Abstract
Glycoprotein hormones (GpHs) produced in the human pituitary act through receptors (GpHRs) in the gonads to support reproduction and in the thyroid for metabolism. GpHs are heterodimeric cystine-knot proteins; their receptors bind cognate hormones at an extracellular domain and signal through a transmembrane domain to heterotrimeric G proteins. GpHs and GpHRs have co-evolved from invertebrate counterparts. Structures of the human receptors as isolated for cryogenic electron microscopy (cryo-EM) are all monomeric despite compelling evidence for their functioning as dimers. Here we characterize the homologous receptor from Caenorhabditis elegans. Its biochemical properties are notably similar to those of the thyroid stimulating hormone receptor (TSHR) of humans. Structurally, it is an asymmetric dimer (protomers screw-transformed by 142°/4.1 Å), composed such that only one hormone could bind. This is compatible with the 1:2 asymmetry of negatively cooperative TSH:TSHR complexes and for the transactivation evident from functional complementation of binding-deficient and signaling-deficient GpHRs. By modeling, a symmetrized dimer can bind two hormones as in the 2:2 complexes that support TSHR switches in G-protein usage.
Similar content being viewed by others
Introduction
There are three glycoprotein hormone receptors (GpHRs) in humans: luteinizing hormone–choriogonadotropin receptor (LHCGR), follicle stimulating hormone receptor (FSHR) and TSHR. Together, they play important roles in reproduction and regulating metabolism1,2. GpHRs belong to a subfamily of class A G protein coupled receptors (GPCRs) called leucine-rich-repeat-containing GPCRs (LGRs). An LGR is characterized by a large extracellular domain (ECD) with multiple leucine-rich repeats (LRRs), a rhodopsin-like transmembrane domain (TMD) of seven transmembrane (TM) helices, and a disulfide-bridged hinge segment that connects the two. The ECD binds the hormone3,4 while the TMD transmits the signal of this binding to cytoplasmic effectors, notably G proteins5.
The structures of signaling complexes for all three human GpHRs have been solved recently using cryogenic electron microscopy (cryo-EM)6,7,8,9. The three hormone-receptor-G-protein complexes are similar to one another; however, in each case the receptor is monomeric, whereas compelling evidence implicates receptor dimers (possibly other oligomers) in GpHR signaling: Förster and bioluminescence resonance energy transfer (FRET and BRET)10,11,12,13 and super-resolution microscopy experiments14 demonstrate associations. Negative cooperativity in hormone binding as well as assays of binding competition for chimeric receptors imply asymmetric 1:2 hormone:receptor association11,15,16. A switch in TSHR signaling from being through Gs to produce cAMP to being through Gq to produce inositol phosphate requires the formation of 2:2 TSH:TSHR complexes15; and, similarly at higher TSH levels, a switch to Gi/o usage occurs in cAMP responses17,18. And, finally, functional complementation of mutants defective in binding by mutants defective in signaling requires transactivation from one protomer to another11,19,20,21. Most impressively, transgenic mice co-expressing binding-deficient and signaling-deficient LHCGRs reinstate functional hormone action in the absence of wild-type receptors22. Such complementation is intrinsic to the natural functioning of class C heterodimeric GABAB receptors23,24.
The glycoprotein hormones and their receptors co-evolved from homologs in invertebrate metazoans25, in which the hormones are α2/β5 heterodimers and their type A LGR receptors are already distinguished as having 12 LRRs bounded by disulfide-bridged regions at the N-terminus and at the hinge region26. The Caenorhabditis elegans genome includes a single LGR gene27 and genes for the α228 and β529 chains encode the CeLGR ligand (Ceα2β5); gene knockouts of any one of these produce the same growth defective phenotype in the nematode30. The human homolog of the CeLGR ligand can activate human TSHR, and it is called thyrostimulin (Hsα2β5)31.
We previously solved the crystal structure of Ceα2β532, which has expected similarity to human glycoprotein hormones but is unglycosylated. In this study, we report a nearly full-length cryo-EM structure of CeLGR. The structure is that of an asymmetric dimer; and, in solution, the CeLGR dimer binds Ceα2β5 in 1:2 hormone:receptor stoichiometry as for the negatively cooperative complexes of TSH with TSHR. Moreover, also similarly as in TSH activation of TSHR, we show that Ceα2β5 activates CeLGR in human embryonic kidney (HEK) cells to produce both cyclic adenosine monophosphate (cAMP) and, at higher hormone concentration, inositol triphosphate (IP3) as well. TSHR dimers modeled on the CeLGR structure are compatible with the observed hormone:receptor stoichiometries.
Results
Characterization of the CeLGR receptor
The full-length CeLGR contains 929 residues, including a C-terminal tail extending beyond the limits of hGpHRs and predicted by AlphaFold to be unstructured (sequence alignment in Supplementary Fig. 1). For protein production, we truncated CeLGR after the predicted end of helix 8 at residue 712 (CeLGR-ΔC), and replaced its native signal peptide (residues 1 to 30) with an influenza hemagglutinin (HA) signal peptide to improve the expression of CeLGR in HEK293S cells. We also produced and purified human TSHR (hTSHR) and characterized both proteins by size-exclusion chromatography and SDS-PAGE electrophoresis (Supplementary Fig. 2). The receptor from C. elegans is dimeric in solution, whether at full length (CeLGRwt) or when much better expressed as truncated (CeLGR-ΔC); conversely, hTSHR behaves as a monomer and with much better yields when produced at full length (Supplementary Fig. 2e). Functional consequences from the apparently disordered C-terminal extensions are unclear. Although their involvement in receptor-receptor interactions cannot be ruled out; we find no evidence for such roles in either case. For simplicity here, we mostly use CeLGR in place of CeLGR-ΔC for the former and hTSHR for the latter at full length.
Complete cleavage into two subunits was observed for TSHR from human thyroid membranes33; however, we found incomplete cleavage of hTSHR as produced from HEK293S cells, which could be completed by limited trypsin digestion (Supplementary Fig. 3). Based on evidence that a double cleavage had excised a segment of the hinge region34, both cryo-EM structures of hTSHR employed constructs that deleted residues Ala317-Phe3667,8.
We used proteolytic mass spectrometry to delimit the cleavage site that generated the two polypeptides found in our preparations of CeLGR and of hTSHR (Supplementary Fig. 4). We analyzed each protein after reduction and subsequent digestion with three proteases, coupling the observed coverages from identified proteolytic peptides with knowledge of contiguously ordered cryo-EM structure on either side of a disordered segment, which is where the natural cleavages must have occurred. These considerations delimit the cleavage of CeLGR to within residues Arg343-Arg354 and that of hTSHR to within its residues Arg310-Ser314 (Supplementary Fig. 4). This is surprising for CeLGR since cleavage might have been expected after Arg354 at a canonical furin site (RRKR ↓ ) and surprising for hTSHR in that it excludes the previously suggested cleavages after Asn316 and Phe36634. The severances that we observe cut at similar sites in CeLGR and hTSHR, between the disulfide-bridged cysteine residues of the hinge-region and just beyond HH2 at the C-terminal end of ordering in the hormone-binding ECD (Supplementary Fig. 1). The highly conserved P10 segment joins the ECD to the TMD.
Cryo-EM structure of the unliganded CeLGR dimer
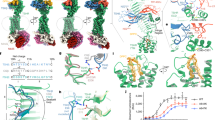
The structure of CeLGR was determined by single-particle cryo-EM to an overall resolution of 3.79 Å (Fig. 1a and Supplementary Figs. 5–7). The cryo-EM data collection, refinement and validation statistics are summarized in Supplementary Table 1. The structure of CeLGR is an asymmetric homodimer. By comparison to the known structures of hGpHRs6,7,8,9, the ECDs of both CeLGR protomers are tilted down towards the cell membrane, occupying an inactive conformation. Overall, the two protomers are similar in structure (Supplementary Fig. 8a-c); but protomer B embraces protomer A after an anti-clockwise rotation of 142° and a translation of 4.1 Å toward the ECD along the rotational screw axis that penetrates the dimeric interface (Fig. 1b, c). The angle between the rotation axis and the normal to cell membrane is 8.3° (Fig. 1b) based on a membrane boundary calculated using the Positioning of Proteins in Membrane (PPM 3.0) server35. The two protomers are not identical; rather, ECD and TMD are rigid bodies linked such that the ECD of protomer A is rotated 7.3° further relative to the TMD (Supplementary Fig. 8b).
a Cryo-EM density map of the CeLGR homodimer at an overall resolution of 3.79 Å from the consensus refinement. The map is sharpened using DeepEMhancer and shown at 3.6 sigma contour level. b Lateral view of a ribbon diagram of the atomic model fitted to the cryo-EM density for the CeLGR structure (same view as in a). The orange stick is the screw axis relating protomer B to protomer A. N-acetylglucosamines (NAGs) and cholesterol moiety are shown as sticks. c Top view of the CeLGR structure in ribbon diagram, looking directly down along the screw axis (orange stick). Coloring has protomer A (green), protomer B (light purple) and assigned cholesterol (pink). b reproduced from (Hendrickson WA, Gong Z. Structural and evolutionary insights into the functioning of glycoprotein hormones and their receptors. Andrology, online ahead of print (2025))49.
The density map is well resolved for both protomers from near the N termini through TM5, except for a disordered span in the cleavage-susceptible hinge region. Side chains of residues in those ordered regions could be assigned with certainty. However, despite extensive attempts at classification to resolve ambiguities, the map remains poor at TM6, TM7 and helix 8 for both protomers with little side chain definition. This may be due to a dynamic character for the TM6-TM7 interface. Therefore, the model for TM6 and TM7 in both protomers was built by rigid-body fitting of the AlphaFold-predicted model into the density map.
Five potential N-linked glycosylation sites (Asn80, Asn92, Asn155, Asn372 and Asn410) in CeLGR are predicted by the NetNGlyc-1.0 server36. Density was observed to model carbohydrate residues at three potential glycosylation sites (Asn80, Asn92 and Asn155); Asn 372 is in a disordered segment, and density observed at Asn 410 in protomer A was too weak to model.
Since the overall structures of the two protomers are similar and protomer A has the better density, we use it to illustrate structural features in the protomer. The span of ordered residues in protomer A is shown in Supplementary Fig. 1; and the spans of modeling for both protomers are cited in Methods.
The N-terminal cysteine-rich and C-terminal cysteine-rich hinge regions
As for the hGpHRs, the extracellular ligand-binding domain of CeLGR consists of 12 LRRs flanked by N-terminal and C-terminal cysteine-rich regions (NCR and CCR, respectively). Disulfide-bridged flanking regions are common features of LRR proteins, thought to serve LRR integrity by capping its ends, and those of CeLGR conform to cysteine motifs that characterize GpHRs37. LRRs belong to an archaic prokaryotic protein architecture that is widely used in protein-protein interactions. In eukaryotes, LRR domains developed into key recognition modules in innate immunity38.
As for the hGpHRs, the NCR region of CeLGR has an additional β strand, named β0, arranged antiparallel to the LRR β strands that form the inner concave binding surface (Fig. 2a). The NCR region of CeLGR includes three disulfide bridges (Fig. 2a, c). The β strand in LRR1 includes three consecutive cysteine residues (Cys67, Cys68 and Cys69), which connect to β0 through the disulfide bonds of Cys69:Cys54 (as for hGpHRs) and Cys67:Cys56; and to the unique N-terminal helix α0 through the disulfide bond of Cys68:Cys43 (Fig. 2a, c).
For the ribbon diagram in (a and b), N-acetylglucosamines (NAGs) and CeLGR protomer B are not displayed for clarity. Structural elements and cysteine residues are labeled. The P10 region is colored in red. For the sequence alignment in (c and d), the secondary structural elements are indicated by cylinders for α helices and arrows for β strands. Cysteine residues are highlighted with yellow background, and disulfide bridges are indicated by orange connections. The conserved residues are underscored by asterisks (*). Regions that are unstructured in the model are indicated with green dash lines.
As first described by Jiang et al. for FSHR4 and recently confirmed with structures of full-length hGpHRs6,7,8,9, the CCR hinge region is an integral component of LGR ECDs. Mutagenesis and chimeric receptor studies show importance of the CCR hinge region in GpHR function39, seeming to act as a fulcrum40 in transmitting the signal from hormone binding to the ECD to G-protein activation by the TMD. In essence, the CCR of CeLGR arises as an elaborated return loop between the β strands of LRR11 and LRR12. For CeLGR, the CCR hinge region includes three hinge helices (HH1, HH2 and HH3), LRR12 and the highly conserved 10-residue fragment (P10, residues Leu420 to Tyr429) (Fig. 2b, d); this hinge region is disordered between Arg343 and Ile392. It is noteworthy that HH1, P10 and the six cysteine residues in the CCR hinge region are highly conserved in hGpHRs (Fig. 2b, d), indicating a common and important biological function of this region. The HH1 of CeLGR links with LRR12 and P10 through two conserved disulfide bridges Cys311:Cys413 and Cys312:Cys423, while HH2 and HH3 of CeLGR are linked by disulfide bridge Cys340:Cys397 (Fig. 2b, d). All three disulfides bridge the cleaved segments.
Interactions between the ECD and TMD domains of CeLGR
The TMD of each CeLGR protomer arranges itself in the seven-transmembrane-helix conformation of canonical GPCRs, with ECL2 covering the extracellular end in this case (Fig. 3a). P10 in the CCR hinge region of CeLGR directly connects the ECD to the TMD, and this covalent linkage is buttressed by interactions with HH1, ECL1, ECL2 and TM2. The backbone carbonyl group of Leu420P10 forms hydrogen bonds with the backbone NH and the side chain hydroxyl group of Ser582ECL2 (Fig. 3b). The side chain of Asn421P10 forms a hydrogen bond with the side chain of Gln505ECL1 (Fig. 3b). As mentioned previously, P10 interacts with HH1 through a disulfide bridge Cys423P10:Cys312HH1 (Fig. 3b). The backbone carbonyl group of Glu424P10 forms a hydrogen bond with the backbone NH of Asp495ECL1 (Fig. 3b). In addition, the backbone NHs of Ile426P10 and Val427P10 form hydrogen bonds with the side chain of Asp490TM2 (Fig. 3b).
a P10 in the CCR hinge region of CeLGR plays a major role in linking the ECD with the TMD through interactions with HH1, ECL1, ECL2 and TM2. P10, ECL1 and ECL2 are colored in red, cyan and purple, respectively. b A magnified illustration of the region boxed in (a) to show atomic interactions between HH1, P10, TM2, ECL1 TM3 and ECL2. The ECL3 and TM7 are not shown for clarity. Hydrogen bonds are shown in black dashed lines. Disulfide bridges are represented as orange sticks. The carbon, oxygen and nitrogen atoms are shown in green, red and blue, respectively. c A summary of interactions shown in (b). Conserved residues among CeLGR and hGpHRs are underscored. Hydrogen bonds and disulfide bridges are indicated by black and orange arrow, respectively. d Sequence alignments in the vicinity of ECL1 and ECL2 comparing CeLGR with the hGpHRs. Cysteine residues are highlighted with yellow background, and disulfide bridges are indicated by orange connections. The conserved residues are underscored by asterisks (*).
Residue Gln505 in ECL1 is essential in interaction with HH1, P10 and ECL2. The side chain of Gln505ECL1 forms hydrogen bonds with the side chain of His309HH1 and Asn421P10, and the carbonyl backbone of Ser579ECL2 (Fig. 3b). The ECL2 of CeLGR interacts with TM2 through a hydrogen bond between the carbonyl backbone of V583ECL2 and side chain of Y482TM2, and TM3 through a disulfide bridge Cys584ECL2:Cys510TM3 (Fig. 3b, d).
The interactions mentioned above are summarized in Fig. 3c. Most residues involved are conserved between CeLGR and all three hGpHRs (Figs. 2d and 3d). The ones which are not conserved (Leu420P10, Ser579ECL2, Val427P10 and V583ECL2) interact through backbone atoms; thus, variations at these residues will not affect their interactions. His309HH1 in CeLGR has evolved to serine in all three hGpHRs (Fig. 2d). A systematic study of substitutions of Ser277 in hLHCGR, corresponding to His309HH1 in CeLGR, indicated that the activity of S277H LHCGR did not change significantly from the wild-type receptor41.
In summary, the ECD and TMD of CeLGR interact with each other through the CCR hinge region and extracellular loops in a delicate arrangement, which keep the pore to the TMD cavity ‘blocked’. The ECD of CeLGR could be viewed as a tethered antagonist from this point.
Dimer interface between CeLGR protomers A and B
The CeLGR protomers A and B form a compact homodimer through an extensive dimer interface that primarily involves their TMDs. In particular, TMs1, 6 & 7 from protomer A contact TMs1, 5, 6 & 7 from protomer B, with a buried surface area of 2075.2 Å2 from the TMDs (Fig. 4a–d). As mentioned earlier, the density map of TM6 and TM7 in both protomers is poor with no density observed for many side chains. The electron density for half of TM6 in protomer B could not be resolved at all, and it is indicated in grey in Fig. 4a–c. In addition, the central layers of direct homodimer contacts are flanked by an assigned cholesterol molecule, which facilitates the interaction between TM6 of protomer A and TM1 of protomer B (Fig. 4a–d). From differences in monomer-dimer equilibration of the GFP-labeled receptor after detergent extraction in the presence or absence of cholesterol hemisuccinate (CHS), cholesterol appears to stabilize the CeLGR dimer (Supplementary Fig. 9). The succinyl modification also adversely affects the activation of TSPO1 enzyme activity by cholesterol42.
a Dimer interface between the TMDs of CeLGR protomer A (green) and B (light purple). The transmembrane helices (TMs) are illustrated as cylinders and numbered. Half of TM6 in protomer B is shown in grey because its density could not be resolved for modeling. The orange stick is the screw axis from protomer B to protomer A. The cholesterol is shown as sticks (pink). b View of the TMD dimer interface from outside the cell. c View of a cross section of the homodimer as circled in (a), but in the same view orientation as (b). d Molecular surface of the homodimer (center) and then of protomer A (left) and protomer B (right) after opening by rotations as indicated and left with the imprint of the surface buried into the interface as colored by the partner protomers or by the assigned cholesterol outlined in pink. e Direct interaction between the CCR hinge region of protomer A and the ECD of protomer B. A hydrogen bond is shown as a black dashed line, and an ionic interaction is indicated by a double-headed arrow colored as for the contacting charged atoms.
Direct contacts are also observed between the CCR hinge region of protomer A and the ECD of protomer B (Fig. 4e) with a buried surface area of 185.3 Å2. The backbone carbonyl group of Ile392 in protomer A forms a hydrogen bond with the side chain amino group of Gln230 in protomer B (Fig. 4e). The side chains of Glu395 in protomer A and Lys233 in protomer B make an ionic interaction (Fig. 4e). In addition, favored van der Waals contacts are observed between Leu232 in protomer B and Gly393, Ala394, Glu395 in protomer A, Gln230 in protomer B and Ile392 in protomer A, as well as Arg209 in protomer B and Pro396 in protomer A (Fig. 4e).
Urizar et al. (2005) have reported that hTSHR and hLHCGR form homo- and heterodimers via interactions involving primarily their TMDs and their ECDs are dispensable for dimerization. However, the ECDs must play a role in the dimeric interaction because differences were observed in the FRET signals obtained from dimers made of intact or truncated hTSHR devoid of ECD11. Our CeLGR structure supports their observation that dimeric interactions involve primarily the TMDs. It is unlikely that the spare interactions between ECDs of the protomers (Fig. 4e) will affect receptor dimerization very much since the major dimer interface resides in the TMD; however, hormone binding may generate greater interaction.
Conformational dynamics
Various features of the analysis indicate that CeLGR in these preparations is highly dynamic. Distinct sub-populations could not be detected. Thus, we undertook a 3D variability analysis (3DVA) of the particles by cryoSPARC43, which corroborates and quantifies this impression (Supplementary Movies 1, 2). The CeLGR protomers appear to be in complex oscillatory motions about the interprotomer screw axis. Rotational orientations of the protomers vary smoothly through the trajectory, most obviously for the ECDs; each protomer contracts and extends along the screw axis; and the TMD micelle appears to expand and contract in diameter.
To further analyze these dynamics, we built models at the motional extrema by rigid body fittings of the ECD (from N terminus to LRR12) and TMD (TM1 to TM7) from each protomer into the initial and final frame of the computed 3DVA progression. These two models (conformers 1 and 2, respectively) were then compared with the centroid model built with the consensus frame (same with Fig. 1b). Protomers A and B fluctuate in opposite directions asymmetrically about the interprotomer screw axis (Fig. 5a, b). As a result, relative to the centroid state, the angle between the ECDs decreases by 6.9° for conformer 1 and increases by 11.1° for conformer 2. Similarly, the interprotomer angle for the TMDs decreases by 5.3° for conformer 1 and increases by 10.8° for conformer 2.
a ECDs as viewed down the indicated screw axes with interprotomer rotations indicated. b TMDs as viewed into the membrane from extracellular side with TMs drawn as cylinders. The centroid model (center) is superimposed on the extreme conformers (1, left; 2, right) with excursion rotations indicated by directed arrows. Measures of expansion and contraction are given as distances between markers Cα 573 in protomer A and Cα 502 in protomer B. c Side views of the CeLGR homodimer as in Fig. 1b. The angles between the ECD axis indicator (Cα 55 and Cα 413) and the plane normal to the screw axis are given for each chain, and excursion rotations are indicated by directed arrows.
Concomitant with the rotations, the ECD of each protomer also moves relative to the deduced membrane surface (Fig. 5c). To measure this angle, we defined a pseudo axis from Cα 55 in β0 to Cα 413 in LRR12 and determined the angle between this axis and the plane normal to the screw axis. Taken again relative to the centroid state, this angle decreases by 2.2° and 3.6° in protomers A and B, respectively, on going to conformer 1 and it increases by 5.0° and 6.1° in A and B, respectively on going to conformer 2. TMD positions relative to the membrane plane are relatively constant through the oscillations (Fig. 5c); however, screw dispositions of the TMDs do shift and they do so asymmetrically [(Δχ, Δtχ) = (−3.2°, − 0.5 Å) and ( + 10.1°, −1.6 Å) respectively for conformers 1 and 2 relative to the centroid screw (χ = 140.0°, tχ = 5.1 Å) for TMDB relative to TMDA]. At the same time, the TMD separations also vary (Fig. 5b), and to quantify these changes, we define an outer surface width by the distance between Cα 573 in TM4 of protomer A and Cα 502 in TM3′ of protomer B. On going from conformer 1 to conformer 2, this distance decreases from 50.5 Å to 48.7 Å through the centroid distance of 49.3 Å.
The extents of rotational displacements and ECD elevations are appreciably greater in protomer B than in protomer A. This is consistent with the observation that the density map quality is worse in protomer B than in protomer A. The extensive TMD interface is both intimate and highly dynamic, seeming to involve correlated movements between the two protomers.
Cellular signalings through Ceα2β5-activated CeLGR and TSH-activated hTSHR
The C. elegans genome contains only one LGR sequence27, the type A LGR that is the subject of this study, and only one LGR ligand, Ceα2β544. The Ceα2β5:CeLGR pathway (also called GPA2/GPB5:FSHR-1) has been reported to control germline development and fertility45 and growth and gut formation30 in C. elegans. Ceα2β5 signaling is required for normal body size and intestinal lumens, acting through activation of CeLGR for cAMP production30. In addition, CeLGR (FSHR-1) has been implicated in responses to infection, oxidative and freezing-thawing stress in C. elegans30, which suggests that the thyrostimulin (alias for α2β5 hormone) signaling pathway may also regulate protective immune and stress responses in C. elegans. How the varied activities of CeLGR in the worm might relate to those of GpHRs in mammals is unclear.
On the presumption that CeLGR signaling might be biochemically similar to mammalian GpHR signaling, we sought to ascertain whether Ceα2β5 can activate CeLGR when expressed in HEK293 cells. Such heterologous activity seemed plausible since the Gα proteins of mammals and C. elegans are highly similar with 66% sequence identity for Gαs46 and more than 80% identity for Gαq47. HEK293S cells transiently transfected with cDNA for full-length CeLGR were incubated with serially diluted Ceα2β5 hormone for second messenger assays quantified by the half-maximal effective concentration (EC50). We thus measured the production of cAMP (Fig. 6a) and IP3 production (Fig. 6c), in separate experiments via IP3 breakdown to IP1; and we made similar measurements for hTSHR activity (Fig. 6b, d).
a, b Dose-dependent Ceα2β5-stimulated CeLGRwt response (a) and hTSH-stimulated hTSHRwt response (b) measured using cAMP accumulation. c, d Dose-dependent Ceα2β5-stimulated CeLGRwt response (c) and hTSH-stimulated hTSHRwt response (d) measured using IP1 accumulation. Data points (mean ± SEM) for cAMP and IP1 assays are plotted for measurements from an experiment of n = 3 biological replicates. e The equilibrium dissociation constant (Kd) of the Ceα2β5:CeLGR-ΔC complex was determined using MST. Data points (mean ± SEM) are plotted for measurements from an experiment of n = 4 biological replicates (see Methods for details). f–h Mass photometry measurements of CeLGR-ΔC alone (f), Ceα2β5 alone (g) and a hormone:receptor complex (h).
We found that Ceα2β5 activates CeLGR through Gαs to produce cAMP and through Gαq to produce IP3 with EC50 values of 0.74 μM and 3.6 μM, respectively. These concentrations are respectively 99- and 7-fold higher than those of the EC50 values needed for TSH stimulation of hTSHR signaling through Gαs and Gαq (7.5 nM and 510 nM). Our measurement of the EC50 from recombinantly expressed and purified hTSH is comparable to that reported previously from bovine TSH (3 nM)48. The lower potencies through CeLGR than through hTSHR may in part reflect the imperfect ‘match’ between CeLGR and the mammalian G proteins; however, the biophysical dissociation constant (Kd), determined by microscale thermophoresis (MST), also indicates lower potency through the nematode receptor (Kd = 170 nM; Fig. 6e). We additionally compared the cAMP production through truncated CeLGR-ΔC with that of the full-length receptor (Supplementary Fig. 10), showing that the truncated form is as functional as wild type (EC50 of 0.17 μM vs 0.74 μM).
To assess the stoichiometry of Ceα2β5:CeLGR complexes, we turned to mass photometry. Mass histograms from CeLGR alone (Fig. 6f), Ceα2β5 alone (Fig. 6g) and a hormone:receptor complex (Fig. 6h) have principal peaks that we assign respectively to the receptor in a detergent micelle, the heterodimeric hormone, and the complex in its detergent micelle. The known masses of the Ceα2β5 heterodimer and of the glycosylated CeLGR-ΔC polypeptide are 24.2 kDa and 85.0 kDa, respectively. The mass measured for the receptor (195 ± 29 kDa; Fig. 6f) is compatible with the CeLGR dimer in a 25-kDa micellar coat; but a monomeric component is excluded. The measured mass of the hormone:receptor complex (227 ± 29 kDa; Fig. 6h) is compatible with a 1:2 complex in a similar 33-kDa micellar coat; but 0:2 or 2:2 stoichiometries are excluded. Our effort to achieve a 2:2 complex at higher hormone concentrations led to uninterpretable results at low particle counts.
Relationship of CeLGR to mammalian glycoprotein hormone receptors (GpHRs)
CeLGR and the mammalian GpHRs are similar in sequence, structure, and biochemical activity; and the relationship is closest to TSHR. The similarities are manifest in several attributes, including some described in this study: (1) As described just above, CeLGR selectively activates G proteins to produce cAMP and IP3 with a hormone-dose dependence similar to that for hTSHR, which was shown to depend on Gαs-to-Gαq switch associated with a 1:2-to-2:2 hormone:receptor stoichiometry15. (2) The co-evolution of type A LGRs and their activating ligands is thought to have proceeded in early vertebrates in two rounds of whole genome duplication from invertebrate counterparts, the first producing progenitors of TSH and TSHR and the second adding FSH and LH and their receptors49; thus, by evolution, CeLGR is expected to be most closely related to TSHR. Moreover, just as CeLGR binds Ceα2β5, human TSHR uniquely retained high affinity for Hsα2β5 (thyrostimulin) while it acquired affinity for hTSH (thyrotropin) as well. (3) CeLGR is like hTSHR in having a sizable insertion, relative to hFSHR and hLHCGR, within its CCR hinge region and, also, in being cleaved just beyond HH2 between the disulfide-bridged cysteine residues of the CCR (Supplementary Fig. 4). As for CeLGR, helices HH1, HH2 and HH3 are all ordered in structures of TSHR7,8, whereas only HH1 is ordered in the structures of LHCGR6 and FSHR9. (4) Finally, the asymmetric CeLGR dimer structure is consistent with abundant evidence for functional GpHR dimerization, as recently reviewed49; and which is particularly compelling for hTSHR10,11,15,16,18.
The CeLGR dimer differs from other GpHR dimers in the strength of its self-association. Whereas we found no monomers in our 2D classifications of cryo-EM particles, and only a small fraction (1.5%) of trimers (Supplementary Fig. 4), only monomer classes were reported for the cryo-EM analyses of human GpHRs6,7,8,9. Monomers also predominate (70%) in imaging analyses of FSHR as expressed in HEK293 cells; however, a substantial fraction of dimers (15.5%) and other oligomers are also present in basal conditions, with some variation in the presence of hormone variations50. Similar oligomerization levels (41.4%) were found for LHR expressed in HEK293 cells14. Of course, dimerization is concentration dependent; and, in the case of TSHR, the expression level in thyrocyte cultures needed to be at the thyroid level in situ to exhibit physiological biphasic responses reflective of dimerization17.
The molecular interactions in complexes of GpHR monomers with their cognate hormones and G proteins6,7,8,9 would appear to be functionally relevant; and yet the evidence that these GpHRs actually function as dimers in the plasma membrane is very compelling. To our knowledge, functional tests for activity as monomers, such as those performed for the β adrenergic receptor in nanodiscs51, have not been carried out for these GpHR monomers. Membrane-bound dimers may well account for the demonstrations of cellular activity from constructs that yield monomers when detergent solubilized.
The puzzle as to why detergent-extracted CeLGR is dimeric, whereas similarly purified human GpHRs are monomeric may be explained by properties of the dimer interface of CeLGR. Nearly all of the surface area buried into that interface comes from the transmembrane helices (91.8%), for which the sequences are highly conserved among the three human GpHRs (68–72% identity overall) and especially so for residues at the TMD interior (84–88% identity). The respective identity levels between the CeLGR and human GpHR sequences, while appreciably lower, are also quite high (35-37% overall and 58-63% for interior residues). The dimer-interface residues of CeLGR are more conserved than outward-facing residues generally (29–37% identity with human GpHR homologs as compared to 25–28% overall). Similarly, among human GpHRs, the homologs of dimer-interface residues are more conserved than all outward-facing residues (63-74% compared to 61-67% identity overall). The CeLGR interface includes seven residues that are distinguished from counterparts that are identical in human GpHRs and in Drosophila LGR1 as well (A442L, T637K, V644F, L647F, V675L, F685C and V692A). As for the human GpHRs, detergent-extracted DmLGR1 purifies as a monomer (Supplementary Fig. 11). We postulate that the CeLGR interface distinctions stabilize these dimers, whereas the similar but distinctive interfaces of the homologous receptors are more labile and do not survive detergent solubilization from the lipid bilayer.
Implications for receptor activation
The structure of the CeLGR dimer sheds light on the activation mechanism of GpHRs. Its asymmetric assembly suggests a structural basis for the negative cooperativity observed in hormone binding11,15,16, for its corollary in switching from Gs signaling from 1:2 complexes to Gq or Gi/o signaling in 2:2 complexes15,17,18, and for the functional complementation provided by the co-expression of binding-deficient and signaling-deficient receptors11,19,20,21,22. The current activation model based on structures of monomeric GpHRs6,7,8,9 could not explain these effects, whereas the compactly associated CeLGR dimer makes allosteric modulation and rescue of signaling possible. Atomic details of the conformational changes found in the full-length GpHR monomers upon binding of their hormones coupled with constraints from the dimer structure allow us to contemplate how this might happen.
As found for the hTSHR, hFSHR and hLHCGR monomers in their inactive hormone-free states, both CeLGR protomers have their ECDs in a ‘down’ orientation. Upon activation for hormone binding, each GpHR then adopts an ‘up’ conformation with its ECD rotated upward by 45–55° relative to the ligand-free state6,7,8,9. We presume that CeLGR similarly adopts an ECD-up conformation when hormone associated and that the CeLGR asymmetry is retained as it forms a 1:2 complex. Whether such dimers have both protomers ECD-up or only the one that is hormone-bound is uncertain; however, we successfully modeled both alternatives. We built Ceα2β5-activated CeLGR dimers by first superimposing the TMD of hTSHR in a hTSH:hTSHR:Gs complex (PDBid 7UTZ) onto each CeLGR protomer, then moving one or both of the CeLGR ECDs into superposition with the ECDs of this TSHR ‘dimer’, and finally replacing hTSH on protomer A with the Ceα2β5 ligand (Supplementary Fig. 12a).
Ceα2β5 could only be fitted to protomer A because the binding site in protomer B, whether ECD-up or ECD-down, is blocked by the CCR hinge region of protomer A as it is for both ECDs down (Fig. 4e). As noted for hGpHRs6,7,8,9, Ceα2β5 would be expected to collide with the cell membrane if bound to CeLGR in the down conformation (Supplementary Fig. 12b). Moreover, there is no room for a second Ceα2β5 unless the subunits are rotated, with both ECDs up, from asymmetry (142° apart) to symmetry (180°). In accord with the observed negative cooperativity, this symmetrization entails an energetic penalty for the second-site binding event, which is lessened when the receptor is pre-disposed by activating mutation16 to having both protomer ECD-up for the 2:2 state.
Another question is how many heterotrimeric G proteins couple to a CeLGR dimer? There is sufficient room for only a single heterotrimeric G protein at a time to bind to the 1:2 CeLGR dimer; however, G-protein coupling with either protomer A (cis activation) or B (trans activation) seems possible (Supplementary Fig. 12a). In light of functional complementation experiments for hGpHRs and structural results from class C GPCRs, trans-activation would be expected. The actual activation mechanism for dimeric CeLGR is complicated and remains to be determined experimentally.
Discussion
This study reveals several structural features of the LGR from C. elegans that are pertinent to functioning of the nematode receptor CeLGR and, by analogy, to the activity of its mammalian counterparts. Most prominently, this nearly full-length LGR is dimeric, both in solution after detergent extraction and as imaged on cryo-EM grids. These dimers show pronounced asymmetry, and they are highly dynamic in the apo state, where the cognate α2/β5 hormone and heterotrimeric G protein are absent. Functionally, CeLGR is similar to hTSHR in that it activates both cAMP through Gαs and IP3 through Gαq. By evolution, TSHR is closest to CeLGR among the GpHRs, and it retains efficient activation by the progenitor α2/β5 hormone (thyrostimulin) while acquiring potentiation by TSH. Structurally, these two receptors are also uniquely similar in having cleaved insertions at the ECD-to-TMD juncture.
We find a stoichiometry of 1:2 for the Ceα2/β5:CeLGR complex, which is as for the negatively cooperative TSH:hTSHR complex. From our modeling, based on combining the apo CeLGR structure with the activating transition from apo TSHR to its complex with TSH7,8, the single hormone that can be fitted to this asymmetric state blocks the second site. A receptor change is required to accommodate a second Ceα2/β5 hormone; and this can be accomplished by a symmetrizing rotation by which each receptor site becomes equally accessible. This is as found for fully constitutive mutants of hTSHR16. Taken together, modeling based on the CeLGR structure can explain the succession of stoichiometries for these receptors (Fig. 7).
The receptor ECD and TMD domains are depicted as yellow rectangles; the heterodimeric hormones are depicted as red rectangles; and the heterotrimeric G proteins are depicted respectively as green and blue rectangles for Gs and Gq, but not to scale. (Left pair) An asymmetric receptor dimer (142° rotation; 4 Å translation) in the apo state (0H:2 R) is shown oriented as for CeLGR in Fig. 1a (lower) and 1c (upper). (Center pair) An asymmetric dimer having one hormone bound (1H:2R) is shown as for the Aup/Bdown model in Supplementary Fig. 12, which has protomer A in its upright pose as for the TSH:TSHR complexes but with Ceα2β5 replacing TSH. The hormone bound to protomer A blocks the binding site on protomer B; thus, only one hormone can bind. (Right pair) The receptor model accommodates two hormone molecules equivalently after symmetrization to a simple 180° rotation. The equilibria defined by KEq1 and KEq2 refer to intrinsic conformational transitions of the receptor. The hormone binding equilibria, defined by Kd1 for the first hormone binding event and by Kd2 for the second, incorporate penalties that must be met in surmounting energetic barriers for achieving the respective hormone-receptive conformations.
The CeLGR dimer as related to dimerization of other GPCRs
Whether the CeLGR structure truly reflects the nature of functional dimers of TSHR and other GpHRs remains to be seen. Meanwhile, since the evidence for physiological relevance of GpHR dimers is compelling, the CeLGR dimer provides us with hypotheses on the functioning of GpHRs. The growing body of evidence on the obligate dimers of class C and class D GPCRs52 supplements this structural foundation. In particular, functional complementation such as that employed in GpHR experiments11,18,19,20,21,22 is intrinsic to GABAB receptors, which are heterodimeric class C GPCRs wherein the B1 subunit binds the ligand but does not contact Gα and the B2 subunit does not interact with GABA but does bind Gα23,24. The homodimeric class C GPCRs, metabotropic glutamate receptor53 and calcium-sensing receptor (CaSR)54, also have only one G protein coupled to the receptor dimer.
The TMD interfaces in the known class C structures are more symmetric than that in the CeLGR structure; but they nevertheless involve similar TMD surfaces. For the active GABAB and CaSR receptors, these are centered at TM6-TM6′ interactions with only peripheral involvement of the adjacent TM5 and TM7 helices; for CeLGR, being off center, TM6 and TM7 of protomer A contact the TM5′-TM7′ surface of protomer B. Also, whereas direct contacts are appreciable in the active states for class C receptors, and they vary but are typically minimal in inactive states23,53,55,56, the CeLGR dimer interface in the hormone-free (presumably inactive) state here is intimate, albeit mobile. Such TM6-centered dimers may not be universal, however; cross-linking evidence for 5HT2c and some other class A receptors contacts involving TM4 and TM557.
Implications for physiology
Little is known about the molecular physiology of the LGR-α2β5 system in nematodes, but much is known about the GpHR-hormone systems of humans. What role the dimer may play in the observed impact of the LGR system in worm physiology, and whether the tight association in the CeLGR dimer is important for its functioning is unknown. Some of the diverse phenotypic consequences from disruption of the fshr-1 gene of C. elegans may be distinguished by the Gαs vs. Gαq signaling that we observed; but none of this has been explored biochemically.
As discussed above, there is considerable evidence for the role of GpHR dimers in human physiology; however, these dimers are relatively loosely self-associated since they do not survive detergent-extraction from cell membranes. Moreover, TSHR dimerization in cells requires expression at the level of thyrocytes in vivo17. How labile GpHR dimerization impacts biological function is unclear; but the resulting effects of negative cooperativity are evident: whereas the 1:2 hormone:dimer complex promotes Gs signaling and cAMP production, the 2:2 complex from higher TSH levels promotes Gq signaling to elicit IP3 production15 and a switch to Gi/Go signaling that decreases the cAMP production15,17,18.
Negative cooperativity, resulting from allosteric communication between protomers of the dimer, is essential for the function of GpHRs. For instance, while the hCG level is very low (0.04–5.5 ng/ml) for unpregnant women, it rises rapidly during pregnancy and can reach 1952 to 19,958 ng/ml at a peak around 8–10 weeks58. Negative cooperativity provides exquisite sensitivity at low hormone concentrations while buffering against acutely elevated hormone levels59.
Methods
Homolog screen and construct design
Homologs of GpHR from the following species were cloned into a BacMam vector with oxidizing environment-optimized GFP (oxGFP)60 fused at the C-terminus for fluorescence-detection size exclusion chromatography (FSEC) screen61: Caenorhabditis elegans (UniProt code: G5EG04), Drosophila melanogaster (Fruit fly; UniProt code: Q9VEG4), Petromyzon marinus (Sea lamprey, UniProt code: Q0P6K8), Danio rerio (Zebrafish; UniProt code: F1R4X9), Xenopus laevis (African clawed frog; UniProt code: G9M6I0), Bothrops jararaca (Jararaca; UniProt code: Q6K0L4), Gallus gallus (Chicken; UniProt code: P79763), Rattus norvegicus (Rat; UniProt code: P20395), Mus musculus (Mouse; UniProt code: P35378), Bos taurus (Bovine; UniProt code: P35376) and Homo sapiens (Human; UniProt code for hFSHR: P23945, hLHCGR: P22888 and hTSHR: P16473). The codons from each species other than human have been optimized for expression in human cells. An influenza hemagglutinin (HA) signal peptide was fused at the N terminus of each gene in place of its original signal peptide to enhance protein insertion into cellular membranes. CeLGR was identified as the best candidate for structural studies because of its large expression yield and good solubility in detergent.
CeLGR contains a large intracellular domain (Ile713 to Ser929) at its C-terminus compared with hGPHRs (Supplementary Fig. 1), which is predicted by AlphaFold to be unstructured. In order to reduce structural flexibility potentially introduced by the unstructured C-terminus, CeLGR was truncated after helix 8. Therefore, the final construct contains an HA signal peptide replacing the natural signal peptide (CeLGR residues 1-30) at the N-terminus, followed with a Flag tag, residues Gln31 to Arg712 of CeLGR, a triple alanine linker and a Flag tag at the C-terminus for affinity purification. The oxGFP was removed by inserting a stop codon after the C-terminal Flag tag for large-scale expression and purification of CeLGR. The presence of both N-terminal and C-terminal Flag tag is because a proteolytic posttranslational cleavage was observed during the maturation event of CeLGR. The original plan was to purify the N-terminal Flag-tagged ECD with anti-Flag M1 antibody affinity resin and the C-terminal Flag-tagged TMD with anti-Flag M2 antibody affinity resin. Actually, the cleaved ECD and TMD of CeLGR are linked by three disulfide bridges in the CCR hinge region. Therefore, either one of the two Flag tags should be sufficient to purify the CeLGR.
Expression and purification of CeLGR
The gene of the expression construct was cloned into a BacMam expression vector containing a human cytomegalovirus (CMV) promoter. Baculovirus was made in Sf9 cells. For large-scale expression, suspension-adapted HEK293S, which lack N-acetyl-glucosaminyltransferase I (GnTI), were grown in Freestyle 293 expression medium supplemented with 2% Fetal Bovine Serum (FBS) at 37 °C in the presence of 8% CO2. The culture was transduced with P3 baculovirus once cell density reached 2.0 × 106 to 3.5 × 106 cells per milliliter. After incubation for 8–16 h at 37 °C, 10 mM sodium butyrate was added to enhance the expression level, and the temperature was changed to 30 °C for protein expression. Cells were harvested 72 h after adding sodium butyrate and resuspended in a buffer containing 20 mM HEPES, pH 7.5, 300 mM NaCl and 10 mM MgCl2, supplemented with EDTA-free protease inhibitor cocktail tablet (Sigma) and 1 mM phenylmethylsulfonyl fluoride (PMSF). Cell pellets were stored at −80 °C until use or lysed immediately for protein purification.
Cells were lysed using an EmulsiFlex-C3 high pressure homogenizer (Avestin) with three passes at 10,000-15,000 psi. Cell debris was removed by centrifugation at 2850 x g for 15 min. Cell membranes were collected by ultracentrifugation for 1 h in a Beckman 45 Ti rotor at 125,440 x g. The membranes were homogenized and subsequently solubilized for 2 h in a buffer composed of 20 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM MgCl2, 1% lauryl maltose neopentyl glycol (LMNG), and 0.2% cholesteryl hemisuccinate tris (CHS), supplemented with protease inhibitor cocktail tablet (Sigma) and 1 mM (PMSF). After insoluble material was removed by ultracentrifugation for 1 h at 93,471 x g in a Beckman 50.2 Ti rotor, the supernatant was incubated with anti-FLAG M2 resin (Sigma) by rotating overnight. The resin was washed with 30 column volumes of wash buffer containing 50 mM Tris HCl, pH 7.4, 150 mM NaCl, 0.01% LMNG and 0.002% CHS and the protein was eluted using wash buffer supplemented with 0.2 mg/ml Flag peptide (Sigma). The eluted protein was concentrated using a 100-kDa cutoff concentrator and injected into a Superose 6 Increase size exclusion column (Cytiva) equilibrated in a buffer composed of 20 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.002% LMNG, and 0.0004% CHS (Supplementary Fig. 2a). The peak fraction was collected for imaging. All purification steps were conducted on ice or at 4 °C.
Construct design, expression and purification of hTSHR and DmLGR1
The wild type hTSHR (UniProt code: P16473, residues 21-764) and C-terminal truncated DmLGR1 after Helix 8 (UniProt code: Q9VEG4, residues 48-777) were cloned into a BacMam expression vector containing a CMV promoter with an HA signal peptide at its N-terminus in place of its native signal peptide and a Flag tag at its C-terminus. The expression and purification procedures for hTSHR and DmLGR1-ΔC were the same as those used for CeLGR (Supplementary Fig. 2c and Supplementary Fig. 11a).
Characterization of cellular proteolysis of expressed CeLGR and hTSHR
The purified CeLGR and hTSHR receptors were analyzed by SDS polyacrylamide gel electrophoresis (SDS PAGE) with or without the presence of a reducing agent DTT to break the disulfide bridges. To test whether residual monomer bands reflected intact receptors or other effects, the purified proteins also went through limited trypsin digestion by adding trypsin to a final concentration of 0.005% to 0.01% followed with incubation at room temperature for 15 min. A 2-fold excess of trypsin inhibitor (Sigma) was then added, and incubation continued for 10 min before adding DTT (Supplementary Fig. 3a, b).
Purified CeLGR without reducing agent appears in monomer and ‘dimer’ bands on an SDS-PAGE gel. In the presence of DTT, the dimer band almost disappears, residual monomer bands persist, and two new bands corresponding to lower molecular weight appear. Trypsinolysis eliminated the residual monomer band, indicating incomplete natural cleavage in HEK cells (Supplementary Fig. 3a). Purified hTSHR shows two ‘monomer’ bands, as well as diffuse densities at larger sizes; and, similarly as for CeLGR, new bands appear in the presence DTT and again residual monomer bands persist but are mostly eliminated by trypsinolysis (Supplementary Fig. 3b). We attribute the dimer band in Supplementary Fig. 3a to native-like CeLGR that remains undissociated by SDS. Glutaraldehyde cross-linking promotes the dimer (Supplementary Fig. 3c). Although intact monomers remain in SDS-PAGE after the DTT reduction that separates cleaved molecules, structural disruptions after disulfide breakage must preclude dimerization of those molecules that stay uncleaved. Dimers (169,402 Da) of glycosylated CeLGR (84,979 Da) also persist in the MALDI mass spectrogram of CeLGR (Supplementary Fig. 3d). The MALDI mass spectrogram of hTSHR reveals two components (Supplementary Fig. 3e), which we attribute to an unglycosylated component (86,263 Da; theoretical 85,808 Da) and a glycosylated component (93,257 Da) to explain the presence of two ‘monomer’ bands in Supplementary Fig. 3b.
The site of natural protease cleavage was delimited by mass spectrometric (MS) analysis of proteolytic peptides after digestion of the denatured receptors (Supplementary Fig. 4). Because natural cleavage was incomplete for the receptors as expressed in HEK293S cells, we used proteolytic MS analysis of only the post-cleavage bands from SDS PAGE (highlighted by red boxes in Supplementary Fig. 3a, b). The gel band was excised, and in-gel digestion was performed as previously described62 with minor modifications. Briefly, the proteins were digested using trypsin, chymotrypsin or GluC. The resulting peptides were dissolved in a solution of 3% acetonitrile and 0.1% formic acid. Peptides were separated using a reversed-phase C18 column (25 cm × 75 µm, 1.6 µm, IonOpticks). Peptide MS/MS analysis was performed using a Thermo Scientific™ Orbitrap Fusion™ Tribrid™ mass spectrometer.
Raw mass spectrometric data were analyzed using the MaxQuant environment v.2.6.1.0, utilizing Andromeda for database searching with default settings with a few modifications. The default tolerances were used for the first search (20 ppm) and the main search (6 ppm). MaxQuant was configured to search against the reference human/C. elegans proteome database, which was downloaded from UniProt. The search parameters included trypsin/ chymotrypsin /GluC digestion with up to 2 missed cleavages. The false discovery rates (FDRs) for peptides, sites, and proteins were all set to 1%. The output combined folder was uploaded into Scaffold 5.0 (Proteome Software). Spectral counting was employed for comparative sample analysis.
Expression and purification of Ceα2β5 and hTSH
Ceα2β5 was expressed and purified as previously described32 with a brief summary here. The genes coding for Ceα2 (UniProt code: A0T3A2, residues 29-120) and Ceβ5 (UniProt code: A7DT38, residues 20-125) were cloned into two BacMam expression vectors. Both Ceα2 and Ceβ5 have an N-terminal HA signal peptide in place of the original signal peptide. A Flag affinity tag was engineered between the HA signal peptide and Ceα2, while an HA affinity tag was engineered between the HA signal peptide and Ceβ5 to facilitate affinity purification. Suspension-adapted HEK293S (GnTI-) cells were used for co-expression of Ceα2β5 using baculovirus produced by Sf9 cells. The Ceα2β5 protein was purified using anti-Flag M2 antibody affinity column and size exclusion chromatography (Supplementary Fig. 2b).
The hTSH construct comprises the native signal peptide from hTSHβ (UniProt code: P01222, residues 1–20), followed by an HA affinity tag, the mature sequence of hTSHβ (UniProt code: P01222, residues 21-138), a 15-residue linker (GGGSGGGSGGGSGGG), the mature sequence of hα (UniProt code: P01215, residues 25-116), a triple alanine linker and a Flag affinity tag at C-terminus, which is analogous to the construct used by Fan and Hendrickson for the structural determination of hFSH bound to the extracellular hormone-binding domain of its receptor using X-ray crystallography3. Suspension-adapted HEK293S (GnTI-) cells infected with baculovirus secreted the hTSH into the cell medium. Both anti-HA (Sigma) and anti-Flag M2 antibody affinity resins were tested to purify the hTSH. No protein could be purified using the anti-HA affinity resin. It is very likely that the anti-HA resin could not bind to HA-tagged proteins well (not limited to hTSH) according to our experience. Finally, the hTSH was purified using an anti-Flag M2 antibody affinity column and Superdex 75 size exclusion chromatography (Supplementary Fig. 2d).
Expression and purification of GFP
Green fluorescent protein (GFP, Uniprot code: P42212) was added into the FSEC samples as an internal marker to align the elution peaks. GFP was expressed and purified according to a previously published protocol63, with modifications summarized as follows. GFP with an N-terminal 7xHis tag was cloned into a pSMT3 vector64 and expressed in E. coli BL21-CodonPlus (DE3)-RIL cells (Invitrogen). Cells were cultured in terrific broth and induced with 0.3 mM IPTG at an OD600 of 0.6–0.8, followed by overnight expression at 30 °C. Cells were harvested and the protein was purified using anti-His affinity chromatography.
Cryo-EM sample preparation and data collection
EM grids (Quantifoil R0.6/1, 400 mesh, holey gold) were glow discharged for 25 s (6.4 sccm H2, 27.5 sccm O2, 10 watts) using a Gatan Solarus model 950 Advanced Plasma Cleaning System prior to sample application. 3 μL of protein solution at 0.3–0.4 mg/ml was applied onto a grid and then plunge-frozen in liquid ethane cooled by liquid nitrogen using a Vitrobot Mark IV (FEI). The Vitrobot chamber was set to 100% humidity at 4 °C. The sample was blotted for 8 s with a wait time of 30 s and a blot force of 3.
Cryo-EM imaging was performed on a Titan Krios electron microscope (FEI) operating at 300 kV, equipped with a post-column GIF Quantum energy filter with a slit width of 20 eV, and a K2 summit direct electron camera (Gatan) in counting mode. Micrographs were collected with 70 μm C2 aperture at a calibrated magnification of 130,000 corresponding to a magnified pixel size of 1.06 Å. Each micrograph comprises 50 sub-frames with a total accumulated dose of 71.01 e-/Å2, recorded for a total exposure time of 10 s at a dose rate of 8 e-/pixel/sec. Data acquisition was done using Leginon across a nominal defocus range of −1.2 μm to −2.0 μm. A total of 8,028 micrographs were acquired as does-fractionated image stacks.
Cryo-EM image processing
The cryo-EM data processing workflow is summarized in Supplementary Fig. 5. Maps and raw videos have been deposited at EMDB (Supplementary Table 1) and EMPIAR (EMPIAR-11917). Subsequent steps were performed in cryoSPARC (v4.5.0) unless otherwise indicated65.
Patch-based motion correction and dose weighting of the 8028 cryo-EM movies were executed in cryoSPARC using the Patch Motion job type. Patch-based CTF estimation was conducted on the aligned averages utilizing Patch CTF. Out of these, 7031 micrographs were selected based on a CTF estimate resolution of <4 Å, relative ice thickness between 0.9–1.1, total motion distance of <50 pixels, and a defocus range of <1200.
The initial round of particle picking employed Blob Picker, extracting particles in a 256-pixel box. Six typical classes emerged post 2D classification. The secondary round of particle picking used template picking with the six identified classes, resulting in 5,634,393 particles being picked. Subsequent multiple rounds of 2D classification yielded 997,598 selected particles. One well-defined class, consisting of 185,961 particles, was identified and selected after 3D classification. Nonuniform refinement of this class achieved a resolution of 3.87 Å. For the third-round particle picking, 75 exemplary micrographs, containing 70–90 good particles each (totaling 5,538 particles), were selected as the training dataset.
A tertiary round of particle picking utilized Topaz66, initially training the Topaz neural network with the 75 micrographs in the training dataset. In total, 965,789 particles were selected using the Topaz training model. Post 2D classification, 940,614 particles were chosen, with one well-defined class being selected after 3D classification. Subsequently, 286,046 particles corresponding to CeLGR were re-extracted with re-centering in a 256-pixel box.
An initial refinement of all 286,046 CeLGR particles, using nonuniform refinement, yielded a consensus refinement with a resolution of 3.82 Å. The application of dynamic masking and real-space windowing of particle images during refinement was not enabled. Further 3D classification without alignment resulted in the best class with complete ECD and TMD, consisting of 72,220 particles, achieving a nonuniform refinement resolution of 3.79 Å.
3D variability analysis
After 3D classification, 3D Variability Analysis43 (3DVA) was carried out with 286,046 selected particles in cryoSPARC, solving in three modes. Reconstructions were calculated along each mode. A mode corresponding to rotation of each protomer, in combination of contraction and extension of the CeLGR dimer was identified. This mode was then used (using the 3D Variability Display job type) to split the particles into 20 clusters, and a reconstruction calculated for each to display the 3D variability.
Atomic model building and refinement
An initial model for the CeLGR homodimer, which includes residues from the N-terminus to TM5 in each protomer, was generated using phenix.predict_and_build67. This model was then extended manually and completed in Coot68. The cholesterol molecule and glycans were added where justified by the density map and chemical environment. The model was finalized by rebuilding in ISOLDE69 and refinement with phenix.real_space_refine70. This model includes residues H41 - R343 and I392 - V692 from protomer A and H41 - R343, S399 - A660, and S670 - V692 from protomer B. Figures were prepared using ChimeraX71. Refinement and validation statistics are provided in Supplementary Table 1.
cAMP assay
The full-length CeLGR (31-929), CeLGR-ΔC (31-712) and full-length hTSHR (21-764) were cloned into a pcDNA3.1 vector for the cAMP assay. HEK293 T/17 cells grown in Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 supplemented with 10% fetal bovine serum (FBS) were seeded onto a 6-well plate at 1.0 ×106 cells per milliliter. Two hours later, cells were transiently transfected with plasmids using Lipofectamine 3000 (Invitrogen). Transiently transfected cells were incubated at 37 °C in the presence of 5% CO2 for one day. Next, cells were harvested with centrifugation at 200 x g for 5 min, washed with phosphate-buffered saline (PBS), resuspended in stimulating buffer (Cisbio), seeded onto a 384-well plate and incubated with serially diluted ligand for 1 h at 37 °C in the presence of 5% CO2. The fluorescence emissions at two different wavelengths (665 nm and 620 nm) were read on the EnVision 2104 Multilabel Microplate Reader (Perkin Elmer). Data were analyzed using GraphPad Prism 10.1 and were presented as mean ± Standard Error of the Mean (SEM) of measurements from an experiment of n = 3 biological replicates.
Inositol phosphate accumulation assay
We measured the CeLGR/hTSHR-mediated Gq activation through the accumulation of inositol phosphate-one (IP1) in transfected cells. HEK293 T/17 cells were transfected using the same protocol as described for the cAMP assay. IP1 production was determined using the homogenous time-resolved fluorescence (HTRF) IP-one Terbium (Tb) kit (Cisbio Bioassays, Codolet, France) according to the manufacturer’s instructions. The assay monitors the production of inositol 1-monophosphate (IP1), a downstream metabolite of inositol 1,4,5-triphosphate (IP3). The data analysis was performed identically to that for the cAMP assay.
MST measurements
The dissociation constant (Kd) of Ceα2β5 to CeLGR was measured using Microscale Thermophoresis (MST). Ceα2β5 and CeLGR were purified separately as described above. Purified CeLGR was labeled with the RED fluorescent dye NT-647-MALEIMIDE using the Monolith NT Protein Labeling Kit RED - MALEIMIDE (NanoTemper Technologies) following manufacturer’s instructions. 50 nM of labelled CeLGR dimer was incubated with serially diluted Ceα2β5 with a 1:1 (v:v) ratio. The normalized fluorescence (Fnorm) of the mixture was read on the Monolith NT.115 instrument (NanoTemper Technologies) selecting LED color in red. Data were analyzed using GraphPad Prism 7.0 and were presented as mean ± s.d. of measurements from an experiment of n = 4 biological replicates.
Mass photometry experiments
Mass photometry measurements were performed using a OneMP mass photometer (Refeyn) with glass coverslips. Prior to measurements, coverslips were cleaned by sequential rinsing with H2O, isopropanol and H2O, followed by drying under a clean stream of nitrogen. The sample buffer (20 mM HEPES, pH 7.5, 150 mM NaCl) was filtered through a 0.22 μm membrane (Millipore), and protein samples were diluted in the filtered sample buffer. A clean coverslip and gasket were assembled and placed onto the mass photometer. Focal calibration was performed using the sample buffer. Data were acquired using AcquireMP software (v2.2, Refeyn), and the resulting contrast distributions were analyzed by Gaussian fitting using a maximum-likelihood procedure. For each acquisition, 1 μL of protein sample - Ceα2β5 (645 nM), CeLGR (130 nM), or a mixture of Ceα2β5 and CeLGR (1075.3 and 181.7 nM, respectively) - was applied to a 10 μL of sample buffer on the coverslip, resulting in an approximate 10-fold dilution. After autofocus stabilization, mass photometry videos were recorded for 60 s. Individual particle binding events and their interferometric scattering contrast were detected and quantified from the analysis of each individual video and converted to mass using the contrast-to-mass calibration.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM density maps have been deposited in the Electron Microscopy Data Bank (EMDB) under accession code EMD-43735 (CeLGR in the apo state). The atomic coordinates have been deposited in the Protein Data Bank (PDB) under accession code 8W1Z (CeLGR in the apo state). Raw cryo-EM images have been deposited in the Electron Microscopy Public Image Archive (EMPIAR) under accession code EMPIAR-11917 (CeLGR in the apo state). The source data underlying Fig. 6a-h, and Supplementary Figs. 2a-d, 3a-c, 10 and 11a are provided as a Source Data File. The following atomic coordinate sets have been used from PDB depositions: 1XWD (hFSH in complex with its receptor), 4AY9 (hFSH in complex with the entire ectodomain of its receptor), 7FIJ (hLHCGR), 7FIH (hLHCGR-CG-Gs-Org43553 complex), 7T9M (hTSHR by CS-17 inverse agonist Fab/Org 274179-0 antagonist), 7UTZ (hTSH analog TR1402 bound to hTSHR in complex with miniGs399), 7XW7 (hTSHR-K1-70 complex), 7XW5 (hTSHR-TSH-Gs-ML109 complex), 8I2H (hFSHR), 8I2G (hFSHR-FSH-compound 716340-Gs complex). Source data are provided with this paper.
References
Pierce, J. G. & Parsons, T. F. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50, 465–495 (1981).
Querat, B. Unconventional actions of glycoprotein hormone subunits: a comprehensive review. Front. Endocrinol. 12, 731966 (2021).
Fan, Q. R. & Hendrickson, W. A. Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433, 269–277 (2005).
Jiang, X. et al. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc. Natl. Acad. Sci. USA 109, 12491–12496 (2012).
Vassart, G., Pardo, L. & Costagliola, S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem. Sci. 29, 119–126 (2004).
Duan, J. et al. Structures of full-length glycoprotein hormone receptor signalling complexes. Nature 598, 688–692 (2021).
Duan J. et al. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 609, 854–859 (2022).
Faust B. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature, 609, 846–853 (2022).
Duan, J. et al. Mechanism of hormone and allosteric agonist mediated activation of follicle stimulating hormone receptor. Nat. Commun. 14, 519 (2023).
Latif, R., Graves, P. & Davies, T. F. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J. Biol. Chem. 276, 45217–45224 (2001).
Urizar, E. et al. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 24, 1954–1964 (2005).
Guan, R. B. et al. Bioluminescence resonance energy transfer studies reveal constitutive dimerization of the human lutropin receptor and a lack of correlation between receptor activation and the propensity for dimerization. J. Biol. Chem. 284, 7483–7494 (2009).
Zhang, M., Feng, X., Guan, R., Hebert, T. E. & Segaloff, D. L. A cell surface inactive mutant of the human lutropin receptor (hLHR) attenuates signaling of wild-type or constitutively active receptors via heterodimerization. Cell Signal 21, 1663–1671 (2009).
Jonas, K. C., Fanelli, F., Huhtaniemi, I. T. & Hanyaloglu, A. C. Single molecule analysis of functionally asymmetric G protein-coupled receptor (GPCR) oligomers reveals diverse spatial and structural assemblies. J. Biol. Chem. 290, 3875–3892 (2015).
Allen, M. D., Neumann, S. & Gershengorn, M. C. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling. Faseb J. 25, 3687–3694 (2011).
Zoenen, M., Urizar, E., Swillens, S., Vassart, G. & Costagliola, S. Evidence for activity-regulated hormone-binding cooperativity across glycoprotein hormone receptor homomers. Nat. Commun. 3, 1007 (2012).
Boutin, A. et al. TSH Receptor Homodimerization in Regulation of cAMP Production in Human Thyrocytes in vitro. Front. Endocrinol. 11, 276 (2020).
Neumann, S. et al. Thyrotropin causes dose-dependent biphasic regulation of cAMP production mediated by G(s) and G(i/o) proteins. Mol. Pharm. 97, 2–8 (2020).
Osuga, Y. et al. Co-expression of defective luteinizing hormone receptor fragments partially reconstitutes ligand-induced signal generation. J. Biol. Chem. 272, 25006–25012 (1997).
Ji, I., Lee, C., Song, Y., Conn, P. M. & Ji, T. H. Cis- and trans-activation of hormone receptors: the LH receptor. Mol. Endocrinol. 16, 1299–1308 (2002).
Ji, I. et al. Trans-activation of mutant follicle-stimulating hormone receptors selectively generates only one of two hormone signals. Mol. Endocrinol. 18, 968–978 (2004).
Rivero-Muller, A. et al. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc. Natl. Acad. Sci. USA 107, 2319–2324 (2010).
Park, J. et al. Structure of human GABAB receptor in an inactive state. Nature 584, 304–309 (2020).
Shen, C. et al. Structural basis of GABA(B) receptor-G(i) protein coupling. Nature 594, 594–598 (2021).
Dos Santos, S., Mazan, S., Venkatesh, B., Cohen-Tannoudji, J. & Querat, B. Emergence and evolution of the glycoprotein hormone and neurotrophin gene families in vertebrates. BMC Evol. Biol. 11, 332 (2011).
Van Hiel, M. B., Vandersmissen, H. P., Van Loy, T. & Vanden Broeck, J. An evolutionary comparison of leucine-rich repeat containing G protein-coupled receptors reveals a novel LGR subtype. Peptides 34, 193–200 (2012).
Kudo, M., Chen, T., Nakabayashi, K., Hsu, S. Y. & Hsueh, A. J. The nematode leucine-rich repeat-containing, G protein-coupled receptor (LGR) protein homologous to vertebrate gonadotropin and thyrotropin receptors is constitutively active in mammalian cells. Mol. Endocrinol. 14, 272–284 (2000).
Park, J. I., Semyonov, J., Chang, C. L. & Hsu, S. Y. Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine 26, 267–276 (2005).
Hsu, S. Y., Nakabayashi, K. & Bhalla, A. Evolution of glycoprotein hormone subunit genes in bilateral metazoa: identification of two novel human glycoprotein hormone subunit family genes, GPA2 and GPB5. Mol. Endocrinol. 16, 1538–1551 (2002).
Kenis, S. et al. Ancestral glycoprotein hormone-receptor pathway controls growth in C. elegans. Front Endocrinol. 14, 1200407 (2023).
Nakabayashi, K. et al. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J. Clin. Investig. 109, 1445–1452 (2002).
Gong, Z. et al. Crystal structure of LGR ligand alpha2/beta5 from Caenorhabditis elegans with implications for the evolution of glycoprotein hormones. Proc. Natl. Acad. Sci. USA 120, e2218630120 (2023).
Loosfelt, H. et al. Two-subunit structure of the human thyrotropin receptor. Proc. Natl. Acad. Sci. USA 89, 3765–3769 (1992).
Chazenbalk, G. D. et al. Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology 138, 2893–2899 (1997).
Lomize, A. L., Todd, S. C. & Pogozheva, I. D. Spatial arrangement of proteins in planar and curved membranes by PPM 3.0. Protein Sci. 31, 209–220 (2022).
Gupta R., Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 310-322 (2002).
Kobe, B. & Kajava, A. V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struc Biol. 11, 725–732 (2001).
Ng, A. C. et al. Human leucine-rich repeat proteins: a genome-wide bioinformatic categorization and functional analysis in innate immunity. Proc. Natl. Acad. Sci. USA 108, 4631–4638 (2011).
Mueller, S., Jaeschke, H., Gunther, R. & Paschke, R. The hinge region: an important receptor component for GPHR function. Trends Endocrinol. Metab. 21, 111–122 (2010).
Mueller, S. et al. Significance of ectodomain cysteine boxes 2 and 3 for the activation mechanism of the thyroid-stimulating hormone receptor. J. Biol. Chem. 281, 31638–31646 (2006).
Nakabayashi, K., Kudo, M., Kobilka, B. & Hsueh, A. J. Activation of the luteinizing hormone receptor following substitution of Ser-277 with selective hydrophobic residues in the ectodomain hinge region. J. Biol. Chem. 275, 30264–30271 (2000).
Qiu, W. et al. Cholesterol-dependent enzyme activity of human TSPO1. Proc. Natl. Acad. Sci. USA 122, e2323045122 (2025).
Punjani, A. & Fleet, D. J. 3D variability analysis: Resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Dos Santos, S. et al. Distinct expression patterns of glycoprotein hormone-alpha2 and -beta5 in a basal chordate suggest independent developmental functions. Endocrinology 150, 3815–3822 (2009).
Cho, S., Rogers, K. W. & Fay, D. S. The C. elegans glycopeptide hormone receptor ortholog, FSHR-1, regulates germline differentiation and survival. Curr. Biol. 17, 203–212 (2007).
Park, J. H., Ohshima, S., Tani, T. & Ohshima, Y. Structure and expression of the gsa-1 gene encoding a G protein alpha(s) subunit in C. elegans. Gene 194, 183–190 (1997).
Brundage, L. et al. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron 16, 999–1009 (1996).
Nagayama, Y. et al. High affinity binding of thyrotropin (TSH) and thyroid-stimulating autoantibody for the TSH receptor extracellular domain. Thyroid 4, 155–159 (1994).
Hendrickson, W. A. & Gong, Z. Structural and evolutionary insights into the functioning of glycoprotein hormones and their receptors. Andrology, online ahead of print (2025).
Agwuegbo, U. T. et al. Differential FSH Glycosylation Modulates FSHR Oligomerization and Subsequent cAMP Signaling. Front. Endocrinol. 12, 765727 (2021).
Whorton, M. R. et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 104, 7682–7687 (2007).
Gusach A., García-Nafría J. & Tate C. G. New insights into GPCR coupling and dimerisation from cryo-EM structures. Curr. Opin. Struc. Biol. 80, (2023).
Seven, A. B. et al. G-protein activation by a metabotropic glutamate receptor. Nature 595, 450–454 (2021).
Zuo, H. et al. Promiscuous G-protein activation by the calcium-sensing receptor. Nature 629, 481–488 (2024).
Du, J. et al. Structures of human mGlu2 and mGlu7 homo- and heterodimers. Nature 594, 589 (2021).
Park, J. et al. Symmetric activation and modulation of the human calcium-sensing receptor. Proc. Natl. Acad. Sci. USA 118, e2115849118 (2021).
Mancia, F., Assur, Z., Herman, A. G., Siegel, R. & Hendrickson, W. A. Ligand sensitivity in dimeric associations of the serotonin 5HT2c receptor. EMBO Rep. 9, 363–369 (2008).
Cole, L. A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 10, 24 (2012).
Koshland, D. E. Jr. The structural basis of negative cooperativity: receptors and enzymes. Curr. Opin. Struct. Biol. 6, 757–761 (1996).
Costantini, L. M. et al. A palette of fluorescent proteins optimized for diverse cellular environments. Nat. Commun. 6, 7670 (2015).
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Noubhani, A. M., Dieryck, W., Chevalier, S. & Santarelli, X. On-line purification of His-tag enhanced green fluorescent protein taken directly from a bioreactor by continuous ultrasonic homogenization coupled with immobilized metal affinity expanded bed adsorption. J. Chromatogr. A 968, 113–120 (2002).
Mossessova, E. & Lima, C. D. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell 5, 865–876 (2000).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Terwilliger, T. C. et al. Improved AlphaFold modeling with implicit experimental information. Nat. Methods 19, 1376–1382 (2022).
Casanal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci. 29, 1069–1078 (2020).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D. 74, 519–530 (2018).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
Cryo-EM data were collected at the Columbia Cryo-EM facility and the Simons Electron Microscopy Center (SEMC), with the assistance of staff from both SEMC and the Columbia University Cryo-Electron Microscopy Center. R. Grassucci and Z. Zhang from the Columbia Cryo-EM Center assisted with data collection. Some of the work was performed using equipment from the Center for Membrane Protein Production and Analysis (COMPPÅ; grant no. NIH P41 GM116799 to W.A. Hendrickson). We thank Dr. R. Bruni for the gift of BacMam-oxGFP vector. This project is partially supported by the NIH grant (NIGMS R35GM141871) to Q. R. Fan. Z. Gong was partially supported by Endocrinology Training grant from NIH (grant number 5 T32 DK 7328-40).
Author information
Authors and Affiliations
Contributions
Z.G., Q.R.F. and W.A.H. designed the expression constructs. Z.G. performed gene cloning, protein purification, functional assay and MST measurement. B.K. J.K. and Z.G. carried out FSEC screens. S.C., Z.F. and Z.G. screened and optimized sample vitrification and generated cryo-EM data. Single-particle cryo-EM data analysis was conducted by S.C., O.B.C., C.W. and Z.G. 3D Variability Analysis was carried out by Z.G. and S.C. Model building was conducted by Z.G. The project was supervised by Q.R.F. and W.A.H. The manuscript was written by Z.G. and W.A.H. with input from S.C., O.B.C., and Q.R.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gong, Z., Chen, S., Fu, Z. et al. Structure of an LGR dimer, an evolutionary predecessor of glycoprotein hormone receptors. Nat Commun 16, 11716 (2025). https://doi.org/10.1038/s41467-025-66676-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-66676-x