Abstract
Obesity is a global public health crisis that is aggravated and mirrored by the high prevalence of physical inactivity. This single-center, assessor-blinded, three-group, randomized controlled trial in Hong Kong examined the therapeutic value of high-intensity interval training (HIIT) delivered in one session per week via a “weekend warrior” approach versus three sessions per week for reducing body adiposity in adults with overweight and central obesity. Participants were randomly assigned in a 1:1:1 ratio to once-weekly HIIT (H1), thrice-weekly HIIT (H3), and control (CON) groups (n = 105 per group). The interventions lasted for 16 weeks. The HIIT groups performed a total of 75 minutes of HIIT per week (either in one session or three sessions), while the control group received biweekly health education classes. Outcomes were assessed at baseline, week 16, and week 32. The primary outcome was the change in total body fat mass from baseline to week 16. Secondary outcomes were reported in the main text. Compared to CON at week 16, both HIIT groups showed decreased fat mass (adjusted mean differences: H1 vs. CON: −0.8 kg [95% CI: −1.4 to −0.2], P = 0.0107; H3 vs. CON: −1.0 kg [95% CI: −1.6 to −0.5], P = 0.0003). No study-related adverse events were reported. Here, we show that HIIT, performed once- or thrice-weekly, is safe and reduces fat mass in adults with overweight and central obesity. Trial Registration: ClinicalTrials.gov identifier: NCT04887454.
Similar content being viewed by others
Introduction
Obesity is a global epidemic. From 1990 to 2022, the global prevalence of obesity more than doubled in women and tripled in men, resulting in nearly 880 million adults living with obesity1. The excessive accumulation of body fat impairs health and is well-established as a gateway disease that precipitates and aggravates a host of non-communicable and communicable diseases2. Central obesity, the excess deposition of adipose tissue around the abdomen, is of particular concern, as it is strongly associated with increased health risks. Indeed, central obesity is linked to the development of an atherogenic pro-inflammatory environment that contributes to the pathogenesis of atherosclerosis and cardiovascular diseases (CVD) and is highly predictive of mortality3,4. In fact, a meta-analysis of over 2.5 million participants showed that for every 10 cm gain in waist circumference, there was an 11% greater risk of premature death4.
The management of obesity is multifaceted, with physical activity serving as a cornerstone of lifestyle modification5. In agreement, global obesity management guidelines endorse physical activity as an essential component of any weight reduction program6,7,8,9. These guidelines align their recommendation of physical activity with the guidelines of the World Health Organization (WHO)10, citing the widespread health benefits of exercise in people with obesity, which range from reducing adiposity to improving cardiovascular health and cardiorespiratory fitness (CRF)11,12. Of note, the robust improvements in CRF resulting from physical activity, which cannot be achieved through medication or dietary modification alone, attenuate the mortality risks associated with obesity irrespective of weight status13,14. However, despite the established and essential benefits of exercise, regular participation remains insufficient, especially among people with obesity15,16,17. In recent years, the growing acknowledgment of the physical inactivity public health challenge has highlighted the need to identify novel exercise strategies that enhance adherence whilst having comparable effectiveness to that of conventional exercise strategies to aid in the comprehensive care of obesity. Accordingly, exercise strategies that attenuate time commitment play a critical role in tackling the obesity epidemic, given that from a public health perspective, lack of time is one of the primary barriers hindering exercise adherence18.
Two exercise strategies that hold promise for alleviating obesity and circumventing time-related barriers are high-intensity interval training (HIIT) and the “weekend warrior” exercise pattern. The application of HIIT as a therapeutic exercise strategy to reduce total body and visceral adiposity is well substantiated19. Indeed, studies have reported that HIIT achieves comparable reductions in body fat mass as moderate-intensity continuous training, but in less time20. Likewise, the weekend warrior exercise pattern is a convenient exercise strategy as it condenses a person’s weekly exercise volume into 1 to 2 days. This attenuates time commitment and increases feasibility as a result of reduced commute time to the exercise facility, and allows for greater flexibility in choosing which day to exercise. Moreover, this exercise pattern has substantial practical and clinical value for people with obesity, as findings from epidemiological research point towards its efficacious effects on reducing the relative risk of all-cause mortality, CVD mortality, and even adiposity21,22,23,24. Intriguingly, no randomized controlled trials have rigorously explored the combination of this therapeutic exercise modality and convenient exercise pattern on obesity, namely HIIT in a weekend warrior exercise pattern. Therefore, rigorous and robust evidence on the practical and clinical value of HIIT administered in a weekend warrior exercise pattern in adults with central obesity is lacking.
Here, we compare the effects of 16 weeks of duration-matched once-weekly and thrice-weekly HIIT with health education on total body adiposity in inactive adults with overweight and central obesity, and hypothesize that 16 weeks of duration-matched once-weekly HIIT and thrice-weekly HIIT in inactive adults with overweight and central obesity would reduce total body adiposity compared to the health education control group. In this study, once- and thrice-weekly HIIT demonstrated reductions in adiposity, together with improvements in cardiorespiratory fitness. Undertaking HIIT in a weekend warrior exercise pattern can mitigate time-related barriers and is a practical alternative to thrice-weekly HIIT.
Results
Baseline characteristics of participants
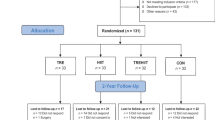
After assessing 495 responding individuals for eligibility, 315 participants were enrolled in the trial and randomized in a ratio of 1:1:1 to the control (CON), once-weekly HIIT (H1), and thrice-weekly HIIT (H3) groups (Fig. 1). Among the 315 participants, 204 (64.8%) were female, mean age was 48.2 (12.5) years, mean body mass index (BMI) was 28.7 (3.8) kg/m2, and mean waist circumference was 94.8 (9.3) cm. The baseline characteristics of the participants were comparable across the groups (Table 1). Participants completed the 16-week follow-up assessments in 8.6 (7.6) days after the last intervention session (Supplementary Table 2). A total of 297 (94%) participants completed the 16-week follow-up, and 18 participants were lost to follow-up at week 16, and 15 were lost to follow-up at week 32. Similar rates of dropout were observed across the groups.
Primary outcome
The results for the primary and secondary outcomes are presented in Table 2. At week 16, the H1 and H3 groups showed statistically significant reductions in total body fat mass compared to the CON group (H1 vs. CON: adjusted mean differences, −0.8 kg [95% CI: −1.4 to −0.2], P = 0.0107; H3 vs. CON: adjusted mean differences, −1.0 kg [95% CI: −1.6 to −0.5], P = 0.0003), with no significant differences between the HIIT groups (H3 vs. H1: adjusted mean differences, −0.3 kg [95% CI: −0.8 to 0.3], P = 0.3586). The relative changes in total body fat mass from baseline to week 16 are presented in Table 2. At week 16, the H1 and H3 groups exhibited a relative change of −2.6% and −3.5% in fat mass, respectively. In the CON group, fat mass remained stable compared to baseline (relative change: +0.2%).
Secondary outcomes
At week 16, both the H1 and H3 groups showed statistically significant reductions in total body fat percentage and waist circumference compared to the CON group, with no significant differences between the HIIT groups (Table 2). Modest improvements in body weight and BMI were observed in the HIIT groups at week 16. However, only the H3 group showed statistically significant reductions in both outcomes compared to the CON group. The H1 and H3 groups also showed statistically significant increases in CRF compared to the CON group at week 16, with no significant differences found between the HIIT groups. At week 32, both the H1 and H3 groups showed statistically significant reductions in waist circumference compared to the CON group. The H3 group also showed statistically significant increases in CRF compared to the CON group. There were no significant differences in total body fat mass, total body fat percentage, body weight, and BMI between groups at week 32. Total body lean mass, blood pressure (systolic and diastolic), and blood biomarkers (fasting plasma glucose [FPG], total cholesterol, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglycerides) remained relatively stable for all groups throughout the study, with no significant differences found between the groups at any timepoint.
Sensitivity analyses
To assess the robustness of the results, sensitivity analyses were conducted for all outcomes by adjusting for sex, age, BMI, socioeconomic status, attendance rate, habitual physical activity (energy expenditure), and diet (energy intake) in the statistical model. The results of the sensitivity analyses were consistent with those from the primary analyses (Table 3), with the exception that the improvement in CRF in the H3 group was statistically significant when compared to the H1 group at week 16. Further, the results remained consistent with the primary analyses when including the number of days after the last intervention session to complete the assessments at week 16 as an additional covariate (Supplementary Table 3).
Intervention fidelity
Overall, attendance and adherence were high (Supplementary Table 4). The mean attendance rates in the CON, H1, and H3 groups were 88%, 86%, and 83%, respectively. According to the pre-specified attendance criteria, 93 (89%), 90 (86%), and 85 (81%) participants in the CON, H1, and H3 groups met the criteria of attending 70% or more of the prescribed sessions, respectively. Moreover, 74% and 79% of participants in the H1 and H3 groups adhered to the intensity criteria, and 98% and 100% of participants in the H1 and H3 groups adhered to the duration criteria, respectively. Energy expenditure, expressed as weekly metabolic equivalents of task in minutes (MET-min/wk) and estimated based on the participant’s oxygen consumption–heart rate relationship at the baseline exercise test, was 488 MET-min/wk and 480 MET-min/wk for the H1 and H3 groups, respectively (Supplementary Table 5)25. The mean treadmill speed and incline at each week for the H1 and H3 groups are described in Supplementary Table 6. Compliance with maintaining habitual physical activity levels and diet was similar among the CON, H1, and H3 groups before and after the intervention (Supplementary Table 7).
Adverse events
There were no study-related adverse events (serious and non-serious). Adverse events unrelated to the study are reported in Supplementary Table 8.
Discussion
Herein, we report results from a prospective, randomized controlled trial that investigated the effects of duration-matched HIIT performed once-weekly and thrice-weekly on adiposity in adults with central obesity. Our findings show that HIIT performed in a once-weekly and thrice-weekly frequency reduces fat mass, fat percentage, and waist circumference, and improves cardiorespiratory fitness. However, blood pressure and blood biomarkers were not modified by the HIIT interventions. The retention at post-intervention was 94% and the average exercise attendance was 85% (H1: 86%; H3: 83%). No study-related adverse events were reported. Collectively, our findings provide evidence-based data that HIIT, performed either once-weekly or thrice-weekly, is safe, feasible, and effective for reducing body fat and improving CRF, both of which are integral to the comprehensive management of obesity. These findings further inform obesity management guidelines, healthcare professionals, and stakeholders on the practical and therapeutic value of performing HIIT in a weekend warrior (once-weekly) exercise pattern in adults with central obesity, and are especially relevant to individuals with time constraints.
Obesity, by convention, is classified using BMI, a measure of body weight relative to height. However, the adverse health consequences associated with overweight and obesity primarily stem from excess adipose tissue26, and not excess body weight. Indeed, excess adipose tissue deposited subcutaneously or centrally at the abdominal region is highly associated with health complications27,28, and is a more clinically meaningful marker of obesity29. In an analysis of over 6000 adults from the NHANES-III, normal-weight adults in the highest tertile of body fat (>23% for men; >33% for women) were found to have a four-fold higher prevalence of metabolic syndrome, with women in the highest tertile being twice as likely to die at follow-up as women in the lowest tertile27. Therapies that can effectively ameliorate adiposity are therefore pivotal for managing obesity.
In the present study, the H1 and H3 groups decreased fat mass by −0.8 kg (95% CI: −1.4 to −0.2) and −1.0 kg (95% CI: −1.6 to −0.5), and fat percentage by −0.8% (95% CI: −1.3 to −0.2) and −0.9% (95% CI: −1.4 to −0.3), respectively. Relative to baseline, both HIIT frequencies produced a modest reduction in fat mass, with the H1 and H3 groups losing −2.6% and −3.5%, respectively (Table 2). These results are consistent with the literature30,31, and consolidate HIIT as a therapeutic component to manage excess adiposity. There is, however, no consensus on what constitutes a clinically relevant reduction in fat. Referring to a population-based cohort study that demonstrated a dose-response relationship between reductions in the fat mass index and CVD risk32, we may infer that the HIIT-induced modest reductions in fat mass (absolute mean change of –0.3 kg/m2 and –0.5 kg/m2 in the H1 and H3 groups, respectively) can lead to CVD risk reductions in adults with central obesity. With regards to the HIIT frequencies, the H3 group had a greater absolute mean change in fat mass compared to the H1 group (mean change of –1.3 kg and –0.7 kg in the H3 and H1 groups, respectively), which may suggest that exercising three times a week can be more beneficial. However, our study was not powered to detect the differences between the H1 and H3 groups. Nevertheless, as both the H1 and H3 groups decreased fat mass compared to the control group at week 16 (post-intervention), the results of this study portray that HIIT performed in a once-weekly exercise pattern can be a practical and appealing alternative to exercising thrice-weekly. Indeed, participants’ weekly commute time to our center plus time spent exercising was around 4.5 h per week for the H1 group and 8.2 h per week for the H3 group (Supplementary Table 9). Correspondingly, the practicality of once-weekly HIIT in our study was largely due to a reduced commute time, which is especially relevant to individuals with many time commitments, demanding weekly schedules, or those with long commutes to exercise facilities, which prevent them from allocating multiple days per week to exercise. However, the feasibility of once-weekly HIIT may vary in other settings and depends on intersectional factors, such as employment status, marital status, socioeconomic status, transport infrastructure, and/or other contextual factors specific to different countries, cities, and cultures, which affect the daily lives of individuals and consequently their willingness to commute to a fitness center. Conversely, performing HIIT in a thrice-weekly pattern also holds practical implications for individuals who are more deconditioned or whose co-existing medical conditions (e.g., individuals with diabetes who have a risk of exercise-induced hypoglycemia) contraindicate them from completing longer exercise durations per session. This is particularly relevant for individuals with adiposity-related metabolic complications, such as insulin resistance and type 2 diabetes, as a higher exercise frequency may be preferred to achieve continuous residual effects of the last exercise bout performed33,34. However, our study did not observe such an effect for fasting plasma glucose, likely due to participants having relatively normal levels at baseline. Thus, depending on the individual’s availability, functional capacity, and health condition, stakeholders may consider performing HIIT either once or three times a week to reduce adiposity. Of note, although the reductions in fat are not as marked as those achieved by dietary and pharmacological approaches, the HIIT interventions offer important health benefits that cannot be achieved by drug or diet alone and should not be overlooked, such as CRF improvements34.
In this study, significant improvements in CRF and waist circumference were observed in the HIIT groups when compared to the control group at week 16 (post-intervention). These improvements are important, as both CRF and waist circumference are independent predictors of all-cause and CVD mortality28,35. In fact, modest improvements in CRF and waist circumference are meaningful, as dose-response relations with all-cause mortality have been established for both4,36. Furthermore, in patients with obesity, better CRF is linked to substantially less disability37, with a 10% improvement in CRF considered clinically relevant and linked to significantly lower risks of developing adverse health outcomes, CVDs, and mortality35. Notably, in the H1 and H3 groups, there was a mean increase of ~6% and ~11% in CRF, respectively. Indeed, although our study was not powered to detect the differences between the H1 and H3 groups, in the adjusted analysis, the H3 group had a significantly greater increase in CRF than the H1 group at post-intervention (week 16). This implies that undertaking HIIT thrice a week can offer more robust improvements in CRF, and may confer more holistic health benefits when accounting for other key factors, such as demographics, habitual physical activity, and attendance. Other investigated cardiometabolic parameters, including blood pressure, FPG, total cholesterol, HDL-C, LDL-C, and triglycerides, did not change (Tables 2 and 3). This is likely due to participants being in the relatively healthy range to begin with, thus resulting in a ceiling effect.
This study has limitations that need to be considered. First, whether a modest reduction in fat mass induced by the HIIT interventions results in clinically important health benefits (e.g., reduced risk of all-cause mortality) in patients with obesity in the long term is unknown and warrants investigation. Second, despite the use of standardized and objective measures to monitor habitual diet (3-day food diary with weighted measurements) and physical activity (tri-axial accelerometer) at each assessment time point, we were unable to monitor the daily fluctuations in diet and physical activity during the time between each assessment time point, as it would place undue burden on the participants. Moreover, while we objectively measured diet and physical activity, we cannot rule out the possibility of bias caused by participants’ knowledge of being monitored in a research setting, which may have influenced their usual eating and activity habits. Nonetheless, objective monitoring of their habitual diet and physical activity illustrates that the change between baseline and post-intervention (week 16) did not exceed 3% for energy intake and 10% for energy expenditure for all three groups. Third, due to the intermittent nature of HIIT, exercise duration (75 min a week) was used to equalize the dose between HIIT groups instead of energy expenditure. This also allows for more direct public health implications, as the HIIT prescription aligns with the WHO recommendation of 75 min of vigorous-intensity physical activity per week (450 MET-min/week). In this study, a HIIT duration of 75 min a week had an average exercise intensity of 6 METs (vigorous-intensity) and a weekly MET-min that meets the WHO recommendations (Supplementary Table 5). Fourth, given the participants’ relatively healthy levels of glucose, lipids, and blood pressure at inclusion, the results may not be generalizable to individuals with adiposity-related metabolic complications, such as individuals with type 2 diabetes. Fifth, the generalizability of the trial results is limited to the population group investigated, that is, Chinese adults. Further research is needed to validate the effects of the HIIT frequencies in more ethnically and metabolically diverse samples.
This study shows that undertaking HIIT once or thrice-weekly is safe and beneficial for reducing body fat and improving CRF in inactive adults with central obesity. Furthermore, implementing HIIT in a weekend warrior (once-weekly) exercise pattern can enhance practicality and offer convenience, thereby potentially serving as a favorable public health strategy to aid in the comprehensive management of obesity.
Methods
Study design
This single-center, assessor-blinded, three-group, parallel-group randomized controlled trial (ClinicalTrials.gov ID: NCT04887454) is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement and the multi-arm trials extension38,39. Participant enrolment began on September 1, 2021, and was concluded on November 23, 2023. Data collection was subsequently completed on September 7, 2024. The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (reference number: UW20-066). The study protocol has been previously published40.
Participants
Participants were recruited through a poster and leaflet mailing campaign conducted in the local community. Participants were eligible if they were ethnic Chinese adults aged 18 years or older who were overweight (BMI ≥23) and centrally obese (waist circumference ≥90 cm for men and ≥80 cm for women [sex was self-reported and verified against their Hong Kong Identity Card])41. Major exclusion criteria included any absolute or relative contraindications to exercise testing42, medical or somatic conditions that preclude brisk walking, regular exercise training in the past 3 months, or weight loss interventions initiated in the past 6 months. Written informed consent was provided by all participants. Participants did not receive monetary compensation for participating in this study, but the health benefits and the chance to receive free health assessments were proposed to incentivize enrollment.
Randomization
After baseline assessments, participants were randomly assigned in a 1:1:1 ratio to the H1, H3, or CON groups by the blinded study statistician using block randomization with randomly varied block sizes between 6 and 12. The computer-generated randomization sequence was prepared by the study statistician and kept in sealed opaque envelopes. The study statistician was not involved in the recruitment of participants, the provision of the interventions, or outcome assessments.
Interventions
Participants randomized to the CON group attended a 150-min health education class in a group setting every 2 weeks for 16 weeks. The contents of the health education program were based on publicly available obesity-related health information from the Hong Kong SAR Government. The attention received by the CON group was time-matched with the HIIT bouts.
Participants randomized to the HIIT groups were prescribed 12 high-intensity intervals per week on a motorized treadmill in a group setting for 16 weeks. The first 4 weeks were used as the familiarization phase, whereby participants progressively increased their weekly duration of HIIT, with the goal of achieving 50% of the prescribed weekly duration by the second week and 100% by the fourth week. To further facilitate adaptation to the HIIT and to minimize the risk of injury, participants completed a structured protocol during the familiarization phase, which involved lower-body muscle-strengthening exercises (e.g., bodyweight squats, bodyweight lunges, and lateral band walks) and stretching of major lower-body muscle groups. Following the 4-week familiarization phase, participants then performed 12 high-intensity intervals weekly for the remainder of the intervention. The total HIIT duration of 75 min a week was matched between H1 and H3 groups in one and three training sessions, respectively. The H3 group performed the HIIT bouts in three sessions. Each session comprised 5 min of warm-up at an intensity of 50–60% peak heart rate (HRpeak), a 25-min HIIT bout, and 5 min of cool-down at 50–60% HRpeak. The 25-min HIIT bout consisted of four 4-min high-intensity intervals at 85–95% HRpeak interspersed with 3-min active recovery intervals at 50–70% HRpeak on a motorized treadmill supervised by a certified trainer. The H1 group performed the 25-min HIIT bout three times in one session, with breaks lasting 15 to 30 min in between each HIIT bout. Warm-up and cool-down periods were as above. Exercise intensity was individually determined using a maximal cardiopulmonary exercise test at baseline and was continuously monitored during the training sessions using a Polar A300 monitor with an OH1 heart rate sensor. Treadmill speed and incline were also individually determined to enable participants to exercise appropriately at the target intensity, with guidance from the trainers to prioritize adjusting one variable at a time (typically speed first, then incline if needed) to reach the target heart rate. Participants were not provided the intervention or access to the exercise equipment during the follow-up period after the 16-week intervention.
Participants’ attendance to their randomized condition and adherence to their prescribed exercise intensity and duration were recorded. All participants were instructed to maintain their usual physical activity and dietary habits during the intervention period.
Outcome measures
All participants were assessed at baseline, after the completion of the intervention (week 16), and at the 4-month post-intervention follow-up (week 32). Outcome assessments were performed according to standardized procedures. Trained research personnel conducting the assessments were blinded to the treatment allocation. Participants were instructed not to disclose their group assignment to the blinded assessors during the outcome assessments. Intervention providers, however, were not blinded to group assignments due to the nature of exercise interventions.
Primary outcome
The pre-specified primary outcome was the change in total body fat mass from baseline to 16 weeks. Body fat mass was measured using a whole-body dual-energy X-ray absorptiometry (DXA) scanner (Horizon A, Hologic Inc.) with a standardized preparation and scanning procedure. Participants wore minimal and loose-fitting clothing with no metallic materials and were instructed to fast for 4 h before the scan, but were allowed to drink water and take their usual medication. Immediately before the measurement, participants were asked to empty their bladder and remove any external objects. Participants were positioned on the DXA scanner according to the recommended positioning and alignment of the National Health and Nutrition Examination Survey (NHANES) method43.
Secondary outcomes
The pre-specified secondary outcomes were the changes in total body fat mass from baseline to 32 weeks, and the changes in total body fat percentage (measured using a DXA scanner), total body lean mass (measured using a DXA scanner), body weight (measured using a calibrated electronic weighing scale [UC-321, A&D Medical]), BMI, waist circumference (measured using an inelastic measuring tape [seca 201, seca]), blood pressure (systolic and diastolic, measured using a digital blood pressure monitor [HEM-907, Omron]), blood biomarkers (FPG, total cholesterol, HDL-C, LDL-C, and triglycerides), and CRF (measured using a gas analysis system [Quark CPET, COSMED]) from baseline to 16 and 32 weeks.
Daily physical activity energy expenditure and dietary intake were monitored at baseline, 16 weeks, and 32 weeks as potential confounding factors for the primary outcome of total body fat mass, but were not investigated as efficacy endpoints. Daily physical activity energy expenditure was measured at each assessment time point using a three-axis accelerometer (GT9X Link, Ametris, USA) worn on the non-dominant wrist for 24 h for 7 consecutive days. Daily dietary intake was assessed using a 3-day food diary with weighted measurements.
Statistical analyses
All statistical analyses were performed using SAS OnDemand for Academics (SAS Institute). Statistical significance was set at P < 0.05 for two-sided tests.
Sample size
Sample size estimation was based on our previous preliminary work44, in which the 8-week thrice-weekly HIIT intervention significantly reduced total body fat mass compared to the control group with a between-group effect size of 0.6 (Cohen’s d). A sample size of 84 participants per group was necessary to achieve the stringent criterion of 90% statistical power (α = 0.01) with a moderate effect size of 0.6. Accounting for a 20% attrition rate, a total of 315 participants were required.
Analytical approach
Statistical analyses were conducted by the blinded statistician, with double data entry and verification performed by two independent research personnel to ensure data quality. Data were summarized as mean (SD). The intention-to-treat principle was followed, we included all randomized participants as randomized in the analyses. Multiple imputation using the fully conditional specification method was employed to handle missing values with 25 imputations imputed45. The available primary and secondary outcome data were used to impute for the missing values46. The imputed datasets were pooled using Rubin’s rule45. Data from outcome measures were analyzed by the generalized estimating equations (GEE) using group (3 levels) as the main effect and baseline measurements as a covariate at week 16 and week 32, respectively. Between-group pairwise comparisons (H1 vs. CON, H3 vs. CON, H3 vs. H1) were performed using linear contrasts. The Holm-Bonferroni correction was applied only to the between-group pairwise comparisons of the primary outcome to adjust for multiplicity47. To conduct the Holm-Bonferroni procedure, we first obtained the raw P values for the 3 pairwise comparisons (H1 vs. CON, H3 vs. CON, H3 vs. H1) for fat mass at week 16. These were then ranked from smallest to largest, with their significance examined sequentially. Specifically, the smallest P value was determined to be significant if it was smaller than (0.05 ÷ 3). If it was significant, the second-smallest P value was determined to be significant if it was smaller than (0.05 ÷ 2), and so on. If a P value was insignificant, the procedure was stopped, and the rest of the P values were considered insignificant. Sensitivity analyses for all outcomes were performed by including the following variables: sex, age, BMI, socioeconomic status, attendance, physical activity, and dietary intake as additional covariates in the GEE model.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The de-identified participant data generated in this study have not been publicly deposited to ensure participant confidentiality. The de-identified data, including study protocol and statistical analysis plan, are available under restricted access for privacy reasons and will be shared beginning 3 months and ending 5 years after publication of this article. Data will be shared with researchers who provide a methodologically sound proposal with achievable aims. Proposals should be directed to pmsiu@hku.hk, and those requesting access to the data will need to sign a data access agreement.
Code availability
Analyses were conducted on SAS OnDemand for Academics (SAS Institute, Engine V9) using standard syntax. No custom code was generated for the present analyses.
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Busetto, L. et al. A new framework for the diagnosis, staging and management of obesity in adults. Nat. Med. 30, 2395–2399 (2024).
Després, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887 (2006).
Jayedi, A. et al. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 370, m3324 (2020).
Bray, G. A., Frühbeck, G., Ryan, D. H. & Wilding, J. P. Management of obesity. Lancet 387, 1947–1956 (2016).
Jensen, M. D. et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 129, S102–S138 (2014).
Yumuk, V. et al. European guidelines for obesity management in adults. Obes. Facts 8, 402–424 (2015).
Garvey, W. T. et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 22, 1–203 (2016).
Lau, D. C. et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 176, S1–S13 (2007).
World Health Organization. WHO Guidelines on Physical Activity And Sedentary Behavior (World Health Organization, 2020).
Recchia, F. et al. Dose-response effects of exercise and caloric restriction on visceral adiposity in overweight and obese adults: a systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 57, 1035–1041 (2023).
Myers, J. Cardiology patient pages. Exercise and cardiovascular health. Circulation 107, e2–e5 (2003).
Weeldreyer, N. R. et al. Cardiorespiratory fitness, body mass index and mortality: a systematic review and meta-analysis. Br. J. Sports Med. 59, 339–346 (2025).
Jobanputra, R. et al. The effects of weight-lowering pharmacotherapies on physical activity, function and fitness: a systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 24, e13553 (2023).
World Health Organization. Global Status Report on Physical Activity 2022 (World Health Organization, 2022).
Silveira, E. A. et al. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: a systematic review and meta-analysis. Clin. Nutr. 50, 63–73 (2022).
López-Gil, J. F., Calatayud, J. & López-Bueno, R. Trends in adherence to physical activity guidelines from 1997 to 2018 among adults with obesity: an analysis from the US National Health Interview Survey. Obes. Rev. 26, e13866 (2025).
Lee, D. C. et al. Aerobic, resistance, or combined exercise training and cardiovascular risk profile in overweight or obese adults: the CardioRACE trial. Eur. Heart J. 45, 1127–1142 (2024).
Maillard, F., Pereira, B. & Boisseau, N. Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Med. 48, 269–288 (2018).
Wewege, M., van den Berg, R., Ward, R. E. & Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes. Rev. 18, 635–646 (2017).
O’Donovan, G., Lee, I. M., Hamer, M. & Stamatakis, E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern. Med. 177, 335–342 (2017).
Lei, L. et al. The associations of “weekend warrior” and regularly active physical activity with abdominal and general adiposity in US adults. Obesity 32, 822–833 (2024).
Kany, S. et al. “Weekend warrior” physical activity and adipose tissue deposition. JACC Adv. 4, 101603 (2025).
Hui, S. S. et al. Association of ‘weekend warrior’ and other leisure time physical activity patterns with obesity and adiposity: a cross-sectional study. Diabetes Obes. Metab. 27, 482–489 (2025).
Achten, J. & Jeukendrup, A. E. Heart rate monitoring: applications and limitations. Sports Med. 33, 517–538 (2003).
Cornier, M. A. et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation 124, 1996–2019 (2011).
Romero-Corral, A. et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 31, 737–746 (2010).
Ross, R. et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 16, 177–189 (2020).
Rubino, F. et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 13, 221–262 (2025).
Poon, E. T. et al. Efficacy of interval training in improving body composition and adiposity in apparently healthy adults: an umbrella review with meta-analysis. Sports Med. 54, 2817–2840 (2024).
Khodadadi, F. et al. The effect of high-intensity interval training type on body fat percentage, fat and fat-free mass: a systematic review and meta-analysis of randomized clinical trials. J. Clin. Med. 12, 2291 (2023).
Kim, S. R. et al. Changes in predicted lean body mass, appendicular skeletal muscle mass, and body fat mass and cardiovascular disease. J. Cachexia Sarcopenia Muscle 13, 1113–1123 (2022).
Colberg, S. R. et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 33, e147–e167 (2010).
Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17, 162–184 (2013).
Ross, R. et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134, e653–e699 (2016).
Mandsager, K. et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw. Open 1, e183605 (2018).
Henriksson, H. et al. Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: a cohort study of 1 million men. Eur. Heart J. 41, 1503–1510 (2020).
Hopewell, S. et al. CONSORT 2025 statement: updated guideline for reporting randomized trials. Nat. Med. 31, 1776–1783 (2025).
Juszczak, E., Altman, D. G., Hopewell, S. & Schulz, K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA 321, 1610–1620 (2019).
Leung, C. K. et al. Effects of volume-matched once-weekly and thrice-weekly high-intensity interval training (HIIT) on body adiposity in adults with central obesity: Study protocol for a randomized controlled trial. J. Exerc. Sci. Fit. 22, 329–340 (2024).
Alberti, K. G., Zimmet, P. & Shaw, J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 23, 469–480 (2006).
Fletcher, G. F. et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 128, 873–934 (2013).
Hangartner, T. N. et al. The Official Positions of the International Society for Clinical Densitometry: Acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J. Clin. Densitom. 16, 520–536 (2013).
Chin, E. C. et al. Low-frequency HIIT improves body composition and aerobic capacity in overweight men. Med. Sci. Sports Exerc. 52, 56–66 (2020).
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 30, 377–399 (2011).
Little, R. J. et al. The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 367, 1355–1360 (2012).
Li, G. et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int. J. Epidemiol. 46, 746–755 (2017).
Acknowledgements
The authors would like to thank all of the participants in the study, the fitness instructors (Mr. Tang Wai Hung and Mr. Fan Ka Tung), and the assessment technicians. This study is supported by the General Research Fund of Research Grants Council (RGC), Hong Kong University Grants Committee (project numbers: 17105920, 17110722, and 17112223), and the Seed Fund for Basic Research of the University of Hong Kong, which were awarded to P.M. Siu. The funders had no role in the design, data collection, data analysis, and reporting of this study.
Author information
Authors and Affiliations
Contributions
P.M.S. designed and conceptualized the study. P.M.S., D.Y.F., D.K.C., H.H.N., C.H.L., P.S.Y., S.H.W., and M.G. were involved in funding acquisition. P.M.S., C.K.L., J.B., and F.R. oversaw the execution of this study. P.M.S., C.K.L., and J.B. wrote and edited the manuscript. A.P.Y. and D.Y.F. provided statistical expertise. P.M.S., C.K.L., J.B., A.P.Y., F.R., B.T.T., D.Y.F., D.K.C., H.H.N., C.H.L., P.S.Y., S.H.W., and M.G. contributed to the critical revision of the article for intellectual content and gave final approval to its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Raaj Biswas and the other anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Siu, P.M., Leung, C.K., Bernal, J.D.K. et al. Once and thrice weekly interval training in adults with central obesity: a randomized controlled trial. Nat Commun 17, 1410 (2026). https://doi.org/10.1038/s41467-025-68149-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-68149-7