Project of State Key Laboratory of Natural Medicines (SKLNMKF202401, A.L.). We thank Dr. Hui-Min Xu of the Public Laboratory Platform at China Pharmaceutical University for assistance with NMR techniques, and Zaiyong Zhang from Shanghai Institute of Materia Medica for analysis of the crystal structures. The authors thank the Hefei Advanced Computing Center for the computational resources.
Author information
Authors and Affiliations
Contributions
J.Z., M.L., C.W. and H.L. planned and conducted most of the experiments; H.D. prepared some substrates for the reaction scope evaluation; H.L. and C.C. performed the activity tests; A.L. and H.Y. directed the projects and cowrote the manuscript. All authors contributed to the discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhikun Yang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhong, J., Li, M., Wu, C. et al. Catalytic asymmetric tandem Heck/Sonogashira reaction enabling access to versatile chiral bridged ring scaffolds. Nat Commun 16, 11373 (2025). https://doi.org/10.1038/s41467-025-66363-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-66363-x