Abstract
Ultrasound stimulation is a promising non-invasive strategy for neuropathic pain, yet its sustained effects and underlying mechanisms remain poorly understood. We investigated brainwave-patterned low-intensity continuous theta-burst ultrasound stimulation (LI-cTBUS) in a mouse model of partial sciatic nerve crush injury (PCI). LI-cTBUS substantially alleviated mechanical allodynia during and after treatment. Mechanistically, PCI upregulated brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrkB) signaling, while LI-cTBUS enhanced extracellular BDNF uptake by spinal astrocytes, thereby normalizing the BDNF/TrkB pathway and restoring potassium chloride cotransporter 2 (KCC2) function. Furthermore, LI-cTBUS attenuated reactive astrogliosis via activation of the transient receptor potential ankyrin 1 (TRPA1) channel, indicating a glial mechanism for ultrasound-induced analgesia. Transcriptomic profiling revealed that PCI altered the spinal transcriptome, whereas LI-cTBUS reversed inflammatory signatures, corrected aberrant BDNF/TrkB signaling, and restored GABAergic transmission. Collectively, these findings demonstrate that LI-cTBUS reprograms reactive astrocytes, suppresses nociceptive signaling, and provides sustained relief from neuropathic pain, underscoring its therapeutic potential for non-invasive spinal neuromodulation.
Similar content being viewed by others
Introduction
Therapeutic ultrasound has been emerging as a potential non-invasive treatment in recent decades1,2. By utilizing sound waves and mechanical vibration, ultrasound stimulation can effectively penetrate deep into the tissue, providing benefits such as increased blood flow, soft tissue healing, anti-inflammation, and pain reduction3,4,5. Early studies have shown promising clinical outcomes in patients with chronic low back pain6 or knee osteoarthritis7,8. However, further research is essential to comprehensively unravel the mechanisms and sustainability of the effects of ultrasound therapy in order to determine the optimal parameters, notably focusing on intensity, pulse width, frequency, and particularly, pattern. Low-intensity focused ultrasound (LIFU) stands out as a pivotal non-invasive method for deep brain stimulation, allowing precise modulation of brain activity essential for investigating psychiatric conditions. Understanding the operational mechanisms of LIFU and optimizing parameters, especially temporal stimulation patterns, is critical to enhance its efficacy9,10,11. The temporal patterns of stimulation are closely linked to the dynamic synaptic and neuronal activities both in vivo and in vitro12,13. Originally designed for repetitive transcranial magnetic stimulation (rTMS), the theta-burst stimulation (TBS) pattern has shown promise in modulating neural activity across various neurological and psychiatric disorders14. However, the sustainable impact of rTMS-based TBS is limited due to constraints in spatial resolution, emphasizing the need for innovative techniques that offer prolonged pain relief. Based on prior evidence that temporally patterned ultrasound stimulation, including theta-gamma burst paradigms, can modulate neural activity and induce sustained plastic changes in the brain15, we hypothesized that similar patterns may also modulate spinal circuits relevant to chronic pain. Although the present study is confined to spinal mechanisms and does not directly examine supraspinal plasticity, it seeks to establish patterned ultrasound as a viable neuromodulatory tool for pain processing.
A comprehensive understanding of pain mechanisms is essential for developing innovative treatments that target neurological disorders, particularly chronic pain. Brain-derived neurotrophic factor (BDNF) signaling pathways have gained attention for their role in neuropathic pain. BDNF notably influences sensory neurotransmission and contributes to central sensitization16,17,18. Elevated BDNF levels lead to the downregulation of the neuronal potassium-chloride co-transporter (KCC2), reducing GABAergic inhibition in the dorsal horn of the spinal cord and contributing to neuropathic pain19,20. Notably, astrocytes—increasingly recognized as active modulators of synaptic function—are one of the primary sources of BDNF. Under chronic pain conditions, neurons become reactive and release gliotransmitters such as GABA at aberrant levels21,22. Intriguingly, astrocytes express specific transporters that facilitate the uptake of BDNF from the extracellular space, thereby regulating its availability to neurons and contributing to homeostasis as well as the regulation of neural activity23,24. Emerging evidence indicates that astrocytes regulate neuronal excitability via gliotransmitter release and ion channel-dependent signaling, with TRPA1 playing a key role in glial neuromodulation25,26. Mechanosensitive ion channels, particularly TRPA1, have emerged as key mediators of cellular responses to ultrasound15,27. Activated by mechanical and oxidative stimuli, TRPA1 is expressed in both nociceptive neurons and astrocytes and is implicated in calcium-dependent gliotransmission and neuroinflammation. While its role in peripheral pain is well known, its contribution to central modulation and glial–neuronal signaling during ultrasound neuromodulation remains unclear.
In this study, we investigated the potential of non-invasive low-intensity (<3 W/cm2) theta burst ultrasound stimulation with a continuous paradigm (LI-cTBUS) targeted at spinal cord astrocytes during neuropathic pain development. Additionally, by incorporating the role of the transient receptor potential ankyrin 1 (TRPA1) in low-intensity ultrasound stimulation-mediated neuromodulation15,27, we aim to explore the application of non-invasive ultrasound following brain-wave patterns as a promising modality for alleviating neuropathic pain. Emphasizing these aspects underscores the importance of comprehending related mechanisms and highlights the potential of brain-wave-patterned ultrasound to induce sustained neuromodulatory effects while minimizing adverse consequences.
Results
Alleviating mechanical allodynia through patterned cTBUS ultrasound stimulation
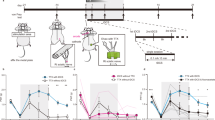
To investigate non-invasive ultrasound as a therapeutic strategy for chronic pain, we employed a partial sciatic nerve crush injury (PCI), which induces peak mechanical allodynia by day 7 and sustained tactile hypersensitivity through day 64 (Supplementary Fig. 1a, b)28. Ultrasound stimulation was applied daily for 10 days consecutively to the lumbar-sacral region through intact skin on day 17 post-sciatic nerve injury, which presents the persistent and chronic manifestation of tactile allodynia (Fig. 1a). We developed an ultrasound-based burst spinal cord stimulation (USCS, 40 Hz train of 5-pulse bursts at 500 Hz) that mimic gold standard for neuropathic pain treatment29,30, and two other ultrasound paradigms based on continuous theta-burst stimulation (cTBUS), incorporating theta-frequency (5 Hz) trains of high gamma bursts at 100 or 200 Hz (Fig. 1b, see the “Methods” section). Higher frequencies as of 200 Hz closely matched the burst structure of continuous theta burst stimulation (cTBS) protocols used in brain stimulation, which are known to induce stronger synaptic plasticity and inhibitory effects31,32. Notably, while all paradigms contained gamma-frequency components, only cTBUS patterns retained the theta frequency to comprehensively investigate the impact of specific frequency ranges on achieving long-lasting pain relief. To determine appropriate stimulation intensity, motor evoked potential (MEP) thresholds were established for the motor cortex (0.77 W/cm²) and spinal cord (77 W/cm²) (Supplementary Fig. 1c, d). Ultrasound stimulation with an intensity below 1 W/cm² was classified as ultra-low-intensity27, while intensities below 3 W/cm² were defined as low-intensity (LI) according to the criteria used in this study. Unless otherwise noted, all in vivo ultrasound experiments used 0.77 W/cm². The paw withdrawal threshold (PWT) of the ipsilateral nociceptive threshold considerably increased with the LI-cTBUS-200 Hz pattern (0.69 ± 0.05), and LI-cTBUS-100 Hz pattern (0.56 ± 0.04), compared to the PCI group (0.13 ± 0.03) (Fig. 1c), while no changes were observed contralaterally (Supplementary Fig. 1e). In contrast, the USCS did not produce notable changes in the withdrawal threshold (0.23 ± 0.04). Additionally, adapting the USCS to 301 Hz with a 3 ms pulse width to match the temporal envelope of cTBUS did not result in appreciable changes (Supplementary Fig. 1f). These findings indicate that, unlike electrically based spinal stimulation systems, the ultrasound-based LI-cTBUS pattern effectively alleviates neuropathic pain in mouse models by incorporating both theta and high gamma frequencies. Intriguingly, the analgesic effect of LI-cTBUS-200 Hz persisted for ~5 days after treatment cessation, before gradually returning to baseline (Fig. 1c).
a Experimental paradigm of pain threshold test and ultrasound (US) treatment. Arrows indicate each von Frey test. Partial crush injury (PCI) was induced, followed by 10 consecutive days of US treatment targeted at the lumbar-sacral spinal cord region. b Ultrasound patterns for spinal cord stimulation: ultrasound-based burst spinal cord stimulation (USCS), continuous theta burst ultrasound stimulation (cTBUS) at 100 Hz and cTBUS at 200 Hz (see the “Methods” section). c Top: Paw withdrawal threshold (PWT) in PCI model mice treated with USCS, cTBUS-100 Hz, and cTBUS-200 HZ at low intensity. Bottom: Area under curve (AUC) of PWT during and after treatment, N = 6 per group; data are presented as box-and-whisker plots, *p = 0.02, **p = 0.003, ***p = 0.000003, one-way ANOVA with Tukey’s multiple comparison test. d Top: Intensity screening for cTBUS-200 Hz protocol: 0.77, 0.88, 3.4, and 10.8 W/cm2. Bottom: Area under curve (AUC) of PWT during and after treatment. N = 6; data are presented as box-and-whisker plots, *p = 0.01, **p = 0.001, ***p = 0.000024, one-way ANOVA with Tukey’s multiple comparison test. e Top: PWT during two stimulation periods in PCI mice receiving LI-cTBUS 200 Hz. Bottom: Total AUC differences between PCI and PCI + LI-cTBUS 200 Hz groups. N = 6; data are presented as box-and-whisker plots, *p = 0.03, **p = 0.002, ***p = 0.00032, unpaired two-tailed t-test. f Schematic of CatWalk gait analysis, PCI mice were treated with LI-cTBUS 200 Hz for 10 consecutive days starting at post-injury day 17. The gait test was conducted after 4 h of the last LI-cTBUS 200 Hz stimulation. Injury was induced in the right hind limb, RH: Right-hind (magenta), RF: Right-front (cyanine blue), LH: Left-hind (green), LF: Left-front (yellow). g Representative CatWalk step cycles and h footprint appearance. i Analysis of right hind limb/left hind limb (RH/LH) ratio shows differences in Stand (s) and Print Width (cm) in PCI vs. PCI + LI-cTBUS groups. N = 8; data are presented as box-and-whisker plots, *p = 0.02, **p = 0.001, ***p = 0.0000014; ns: not significant, one-way ANOVA with Tukey’s multiple comparison test. All data are presented as line graphs with mean ± SEM, or as box-and-whisker plots with the median (center line), interquartile range (box), and minimum to maximum values (whiskers). N number of mice. Schematic illustrations were created in BioRender. Sung, Y. (2025) https://BioRender.com/nm1aok3.
To assess whether this effect was intensity-dependent, we increased the stimulation intensity of cTBUS-200 Hz to 3.4 or 10.8 W/cm². This abolished the analgesic benefit, indicating that excessive energy delivery disrupts the therapeutic effect (Fig. 1d and Supplementary Fig. 1g, h). We also varied stimulation duration and found that 800 pulses produced more durable analgesia than 400, 1200, or 1600 pulses, suggesting that longer stimulation does not linearly translate to better outcomes (Supplementary Fig. 1i).
Next, we evaluated whether analgesia could be re-established after its initial effects subsided. Mice treated with LI-cTBUS-200 Hz from day 17 to 26 displayed improved withdrawal thresholds, which waned by day 39. A second 4-day stimulation series (day 39–42) reinstated analgesia, lasting over three days (Fig. 1e). These findings demonstrate both the long-lasting and re-inducible nature of LI-cTBUS-mediated analgesia.
To evaluate motor functional relevance, we assessed gait recovery using the CatWalk system. Following the injury in the right hind limb, the mice received a 10-day course of LI-cTBUS-200 Hz stimulation (Fig. 1f). PCI mice exhibited reduced right hind limb print width and standing time, indicative of pain-induced motor avoidance (Supplementary Movies S1, S2). Notably, mice treated with LI-cTBUS-200 Hz demonstrated improvement in both walking speed and standing time compared to the untreated PCI group (Fig. 1g and Supplementary Movie S3). Moreover, recovery of hindlimb print width was observed in the treated group (control: 0.92 ± 0.05, PCI: 0.42 ± 0.02, PCI + LI-cTBUS: 0.63 ± 0.03), further indicating functional restoration (Fig. 1h, i). These findings suggest that spinal LI-cTBUS effectively enhances gait-related motor function in a neuropathic pain model.
To assess whether the analgesic effects of LI-cTBUS generalized across pain modalities, we evaluated thermal nociception using the hot plate test. Despite its robust efficacy against mechanical allodynia in both male and female mice, LI-cTBUS did not alter thermal nociception in the hot plate test (Supplementary Fig. 2a, b). These findings are consistent with prior studies demonstrating that mechanical and thermal hypersensitivity involves distinct mechanisms—for instance, mechanosensitive channels such as TRPA1 are critically involved in mechanical allodynia, whereas thermal pain is primarily mediated by heat-sensitive channels such as TRPV1 and TRPM8. The lack of effect of LI-cTBUS on thermal thresholds suggests that its analgesic action is specifically linked to ultrasound-induced mechanical bioeffects-likely mediated through mechanosensitive pathways such as TRPA1 signaling33,34,35.
LI-cTBUS suppresses spinal hyperexcitability
To directly examine how cTBUS influences spinal excitability in chronic pain, we quantified neuronal activation using c-Fos, a marker of activity-dependent gene expression. In PCI mice, the proportion of c-Fos⁺/NeuN⁺ neurons in the ipsilateral dorsal horn was notably higher than in sham-operated controls, 10.27 ± 0.24 versus 6.1 ± 0.73 (Fig. 2a and Supplementary Fig. 4a). Remarkably, delivering LI-cTBUS (0.77 W/cm²) markedly reduced this activation, whereas high-intensity HI-cTBUS (77 W/cm²) produced the opposite effect, leading to a substantial increase in neuronal activation. These findings parallel our behavioral data (Fig. 1d): cTBUS below 3 W/cm² elevates the mechanical withdrawal threshold and produces analgesia, while higher intensities progressively lower the threshold and diminish analgesic efficacy. Consistent with this specificity, LI-cTBUS enhanced the withdrawal threshold in response to von Frey filaments and suppressed spinal c-Fos expression in PCI mice, yet caused no notable change in dorsal-horn c-Fos expression in control mice (Supplementary Fig. 4a). Thus, LI-cTBUS appears to act selectively under neuropathic pain conditions. Furthermore, c-Fos levels in dorsal root ganglia (DRG) remained unchanged following both low- and high-intensity stimulation (Supplementary Fig. 4b). Together, these results highlight a differential, state-dependent modulation of spinal—but not DRG—neuronal activity by LI-cTBUS, supporting its targeted efficacy in neuropathic pain.
a Left: Representative immunohistochemistry images showing c-Fos and NeuN expression in the spinal cord of PCI mice treated with high-intensity (77 W/cm2) or low-intensity (0.77 W/cm2) ultrasound. Scale bar: 500 µm; magnified image scale bar: 50 μm. Right: Quantification of c-Fos+/NeuN+ (percentage). *p = 0.04, ***p = 0.00015, N = 4 per group, data are presented as box-and-whisker plots, two-way ANOVA with Tukey’s multiple comparison test. ns: not significant. b Left: Representative traces of evoked EPSCs from each group: control, PCI, and PCI + LI-cTBUS. Right: Summary data showing the cumulative probability distributions of sEPSC amplitudes and frequencies across groups. Data are shown as mean ± SEM. *p = 0.01; ns: not significant, n = 7–13 per group (control = 8, PCI = 13, PCI + LI-cTBUS = 7). One-way ANOVA with Tukey’s multiple comparison test. N number of mice, n number of independent experiments.
Previous studies demonstrated that single-pulse low-intensity ultrasound can transiently activate neuronal NMDARs via astrocytic TRPA1-mediated glutamate release25,27; however, whether patterned stimulation induces lasting synaptic plasticity remained unknown. Given the reduced c-Fos levels following LI-cTBUS, we hypothesized that LI-cTBUS-induced activation of astrocytic TRPA1 may trigger the release of gliotransmitters (e.g., glutamate, D-serine), which could drive LTD-like synaptic plasticity in adjacent dorsal horn neurons. To test this, we performed whole-cell voltage-clamp recordings of spontaneous excitatory postsynaptic currents (sEPSCs) mediated by AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors in lamina II neurons from acute spinal cord slices, in order to assess potential LTD-like changes in excitatory synaptic transmission (Fig. 2b). PCI mice exhibited a significant increase in sEPSC amplitude, consistent with enhanced postsynaptic excitatory strength. LI-cTBUS treatment reversed this enhancement, restoring sEPSC amplitude to near-control levels (WT: 19.71 ± 1.43 pA; PCI: 27.19 ± 1.21 pA; PCI + LI-cTBUS: 20.9 ± 1.51 pA; p < 0.05). Cumulative probability analysis of sEPSC amplitudes further confirmed this normalization. In contrast, sEPSC frequency, as measured by inter-event intervals (IEIs), presented no significance among groups (WT: 1.31 ± 0.09 s; PCI: 1.13 ± 0.08 s; PCI + LI-cTBUS: 1.60 ± 0.12 s), suggesting that LI-cTBUS modulates postsynaptic, but not presynaptic, components of excitatory transmission. Together, these findings suggest that LI-cTBUS induces a suppression of excitatory transmission via postsynaptic LTD-like plasticity, potentially mediated by TRPA1-dependent astrocyte–neuron interactions. This synaptic depression likely contributes to the reduction in spinal hyperexcitability and behavioral hypersensitivity observed in PCI mice.
To ensure the safety of LI-cTBUS stimulation and exclude potential cellular stress or thermal damage, we conducted a series of safety assessments. TUNEL assays and in situ hybridization confirmed no increase in apoptotic cell death in spinal tissue following LI-cTBUS exposure (Supplementary Fig. 3a and b). Temperature monitoring confirmed that LI-cTBUS did not induce appreciable temperature increases at therapeutic intensities (Supplementary Fig. 3c). Ultrasound beam profiling further validated efficient penetration and accurate targeting through the vertebral column (Supplementary Fig. 3d).
Together, these results demonstrate that LI-cTBUS safely attenuates neuronal hyperactivity and induces synaptic depression in neuropathic circuits, while preserving tissue integrity and normal physiological neuronal function.
Rebalancing the spinal BDNF/KCC2 for attenuating mechanical allodynia following LI-cTBUS
BDNF upregulation in the spinal dorsal horn has been shown to promote neuropathic pain by impairing GABAergic inhibition36. BDNF exerts both acute and long-term excitation on dorsal horn neurons17,18,37, suppresses KCC2 expression, leading to intracellular Cl− accumulation, depolarizing shift in the chloride reversal potential (Eanion), and ultimately disinhibition of nociceptive circuits20. To determine whether LI-cTBUS-induced analgesia involves modulation of this BDNF-KCC2 axis, we first assessed BDNF protein levels in the lumbar spinal cord. Compared to controls, PCI mice exhibited elevated BDNF level, which was markedly reduced following 10 days of LI-cTBUS treatment (Fig. 3a). Concurrently, KCC2 protein expression in the ipsilateral side of dorsal spinal horn—suppressed in the PCI group—was restored to near-control levels by LI-cTBUS (Fig. 3b), indicating rebalancing of the BDNF–KCC2 signaling cascade. This rebalancing effect was consistent across sexes and specific to the spinal cord, with no notable changes in dorsal root ganglia (Supplementary Figs. 6 and 7).
a Left: Experimental schematic for measuring total BDNF protein in the lumbar spinal cord (whole segment); center: representative immunoblots; right: group quantification. N = 6 independent experiments; data are presented as box-and-whisker plots; one-way ANOVA with Tukey’s post-hoc test, *p = 0.02. b Experimental schematic for measuring KCC2 protein levels in the ipsilateral dorsal lumbar spinal cord; center: representative immunoblots; right: quantification. N = 6 independent experiments; data are presented as box-and-whisker plots; *p = 0.03, ***p = 0.000094, one-way ANOVA with Tukey’s multiple comparison test. c Intrathecal injection of recombinant human BDNF (1 ng/10 μL) transiently lowered the withdrawal threshold, lasting for <3 h, N = 4 (BDNF), N = 5 (Saline); mean ± SEM; **p = 0.003, two-way ANOVA with Tukey’s multiple comparison test. d Timeline for LI-cTBUS (200 Hz, 0.77 W/cm²) applied daily after BDNF injection. Center: time-course of withdrawal threshold; right: PWT change on days 3 and 4. N = 6; data are presented as mean ± SEM and box-and-whisker plots; *p = 0.05, **p = 0.005, two-way ANOVA with Tukey’s multiple comparison test. e Total BDNF protein 5 days after BDNF injection with or without LI-cTBUS. Top: blot; bottom: quantification. N = 4 independent experiments; data are presented as box-and-whisker plots; *p = 0.01; ns: non-significant test. f Perforated patch-clamp recordings from lamina II neurons. Left: GABA-evoked current–voltage (I–V) representative traces; center: representative traces; right: summary of chloride reversal potential (Eanion). PCI caused a right-shifted Eanion that was normalized by LI-cTBUS. Data are presented as mean ± SEM. **p = 0.002, ***p = 0.000002; n = 3–10 (control = 3, PCI = 5, PCI + LI-cTBUS = 10), one-way ANOVA with Tukey’s multiple comparison test. N number of mice, n number of recorded cells. Schematic illustrations were created in BioRender. Sung, Y. (2025) https://BioRender.com/figix4a.
To examine the functional consequences of this molecular restoration, we performed perforated patch-clamp recordings from lamina II neurons using a gramicidin-based internal solution. In control mice, GABA-evoked currents revealed a hyperpolarized chloride reversal potential (Eanion)−65.4 ± 0.86 mV, consistent with effective Cl− extrusion. In contrast, PCI mice showed a substantially depolarized Eanion (−63.1 ± 0.29 mV), reflecting impaired GABAergic inhibition due to intracellular Cl− accumulation. Importantly, LI-cTBUS treatment shifted Eanion back toward control levels (−66.5 ± 0.12 mV), suggesting functional recovery of KCC2-mediated chloride extrusion (Fig. 3f). These findings indicate that LI-cTBUS restores inhibitory tone in dorsal horn neurons by normalizing the chloride gradient, which represents one of the key mechanisms underlying its analgesic effect.
To directly test the contribution of elevated BDNF to mechanical hypersensitivity, we intrathecally injected recombinant human BDNF into naïve mice. BDNF injection acutely lowered mechanical withdrawal thresholds, confirming its sufficiency to induce allodynia (Fig. 3c). Notably, subsequent LI-cTBUS treatment restored threshold levels and reduced BDNF protein (Fig. 3d, e), suggesting that LI-cTBUS counteracts BDNF-induced sensitization. Interestingly, BDNF mRNA levels remained unchanged between PCI and LI-cTBUS-treated mice (Supplementary Fig. 8), implicating post-transcriptional mechanisms—such as uptake and degradation—in the reduction of BDNF protein.
Finally, we extended these findings to another neuropathic pain model and across sexes. In the spared nerve injury (SNI) model, LI-cTBUS applied during the early pain phase similarly reduced BDNF expression and restored KCC2 levels (Supplementary Fig. 5a, b). Immunostaining revealed a corresponding reduction in glial BDNF expression and gliosis (Supplementary Fig. 5c, d). To assess microglial responses, we utilized TMEM119 transgenic reporter mice, in which resident microglia are selectively labeled, enabling clear visualization of microglial dynamics both in vivo and in vitro. Immunostaining revealed a marked increase in BDNF expression within TMEM119⁺ microglia in the ipsilateral dorsal horn at day 10 post-SNI, which was significantly attenuated by LI-cTBUS (Supplementary Fig. 5c, d). Although TMEM119 is generally considered a homeostatic marker of resident microglia, its upregulation has also been reported in models of peripheral nerve injury38, supporting our observation that TMEM119 expression can increase under neuropathic conditions and is reversed by LI-cTBUS.
Additionally, LI-cTBUS restored BDNF/KCC2 balance in both male and female PCI mice (Supplementary Fig. 6), suggesting that its effects are sex-independent. Antibody specificity was validated using BDNF exon IV knockout mice, further confirming the accuracy of BDNF protein quantification (Supplementary Fig. 9).
Together, these results demonstrate that LI-cTBUS alleviates mechanical allodynia by reducing BDNF protein expression and restoring KCC2-mediated chloride extrusion in the spinal cord. This mechanism likely involves post-transcriptional modulation of BDNF, including uptake and lysosomal degradation, leading to normalization of inhibitory tone and suppression of pathological excitation in dorsal horn neurons.
LI-cTBUS reduces astrogliosis and suppresses pathological astrocytic GABA signaling
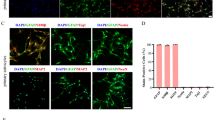
Given that LI-cTBUS lowers spinal BDNF levels in PCI mice and restores inhibitory balance via KCC2 upregulation, we next investigated its effects on astrocytes, a key mediator of GABA release in neuropathic pain39,40. To elucidate how LI-cTBUS acts on these cells, we analyzed astrocytic morphology and function after 10 days of stimulation (Fig. 4a). Immunohistochemistry and IMARIS-based 3-D reconstructions revealed that LI-cTBUS reduced astrocyte reactivity, bringing cell volume and branch complexity closer to levels observed in control mice (Fig. 4b, c). Functionally, LI-cTBUS also lowered the proportion of GFAP⁺/GABA⁺ astrocytes, indicating suppression of pathological tonic GABA release (Fig. 4d), whereas neuronal GABA levels (NeuN+/GABA+) remained unchanged (Supplementary Fig. 10). These results align with the notion that elevated spinal BDNF decreases paw-withdrawal thresholds by depolarizing Eanion, thereby converting GABAergic signaling from inhibitory to excitatory in neuropathic pain20,41.
a Experiment schematic: Lumbar-sacral spinal cords were collected after 10 consecutive days of LI-cTBUS-200 Hz. b Representative GFAP immunostaining of lumbar spinal cords at the acute phase (day 7), chronic phase (day 17), and after 10 days of LI-cTBUS. Scale bar: 200 µm. c Top: 3D reconstructions of dorsal-horn astrocytes. Scale bar: 20 µm. Bottom: Average GFAP area and dendrite volume analysis. N = 3–6 per group (control, PCI d7, PCI = 3, PCI + LI-cTBUS = 6); **p = 0.004, ***p = 0.000000018, two-way and one-way ANOVA with Tukey’s multiple comparison test. d Representative images of astrocytic GABA in ipsilateral spinal dorsal horn under LI-cTBUS stimulation. Scale bar: 20 and 200 µm. Percentage of GFAP⁺/GABA⁺ cells in PCI vs. PCI + LI-cTBUS groups. N = 6. *p = 0.03, Unpaired two-tailed t-test. e Schematic of in-vitro ultrasound stimulation of primary spinal astrocytes. f Immunocytochemistry of GFAP and GABA in cultured astrocytes. Scale bar: 10 µm. g IMARIS reconstruction of cultured spinal astrocytes. Scale bar: 20 µm. h Statistical analysis of spinal astrocyte dendrite volume (µm3). n = 10–18 per group (control = 14, control + cTBUS = 18, H2O2 200 µM = 12, H2O2 200 µM + cTBUS = 17, H2O2 400 µM = 10, H2O2 400 µM + cTBUS = 16), ***p = 0.000069, one-way ANOVA with Tukey’s multiple comparison test. i Quantification of GFAP⁺/GABA⁺ cells after H₂O₂ (400 µM) and LI-cTBUS (2.4 or 2.8 W/cm²) applied once daily for three consecutive stimulations (see the “Methods” section), n = 15. j Electron-microscopy images of endoplasmic reticulum (ER) in control, H2O2-only, and H2O2 + cTBUS-treated astrocytes. Scale bar: 5 μm. Right: ER area quantification. Data represent mean ± SEM. n = 100 recorded cells from their independent replicates. ***p = 0.0000000175, one-way ANOVA with Tukey’s multiple comparison test. k Electron-microscopy images of lysosomes. Scale bar: 2 μm. Right: Quantification of lysosome number per cell. n = 5 recorded cells from there independent replicates; **p = 0.0025, ***p = 0.000188, one-way ANOVA with Tukey’s multiple comparison test. All data are presented as box-and-whisker plots. N number of mice, n number of recorded cells. Schematic illustrations were created in BioRender. Sung, Y. (2025) https://BioRender.com/wbn112g.
In contrast, microglial morphology was unaffected during the chronic stage, despite early post-injury activation (Supplementary Fig. 11). These findings suggest that while microglia may contribute to the initial development of neuropathic pain, they play a minimal role—at least morphologically—in the maintenance of allodynia in the PCI model. Importantly, the analgesic effects of LI-cTBUS during the chronic phase appear to be closely associated with the reversal of astrogliosis and modulation of GABAergic astrocytes, rather than microglial activation.
Reactive astrocytes are often associated not only with neurodegenerative conditions characterized by elevated levels of reactive oxygen species (ROS), but also with chronic pain, where oxidative stress contributes to sustained glial activation and pathological signaling42,43. Therefore, we investigated the effects of LI-cTBUS on astrogliosis induced by hydrogen peroxide in a primary spinal astrocyte culture model (Fig. 4e). Exposure to hydrogen peroxide induced a reactive astrocyte phenotype, increasing astrocytic GABA levels and pain hypersensitivity (Supplementary Fig. 12a, b). Given that LI-cTBUS at intensities below 3 W/cm² elicited clear analgesic effects in vivo (Fig. 1d), we applied in vitro LI-cTBUS at 2.4 and 2.8 W/cm²—two closely related intensities—to assess reproducibility across similar stimulation conditions (see the “Methods” section). This treatment attenuated astrocyte hypertrophy and reduced the proportion of GFAP⁺/GABA⁺ cells (Fig. 4f–i). These findings suggest that low-intensity cTBUS can attenuate ROS-induced astrogliosis and suppress aberrant astrocytic GABA expression, supporting its therapeutic potential for ROS-related neurodegenerative and chronic pain conditions. While the in vitro model allowed us to investigate astrocyte-specific mechanisms in a controlled and high-throughput manner, we acknowledge that it does not fully capture the cellular and network complexity of the intact spinal cord.
In neuropathic pain conditions, oxidative stress contributes to the transformation of astrocytes into a reactive phenotype, characterized by ER stress and increased lysosomal burden—hallmarks of astrogliosis that are associated with aberrant gliotransmission and pro-nociceptive signaling39,44. We performed Electron microscopy (EM) analysis to assess ultrastructural changes in primary spinal astrocyte cultures, focusing on lysosome abundance and endoplasmic reticulum (ER) morphology. Astrocytes exposed to H2O2 showed pronounced endoplasmic reticulum (ER) swelling and increased lysosome abundance, hallmarks of reactive astrocytes under stress (Fig. 4j, k). In contrast, LI-cTBUS reversed both ER swelling and lysosomal accumulation, restoring ultrastructural homeostasis.
These results suggest that cTBUS alleviates oxidative stress–induced ER and lysosomal dysfunction in spinal astrocytes, which may contribute to the suppression of reactive astrogliosis and the restoration of glial homeostasis under pathological conditions. While the causal relationship between ER stress reduction and astrogliosis remains to be determined—whether cTBUS directly relieves organelle stress or acts via upstream mechanisms such as TRPA1 activation or BDNF uptake—our data provide strong evidence that targeting astrocytic stress responses may be a key component of the sustained analgesic effects of LI-cTBUS.
LI-cTBUS mitigates BDNF-induced mechanical allodynia by enhancing astrocytic BDNF uptake
Astrocytes have been implicated in regulating extracellular BDNF levels, particularly through uptake mechanisms mediated by the p75NTR/clathrin endocytic pathway24,45,46. Additionally, both proBDNF and mature BDNF can bind p75NTR, suggesting a shared entry route into astrocytes47,48. To determine whether the reduction of spinal BDNF levels by LI-cTBUS stimulation is associated with astrocytic uptake of BDNF, we performed fluorescence-activated cell sorting (FACS) analysis on spinal cord tissue after stimulation. Using surface markers for astrocytes (APC-A) and microglia (FITC-A) (Supplementary Fig. 13), we quantified intracellular BDNF (PE-A) levels. Consistent with protein-level analyses, intracellular BDNF levels in astrocytes and microglia were elevated during early phases of neuropathic pain. However, by the chronic phase, intracellular BDNF levels declined in microglia but remained high in astrocytes (Fig. 5a). Importantly, LI-cTBUS reduced astrocytic BDNF levels in the PCI model, whereas microglia—whose BDNF expression had already declined after day 7—exhibited only a mild reduction. These results suggest that during the chronic pain stage, the LI-cTBUS-induced decrease in spinal BDNF is primarily attributable to astrocytes (Fig. 5a).
a Left: Schematic of experimental design for flow cytometric quantification of astrocytic and microglial BDNF in the spinal cord after LI-cTBUS treatment. Middle: Representative FACS plots showing gating for astrocytes (APC-A), microglia (FITC-A), and intracellular BDNF (PE-A). Group colors: Control (Blue), PCId7 (red), PCI (yellow), and PCI+cTBUS (green). Right: Quantification shows a significant increase in BDNF-positive astrocytes in the LI-cTBUS group. Data are presented as box-and-whisker plots, *p = 0.02, **p = 0.007; one-way ANOVA with Tukey’s multiple comparison test, N = 6. b Left: Experimental schematic for in vivo assessment of astrocytic uptake of exogenous BDNF-GFP protein following intrathecal injection (i.t.). Middle: Representative FACS plots show GFP-positive astrocytes (Q2: FITC-A + APC-A). Right: LI-cTBUS stimulation increased the percentage of double-positive cells. Data are presented as box-and-whisker plots, **p = 0.04; unpaired two-tailed t-test, N = 4 (BDNF-GFP control), N = 6 (BDNF-GFP + LI-cTBUS). c Left: Schematic of in vitro assay using cultured primary spinal astrocytes transfected with BDNF-GFP or control proteins. Middle: FACS plots show elevated GFP signal in astrocytes following LI-cTBUS stimulation. Right: Quantification indicates a specific increase in BDNF-GFP uptake relative to non-GFP and SAR-GFP controls, indicating selective facilitation by LI-cTBUS. All data are presented as box-and-whisker plots, ***p = 0.000205; ns: not significant. n = 7, one-way ANOVA with Tukey’s multiple comparison test. N number of mice, n number of independent experiments. Schematic illustrations were created in BioRender. Sung, Y. (2025) https://BioRender.com/gfmovyn.
To directly assess astrocytic uptake of extracellular BDNF, we intrathecally delivered a GFP-tagged BDNF fusion protein (BDNF-GFP) and analyzed spinal tissues after LI-cTBUS stimulation. The proportion of astrocytes+/GFP+ (APC-A+/FITC-A+) increased following LI-cTBUS, indicating enhanced internalization of exogenous BDNF by astrocytes (Fig. 5b). These findings were further validated in a primary astrocyte culture system. Following LI-cTBUS stimulation, the proportion of GFP-positive cells in the BDNF-GFP-treated group was markedly higher than in the non-GFP control group. In contrast, no increase in GFP-positive cells was observed in cultures treated with untagged GFP or a SARS-CoV-2-derived fusion protein (SAR-GFP), underscoring the specificity of BDNF uptake induced by LI-cTBUS (Fig. 5c and Supplementary Fig. 14b). These results suggest the possibility that LI-cTBUS specifically promotes the active uptake of BDNF in spinal astrocytes.
To investigate the intracellular fate of BDNF, we applied an oxidative stress model using primary astrocyte cultures treated with hydrogen peroxide (H2O2) under LI-cTBUS stimulation for three consecutive days. This led to an increase in the proportion of BDNF⁺/LysoTracker⁺ double-positive cells, indicating enhanced lysosomal trafficking of the internalized BDNF. However, the total BDNF fluorescence intensity remained unchanged (Supplementary Fig. 15), suggesting that LI-cTBUS promotes the lysosomal degradation pathway. Consistent with these findings, transcriptomic analysis revealed that genes associated with BDNF internalization and lysosomal processing—such as Atp6v0v, a subunit of the vacuolar ATPase, and Cltc, encoding clathrin heavy chain—were upregulated in LI-cTBUS-treated PCI mice, further supporting the involvement of astrocytic endolysosomal pathways in mediating BDNF clearance (Supplementary Fig. 16). This further supports the involvement of the astrocytic endolysosomal system in BDNF clearance. Collectively, these results identify astrocytic uptake and lysosomal degradation of BDNF as a key mechanism underlying the LI-cTBUS-induced reduction of spinal BDNF and its associated analgesic effects. These findings suggest that patterned low-intensity ultrasound stimulation alleviates mechanical allodynia in neuropathic pain through a previously unrecognized, astrocyte-mediated cellular regulatory pathway.
Transcriptomic restoration by LI-cTBUS in neuropathic pain
To further investigate whether the functional restoration observed following LI-cTBUS is accompanied by transcriptomic changes in the spinal cord, we performed RNA-sequencing analysis to profile gene expression patterns associated with its analgesic effects (Supplementary Fig. 16a). Specifically, we aimed to determine whether LI-cTBUS modulates key transcriptional pathways involved in BDNF/TrkB signaling, astrocyte-mediated regulation, and long-term synaptic plasticity relevant to sustained pain relief.
Transcriptomic profiling revealed that LI-cTBUS restored gene expression patterns toward homeostatic levels observed in healthy controls, in contrast to the pronounced dysregulation seen in untreated PCI mice (Supplementary Fig. 16b). A total of 563 genes were differentially expressed between PCI and LI-cTBUS groups, with 233 upregulated and 330 downregulated (Supplementary Fig. 16c). Pathway analysis revealed upregulation of the BDNF/TrkB signaling pathway and glial cell differentiation in the PCI group and attenuated following LI-cTBUS (Supplementary Fig. 16d). Notably, TrkB and its downstream effector Akt3 were upregulated in PCI mice, while transcription factors regulating KCC2—such as Egr4 (promoting KCC2 expression)49 and Sp3 (repressing KCC2 through REST-RE1)50—were downregulated and upregulated, respectively, indicating suppression of inhibitory neurotransmission (Supplementary Fig. 16e). Importantly, these transcriptomic alterations were reversed following LI-cTBUS treatment. These findings indicate that LI-cTBUS may help restore inhibitory synaptic transmission by normalizing BDNF/TrkB signaling and reactivating KCC2 transcriptional regulatory mechanisms.
Conversely, GO analysis revealed the upregulation of genes involved in apoptosis, TNFα signaling, and IL-1-induced NFκB activation in the PCI group compared to the LI-cTBUS group (Supplementary Fig. 16d), suggesting that LI-cTBUS exerts anti-inflammatory effects. Furthermore, we identified differential expression of genes associated with reactive astrogliosis, BDNF internalization, and AMPAR trafficking across the three groups (Supplementary Fig. 16e). These findings suggest that the analgesic effects of LI-cTBUS may be mediated through modulation of BDNF/TrkB/KCC2 signaling, mechanosensitive ion channels, and astrocytic activity, ultimately contributing to the long-term attenuation of neuropathic pain.
TRPA1 is essential for LI-cTBUS-induced analgesia via astrocytic calcium signaling and modulation of reactive astrogliosis
Mechanosensitive ion channels such as TRPA1 have emerged as key mediators of glial and neuronal responses to mechanical stimuli, including low-intensity ultrasound stimulation25,26,27. Furthermore, TRPA1 expression in nociceptive sensory neurons within peripheral nerves is implicated in diverse pain modalities51,52,53. Moreover, TRPA1 activation in astrocytes has been shown to play a protective role under chronic cerebral hypoperfusion54 and to mediate tonic GABA release, thereby influencing synaptic plasticity55,56. Given its mechanosensory role and expression in spinal glia, we hypothesized that TRPA1 activation may be essential for the analgesic effect of LI-cTBUS.
To assess this, we first used a pharmacological approach by systemically administering the TRPA1-specific antagonist HC030031 to PCI mice receiving LI-cTBUS. TRPA1 inhibition attenuated the analgesic effects of LI-cTBUS, as reflected by diminished paw withdrawal thresholds across the stimulation period (Fig. 6a). In parallel, spinal fiber photometry in Thy1-GCaMP6f mice revealed that LI-cTBUS-induced robust calcium responses in the dorsal horn, which were completely suppressed by TRPA1 blockade (Fig. 6b). These results indicate that TRPA1-dependent calcium transients are essential for ultrasound-induced antinociception57.
a Top: Experimental paradigm showing intraperitoneal (i.p.) injection of the TRPA1 inhibitor HC030031. PCI mice received HC030031 on day 17 post-injury, followed by LI-cTBUS stimulation one hour later. The procedure was repeated for 8 days. Bottom: The 50% withdrawal threshold revealed that HC030031 partially inhibited LI-cTBUS-induced analgesia in PCI mice. Data are presented as mean ± SEM, *p = 0.04, **p = 0.002, ***p = 0.00018, two-way ANOVA with Tukey’s multiple comparison test, N = 9. b Top: Schematic of fiber photometry recording in the spinal dorsal horn of Thy1-Gcamp6f mice. Scale bar: 200 µm. Bottom: Calcium responses in the spinal dorsal horn were reduced in HC030031-injected mice. Area under the curve analysis confirmed a significant reduction; data are presented as box-and-whisker plots, ***p = 0.0000000853, two-tailed t-test, repeated measurements. n = 26. c Top: Paw withdrawal threshold (PWT) in PCI wild-type and TRPA1 KO PCI mice treated with LI-cTBUS from day 17 to day 26 post-injury, data are represented as mean ± SEM. Bottom: Area under the curve of PWT over the stimulation period. Data are presented as box-and-whisker plots, **p = 0.006, ***p = 0.00044, one-way ANOVA with Tukey’s multiple comparison test, N = 6. d Left: Representative GFAP immunostaining in the spinal dorsal horn of PCI wild-type and TRPA1 KO PCI with and without LI-cTBUS treatment. Spinal cord samples were collected on day 26 following 10 days of stimulation. Scale bar: 200 µm. (Middle) 3D reconstruction of astrocyte morphology in each group. Scale bar: 20 µm. Right: Quantification of astrocyte filament number (number of astrocyte processes) and average dendrite volume. Data are presented as box-and-whisker plots, *p = 0.02, **p = 0.005, ***p = 0.00000001, one-way and two-way ANOVA with Tukey’s multiple comparison test. ns: non-significant, p > 0.99. N = 3-6 per group (WT PCI, WT PCI + LI-cTBUS = 6, TRPA1-KO PCI = 3, TRPA1-KO PCI + LI-cTBUS = 4). e Top: Immunostaining of GFAP and GABA in spinal dorsal horn astrocytes of wild-type PCI and TRPA1 KO PCI with and without cTBUS stimulation. Scale bar: 20 µm. Bottom: Quantification of GFAP/GABA positive cells, n = 4–6 per group (WT PCI, WT PCI + LI-cTBUS = 4, TRPA1-KO PCI, TRPA1-KO PCI + LI-cTBUS = 6). Data are presented as box-and-whisker plots, *p = 0.03, **p = 0.001; ns: not significant. One-way ANOVA with Tukey’s multiple comparison test. f Schematic of TRPA1 agonist (NMM) treatment in H₂O₂-exposed primary spinal astrocyte cultures. Representative immunostaining of GFAP and GABA. Right: Quantification of GFAP+/GABA+ cell proportion and GABA intensity, N = 9. Data are presented as box-and-whisker plots, *p = 0.05, **p = 0.007, ***p = 0.0003; ns: not significant. One-way ANOVA with Tukey’s multiple comparison test. N number of mice, n number of independent experiments. Schematic illustrations were created in BioRender. Sung, Y. (2025) https://BioRender.com/bf9u5xm.
To confirm the involvement of TRPA1 genetically, we examined TRPA1 KO mice subjected to PCI and LI-cTBUS. In line with pharmacological inhibition, LI-cTBUS failed to induce analgesia in TRPA1 KO PCI mice, supporting a critical requirement for TRPA1 in mediating these effects (Fig. 6c). Notably, astrocyte morphology remained unchanged in TRPA1 KO mice with or without LI-cTBUS (Fig. 6d), in contrast to wild-type mice, where LI-cTBUS reduced astrocytic hypertrophy. Furthermore, while LI-cTBUS suppressed spinal astrocytic GABA levels in wild-type PCI mice, no such reduction was observed in TRPA1 KO PCI mice (Fig. 6e), implicating TRPA1 in the regulation of reactive astrogliosis and astrocytic GABA tone during pain development.
To clarify TRPA1 cellular distribution, we performed immunostaining of TRPA1 in the spinal dorsal horn and observed its localization in both NeuN+ neurons and GFAP+ astrocytes (Supplementary Fig. 17a). Quantitative analysis further demonstrated that TRPA1 expression in both cell types was elevated during pain development and was significantly reduced following LI-cTBUS treatment (Supplementary Fig. 17b), further supporting the hypothesis that functional activation—rather than expression level—is key to TRPA1 involvement in LI-cTBUS-induced analgesia effects.
We next explored whether TRPA1 activation mitigates oxidative stress-induced reactive astrogliosis. Previous studies have shown that TRPA1 expression in cancer cells confers protection against oxidative stress by elevating intracellular Ca²⁺ levels and promoting cell survival in response to H2O2 exposure58. Moreover, moderate levels of ROS can trigger intracellular calcium oscillations and modulate cell proliferation, whereas excessive ROS leads to sustained elevations in intracellular calcium ([Ca2+]i), ultimately inducing cell death. We evaluated the effects of TRPA1 agonist administration following H2O2 exposure in a dose- and time-dependent manner. Notably, brief exposure to low-dose TRPA1 agonists (10 μM NMM, AITC and IJ100) attenuated H2O2-induced astrocyte reactivity, whereas high concentrations or prolonged exposure led to toxicity (Supplementary Fig. 18). Acute exposure with 10 μM NMM under oxidative stress attenuated reactive astrogliosis, as indicated by a reduction in astrocytic GABA (12.8 ± 5.25%) compared to untreated groups (21.1 ± 7.7%) (Fig. 6f). These findings highlight a previously unappreciated role of TRPA1 activation in mitigating reactive astrogliosis and suppressing extracellular GABA accumulation, underscoring its potential as a therapeutic target in oxidative stress-associated conditions.
Astrocytic TRPA1 is required for LI-cTBUS-induced calcium signaling and mechanical analgesia
To directly determine whether the analgesic effects of LI-cTBUS are specifically mediated by TRPA1 expressed in astrocytes—given that TRPA1 is also present in neurons—we generated an astrocyte-specific TRPA1 knockdown model. We conducted retro-orbital injection of AAV-pSico-shTrpa1-GFP virus into Gfap-CreERT2 mice to enable Cre-dependent TRPA1 silencing selectively in astrocytes following tamoxifen induction. Immunohistochemistry confirmed reduced astrocytic TRPA1 expression in spinal cords of knockdown animals compared to scrambled controls (Supplementary Fig. 19a, b). To further validate antibody specificity, we confirmed that TRPA1 immunofluorescence was absent in global TRPA1 knockout mice (Supplementary Fig. S19c). Confocal imaging verified co-expression of GCaMP6s and mCherry in the dorsal horn, validating efficient and cell-type-specific targeting (Fig. 7a).
a Top: Schematic illustrating conditional astrocytic TRPA1 knockdown using Gfap-CreERT2 mice and ex vivo calcium imaging in spinal cord slices, created in BioRender; Sung, Y. (2025) https://BioRender.com/1rwu7p9. Bottom: Representative confocal images showing Cre-dependent mCherry expression (red) and astrocytic GCaMP6s (green) expression in the dorsal horn, confirming astrocyte-specific viral targeting. Scale bar: 100 μm. b Top: Calcium transients (ΔF/F₀) from spinal astrocytes from PCI mice expressing scrambled shRNA show robust responses to single-pulse ultrasound (100, 200, 400 ms; pink-shaded bars) and to LI-cTBUS (40 s). Bottom: Astrocytes from Trpa1 knockdown mice show no detectable calcium response to either stimulation paradigm. Traces represent averaged ΔF/F₀ from defined regions of interest (ROIs). c Pharmacological inhibition of TRPA1 with HC030031 in WT mice abolished LI-cTBUS-induced astrocytic calcium elevations. Data are presented as mean ± SEM box-and-whisker plots, n = 5 (LI-cTBUS), n = 7 (LI-cTBUS + HC030031), *p = 0.031, Welch’s t-test. d Behavioral assessment of mechanical withdrawal thresholds in astrocytic Trpa1 knockdown mice treated with LI-cTBUS. LI-cTBUS failed to elicit analgesic responses in knockdown animals, confirming the functional requirement of astrocytic TRPA1. Data are presented as mean ± SEM and box-and-whisker plots, *p = 0.04, N = 3, one-way ANOVA with Tukey’s multiple comparison test. N number of mice, n number of recorded cells.
Consistent with our findings that LI-cTBUS evokes TRPA1-dependent calcium signaling in astrocytes25,27, calcium imaging results confirm that both spinal neurons and astrocytes are intrinsically sensitive to ultrasound, exhibiting robust calcium transients in response to single-pulse and patterned LI-cTBUS stimulation (Supplementary Fig. 20). Calcium imaging in acute spinal cord slices from PCI mice revealed that astrocytes in the scrambled shRNA group exhibited robust calcium transients in response to single-pulse ultrasound (100–400 ms) and LI-cTBUS, whereas astrocytes from TRPA1 knockdown mice showed no calcium responses under either paradigm, despite intact GCaMP6s expression (Fig. 7b). These observations support a broader mechanosensitivity of dorsal horn circuits, suggesting that astrocytic TRPA1 is essential for ultrasound-induced calcium signaling.
Pharmacological inhibition of TRPA1 with HC030031 in WT mice also abolished LI-cTBUS-induced astrocytic calcium activity, confirming that TRPA1 is the mechanosensitive channel mediating astrocytic responses to ultrasound (Fig. 7c).
To determine whether these cellular responses translate into therapeutic effects, we next assessed the behavioral outcomes of LI-cTBUS in PCI mice with astrocyte-specific TRPA1 knockdown. Behavioral analysis revealed that LI-cTBUS elevated the 50% paw withdrawal threshold in PCI mice expressing scrambled shRNA, confirming effective analgesia. In contrast, LI-cTBUS failed to induce analgesia in mice with astrocytic TRPA1 knockdown (AAV-pSico-shTrpa1-GFP into Gfap-CreERT2 mice) (Fig. 7d). These findings indicate that although both neurons and astrocytes exhibit ultrasound-evoked calcium responses, astrocytic TRPA1 is essential for converting these mechanosensitive signals into behavioral analgesic outcomes.
Together, these results demonstrate that LI-cTBUS exerts its analgesic effects through TRPA1-dependent activation of spinal astrocytes and identify astrocytic mechanosensation as a critical cellular mechanism underlying ultrasound-based neuromodulation.
Discussion
In this study, we demonstrate that low-intensity continuous theta-burst ultrasound stimulation (LI-cTBUS; <3 W/cm²), which embeds 5 Hz theta bursts within 100–200 Hz high-gamma trains to mimic physiologically relevant theta–gamma coupling, produces robust and sustained analgesia in mouse models of neuropathic pain. LI-cTBUS outperformed both pregabalin and ultrasound-based burst spinal cord stimulation (USCS), lacking theta components in suppressing mechanical allodynia and restoring gait performance29,30,59. These effects were consistent across sexes and generalized to both partial sciatic nerve crush (PCI) and spared nerve injury (SNI) models, underscoring their strong translational potential.
Mechanistically, LI-cTBUS reversed key molecular features of maladaptive spinal plasticity associated with chronic pain. In the PCI model, neuropathic injury led to a substantial upregulation of spinal BDNF and a reduction in KCC2 protein levels, resulting in a depolarizing shift in the GABA reversal potential and subsequent disinhibition of nociceptive circuits19,20. Transcriptomic profiling (Supplementary Fig. 16) revealed changes that closely mirrored these protein-level alterations: the BDNF/TrkB pathway genes TrkB, also known as Ntrk2, and Akt3 were upregulated, while expression of the chloride-extrusion transporter gene Slc12a5 (Kcc2) was suppressed36,39. Furthermore, the activity-dependent transcription factor Egr4 was downregulated, whereas its repressor Sp3 was elevated, providing a plausible gene regulatory mechanism for the observed KCC2 downregulation. These gene-level changes were in strong agreement with the observed protein-level alterations.
Application of LI-cTBUS reversed these maladaptive changes at both transcriptional and protein levels. LI-cTBUS treatment downregulated TrkB, Akt3, and Sp3, while restoring Egr4 expression, leading to the recovery of KCC2 levels and normalization of chloride homeostasis. These effects were accompanied by a reduction in BDNF protein abundance, suppression of AMPAR-mediated excitatory transmission, and decreased c-Fos activity in dorsal horn neurons, consistent with reduced neuronal hyperexcitability60,61,62,63.
Astrocytes emerged as the primary mediators of these therapeutic effects. Recent single-cell studies and spatial transcriptomic profiling have shown that dorsal horn astrocytes segregate into distinct subpopulations under neuropathic pain, including pro-inflammatory states enriched for cytokines and more homeostatic subtypes maintaining ion and neurotransmitter balance64,65,66. Chronic mechanical allodynia induced by PCI was accompanied by astrogliosis, as confirmed both histologically and by transcriptomic signatures of reactive astrocytes, including elevated expression of Gfap, Timp1, Apoe, and B2m. LI-cTBUS significantly suppressed this reactive transcriptional profile while upregulating astrocyte-enriched genes involved in clathrin-mediated endocytosis and lysosomal degradation, such as Cltc and Atp6v0d1, consistent with enhanced uptake and processing of extracellular BDNF (Supplementary Fig. 16). Beyond gene expression, LI-cTBUS promoted structural restoration of astrocytes, including the suppression of GFAP hypertrophy, alleviation of endoplasmic reticulum and lysosomal stress, and a reduction in pathological GABA accumulation within astrocytes. While our study identifies astrocytic BDNF uptake as a major contributor to LI-cTBUS–induced analgesia, astrocytes can also regulate pain processing via diverse mechanisms such as cytokine release, glutamate clearance, ion buffering, and modulation of inhibitory neurotransmission39,43,67,68,69, which remain to be fully explored. In contrast, microglial activation and BDNF expression were observed primarily during the acute phase following injury and declined during the chronic stage, consistent with prior studies highlighting the temporal specificity of glial involvement in chronic pain36,39. These findings reinforce the view that astrocytes—but not microglia—sustain chronic pain and represent the primary glial targets of LI-cTBUS in the maintenance phase.
A key mechanistic insight from this study is the identification of astrocytic TRPA1 as a mechanosensitive transducer that links patterned ultrasound to intracellular calcium signaling and behavioral analgesia. While TRPA1 transcripts are rarely detected due to their low abundance, our functional assays demonstrate that astrocytic TRPA1 protein is nonetheless present and critically mediates the analgesic effects of LI-cTBUS. Through pharmacological inhibition, global knockout, and astrocyte-specific knockdown, we show that astrocytic—but not neuronal—TRPA1 is essential for LI-cTBUS-induced responses. Importantly, both the cellular context and temporal pattern of stimulation were critical: only low-intensity, theta–gamma patterned ultrasound selectively activated astrocytic TRPA1 and conferred analgesia, avoiding pronociceptive TRPA1 activation in peripheral sensory neurons25,27. In addition to its mechanosensory role, astrocytic TRPA1 also exerted protective effects under oxidative stress in vitro and likely facilitated the release of gliotransmitters such as glutamate and D-serine. These gliotransmitters may act on NMDA receptors to induce LTD-like synaptic depression, consistent with prior studies on theta-burst ultrasound-induced plasticity in cortical circuits15,70,71. Although our astrocyte-specific knockdown approach substantially reduced TRPA1 in GFAP-positive cells, potential low-level off-target recombination or indirect effects on neuronal TRPA1 are acknowledged and warrant further investigation in future studies.
Together, these findings establish a mechanistic framework in which theta–gamma patterned LI-cTBUS activates astrocytic TRPA1, enhances BDNF clearance, restores KCC2 expression, and reverses reactive astrogliosis, thereby rebalancing excitation and inhibition within spinal nociceptive circuits. The normalization of astrocytic ultrastructure and gene expression paralleled behavioral improvements, including alleviation of mechanical allodynia and restoration of gait symmetry, suggesting circuit-level repair in the injured spinal cord. Although the current study focused on astrocyte-mediated mechanisms during the chronic phase of neuropathic pain, we do not exclude the possibility that other cell types, including DRG neurons, interneurons, and microglia, may contribute to pain modulation, particularly during earlier stages of injury progression. By demonstrating that a non-invasive and pattern-specific neuromodulatory approach can durably reprogram spinal glial–neuronal interactions without detectable tissue damage, our study introduces LI-cTBUS as a promising therapeutic strategy for chronic pain and potentially other astrocyte-linked neurological disorders.
Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Institute of Basic Science (Approval number: IBS-2022-029). Mice were housed in groups of five, and a 12:12 h light/dark cycle (7 a.m.–7 p.m.) was maintained. Behavioral testing was conducted between 9 a.m. and 6 p.m., with 8–12-week-old mice. Colonies of C57BL/6J (RRID: IMSR_JAX:000664), BDNF KIV knockout (supported by Dr. Bai Lu, National Institutes of Health, 35 Lincoln Drive, Bethesda), TRPA1 knockout (KO; B6;129P-Trpa1tm1Kykw/J, RRID: IMSR_JAX:006401), TMEM119 (C57BL/6-Tmem119em1(cre/ERT2)Gfng/J, RRID:IMSR_JAX: 031820), GFAP-CreERT2 (B6.Cg-Tg(GFAP-cre/ERT2)505Fmv/J, RRID:IMSR_JAX:012849 mice were purchased from Jackson Laboratory.
Partial sciatic nerve injury model (PCI)
The partial crush injury (PCI) model was established in adult male and female C57BL/6J mice (8–10 weeks old), as described previously28. Under isoflurane anesthesia (3% induction, 1–2% maintenance), the right thigh was shaved, sterilized with iodine, and incised to expose the sciatic nerve. The nerve was carefully isolated from surrounding tissue using blunt dissection. A custom-calibrated ultra-fine hemostat (13020-12, Fine Science Tools), modified with a 30 µm spacer composed of two stacked 15 µm aluminum foils, was used to inflict a reproducible crush injury. The nerve was elevated with a fire-polished glass rod (10061-12, Fine Science Tools) and positioned within the hemostat jaws (2–3 mm from the tip), which were then closed to the first locking position and held for 15 s. After the injury, the nerve was released, and the wound was closed with sutures. Mice were allowed to recover under standard housing conditions. All surgical tools were autoclaved, and aseptic technique was strictly maintained throughout the procedure. Ultrasound stimulation was conducted on day 17 post-injury.
Spared nerve injured model (SNI)
The SNI model was performed as previously described by Decosterd and Woolf (2000). Briefly, adult male C57BL/6J mice (8–10 weeks old) were anesthetized with 2.5% isoflurane and placed in a prone position. A skin incision was made at the mid-thigh level to expose the sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerves. The common peroneal and tibial nerves were tightly ligated with 8-0 silk sutures (Surgipro*II, MV-135-5, 6 mm) and then transected distal to the ligation, removing a 2–3 mm segment to prevent regeneration. The sural nerve was left intact. The muscle and skin were then closed in layers with absorbable sutures. Postoperative care was provided until full recovery. Behavioral testing began after a 1-day recovery period. Ultrasound stimulation was conducted on day 6 until day 10 post-injury.
Ultrasound beam profile measurement
The acoustic beam profile of the H217 transducer (Sonic Concepts, Inc., USA) was measured by scanning the peak negative pressure at every 100 μm points with a motorized stage-mounted needle hydrophone (NH1000, Precision Acoustics, UK). For the measurement, a series of 500 kHz three-cycle bursts with an output intensity of 0.77 W/cm2 was transmitted through the mouse spine while acquiring the beam profile from 0.5 to 5.5 mm away from the spine, respectively.
Ultrasound stimulation patterns
The ultrasound-based spinal cord stimulation (USCS) protocol involved bursts of 500 Hz (5 pulses) with a 40 Hz (25 ms) train pattern, totaling 800 pulses. The ultrasound pulses used in this protocol had a center frequency of 500 kHz with continuous cycles over a 1 ms pulse duration. The continuous theta-burst ultrasound stimulation-200 Hz (cTBUS-200 Hz) consisted of bursts of 200 Hz (4 pulses) with a 5 Hz (theta) train pattern. Similarly, the cTBUS-100 Hz included bursts of 100 Hz (4 pulses) with a 5 Hz (theta) train pattern. Both cTBUS-100 Hz and 200 Hz protocols encompassed a total of 800 pulses. The ultrasound pulses used in these protocols had a center frequency of 500 kHz and were characterized by continuous cycles over a 3 ms pulse duration.
During each stimulation session in vivo, mice were anesthetized with 2% isoflurane and positioned securely in a stereotaxic frame to ensure consistent anatomical alignment. The ultrasound transducer was carefully placed over the lumbar-sacral region of the spinal cord, using the pelvic bone as a key external landmark to target the same spinal segment across all animals. LI-cTBUS stimulation (200 Hz bursts at a 5 Hz theta train) was delivered once daily for the specified experimental duration. To minimize the potential confounding effects of anesthesia on neural activity and behavior, all behavioral assessments were performed at least one hour following the cessation of anesthesia.
In vitro ultrasound stimulation for primary spinal astrocytes was used with a continuous theta-burst ultrasound protocol (cTBUS-200 Hz), consisting of 200 Hz bursts repeated at a 5 Hz train frequency. Ultrasound was delivered using an Olympus V301-SU transducer (500 kHz, single-element, 25 mm diameter), positioned directly above the culture slide within a sterile laminar flow hood. Two stimulation intensities were employed: 2.4 and 2.8 W/cm²—both below the in vivo therapeutic threshold (<3 W/cm²). Each stimulation session lasted 40 s and was administered once daily for three consecutive days. Immediately following stimulation, culture dishes were returned to a 37 °C, 5% CO₂ incubator to minimize environmental stress.
Von Frey hair test
Mechanical sensitivity was assessed between 09:00 and 18:00 in a temperature- and humidity-controlled room (22 ± 2 °C; 50 ± 10% RH). Mice were placed individually in transparent acrylic chambers (7 cm diameter × 20 cm height) positioned on a metal mesh platform with 5 × 5 mm grid openings (Jeung Do Bio & Plant Co.) and allowed to acclimate for at least 1 h prior to testing. Mechanical withdrawal thresholds (PWT) of the ipsilateral hind paw were determined using a set of calibrated von Frey filaments (Touch Test Sensory Evaluation Kit, Cat. No. 58011, Stoelting Co.; filament diameters: 2.44–4.31; forces: 0.04–2 g; North Coast Medical). The 50% paw withdrawal threshold was determined using the up–down method, starting with a 0.4 g filament. A brisk paw withdrawal, lift, or flinch was recorded as a positive response. Stimuli were applied sequentially: in the absence of a response, a filament of greater force was applied; upon a response, a filament of lower force was used. This sequence was continued until six responses surrounding the threshold were collected to calculate the 50% response threshold.
Next-generation sequencing
BCL files generated from the Illumina HiSeq2500 platform were converted to fastq format and demultiplexed according to index primer sequences. The data was then imported into Partek Genomics Suite (Flow ver. 10.0.21.0328; Copyright 2009, Partek, St. Louis, MO, USA) for further processing. Read quality for each sample was assessed using FastQC, and high-quality reads were aligned to the mouse genome assembly (mm10, Ensemble transcripts release 99). The aligned reads were normalized to the median ratio and quantified for analysis with DeSeq2. Differential analysis was performed using DeSeq2, and pathway analysis was conducted with reference to the KEGG database.
Catwalk gait analysis
The CatWalk XT version 10.6 (Noldus Information Technology, Netherlands) consists of a 1.3 m long black corridor on a glass plate with a green LED light inside, and is placed in a dark and silent room (<20 lx of illumination). Using the Illuminated Footprints technology, paws were captured by a high-speed video camera (100 frames per second) that was positioned underneath the glass. Mice walked freely across the runway into their home cage. The experiment was performed sheltered from noise in a darkened room with red light. The Catwalk XT 10.6 software package was used for data analysis. Prior to the experiment, each animal was trained over a two-day training period to become familiar with the task. A trial was regarded as successful if the animal did not show a maximum speed variation >60%, did not exceed a walking speed of 400 mm/s, and did not stop, turn around, or change direction. Three compliant runs made up the testing run. For analysis, gait parameters were automatically generated after each footprint being manually checked and, respectively, labeled Left Front (LF), Left Hind (LH), Right Front (RF), and Right Hind (RH) paws. As the PCI-induced alterations develop in an asymmetrical fashion, we analyzed parameters for the left and right sides together and used the RH/LH ratio for comparison. Gait analysis and footprint images were obtained using the CatWalk XT system.
In-vitro calcium imaging of spinal cord slices
To examine calcium excitability in spinal cord astrocytes and neurons, in vitro calcium imaging was performed using Airyscan-enhanced confocal microscopy. Male C57BL/6J mice received bilateral injections of AAV vectors targeting the dorsal horn of the lumbar spinal cord (segments L1–L3). In both control and PCI mice, dual-color calcium imaging was performed in acute spinal cord slices and evaluated responses to single-pulse ultrasound stimulations of varying durations (100, 200, 400 ms) as well as the patterned 40-s LI-cTBUS protocol. Each side was injected with 450 nL of AAV-GFAP104-GCaMP6s (8.09 × 10¹² GC/mL) for astrocytic calcium imaging and AAV-Syn-jReCo1a (8.09 × 10¹² GC/mL) for neuronal labeling. After 3–4 weeks, mice were sacrificed, and spinal cords were rapidly extracted and sectioned into 250 µm-thick transverse slices. Slices were recovered in oxygenated ACSF at 35 °C for 30 min before imaging.
For conditional knockdown of TrpA1 in astrocytes, B6.GFAP-CreERT2 mice underwent PCI surgery to establish a neuropathic pain model. Tamoxifen was administered to induce Cre recombinase activity, followed by retro-orbital injection of either AAV-GFAP104-TrpA1-shRNA or AAV-GFAP104-TrpA1-scramble RNA (serotype PHP.eB). AAV-GFAP104-GCaMP6s was concurrently injected into the dorsal horn to monitor astrocytic calcium activity.
Calcium imaging was performed using a Zeiss LSM 880 laser scanning confocal microscope equipped with an Airyscan detector (Carl Zeiss, Germany). Slices were imaged using a W Plan-Apochromat ×20/1.0 DIC water-immersion objective. Acquisition parameters were as follows: scan area = 290.5 µm × 290.5 µm, pixel size = 1.13 µm, pixel dwell time = 2.06 µs, and scan time = 158.25 ms/frame. Fluorescence was excited at 488 nm (GCaMP6s) and 543 nm (jReCo1a), with gain set to 800 and pinhole size at 13.47 Airy units for both channels. ZEN 2.3 SP1 software (Carl Zeiss) was used for image acquisition and processing. Data were analyzed using ImageJ (NIH) and MATLAB (MathWorks) with custom scripts. All data were visualized using GraphPad Prism 10 (GraphPad Software, San Diego, CA).
Electrophysiology: perforated and whole-cell patch clamp recording
Perforated and whole-cell patch clamp recordings were performed on transverse spinal cord slices (300 µm thick) from the dorsal horn region of wild-type (Control), PCI, and PCI + cTBUS-treated mice. Slices were recovered in oxygenated ACSF at 35 °C for 30 min prior to recording. ACSF consisted of (in mM): 124 NaCl, 5 KCl, 1.23 NaH₂PO₄, 26 NaHCO₃, 10 glucose, 1 MgCl₂, and 2 CaCl₂, continuously bubbled with 95% O₂/5% CO₂. Patch pipettes (3.5–5.5 MΩ) were pulled from borosilicate glass.
For perforated patch recordings to determine chloride reversal potential, the internal solution contained (in mM): 130 KMeSO₄, 5 CsCl, 2 MgCl₂, 1 EGTA, 1 CaCl₂, 10 HEPES, 4 MgATP, and 0.4 Na₂GTP (pH 7.25, 300 mOsm). Gramicidin B (Sigma-Aldrich) was prepared as a 20 mg/mL stock solution in DMSO and diluted to 300 µg/mL in internal solution immediately before use. Once stable access was established, GABA (100 µM, dissolved in ACSF) was pressure-applied through a second glass pipette using a Picospritzer (100 ms, 4 psi). To measure chloride reversal potentials, holding potentials were stepped from –100 to 30 mV in 5 mV increments, with GABA application occurring 300 ms after each voltage change.
For whole-cell recordings of spontaneous excitatory postsynaptic currents (sEPSCs), pipettes were filled with an internal solution containing (in mM): 120 CsMeSO₃, 8 NaCl, 5 MgCl₂, 10 HEPES, 1 EGTA, 0.001 QX-314, 4 MgATP, and 0.4 Na₂GTP (pH 7.25, 300 mOsm). Recordings were obtained from neurons in the dorsal horn voltage-clamped at –70 mV for 5 min, and sEPSCs were analyzed from the final 3 min of each trace. Cells with access resistance exceeding 30 MΩ or showing >25% variation during recording were excluded.
Signals were amplified using a MultiClamp 700B amplifier (Molecular Devices, Union City, CA), low-pass filtered at 1 kHz, and digitized at 10 kHz. Data were analyzed using Clampfit 11.4 (Molecular Devices), and cumulative probability distributions were generated in MATLAB (MathWorks). All data were analyzed and visualized using GraphPad Prism 10 (GraphPad Software, San Diego, CA).
Primary spinal astrocytes isolation, cell cultures and in vitro ultrasound stimulation
Primary cultured astrocytes were isolated from the spinal cord of C57BL/6J mouse pups at d0. Fine scissors were used to cut along the skin, and the pup's spine and spinal cord were collected with fine forceps and immediately put in cold Hank’s Balanced Salt Solution (HBSS). Tissues were evenly homogenized by pipetting, following a brief centrifugation to remove the HBSS. Homogenized tissues were resuspended in cell culture media contained 25 glucose, 4 L-glutamine, 1 sodium pyruvate (in mM), 10% heat-inactivated horse serum (#26050-088, GIBCO), 10% heat-inactivated fetal bovine serum (#10082-147, GIBCO) and 10,000 units/mL penicillin–streptomycin (#15140-122, GIBCO) before spreading into cell culture dishes. Cultures were maintained at 37 °C in a humidified 5% CO2 incubator. On 3 days in vitro (DIV), cells were vigorously washed with repeated pipetting, and the media was replaced to get rid of debris and other floating cell types. During maintenance of the culture before use, the media were replaced every 3–4 days. Cells were replated onto cover-glass coated with 0.1 mg/mL poly-D-lysine (PDL, #P6407, Sigma-Aldrich) for experiments.
In the in vivo experiments, we used the H217 transducer (0.5 MHz, focused single-element, effective diameter 33.0 mm × radius of curvature 63.2 mm) from Sonic Concepts, Inc., USA. The output intensity was set at 0.77 W/cm2. In contrast, for the in vitro cell culture experiments, we used the Olympus V301-SU transducer (0.5 MHz, single-element, effective diameter 25 mm) due to experimental constraints. Based on the low-intensity (<3 W/cm2) threshold observed for the analgesic effect in the in vivo study, we applied cTBUS-200 Hz stimulation using the Olympus transducer in the in vitro experiments. For this purpose, we selected a minimum stimulation value of 500 mVrms and a maximum stimulation value of 990 mVrms, corresponding to output intensities of 2.4 and 2.8 W/cm2, respectively. Notably, both of these stimulation intensities are lower than the 3 W/cm2 intensity observed in the in vivo experiments.
Primary spinal astrocyte cultures were treated with H₂O₂ to induce oxidative stress, followed by administration of the TRPA1 agonist (NMM, AITC, IJ100 at 10 and 50 μM) in acute time (5–10 min) or long-time (3–12 h) and a washout.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (PFA) at room temperature (RT) for 10 min and subsequently permeabilized with 0.03% Triton X-100 PBS with 2% donkey serum and 2% goat serum. After incubation in blocking solution for 1 h at RT, cells were incubated overnight at 4 °C with primary antibodies: GFAP (1:1000, Millipore AB5541), GABA (1:1000, Sigma-Aldrich AB175), and BDNF (1:1000, abcam ab108319). Cells were washed with PBS 3 times before staining with secondary antibodies (Goat Anti-Chicken Alexa Fluor® 488, Goat Anti-Rabbit Alexa Fluor®647, Donkey Anti-Genuine Pig Alexa Fluor® 594). Cells were washed with PBST 3 times, with the DAPI incubation in the last wash (1:1000, Thermo Scientific 66248). Cover glass was mounted with nail polish on a glass slide and stored at 4 °C overnight.
Immunohistochemistry
Mice were perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) through a peristaltic pump. Spinal cord tissue was immediately collected and incubated in 4% PFA overnight. The tissue was then sectioned at 30 µm into cold PBS using a vibratome (Leica VT1000S) and stored at 4 °C. For spinal cord immunohistochemistry, spinal sections were immunolabeled for GFAP, GABA, BDNF, Iba1 (1:1000, ab225260), NeuN (1:1000, Millipore, MAB377), c-Fos (1:1000, SYSY, 226-008), and TRPA1 (1:500, Alomone lab, ACC037; 1:250, Abcam, ab58844). Briefly, tissue was washed with PBS/0.3% Triton X-100 (PBST) for 4 times, blocked with PBST containing 5% normal goat serum for 2 h at RT, and then incubated overnight with primary antibodies at 4 °C. The next day, tissue was washed with PBST for 4 times followed by 2 h of secondary (Goat anti-Rabbit Alexa Fluor® 488, Goat anti-Mouse Alexa Fluor® 647, Goat Anti-Chicken Alexa Fluor® 647, 1:800; abcam) at RT. Tissue was then washed with PBST for 4 times before being mounted onto glass slides and covered with DAPI-containing mounting medium VECTASHIELD® (Vector Laboratories). Imaging for quantification of GFAP, Iba1, GABA, and BDNF expression was conducted at ×40 on Nikon Ti-e Eclipse with PFS microscopy and whole spinal cord section at ×20 on Axio Scan Z1.
Immunoblotting
Proteins were isolated and pooled from the spinal cord, homogenized with a pestle after freeze and thaw in Tissue Lysis/Extraction Reagent (Sigma-Aldrich). Next, SDS–PAGE and western blotting were performed. Thirty micrograms of proteins were loaded for each lane on a 4–12% Tris-glycine gel (Invitrogen), resolved, and transferred onto polyvinylidene difluoride membrane. On blocking 1 h at RT with 5% milk powder (AppliChem, Darmstadt, Germany) diluted in double distillate water, the blotted proteins were incubated overnight at 4 °C with goat polyclonal anti-BDNF antibody diluted 1:1000 (Abcam, ab60210), KCC2 at 1:1000 (Santa Cruz Biotechnology) and mouse monoclonal anti-GAPDH or β-Actin antibody diluted 1:10000 (Abcam, Cambridge, UK). Next, the membrane was incubated for 2 h at RT with the secondary antibodies ECL peroxidase-labeled anti-goat or mouse antibody (1:2000; Amersham Biosciences, Freiburg, Germany). Labeled proteins were detected by chemiluminescence using the ECL Prime Western Blotting Detection Reagents (Amersham Biosciences) on Image Lab software. Band intensities were analyzed using the open-source software ImageJ and calculated as expression relative to the housekeeping gene (GAPDH or β-Actin). Every lane was analyzed with the plot analysis tool of ImageJ, and the area of the peak corresponding to the band was used as the intensity value. Proteins of interest were probed on separate membranes run from the same experimental sample set; representative bands shown in the figures were selected from these membranes. All raw blot images are provided in the Source Data file.
RNA isolation and RT-PCR
Total RNA was isolated from the tissue samples using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA concentration and purity were assessed using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) with 1 μg of total RNA per reaction. PCR amplification was performed using specific primers for BDNF and GAPDH as an internal control. The sequences of the primers were as follows: BDNF exon 1 forward, 5’-GTG TGA CCT GAG CAG TGG GCA AAG GA-3’, BDNF exon 2 forward, 5’-GGA AGT GGA AGA AAC CGT CTA GAG CA-3’, BDNF exon 3 forward, 5’-GCT TTC TAT CAT CCC TCC CCG AGA GT-3’, BDNF exon 4 forward, 5’-CTC TGC CTA GAT CAA ATG GAG CTT C-3’, BDNF exon 5 forward, 5’-CTC TGT GTA GTT TCA TTG TGT GTT C-3’, BDNF exon 6 forward, 5’-GCT GGC TGT CGC ACG GTT CCC ATT-3’, BDNF exon 7 forward, 5’-CCT GAA AGG GTC TGC GGA ACT CCA-3’, BDNF exon 8 forward, 5’-GTG TGT GTC TCT GCG CCT CAG TGG A-3’, BDNF exon 9 forward, 5’-CCC AAA GCT GCT AAA GCG GGA GGA AG-3’. BDNF reverse, 5’-GAA GTG TAC AAG TCC GCG TCC TTA-3’, GAPDH forward, 5’-GGCTGTATTCCCCTCCATCG-3’, GAPDH reverse, 5’-CCAGTTGGTAACAATGCCATGT-3’. PCR products were visualized on 2% agarose gel stained with ethidium bromide. The relative expression of BDNF mRNA was calculated using the 2−ΔΔCt method. Data were expressed as mean ± SEM of three independent experiments.
FACs analysis
To assess astrocytic uptake of BDNF in vitro, HEK293T (ATCC CRL-3216, RRID: CVCL_0063) cells were transfected with a BDNF-GFP plasmid using the Expi293™ expression system (Thermo Fisher Scientific, Cat. A14635) following the manufacturer’s protocol. Conditioned media containing secreted BDNF-GFP were collected 48 h post-transfection, filtered, and applied to primary spinal astrocyte cultures 5 min prior to low-intensity continuous theta burst ultrasound stimulation (LI-cTBUS). Following stimulation, astrocytes were dissociated with 0.25% trypsin-EDTA and resuspended in ice-cold PBS for flow cytometric analysis (FACSCanto II, BD Biosciences).
For in vivo experiments, spinal cords were collected and enzymatically dissociated using the Adult Brain Dissociation Kit (Miltenyi Biotec, 130-107-677) according to the manufacturer’s instructions. Single-cell suspensions were filtered through a 70 µm mesh and processed immediately for staining. Astrocytes were labeled using APC-conjugated ACSA-2 antibody (1:100, Miltenyi Biotec, 130-116-245). For intracellular BDNF detection, cells were fixed and permeabilized (eBioscience™ IC Fixation and Permeabilization Buffer Set), stained with rabbit anti-BDNF antibody (1:500, Abcam, ab108319), followed by Alexa Fluor® 647-conjugated anti-rabbit secondary antibody (1:1000, Invitrogen). Flow cytometry data were acquired on a FACSCanto II and analyzed using FlowJo™ (BD Biosciences). Cell populations were first gated on forward and side scatter (FSC-A vs. SSC-A) to exclude debris. Single-cell populations were isolated using FSC-H vs. FSC-A gating to exclude doublets. Astrocytes were identified as ACSA-2-positive (APC-A+) and microglia as FITC-positive cells. BDNF-positive populations were detected based on PE-A-positive (Alexa Fluor 647 fluorescence) (Supplementary Fig. S14).
In in vitro assays, GFP fluorescence (FITC channel) was used to quantify BDNF-GFP uptake. Positive gating thresholds were established using fluorescence-minus-one (FMO) controls and unstained cells. (supplementary Fig. S15)
Intrathecal injection
The mice were anaesthetized under 3% of isoflurane for induction and 1–2% for maintenance. Intrathecal injection was performed using a winged infusion set connected to a 50 µL Hamilton syringe. BDNF (50 ng/kg) was injected into the intervertebral space in the lumbar region between L5 and L6 levels of the spinal cord. A reflexive flick of the tail was considered a sign of the accuracy of each injection. Behavioral testing was assessed by the Von-Frey test as described earlier.
Fiber photometry in spinal cord
Thy1_GCamp6f mice were fixed using stereotaxic (using multi-functional adapters #68091, RWD) and anesthetized with 2% isoflurane for a laminectomy. Briefly, an incision was made over the back, and the surrounding tissues and spinal bones were carefully dissected to expose the spinal cord. The mice were positioned on a 38 °C heat pad with a soft cushion for stability. For recording, an optical cannula was positioned on the spinal cord. The ultrasound transducer was positioned below the target area, and the anesthesia level was reduced from 2% to 0.5%. Calcium signals were confirmed prior to the application of cTBUS (200 Hz) stimulation. Light-emitting diodes at 488 nm (GCaMP stimulation wavelength) and 405 nm (control for artifactual fluorescence) (Doric) were coupled to a 400 μm 0.4 N.A. 0.22 optical fiber (ZF1.25, Doric). The emitted light was collected by the same optical fiber, passed through a GFP filter and focused onto a fluorescence detector (fluorescence mini cube, Doric) where the two output signals were separated based on modulation frequency. Samples were collected at a frequency of 330 Hz. Recordings were made in the lumbar sacral region during both ultrasound stimulation (40 s) and no stimulation (40 s).
Data were extracted and analyzed using custom-written scripts. To normalize the data, the control channel was fitted to and then subtracted from the raw trace, resulting in the calculation of ΔF/F.
Electron microscopy analysis
Ultrastructural analysis was performed using transmission electron microscopy (TEM). Astrocytes in each group were grown on coverslips. Cells were exposed to 400 µM hydrogen peroxide (H2O2) for 10 min, followed by a washout and ultrasound stimulation in vitro. One group then received cTBUS-200 Hz for 40 s once daily for 3 consecutive days (H2O2 + cTBUS group), while the other group remained untreated (H2O2-only group). The samples were immediately fixed with 1% glutaraldehyde–1% paraformaldehyde solution for 1 h and postfixed in 1% osmium tetroxide for 1 h at 4 °C. The block was stained with 1% uranyl acetate and dehydrated with a graded ethanol series. The samples were embedded in epoxy medium (EMS, Hatfield, PA) and sectioned into 60-nm sections using an ultramicrotome (Leica Microsystems, Wetzlar, Germany). Then, the sections were double-stained with UranyLess for 2 min and 3% lead citrate for 1 min. The stained sections were viewed on a Tecnai 20 TEM (Thermo Fisher Scientific, Waltham, MA, USA) at 120 kV, and images were captured using a US1000X-P camera 200. The acquired images were stitched together using Photomontage software (Thermo Fisher Scientific, Waltham, MA, USA). The area of the ER and the number of lysosomes were analyzed using ImageJ.
Kainic acid injection
Kainic acid (20 mg/kg) was intraperitoneally injected to induce BDNF elevation in wild-type mice. Mice were euthanized 6 h after the injection, and mPFC and spinal lumbar sacral samples were collected for Western blot experiments to validate the BDNF antibody (Supplementary Fig. 9).
Apoptosis assay
Mice were perfused for spinal cord extraction. Apoptosis cell assay was conducted using the TUNEL assay kit-HPR-DAB (ab206386). Briefly, the spinal cord tissues were fixed with 4% PFA and sliced into 10 µm sections. The tissues were then fixed and hydrated with 4% PFA, followed by permeabilization with Proteinase for 10 min. The tissues were then washed with 1X TBS and immersed in 30% H2O2 diluted in methanol for activating endogenous peroxidases. Tissues were then washed and covered with TdT equilibrium buffer, followed by labeling with the Labeling reaction mix and incubated at 37 °C for 90 min. Tissues were immersed in Stop buffer and blocked for 10 min at RT. Finally, tissues were developed using DAB solution for 15 min. Methyl Green counterstain solution was used for counterstaining, followed by washing with100% ethanol and xylene. The mounted samples were visualized at ×20 on Axio Scan Z1.
Thermal monitoring of spinal cord tissue
To confirm the absence of possible unintended neuromodulation or tissue damage, the temperature within the spinal cord tissue was measured during and after LI-cTBUS. The ultrasound transducer was positioned on top of the spinal bone, where the tip of the acoustic gel-filled beam collimator was placed on the bone surface for targeting the beam focus to the spinal cord tissue. A K-type stick thermocouple with an outer diameter of 1 mm was inserted into the center of the spinal cord tissue, where the tip of the thermocouple was horizontally aligned to the center of the beam collimator tip. For real-time temperature monitoring, the thermocouple was connected to a digital LCD temperature display unit (VX4, Hanyoung Nux), then to the PC through USB communication. To evaluate the temperature rise during and after LI-TBUS, baseline temperature was measured for 39 s following 586 s of recording, which included 40 s of ultrasound stimulation.
Statistical information
Statistical analysis was performed using the GraphPad Prism suite (GraphPad Software Inc., San Diego, CA). The sample size was determined based on significance testing, considering the available number of cells per in vitro experiment or mice from the same litters. Data are presented as mean ± SEM, or as box-and-whisker plots which show the median (center line), interquartile range (box), and minimum to maximum values (whiskers). N indicates the number of animals, and n indicates the number of independent experiments, cells, or tissue samples, as specified in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data are available in the main text, supplementary materials, and supplementary data. Source data are provided with this paper. The raw data generated in this study have been deposited in the Figshare database [https://doi.org/10.6084/m9.figshare.30518636]. RNA sequencing data are available in the NCBI Sequence Read Archive (SRA) under BioProject accession PRJNA1359771, accession number of publicly available sequencing datasets are provided in Supplementary Data 3. The microscope image data is too large to host on public repositories and will be provided upon request to the corresponding author. Source data are provided with this paper.
References
Baker, V. J. R.aK. G. A review of therapeutic ultrasound: effectiveness studies. Phys. Ther. 81, 1339–1350 (2001).
O’Brien, W. D. Jr. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 93, 212–255 (2007).
Prabhala, T. et al. External focused ultrasound treatment for neuropathic pain induced by common peroneal nerve injury. Neurosci. Lett. 684, 145–151 (2018).
Lin, H.-T. et al. Ultrasound therapy reduces persistent post-thoracotomy tactile allodynia and spinal substance P expression in rats. Reg. Anesth. Pain Med. 44, 604–608 (2019).
Hsieh, Y. L., Chen, H. Y., Yang, C. H. & Yang, C. C. Analgesic effects of transcutaneous ultrasound nerve stimulation in a rat model of oxaliplatin-induced mechanical hyperalgesia and cold allodynia. Ultrasound Med. Biol. 43, 1466–1475 (2017).
Ebadi, S., Ansari, N. N., Henschke, N., Naghdi, S. & van Tulder, M. W. The effect of continuous ultrasound on chronic low back pain: protocol of a randomized controlled trial. BMC Musculoskelet. Disord. 12, 59 (2011).
Jia, L., Wang, Y., Chen, J. & Chen, W. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci. Rep. 6, 35453 (2016).
Wu, Y. et al. Effects of therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis. Clin. Rehabil. 33, 1863–1875 (2019).
Tyler, W. J. et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 3, e3511 (2008).
Rezayat, E. & Toostani, I. G. A review on brain stimulation using low intensity focused ultrasound. Basic Clin. Neurosci. 7, 187–194 (2016).
Lin, W.-T., Chen, R.-C., Lu, W.-W., Liu, S.-H. & Yang, F.-Y. Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’s disease rat model. Sci. Rep. 5, 9671 (2015).
Larson, J., Wong, D. & Lynch, G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 368, 347–350 (1986).
Sheridan, G. K., Moeendarbary, E., Pickering, M., O’Connor, J. J. & Murphy, K. J. Theta-burst stimulation of hippocampal slices induces network-level calcium oscillations and activates analogous gene transcription to spatial learning. PLoS ONE 9, e100546 (2014).
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P. & Rothwell, J. C. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (2005).
Kim, J. et al. Long-lasting forms of plasticity through patterned ultrasound-induced brainwave entrainment. Sci. Adv. 10, eadk3198 (2024).
Thakkar, B. & Acevedo, E. O. BDNF as a biomarker for neuropathic pain: Consideration of mechanisms of action and associated measurement challenges. Brain Behav. e2903, https://doi.org/10.1002/brb3.2903 (2023).
Kerr, B. J. et al. Brain derived neurotrophic factor modulates nociceptive sensory input and NMDA evoked responds in the rat spinal cord. J. Neurosci. 19, 5138–5148 (1999).
Lu, V. B., Ballanyi, K., Colmers, W. F. & Smith, P. A. Neuron type-specific effects of brain-derived neurotrophic factor in rat superficial dorsal horn and their relevance to ‘central sensitization. J. Physiol. 584, 543–563 (2007).
Hildebrand, M. E. et al. Potentiation of synaptic GluN2B NMDAR currents by Fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 17, 2753–2765 (2016).
Coull, J. A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Eddleston, M. & Mucke, L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience 54, 15–36 (1993).
Faulkner, J. R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004).
Ohno, Y., Kinboshi, M. & Shimizu, S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19113313 (2018).
Bergami, M. et al. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J. Cell Biol. 183, 213–221 (2008).
Oh, S. J. et al. Ultrasonic neuromodulation via astrocytic TRPA1. Curr. Biol. 30, 948 (2020).
Duque, M. et al. Sonogenetic control of mammalian cells using exogenous transient receptor potential A1 channels. Nat. Commun. 13, https://doi.org/10.1038/s41467-022-28205-y (2022).
Lee, K. et al. Ultrasonocoverslip: in-vitro platform for high-throughput assay of cell type-specific neuromodulation with ultra-low-intensity ultrasound stimulation. Brain Stimul. 16, 1533–1548 (2023).
Davies, A. J. et al. Natural killer cells degenerate intact sensory afferents following nerve injury. Cell 176, 716–728 e718 (2019).
De Ridder, D., Vanneste, S., Plazier, M., van der Loo, E. & Menovsky, T. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery 66, 986–990 (2010).
Deer, T. R. et al. A systematic literature review of spine neurostimulation therapies for the treatment of pain. Pain Med. 21, 1421–1432 (2020).
Huang, Y. et al. Theta-burst direct electrical stimulation remodels human brain networks. Nat. Commun. 15, 6982 (2024).
Sweet, J. A., Eakin, K. C., Munyon, C. N. & Miller, J. P. Improved learning and memory with theta-burst stimulation of the fornix in rat model of traumatic brain injury. Hippocampus 24, 1592–1600 (2014).
Tsagareli, M. G. et al. Thermal hyperalgesia and mechanical allodynia elicited by histamine and non-histaminergic itch mediators: respective involvement of TRPV1 and TRPA1. Neuroscience 449, 35–45 (2020).
Li, W. W. et al. Roles of thermosensitive transient receptor channels TRPV1 and TRPM8 in paclitaxel-induced peripheral neuropathic pain. Int. J. Mol. Sci. 25, https://doi.org/10.3390/ijms25115813 (2024).
Wang, L. et al. IL-33/ST2 drives inflammatory pain via CCL2 signaling and activation of TRPV1 and TRPM8. Commun. Biol. 8, 724 (2025).
Zhou, W. et al. Driving effect of BDNF in the spinal dorsal horn on neuropathic pain. Neurosci. Lett. 756, 135965 (2021).
Garraway, S. M., Petruska, J. C. & Mendell, L. M. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur. J. Neurosci. 18, 2467–2476 (2003).
Huck, N. A. et al. Sex-distinct microglial activation and myeloid cell infiltration in the spinal cord after painful peripheral injury. Neurobiol. Pain. 12, 100106 (2022).
Ji, R. R., Donnelly, C. R. & Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 20, 667–685 (2019).
Phan, T. T., Jayathilake, N. J., Lee, K. P. & Park, J. M. BDNF/TrkB signaling inhibition suppresses astrogliosis and alleviates mechanical allodynia in a partial crush injury model. Exp. Neurobiol. 32, 343–353 (2023).
Ju, Y. H. et al. Tonic excitation by astrocytic GABA causes neuropathic pain by augmenting neuronal activity and glucose metabolism. Exp. Mol. Med. https://doi.org/10.1038/s12276-024-01232-z (2024).
Hou, L. et al. NADPH oxidase-derived H2O2 mediates the regulatory effects of microglia on astrogliosis in experimental models of Parkinson’s disease. Redox Biol. 12, 162–170 (2017).
Cheng, T., Xu, Z. & Ma, X. The role of astrocytes in neuropathic pain. Front. Mol. Neurosci. 15, 1007889 (2022).
Chun, H. et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H(2)O(2)(-) production. Nat. Neurosci. 23, 1555–1566 (2020).
Saha, R. N., Liu, X. & Pahan, K. Up-regulation of BDNF in Astrocytes by TNF-α: A Case for the neuroprotective role of cytokine. J. Neuroimmune Pharm. 1, 212–222 (2006).
Ding, H. et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflamm. 17, 19 (2020).
Gibon, J., Barker, P. A. & Seguela, P. Opposing presynaptic roles of BDNF and ProBDNF in the regulation of persistent activity in the entorhinal cortex. Mol. Brain 9, 23 (2016).
Colucci-D’Amato, L., Speranza, L. & Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21207777 (2020).
Ludwig, A. et al. Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J. Neurosci. 31, 644–649 (2011).
Formisano, L. et al. Sp3/REST/HDAC1/HDAC2 complex represses and Sp1/HIF-1/p300 complex activates ncx1 gene transcription, in brain ischemia and in ischemic brain preconditioning, by epigenetic mechanism. J. Neurosci. 35, 7332–7348 (2015).
Pertovaara, A. & Koivisto, A. TRPA1 ion channel in the spinal dorsal horn as a therapeutic target in central pain hypersensitivity and cutaneous neurogenic inflammation. Eur. J. Pharm. 666, 1–4 (2011).
Pinheiro Fde, V. et al. The involvement of the TRPA1 receptor in a mouse model of sympathetically maintained neuropathic pain. Eur. J. Pharm. 747, 105–113 (2015).
Souza Monteiro de Araujo, D., Nassini, R., Geppetti, P. & De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets 24, 997–1008 (2020).
Kakae, M., Nakajima, H., Tobori, S. & Kawashita, A. The astrocytic TRPA1 channel mediates an intrinsic protective response to vascular cognitive impairment via LIF production. Sci. Adv. 9, https://doi.org/10.1126/sciadv.adh0102 (2023).
Shigetomi, E., Tong, X., Kwan, K. Y., Corey, D. P. & Khakh, B. S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80 (2011).
Cheng, Y. T. et al. Social deprivation induces astrocytic TRPA1-GABA suppression of hippocampal circuits. Neuron 111, 1301–1315 e1305 (2023).
Prado, W. A. Involvement of calcium in pain and antinociception. Braz. J. Med. Biol. Res. 34, 449–461 (2001).
Takahashi, N. et al. Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell 33, 985–1003 e1007 (2018).
Freynhagen, R., Strojek, K., Griesing, T., Whalen, E. & Balkenohl, M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 115, 254–263 (2005).
Gagnon, M. et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 19, 1524–1528 (2013).
Coull, J. A. et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003).
Zhang, W., Liu, L. Y. & Xu, T. L. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience 152, 502–510 (2008).
Sugimoto, T., Ichikawa, H., Hijiya, H., Mitani, S. & Nakago, T. c-Fos expression by dorsal horn neurons chronically deafferented by peripheral nerve section in response to spared, somatotopically inappropriate nociceptive primary input. Brain Res. 621, 161–166 (1993).
Dong, F. L. et al. An atlas of neuropathic pain-associated molecular pathological characteristics in the mouse spinal cord. Commun. Biol. 8, 70 (2025).
Arokiaraj, C. M. et al. Spatial, transcriptomic, and epigenomic analyses link dorsal horn neurons to chronic pain genetic predisposition. Cell Rep. 43, https://doi.org/10.1016/j.celrep.2024.114876 (2024).
Xu, J., Yan, Z., Bang, S., Velmeshev, D. & Ji, R. R. GPR37L1 identifies spinal cord astrocytes and protects neuropathic pain after nerve injury. Neuron 113, 1206–1222 e1206 (2025).
Xu, Q. et al. Astrocyte contribute to pain gating in the spinal cord. Sci. Adv. 7, eabi6287 (2021).
Lee, H.-G., Lee, J.-H., Flausino, L. E. & Quintana, F. J. Neuroinflammation: An astrocyte perspective. Sci. Transl. Med. 15, eadi7828 (2023).
Ju, Y. H. et al. Tonic excitation by astrocytic GABA causes neuropathic pain by augmenting neuronal activity and glucose metabolism. Exp. Mol. Med. 56, 1193–1205 (2024).
Ni, Z., Gunraj, C., Kailey, P., Cash, R. F. & Chen, R. Heterosynaptic modulation of motor cortical plasticity in human. J. Neurosci. 34, 7314–7321 (2014).
Samuel, N. et al. Multi-modal investigation of transcranial ultrasound-induced neuroplasticity of the human motor cortex. Brain Stimul. 15, 1337–1347 (2022).
Acknowledgements
This work was supported by the Institute for Basic Science (IBS-R001-D2, to J.M.P.), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2024-00348893 to J.M.P.), and the Korea Brain Research Institute (KBRI) Basic Research Program funded by the Ministry of Science and ICT (25-BR-07-01). We thank Dr. Song-I Kim and Dr. Jung Moo Lee for providing the TMEM119 mouse line and TrpA1 conventional knockout mice. We also thank Jiwoon Lim for IMARIS 3D reconstruction assistance. We thank Dr. Bai Lu for the transgenic BDNF exon IV knock-out mice generation. We thank the Research Solution Center at the Institute of Basic Science for technical support.
Author information
Authors and Affiliations
Contributions
J.M.P. and T.T.P. developed the conception and wrote the original draft. T.T.P. designed, performed, and analyzed the behavior tests, operated the neuropathic pain model, performed immunohistochemistry with spinal cord and DGR samples, accessed protein and mRNA assays, apoptotic assay, in vivo cell culture studies, confocal imaging, in vivo pharmacological studies, IMARIS analysis, RNA-sequencing analysis, and ultrasonic amplitude and threshold measurement. S.S. designed, performed, and analyzed all electrophysiology experiments, ex vivo cell calcium imaging. H.J.K., N.J.J., and T.T.P. performed calcium imaging in vivo. T.T.P., D.W.K., and H.S. performed gait analysis. C.J.L. generated the TRPA1 knockout mice. H.P. generated the plasmid BDNF-GFP. K.L. and M.K. conducted experiments related to the physical characteristics of ultrasound. T.Y.K. and J.H.L. supported the FACs experiment and conditional knockdown mice. H.E.L. and J.Y.M. performed EM and analyzed data. T.T.P. and S.S. analyzed and visualized data. T.T.P., S.S., C.J.L., K.P.L., Y.J.K., J.P., H.W.K., and J.M.P. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Phan, T.T., Shin, S., Kim, H.J. et al. Harnessing theta-gamma coupled brainwaves using ultrasound for spinal astrocyte revitalization and sustained neuropathic pain relief in mice. Nat Commun 17, 273 (2026). https://doi.org/10.1038/s41467-025-66980-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-66980-6