Abstract
Lipedema predominantly affects women and is characterized by an abnormal distribution of adipose tissue, accompanied by pain or discomfort in affected areas. Despite growing awareness, inconsistent diagnostic criteria and treatment approaches hinder medical care and research. This multi-phase Delphi study was conducted to address the need for internationally accepted consensus on fundamental aspects of the disease. Through online surveys and an in-person discussions, experts representing 19 countries evaluated on 62 original statements regarding (1) clarity, (2) agreement, (3) recommendation for inclusion, (4) strength of evidence, and (5) whether additional evidence was needed. Ultimately, 59 statements reached consensus across eight domains encompassing the definition and management of lipedema. The findings provide a framework to guide internationally applicable recommendations for patients with lipedema that may improve outcomes globally. Limited evidence in several areas highlights the importance of further research, standardization of data reporting, and international collaboration among healthcare providers, researchers, and patient advocates to address this women’s health disparity effectively.
Similar content being viewed by others
Introduction
Lipedema was first described in the 1940s by Drs. Allen and Hines at the Mayo Clinic, who identified it as a clinical syndrome characterized by abnormal and symmetrical accumulation of fat in the lower extremities of women, frequently accompanied by physical ache, feet sparing orthostatic edema, and psychological distress1. For decades, lipedema was not widely recognized and was often confused with obesity or lymphedema, leading to misdiagnosis and inadequate treatment2. Depending on the availability of healthcare professionals and services for affected individuals within various healthcare systems, lipedema remains underdiagnosed today, with a limited understanding continuing within the medical community. In recent years, however, awareness of lipedema as a distinct clinical entity has increased. This improved disease recognition has been driven by a combination of patient advocacy, research, and clinical interest, prompting the development of national guidelines and consensus documents aimed at improving diagnosis and management3,4,5,6,7,8,9,10. Despite these advances, however, variability in diagnostic criteria and treatment approaches persists. The lack of standardized diagnostic criteria not only hinders early detection but also complicates research efforts and the development of evidence-based therapies11. There is therefore an urgent need for a unified, internationally accepted consensus on the fundamental aspects of the disease entity and management approaches to caring for patients with lipedema.
This multi-step Delphi consensus study was conducted to address the need for a standardized definition and management approaches to lipedema. By leveraging the collective expertise of clinicians, researchers, and patient representatives from around the world, 59 statements were ultimately recommended for inclusion and reached consensus across eight domains: 36 reached 90–100% agreement, 17 reached 80–90% agreement, and 6 reached 70% agreement. This position paper aims to advance consensus on a standardized, globally relevant definition of lipedema and to delineate principles for its evidence-based management, integrating both empirical data and expert clinical insight. In addition, where evidence was found to be insufficient, further research is called for to improve the lives of individuals with lipedema.
Results
Overall results of Delphi process
After three online Delphi rounds, 59 statements on the definition and management of lipedema reached consensus according to the pre-defined 70 percent agreement threshold on criterion (ii) and (iii). Thirty-six statements reached 90–100% agreement, seventeen statements reached 80–90% agreement, and six statements reached 70% agreement (Table 1).
In the final Delphi evaluation round 3, 71 out of 103 founding members of the LWA participated (34 female, 37 male), representing 19 countries across 5 continents (Table 2). The majority of respondents reported having extensive experience in working with patients with lipedema (Table 3). The professional backgrounds of the participants were diverse, encompassing medical management, conservative and surgical treatment providers, as well as researchers and patient advocates (Table 4). Patient advocates from seven countries participated, with most (ten of twelve) originating from European nations.
Domain 1: Definition & Leading Symptoms
Statement 1:
“Lipedema is a chronic disease”
Clarity of statement 100.0%
Level of agreement 94.4%
Inclusion Rating 93.0%
Strength of Evidence 72.9%
Additional Evidence Needed 28.2%
Expert Panel Comment:
The definition of ‘chronic’ disease varies but typically encompasses conditions that (1) have complex etiology, (2) persist for three months or longer, (3) require ongoing medical management, and (4) may be associated with functional impairment or significantly impact daily activities12. Patients with lipedema typically report long courses of the disease and symptoms that last many years before diagnosis or initiation of appropriate therapeutic measures9,10,13,14,15,16. However, systematic longitudinal studies on the chronicity of the disease are not yet available.
Statement 2:
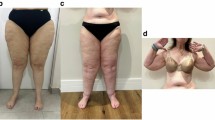
“Untreated lipedema typically presents as symmetrical, bilateral enlargement of subcutaneous adipose tissue in the extremities, accompanied by pain and/or discomfort”.
Clarity of statement 90,0%
Level of agreement 88,6%
Inclusion Rating 91,3%
Strength of Evidence 76,8%
Additional Evidence Needed 33,8%
Expert Panel Comment:
The characteristic presentation of lipedema as symmetrical and bilateral enlargement of subcutaneous adipose tissue (SAT) in the extremities is well-documented in the clinical literature1,17,18. The existing literature consistently describes the unique distribution of SAT predominantly in the legs, typically sparing the feet1, and contributing to the characteristic “column-like” appearance. However, it is important to note that variations in clinical presentation exist, and that some patients may demonstrate asymmetry as the disease progresses or in the presence of comorbidities16,19.
Pain in lipedema is a common and often debilitating symptom, significantly impacting patients’ quality of life. The presence of pain varies widely among individuals, ranging from mild discomfort to severe, chronic pain. It typically manifests as a dull, aching sensation in the affected areas, exacerbated by prolonged standing or walking. In addition, patients may experience tenderness to touch and increased sensitivity in the affected regions20,21,22,23,24,25. More research into the nature of pain and discomfort in lipedema is needed to support disease diagnosis and mechanisms for potential interventions.
Statement 3:
“Lipedema is characterized by a disproportional expansion of the subcutaneous adipose tissue of the extremities compared to that in the torso”.
Clarity of statement 91,0%
Level of agreement 89,6%
Inclusion Rating 89,6%
Strength of Evidence 71,9%
Additional Evidence Needed 37,9%
Expert Panel Comment:
In the absence of comorbid conditions, the characteristic lipedema phenotype is recognized for its hallmark feature of disproportionate fat accumulation in the SAT of the extremities compared to that in the torso. Imaging studies have consistently demonstrated increased fat deposition in the legs, supporting the assertion of disproportionate adipose tissue expansion26,27,28,29,30,31,32. However, inconsistent terminology for anatomical regions in the literature necessitates clarification in order to more clearly discuss the regions affected by lipedema. In this context, the extremities include the shoulder girdle (Cingulum membri superioris) together with the free upper limbs (Pars libera membri superioris) and the pelvic girdle (Cingulum membri inferioris) together with the free lower limbs (Pars libera membri inferioris). The torso or trunk in this context refers to the central part of the body, including the head and neck region, thorax, abdomen and pelvis33,34.
Statement 4:
“Lipedema can involve excess adipose tissue deposition in the upper extremities in a symmetrical and bilateral distribution”.
Clarity of statement 94,1%
Level of agreement 94,0%
Inclusion Rating 89,4%
Strength of Evidence 67,6%
Additional Evidence Needed 28,4%
Expert Panel Comment:
The involvement of the upper extremities (as defined in the “expert panel comment” of statement 3) in lipedema has been observed in both early and advanced stages of the disease. Studies report varying percentages of lipedema patients with arm involvement, ranging from 30% to 80%13,16,19,35.
Statement 5:
“Lipedema typically spares hands and feet of excess fat deposition”.
Clarity of statement 97,0%
Level of agreement 90,9%
Inclusion Rating 92,5%
Strength of Evidence 73,5%
Additional Evidence Needed 30,8%
Expert Panel Comment:
According to the original publication of Allen and Hines, lipedema adipose tissue deposition “usually does not involve the feet”1. Recent publications defining diagnostic criteria for lipedema also confirm this for the arms and often describe a separation between normal and abnormal tissue (“Cuff”) at the ankle or wrist4,5,6,8,9,10,35. However, although not constituting definitive evidence, patient reports suggest that lipedema-related symptoms may manifest in hands and feet as the disease progresses or in the presence of comorbidities16,19.
Statement 6:
“Physical sensitivity to pressure and/or stretch is observed by methods such as palpation, and is mainly reported by patients as pain”.
Clarity of statement 98,5%
Level of agreement 97,0%
Inclusion Rating 98,5%
Strength of Evidence 58,2%
Additional Evidence Needed 40,3%
Expert Panel Comment:
According to the International Association for the Study of Pain (ISAP), pain is defined as an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage36. Pain in response to typically innocuous stimuli (such as pressure, palpation, or stretching applied to lipedema-affected tissue) is a central symptom of lipedema, occurring both superficially and subcutaneously, predominantly throughout the legs or arms. Despite its significance, the literature lacks sufficient investigation and characterization of lipedema-related pain, making it complex and challenging to define23,25,37. The precise pathogenesis of pain in lipedema remains uncertain, with proposed mechanisms including central sensitization, nociceptive pain, and autonomic peripheral neuropathies20,21.
Statement 7:
“Increased sensitivity and pain caused by lipedema seem to be restricted to the body areas with lipedema-related volume increase”.
Clarity of statement 92,6%
Level of agreement 85,3%
Inclusion Rating 83,6%
Strength of Evidence 55,2%
Additional Evidence Needed 39,4%
Expert Panel Comment:
The clinical symptoms of lipedema typically manifest in the areas affected by the enlargement of SAT25,37,38,39. As outlined in statements 2 − 5, this manifestation is typically confined to the extremities, excluding the hands and feet. However, although not constituting definitive evidence, patient reports suggest that lipedema-related symptoms may be present in other body regions as the disease progresses or in the presence of comorbidities16.
Statement 8:
“Patients often report swelling or heaviness in affected areas”.
Clarity of statement 92,6%
Level of agreement 89,7%
Inclusion Rating 88,2%
Strength of Evidence 54,4%
Additional Evidence Needed 36,8%
Expert Panel Comment:
Patients with lipedema frequently describe the affected areas to exert a feeling of swelling or a sensation of heaviness40. Incidence data regarding these symptoms are limited in their availability16.
Statement 9:
“Pitting edema is usually not present in lipedema-affected tissue”.
Clarity of statement 91,2%
Level of agreement 89,7%
Inclusion Rating 86,8%
Strength of Evidence 50,0%
Additional Evidence Needed 44,1%
Expert Panel Comment:
Existing clinical evidence supports the assertion that, in the absence of comorbid conditions, pitting edema is generally absent in lipedema-affected tissue. In this regard, lipedema differs significantly from lymphedema41. As in the rest of the population, some individuals with lipedema may present with lymphedema, however, it is unknown whether this secondary condition represents primary or secondary lymphedema. Regardless, a causative role in lipedema has not been established.
Statement 10:
“Patients with lipedema often experience easy bruising in affected areas”.
Clarity of statement 95,5%
Level of agreement 94,0%
Inclusion Rating 92,5%
Strength of Evidence 58,2%
Additional Evidence Needed 35,8%
Expert Panel Comment:
A frequently reported symptom of patients with lipedema is a tendency to bruise without recollection of preceding trauma within the affected areas, without bleeding from other sites, and in the absence of systemic disorders known to result in bruising. Availability of incidence data is limited, with up to 90.6% of patients reporting easy bruising.16,40,42,43. However, it is important to note that easy bruising is not pathognomonic for lipedema and can have various etiologies.
Statement 11:
“Kaposi-Stemmer’s sign is usually negative in lipedema”.
Clarity of statement 93,9%
Level of agreement 95,5%
Inclusion Rating 95,4%
Strength of Evidence 71,6%
Additional Evidence Needed 22,7%
Expert Panel Comment:
Kaposi-Stemmer’s sign, a clinical indicator of lymphedema characterized by the inability to pinch a fold of skin at the base of the second toe or second finger44,45, is typically considered negative in lipedema46. If there is lymphostasis in addition to lipedema, the sign may be positive.
Domain 2: Pathophysiology
Statement 12:
“Lipedema is a disease involving subcutaneous adipose tissue”.
Clarity of statement 92,4%
Level of agreement 94,0%
Inclusion Rating 90,9%
Strength of Evidence 78,8%
Additional Evidence Needed 19,4%
Expert Panel Comment:
Adipose tissue, commonly known as fat, is a type of connective tissue that consists of lipid-filled cells (adipocytes) surrounded by a matrix of collagen fibers, blood vessels, fibroblasts, and immune cells47. Lipedema has been reported to affect components of the SAT32,38,39,48,49,50,51,52,53,54,55,56.
Statement 13:
“Numerous findings suggest that inflammation may contribute to the pathogenesis of lipedema”.
Clarity of statement 95,2%
Level of agreement 87,7%
Inclusion Rating 83,1%
Strength of Evidence 49,2%
Additional Evidence Needed 60,9%
Expert Panel Comment:
Studies have proposed that inflammatory processes may play a role in the development and progression of lipedema39,48,49,51,53,54,56,57. Nevertheless, it is crucial to note that the exact nature and extent of inflammation25 in lipedema remain areas of active investigation, and the mechanistic underpinnings are not fully elucidated. In particular, it remains unclear whether the observed tissue inflammation is a cause or a consequence of lipedema.
Statement 14:
“Numerous findings suggest that hormonal factors may contribute to the pathogenesis of lipedema”.
Clarity of statement 96,8%
Level of agreement 96,9%
Inclusion Rating 92,1%
Strength of Evidence 58,1%
Additional Evidence Needed 56,3%
Expert Panel Comment:
Hormonal factors are considered to contribute to the regulation of lipedema pathogenesis58,59,60 as lipedema symptoms are reported to worsen during hormonal shifts16. Nevertheless, it is important to emphasize that precise pathophysiological mechanisms for conclusively linking hormonal factors to lipedema have not yet been fully elucidated58,61,62.
Statement 15:
“Several findings suggest that extracellular fluid volume might be elevated in lipedema-affected tissue compared to BMI-matched unaffected controls”.
Clarity of statement 87,1%
Level of agreement 76,2%
Inclusion Rating 72,6%
Strength of Evidence 46,8%
Additional Evidence Needed 66,1%
Expert Panel Comment:
Histological analysis63, bioimpedance spectroscopy64,65, in-vivo MRI imaging27,66, and near-infrared fluorescent lymphatic imaging67 indicate that lipedema might be characterized by an accumulation of excess extracellular fluid (ECF), possibly deriving from impaired capillaries or dysfunctional lymphatic vessels. Notably, clinical observation generally does not reveal visible edema in individuals with lipedema. A hypothesis has been postulated, suggesting that the absence of edema, despite an elevation in ECF, may be attributed to an increase in glycosaminoglycans and proteoglycans, which would bind the increased ECF8.
Domain 3: Epidemiology
Statement 16:
“Lipedema primarily affects biological females. Occurrence in biological males appears to be possible but rare”.
Clarity of statement 96,7%
Level of agreement 88,7%
Inclusion Rating 88,9%
Strength of Evidence 69,8%
Additional Evidence Needed 33,9%
Expert Panel Comment:
The prevailing understanding is that lipedema predominantly manifests in biological females. Limited case reports suggest the occurrence of lipedema in biological males, with cases frequently associated with hormonal abnormalities68,69,70,71,72. Additional research is warranted to explore the underlying factors contributing to this disparity.
Statement 17:
“Hormonal changes may trigger or exacerbate the symptoms of lipedema”.
Clarity of statement 100,0%
Level of agreement 98,4%
Inclusion Rating 98,4%
Strength of Evidence 63,9%
Additional Evidence Needed 36,5%
Expert Panel Comment:
Phases characterized by hormonal fluctuations, such as puberty, pregnancy, or menopause, are frequently temporally associated with the onset or exacerbation of lipedema symptoms16,73. The causality of hormonal changes and their potential to have an impact on lipedema symptoms58,59, including the influence of other hormonal stimuli (such as hormone replacement therapy, contraceptives, etc.), remains uncertain15. Therefore, more comprehensive research, including longitudinal studies and investigations into the underlying biological mechanisms, is needed to establish a clearer understanding of the relationship between hormonal changes and lipedema.
Statement 18:
“Lipedema is hereditary in some cases”.
Clarity of statement 96,8%
Level of agreement 88,7%
Inclusion Rating 88,9%
Strength of Evidence 63,9%
Additional Evidence Needed 47,5%
Expert Panel Comment:
A frequently observed positive family history, with prevalence ranging from 30% to 90%13,42,73,74,75,76, indicates a hereditary nature of lipedema. An examination of familial clusters of lipedema patients has proposed an X-linked dominant inheritance pattern or, more likely, an autosomal dominant inheritance with sex restriction of a single dominant gene77. In addition, other studies have postulated an oligogenic inheritance model, suggesting the involvement of multiple gene variants associated with the phenotypic presentation of lipedema78,79,80,81,82,83,84. It is plausible that lipedema may manifest as either a primary type or as part of a syndromic hereditary condition79.
Statement 19:
“The prevalence of lipedema in the adult female population remains unknown. Estimates range from less than 1% to up to 12%”.
Clarity of statement 96,8%
Level of agreement 79,0%
Inclusion Rating 83,9%
Strength of Evidence 37,1%
Additional Evidence Needed 66,1%
Expert Panel Comment:
Accurately establishing the prevalence of lipedema presents a notable challenge owing to inconsistencies in diagnostic criteria, limited awareness among healthcare professionals and patients, variations in study populations (whether drawn from the general population or specialized institutions), geographical disparities, and differing methodological approaches. Current estimates within the adult female population underscore the considerable variability observed in reported figures18,73,76,77,85,86,87,88,89,90,91.
Domain 4: Comorbidities & concomitant diseases
Statement 20:
“Obesity is a frequently observed concomitant disease in patients with lipedema”.
Clarity of statement 98,4%
Level of agreement 87,3%
Inclusion Rating 92,1%
Strength of Evidence 62,9%
Additional Evidence Needed 44,4%
Expert Panel Comment:
Lipedema is often either misdiagnosed as or coexists with obesity, posing challenges in accurate differentiation. Research consistently demonstrates a higher prevalence of obesity among individuals with lipedema compared to the general population, as assessed by BMI13,40,77,92,93,94. However, BMI is not an ideal tool to assess obesity in patients with lipedema due to the disproportionate body habitus inherent in lipedema95, potentially contributing to divergent clinical evaluations and interpretations of existing literature. Nevertheless, when both conditions coexist, appropriate treatment must be provided for each as a separate disease. In addition, it is essential to recognize that the presence of obesity in lipedema patients may exacerbate symptoms and complicate management strategies. While the precise nature of the relationship between lipedema and obesity warrants further investigation, the recognition of this frequent concomitance is crucial for informing holistic and patient-centered approaches to care.
Statement 21:
“Lipedema is not an obesity-related comorbidity”.
Clarity of statement 98,4%
Level of agreement 92,1%
Inclusion Rating 91,8%
Strength of Evidence 69,4%
Additional Evidence Needed 32,8%
Expert Panel Comment:
Historically, lipedema was often mischaracterized as a simple consequence of obesity. Several studies have demonstrated that lipedema is distinct from obesity and should be regarded as a separate entity to facilitate accurate diagnosis and tailored therapeutic interventions20,96,97,98. Lipedema adipose tissue exhibits distinct morphological, molecular and metabolic characteristics compared to obesity-type adipose tissue32,99. In other words, lipedema is not an invariable comorbidity with obesity; rather, it may coincide with obesity100.
Statement 22:
“Body mass index (BMI) has limited value in distinguishing between lipedema and obesity. Therefore, it is advisable to utilize the Waist-to-Height Ratio (WHtR) to exclude or assess obesity”.
Clarity of statement 95,2%
Level of agreement 77,4%
Inclusion Rating 77,0%
Strength of Evidence 64,5%
Additional Evidence Needed 41,0%
Expert Panel Comment:
The body mass index (BMI) for characterizing obesity is of limited value in lipedema patients, as it leads to falsely high values in the area of overweight or mild obesity due to the increase in adipose tissue in the extremities. Nevertheless, as in all areas of surgery, BMI may have a positive correlation with surgical complication rates. A more accurate assessment of disproportionate fat distribution and metabolic health can be achieved through the waist-to-height ratio (WHtR)95,101,102,103. However, it is essential to acknowledge that while WHtR may provide valuable insights, further research and consensus development are warranted to establish standardized criteria for its application in distinguishing lipedema from obesity across diverse populations9,10.
Statement 23:
“In cases where lipedema coincides with obesity, lipedema symptoms can be expected to persist after bariatric surgery”.
Clarity of statement 91,9%
Level of agreement 88,9%
Inclusion Rating 87,1%
Strength of Evidence 58,7%
Additional Evidence Needed 44,3%
Expert Panel Comment:
Weight loss in patients with lipedema has been reported to reduce leg volume, improve quality of life and alleviate symptoms92,104,105. Conversely, current research indicates that despite significant weight loss achieved through bariatric surgery, symptoms related to lipedema often persist92,100,106,107,108. Studies suggest that the distinctive pathophysiology of lipedema may play a role in the ongoing persistence of these symptoms. In addition, the reduction in volume of the SAT in lipedema-affected extremities may be less pronounced compared to the trunk.
Statement 24:
“Concomitant lymphostasis can develop in lipedema”.
Clarity of statement 90,3%
Level of agreement 88,7%
Inclusion Rating 86,9%
Strength of Evidence 43,5%
Additional Evidence Needed 52,5%
Expert Panel Comment:
Reports suggest that lymphatic impairment leading to lymphostasis or lymphedema may co-occur at any stage of lipedema, further complicating the clinical presentation. The use of multiple modalities to assess lymphatic dysfunction in lipedema is ongoing, as well as the etiology of observed differences27,66,67,109,110. Without definitive evidence supporting a primary lymphatic dysfunction in lipedema, lymphostasis may be related to an imbalance in the production and removal of lymphatic fluid, causing a capacity overload (high-volume transport insufficiency, “Marsch thesis”111) as lymphoscintigraphic findings were not associated to the BMI110,112. Furthermore, lymphostasis may be related to coincident obesity or excessive adipose tissue expansion.
Statement 25:
“Several findings suggest that the prevalence of hypothyroidism might be higher in lipedema patients than in non-lipedema populations with comparable BMI and age”.
Clarity of statement 93,5%
Level of agreement 73,3%
Inclusion Rating 71,0%
Strength of Evidence 39,3%
Additional Evidence Needed 71,0%
Expert Panel Comment:
Recent publications highlighted a possible link between lipedema and hypothyroidism13,14,19,90. The demonstrated prevalence, ranging from 19% to 36%, surpasses that of comparable populations, adjusted for sex, age, and BMI (0.5–2.0%)113. However, the extent of a causal connection or whether hypothyroidism is merely an epiphenomenon of coincident obesity remains unclear114.
Statement 26:
“Lipedema might be associated with connective tissue disorders, such as hypermobility spectrum disorders”.
Clarity of statement 96,8%
Level of agreement 75,8%
Inclusion Rating 72,6%
Strength of Evidence 37,7%
Additional Evidence Needed 65,6%
Expert Panel Comment:
Evidence suggests that women with lipedema exhibit decreased elasticity in both skin115 and aorta116, along with joint hypermobility16,117,118 and muscle weakness119. There is speculation about a link between lipedema and connective tissue disorders, specifically hypermobility spectrum disorders (HSD), although no conclusive underlying mechanism has been identified120. Considering the inconsistency of HSD in lipedema, a hypothesis has proposed categorizing it as a subtype (“rusticanus Moncorps type”) of lipedema115. Further investigation is needed to confirm a definitive association between lipedema and HSD.
Domain 5: Impact on Quality of Life and Symptom Burden
Statement 27:
“Lipedema can negatively impact mental health and overall quality of life”.
Clarity of statement 100,0%
Level of agreement 98,4%
Inclusion Rating 98,4%
Strength of Evidence 70,5%
Additional Evidence Needed 38,7%
Expert Panel Comment:
The available literature consistently highlights the significant psychosocial burden associated with lipedema, emphasizing its adverse effects on mental health and overall quality of life94,121,122,123. The psychosocial implications of lipedema are multifaceted, encompassing impaired daily functioning, social withdrawal, and body image dissatisfaction124,125. Further evidence is required to elucidate how quality of life is affected across broader health domains and in diverse cultural contexts, as the psychosocial burden is exacerbated by insufficient support for affected individuals within many healthcare systems.
Statement 28:
“If present, psychological involvement may be caused by lipedema-related symptoms rather than being the cause of those symptoms”.
Clarity of statement 93,4%
Level of agreement 90,2%
Inclusion Rating 85,5%
Strength of Evidence 48,4%
Additional Evidence Needed 53,2%
Expert Panel Comment:
The intricate interplay between psychological factors and the symptomatology of lipedema has garnered attention123,126. Existing literature suggests that the psychological burden experienced by individuals with lipedema may be a result of the challenges posed by the physical aspects and functional limitations associated with the disease. Conversely, psychological distress (e.g., after traumatic experiences) can potentially exacerbate symptomatology or influence coping mechanisms. Therefore, the relationship between psychological factors and lipedema is complex and warrants careful consideration.
Statement 29:
“Missed or delayed diagnosis or management of lipedema negatively affects a patient’s symptom burden, mental well-being, and overall quality of life”.
Clarity of statement 100,0%
Level of agreement 100,0%
Inclusion Rating 100,0%
Strength of Evidence 67,7%
Additional Evidence Needed 31,1%
Expert Panel Comment:
Lipedema may not consistently progress, yet certain factors, such as overall weight gain42 and hormonal changes (e.g., during pregnancy), can contribute to long-term symptom exacerbation. A progression of the disease, marked by increased pain and disproportionate adipose tissue accumulation, along with unsuccessful therapeutic interventions and diets, heightens the risk of developing depression and has a significant impact on the overall quality of life15,94,121. Therefore, it is advisable to pursue early diagnosis and implement timely treatment interventions127.
Statement 30:
“Missed or delayed diagnosis or management of lipedema increases the cost burden for patients and the healthcare system”.
Clarity of statement 100,0%
Level of agreement 93,3%
Inclusion Rating 91,8%
Strength of Evidence 57,4%
Additional Evidence Needed 49,2%
Expert Panel Comment:
The economic impact of delayed diagnosis and suboptimal management in lipedema is a multifaceted concern, affecting both patients and the broader healthcare system. Research suggests that the complex nature and the fact that the condition is often overlooked contribute to delayed diagnosis, leading in turn to a protracted period of untreated symptoms15. This delay can result in increased healthcare utilization and costs due to the progression of the disease and the development of associated comorbidities13. Furthermore, the economic burden extends beyond direct medical costs, also encompassing expenses related to productivity loss, disability, and diminished quality of life122.
Domain 6: Diagnostic approach
Statement 31:
“The clinical diagnosis of lipedema relies on the patient’s medical history, physical examination and exclusion of differential diagnoses”.
Clarity of statement 98,4%
Level of agreement 98,4%
Inclusion Rating 98,4%
Strength of Evidence 72,1%
Additional Evidence Needed 35,0%
Expert Panel Comment:
Accurate diagnosis of lipedema remains challenging due to its overlapping clinical features with other adipose tissue disorders and lymphatic conditions128. The reliance on comprehensive evaluation, including detailed medical history, thorough physical examination, and exclusion of differential diagnoses, underscores the complexity of diagnosing lipedema in clinical practice4,8,9,46. Notably, the absence of definitive diagnostic criteria and standardized assessment tools may contribute to delays in diagnosis and misidentification of the disease. Collaborative efforts are needed to establish consensus guidelines and improve diagnostic accuracy for the benefit of patients and healthcare providers.
Statement 32:
“Currently, no imaging, serological or genetic tests, or clinical measurement instruments, are officially approved to verify the clinical diagnosis”.
Clarity of statement 98,4%
Level of agreement 93,5%
Inclusion Rating 91,8%
Strength of Evidence 66,1%
Additional Evidence Needed 39,3%
Expert Panel Comment:
Research efforts on different diagnostic modalities are underway to aid the clinical diagnosis of lipedema, including imaging, genetic, serologic, and bedside techniques20,25,38,120,129,130,131. While these investigations show promise in enhancing diagnostic precision and differentiating from other conditions, it is crucial to acknowledge the current lack of official approval for any specific modality in verifying the clinical diagnosis of lipedema.
Statement 33:
“Routine clinical exams should include standardized anthropometric measurements, such as waist-to-height ratio (WHtR), waist-to-hip ratio (WHR), and body mass index (BMI)”.
Clarity of statement 96,7%
Level of agreement 82,3%
Inclusion Rating 75,8%
Strength of Evidence 57,4%
Additional Evidence Needed 43,3%
Expert Panel Comment:
Although anthropometric measurements, including WHtR, WHR, and BMI, taken individually are not useful for diagnosing lipedema, taken together, they are essential in the assessment of overall health and are widely employed in routine clinical examinations132. Despite its limited significance in lipedema133, BMI can support disease monitoring due to its simplicity in measurement and interpretation. While BMI does not reflect regional fat distribution, WHR is subject to age, sex, and ethnic variations. Conversely, WHtR has been identified as a valuable predictor of cardiometabolic risk, with studies suggesting its superiority over other indices, particularly in assessing central obesity95,103,134,135.
Statement 34:
“The clinical classification of lipedema into stages does not reflect the complete symptom severity”.
Clarity of statement 96,7%
Level of agreement 95,1%
Inclusion Rating 93,4%
Strength of Evidence 60,7%
Additional Evidence Needed 52,5%
Expert Panel Comment:
The current clinical stage classification of lipedema is based on the severity of palpation findings in the skin and subcutaneous tissue136. Palpable alterations correspond to a progressive enlargement of nodular and fibrotic tissue structures39, accompanied by an increasing induration of the skin and subcutaneous tissue. While the current staging system primarily focuses on anatomical aspects, it does not adequately correlate with the nuanced and multifaceted nature of symptom severity in individuals with lipedema137.
Statement 35:
“The current clinical classification for lipedema into stages has limited relevance for the disease management”.
Clarity of statement 100,0%
Level of agreement 91,9%
Inclusion Rating 90,3%
Strength of Evidence 54,1%
Additional Evidence Needed 49,2%
Expert Panel Comment:
The existing clinical classification of lipedema into stages does not reflect the individual differences in the total perceived discomfort due to lipedema, presenting challenges in guiding optimal disease management9,10. While stages have been suggested based on phenotypic characteristics and palpation findings136, the practical utility of this classification in guiding therapeutic strategies is limited. To achieve a more nuanced and comprehensive approach to disease management, considerations should extend to factors such as pain intensity, quality of life, and functional impairment98,137.
Statement 36:
“A progression in the severity of lipedema-associated symptoms depends on various factors and is not universal”.
Clarity of statement 93,4%
Level of agreement 95,1%
Inclusion Rating 95,1%
Strength of Evidence 47,5%
Additional Evidence Needed 57,4%
Expert Panel Comment:
The trajectory of lipedema presents considerable heterogeneity, with varying degrees of symptom severity influenced by a complex interplay of factors. While some individuals may experience a progression in symptom severity over time, others may exhibit stable or fluctuating symptomatology. Existing literature suggests that factors such as hormonal changes, individual predisposition, overall weight gain42 and lifestyle factors may contribute to the diversity in symptom progression85. However, it is essential to acknowledge the limited availability of long-term, prospective studies that comprehensively explore the multitude of factors influencing the natural history and disease course of lipedema.
Statement 37:
“The excess limb volume in lipedema is generally not associated with obesity”.
Clarity of statement 93,2%
Level of agreement 79,7%
Inclusion Rating 76,3%
Strength of Evidence 39,0%
Additional Evidence Needed 52,5%
Expert Panel Comment:
The relationship between excess limb volume in lipedema and obesity is a complex and much-debated aspect within the current literature. While some authors suggest that limb volume increase in lipedema is not necessarily correlated with overall obesity100, others propose a potential overlap between lipedema and obesity9,10, indicating that the two conditions may coexist or influence each other. Given the high number of patients with lipedema who also suffer from concomitant obesity, excess limb volume can be attributed to both underlying conditions. The lack of a universally accepted definition for obesity in the context of lipedema and the varied methodologies used to assess limb volume and obesity contribute to the ongoing discourse. Further research and standardized criteria are warranted to elucidate the intricate interplay between limb volume excess in lipedema and obesity.
Statement 38:
“The clinical classifications based on localization have only descriptive significance”.
Clarity of statement 95,1%
Level of agreement 88,5%
Inclusion Rating 86,9%
Strength of Evidence 39,3%
Additional Evidence Needed 43,3%
Expert Panel Comment:
While localization-based phenotype classifications (such as subtypes according to Meier-Vollrath138 or form variant according to Herpertz139) provide a useful framework for characterizing the distribution of adipose tissue in lipedema117,138,139, their clinical utility in predicting disease progression, response to treatments, or specific complications remains limited. Nevertheless, localization-based phenotype classifications serve as a descriptive tool facilitating the contextualization of clinical cases among experts, thus retaining their practical significance. Further research is needed to elucidate the practical implications and prognostic value of these classification systems, taking into account the heterogeneity observed in patients with lipedema.
Domain 7: Treatment modalities
Statement 39:
“All therapeutic interventions of lipedema aim at alleviating symptoms and preventing or delaying progression”.
Clarity of statement 95,1%
Level of agreement 90,2%
Inclusion Rating 90,2%
Strength of Evidence 55,7%
Additional Evidence Needed 45,8%
Expert Panel Comment:
Current therapeutic interventions for lipedema primarily focus on (1) symptom management and (2) disease progression prevention, rather than curative measures9,46,97. It is crucial to note that while existing treatment modalities for lipedema can provide meaningful symptom relief, they do not eliminate the underlying pathology of lipedema altogether.
Statement 40:
“Comprehensive disease management requires a multidisciplinary approach tailored to individual needs, which may involve physicians, physical therapists, dietitians, and mental health professionals”.
Clarity of statement 96,7%
Level of agreement 96,7%
Inclusion Rating 96,7%
Strength of Evidence 63,9%
Additional Evidence Needed 41,7%
Expert Panel Comment:
The significance of a multidisciplinary approach in the comprehensive management of complex conditions, such as lipedema, is widely acknowledged in the literature140. Collaboration among different health care professionals is vital to address the diverse aspects of the disease, encompassing medical, rehabilitative, nutritional, and psychological dimensions. While there is a growing consensus on the importance of multidisciplinary care, further research is warranted to establish standardized protocols and assess the long-term effectiveness of such collaborative interventions in lipedema management.
Statement 41:
“Lipedema pain and physical sensitivity in lipedema-affected areas have been reported to be reduced by bandaging, compression, complete physical decongestive therapy or other physical therapies (such as shock wave therapy), dietary changes, tailored exercise, and lipedema reduction surgery, with varying effect sizes and durations”.
Clarity of statement 86,7%
Level of agreement 86,9%
Inclusion Rating 83,3%
Strength of Evidence 45,9%
Additional Evidence Needed 65,6%
Expert Panel Comment:
Various interventions show potential for symptom relief in lipedema, but the quality of the supporting evidence is variable. The heterogeneity of study designs, patient populations and diagnostic criteria makes direct comparisons between different treatment modalities challenging. In addition, the duration of therapeutic effects remains a subject of investigation, with some interventions demonstrating sustained benefits over time, while others may necessitate ongoing management. It is important to emphasize that a universally applicable therapeutic regimen for lipedema has yet to be established. The choice of treatment modalities often depends on the individual patient’s characteristics, including disease severity, comorbidities, and personal preferences9.
Statement 42:
“Conservative management of lipedema should include lifestyle and nutritional optimization, compression therapy, and exercise to alleviate symptoms and improve quality of life”.
Clarity of statement 95,1%
Level of agreement 93,4%
Inclusion Rating 90,0%
Strength of Evidence 63,3%
Additional Evidence Needed 44,1%
Expert Panel Comment:
Lifestyle and nutritional changes play a pivotal role, aiming to address potential exacerbating factors and enhance overall well-being104,141,142. Compression therapy has demonstrated effectiveness in improving symptoms, contributing to enhanced patient comfort and mobility143,144,145. Tailored exercise regimes, such as aquatic exercise, are often recommended to promote lymphatic flow146 and muscle strength without placing excessive strain on affected limbs145,147. While individual responses to these interventions may vary, a comprehensive strategy incorporating these elements is a cornerstone in the holistic care of lipedema patients.
Statement 43:
“Active self-management can help control lipedema-related symptoms and improve the overall quality of life”.
Clarity of statement 95,1%
Level of agreement 91,7%
Inclusion Rating 90,0%
Strength of Evidence 36,1%
Additional Evidence Needed 59,3%
Expert Panel Comment:
Existing literature suggests that active self-management practices play a crucial role in mitigating lipedema-related symptoms and enhancing the overall quality of life for affected individuals9. Patient education and training are critical components of effective self-management. However, it is important to note that the evidence base for specific self-management strategies is still evolving, and further research is needed to establish comprehensive guidelines for effective self-management in lipedema.
Statement 44:
“Although Complex (also known as Complete) Decongestive Therapy (CDT) can be an important and effective treatment even for early-stage lipedema, not all components are required for every patient”.
Clarity of statement 95,1%
Level of agreement 86,9%
Inclusion Rating 86,9%
Strength of Evidence 39,3%
Additional Evidence Needed 53,3%
Expert Panel Comment:
The key components of Complex (also known as Complete) Decongestive Therapy (CDT) include (1) compression therapy, (2) manual lymphatic drainage (MLD), (3) decongestive exercise, (4) skin care, and (5) empowerment/self-management. Compression therapy for lipedema aims to reduce pain and alleviate subjective symptoms, especially when combined with physical activity, demonstrating positive effects145,148. There is no evidence supporting the sole use of MLD for therapeutic benefits in lipedema, as it has only been studied in various combination forms with other therapies143,144,149. However, it has been shown to have a sympatholytic effect, increase pain tolerance, and elevate pain thresholds9. Not all lipedema patients may require every component of CDT, and tailoring therapeutic interventions based on the specific needs and characteristics of each patient is crucial.
Statement 45:
“Nutritional guidance can help patients manage their weight, optimize overall health, reduce lipedema-associated symptoms and improve their response to therapeutic interventions”.
Clarity of statement 96,7%
Level of agreement 93,4%
Inclusion Rating 93,4%
Strength of Evidence 65,6%
Additional Evidence Needed 47,5%
Expert Panel Comment:
The existing literature suggests that addressing chronic inflammation in lipedema, especially with concomitant obesity, through patient education on pro-inflammatory triggers and recommending an anti-inflammatory Mediterranean or ketogenic diet141,150,151,152,153,154,155,156,157,158. Elevated insulin levels in both conditions can contribute to lipogenesis and inflammation, emphasizing the importance of a diet that avoids blood glucose and insulin spikes159,160,161. Limited studies exist on lipedema-specific diets, but reviews highlight the potential benefits of ketogenic diets, including weight reduction, decreased adipose tissue, and symptom relief105,142,162,163,164,165,166. The absence of pro-inflammatory blood glucose spikes in ketogenic diets is proposed to make them more effective in combating lipedema inflammation151.
Statement 46:
“Although pathological subcutaneous adipose tissue in lipedema is known to be largely resistant to dietary interventions, addressing overall weight loss in coincident obesity may result in symptom improvement”.
Clarity of statement 95,0%
Level of agreement 93,3%
Inclusion Rating 93,2%
Strength of Evidence 62,1%
Additional Evidence Needed 40,7%
Expert Panel Comment:
Although lipedema SAT is considered largely diet-resistant19, improvements in lipedema-associated symptoms have been demonstrated through a combination of dietary modification, weight reduction and exercise141. Conversely, it has been reported that in patients with coincident obesity, bariatric surgery–induced weight loss and reductions in BMI did not result in significant improvement of lipedema-associated symptoms in the absence of dietary modification100. To promote optimal health and mobility, it is advisable to provide nutritional guidance and, when applicable, engage in obesity therapy aligned with clinical guidelines97.
Statement 47:
“Considering that obesity worsens the manifestations of lipedema, disease management should include weight optimization, with a focus on waist-to-height ratio (WHtR) and waist-to-hip ratio (WHR)”.
Clarity of statement 91,8%
Level of agreement 83,3%
Inclusion Rating 80,3%
Strength of Evidence 50,8%
Additional Evidence Needed 51,7%
Expert Panel Comment:
Maintaining a normal weight can help prevent a progression of lipedema-associated symptoms, preserve mobility, and reduce the risk of developing osteoarthritis97. Obesity classification typically relies on BMI132. However, in the context of lipedema and in line with statement 33, utilizing alternative anthropometric measures—such as WHtR—may be more appropriate for assessing or excluding overweight and obesity, particularly in the context of metabolic health categorization95,167.
Statement 48:
“Psychological and social support, addressing body image issues, mental well-being, and coping strategies, can be important to address the symptom burden of patients living with lipedema”.
Clarity of statement 96,7%
Level of agreement 98,3%
Inclusion Rating 96,7%
Strength of Evidence 68,3%
Additional Evidence Needed 37,3%
Expert Panel Comment:
Acknowledging the profound impact of lipedema on patients, addressing psychological and social dimensions is vital for comprehensive care. Studies emphasize the multifaceted psychosocial challenges, including impaired daily functioning, social withdrawal, and mental health implications122,123,168. Interventions targeting these aspects have demonstrated efficacy in improving overall well-being and coping mechanisms121,169.
Statement 49:
“Tailored exercises, such as physical activity in water, walking, and yoga, can help maintain mobility, address lipedema related symptoms and support weight management in individuals with lipedema”.
Clarity of statement 95,0%
Level of agreement 91,7%
Inclusion Rating 90,2%
Strength of Evidence 56,7%
Additional Evidence Needed 42,6%
Expert Panel Comment:
A growing body of literature underscores the potential benefits of exercise interventions for individuals with lipedema, emphasizing improvements in physical function, mobility, symptom management and quality of life143,144,149,170,171,172. Specifically, water-based activities have shown promise in reducing pain and enhancing mobility, possibly due to effects and physical properties of water, such as hydrostatic pressure and buoyancy, and consecutively reduced impact on joints147,173,174. Moreover, structured exercise—such as walking and yoga—have been associated with improved muscular strength, and psychological well-being, factors that are particularly relevant for individuals with lipedema119,175,176.
Statement 50:
“Currently, there is no evidence for the effectiveness of any pharmacological interventions in treating lipedema”.
Clarity of statement 100,0%
Level of agreement 93,3%
Inclusion Rating 93,2%
Strength of Evidence 58,3%
Additional Evidence Needed 44,1%
Expert Panel Comment:
There is no evidence to support the efficacy of pharmaceutical interventions in alleviating lipedema symptoms. Supplements with potential antioxidative, edema-reducing, or immunomodulatory effects have been explored for managing lipedema symptoms. However, the existing evidence supporting their efficacy is limited151,153,155,156,177,178,179.
Statement 51:
“In cases where lipedema coincides with obesity and metabolic disease, it is advisable to prioritize treatment for obesity before considering lipedema reduction surgery”.
Clarity of statement 100,0%
Level of agreement 93,3%
Inclusion Rating 91,7%
Strength of Evidence 66,7%
Additional Evidence Needed 40,7%
Expert Panel Comment:
The relationship between obesity, metabolic disease, and lipedema is complex, necessitating a nuanced approach to treatment decisions. Existing literature highlights the bidirectional interplay between obesity and lipedema, with obesity potentially exacerbating lipedema symptoms180 and vice versa due to impaired mobility, pain or psychological burden linked to lipedema13,40,172,174,181. While addressing obesity is crucial for overall health, it is essential to recognize that lipedema is a distinct condition with unique pathophysiological features.
The sequential or simultaneous management of both conditions may be warranted, acknowledging that successful outcomes often require a multidisciplinary approach9. When considering lipedema reduction surgery, it is generally advisable to address concurrent obesity first182, as a lower BMI correlates with improved outcomes74 and may mitigate perioperative risks183,184. However, in some cases, it may not be universally applicable to postpone lipedema reduction surgery until obesity or metabolic health has been addressed, as the severity of the condition, the patient’s goals and general health may vary from case to case100,185.
Statement 52:
“Lymph vessel-sparing lipedema reduction surgery should be considered when there is potential for a positive impact on lipedema-related symptoms”.
Clarity of statement 86,7%
Level of agreement 89,7%
Inclusion Rating 87,9%
Strength of Evidence 58,6%
Additional Evidence Needed 49,2%
Expert Panel Comment:
Although conservative treatment can alleviate lipedema symptoms, it does not achieve long-lasting benefits and cannot prevent the progression of the disease. Lipedema reduction surgery, or liposuction, is currently the only technique for removing abnormal lipedema tissues and slowing a potential progression of the disease. Recent uncontrolled before-and-after studies reported positive results in reducing extremity size and alleviating lipedema-associated symptoms such as spontaneous pain, easy bruising, sensitivity to pressure, impairment in quality of life, restrictions to mobility, edema, sensation of tightness, and overall improvement in patients’ quality of life186,187,188,189. However, currently, there is a lack of randomized controlled trials and long-term follow-up studies assessing the clinical effectiveness and safety of lipedema reduction surgery compared to no treatment or alternative approaches in managing lipedema. Ongoing studies have not yet yielded definitive results190,191.
Statement 53:
“Surgical interventions should be performed by healthcare providers with extensive knowledge in lipedema management, including conservative treatments, as part of an integral approach”.
Clarity of statement 98,3%
Level of agreement 98,3%
Inclusion Rating 98,3%
Strength of Evidence 53,3%
Additional Evidence Needed 43,1%
Expert Panel Comment:
In contrast to aesthetic liposuctions, the primary goal of lipedema reduction surgery is a medically indicated, functional intervention for symptom reduction through subtotal resection of the presumed pathological, subcutaneous fat tissue in the limbs affected by lipedema. Therefore, lipedema reduction surgery, integrated with conservative management, should be performed according to clinical best practice outlined by medical associations and developed specifically for lipedema4,5,6,8,9,10.
Domain 8: Future directions
Statement 54:
“Raising awareness about lipedema within the medical community and wider society is essential to reduce misdiagnosis and stigma”.
Clarity of statement 100,0%
Level of agreement 98,3%
Inclusion Rating 96,7%
Strength of Evidence 66,7%
Additional Evidence Needed 30,0%
Expert Panel Comment:
Raising awareness about lipedema is critical for timely diagnosis and management, as patients often experience prolonged diagnostic delays and encounter misconceptions surrounding the condition13,15. Adequate education and awareness programs targeted at healthcare professionals can enhance recognition and understanding of lipedema’s distinct features, reducing the likelihood of misdiagnosis. Furthermore, public awareness initiatives play a crucial role in diminishing the stigma associated with lipedema, fostering empathy, and promoting a supportive environment for affected individuals169.
Statement 55:
“A comprehensive standardized case report form (CRF) should be developed to improve consistency in diagnosis, lipedema reporting, follow-up, and research, and to facilitate, for example, both cohort and longitudinal studies”.
Clarity of statement 100,0%
Level of agreement 96,7%
Inclusion Rating 95,0%
Strength of Evidence 55,9%
Additional Evidence Needed 42,4%
Expert Panel Comment:
Standardized data collection tools are crucial for advancing our understanding of lipedema and fostering collaboration across diverse clinical and research domains. Implementation of a CRF can potentially address existing variations in diagnostic criteria, improve accuracy in reporting, and contribute to the creation of a robust foundation for clinical studies or registries.
Statement 56:
“Further research is needed to elucidate the biological mechanisms underlying lipedema, leading to objective diagnostic criteria and targeted therapies”.
Clarity of statement 98,3%
Level of agreement 100,0%
Inclusion Rating 98,3%
Strength of Evidence 57,6%
Additional Evidence Needed 44,8%
Expert Panel Comment:
The call for continued research into the biological mechanisms of lipedema is crucial for advancing our understanding of this complex disease. A comprehensive understanding of these mechanisms is essential for the development of objective diagnostic criteria, robust diagnostic markers and targeted therapeutic interventions. Collaborative efforts between clinicians, researchers, and advocacy groups are integral to driving progress in this field and improving outcomes for individuals affected by lipedema.
Statement 57:
“Studies are required to validate diagnostic modalities for lipedema, which assess their reproducibility, sensitivity, and specificity”.
Clarity of statement 100,0%
Level of agreement 100,0%
Inclusion Rating 96,7%
Strength of Evidence 58,6%
Additional Evidence Needed 41,4%
Expert Panel Comment:
While the need for rigorous validation of diagnostic modalities for lipedema is evident, the current landscape lacks comprehensive studies assessing the reproducibility, sensitivity, and specificity of existing diagnostic tools. The urgency for such research is underscored by the diverse clinical presentations and challenges in accurately identifying lipedema, often leading to delayed or incorrect diagnoses. To enhance diagnostic accuracy, future studies should aim to establish standardized protocols for evaluating the reliability and consistency of diagnostic modalities. These protocols should consider the variability in disease manifestations and demographic factors, ensuring applicability across diverse patient populations. Collaborative efforts, such as multicenter trials, could facilitate the accumulation of robust evidence, fostering a more nuanced understanding of diagnostic performance.
Statement 58:
“Long-term studies are required to assess the efficacy and safety of treatment modalities for lipedema”.
Clarity of statement 100,0%
Level of agreement 100,0%
Inclusion Rating 98,3%
Strength of Evidence 57,9%
Additional Evidence Needed 46,4%
Expert Panel Comment:
While some studies have provided insights into short-term outcomes, a comprehensive understanding of the long-term effects, durability of response, and potential adverse events is crucial for guiding evidence-based clinical decision-making. The call for long-term studies to assess treatment modalities in lipedema is substantiated by the current gaps in knowledge. Future research endeavors should prioritize extended follow-up periods to inform clinicians, researchers, patients, and service providers about the enduring impact and safety considerations associated with various therapeutic approaches.
Statement 59:
“Collaborative efforts between patients, researchers, clinicians and advocacy groups are crucial for advancing knowledge. Translational practice-based application of research knowledge should improve patient care”.
Clarity of statement 100,0%
Level of agreement 98,3%
Inclusion Rating 95,0%
Strength of Evidence 56,9%
Additional Evidence Needed 36,2%
Expert Panel Comment:
Collaborative engagement among patients, researchers, clinicians, and advocacy groups is pivotal for advancing our understanding of complex conditions like lipedema. Such an inclusive approach would foster a more comprehensive perspective, incorporating diverse insights and experiences, which is essential for addressing the multifaceted nature of the disease. Moreover, patient and public involvement in the research process enhances the relevance and applicability of findings to real-world clinical scenarios192,193,194.
The translation of research knowledge into practice is a cornerstone for improving patient care. By bridging the gap between scientific discoveries and clinical applications, we can expedite the integration of evidence-based interventions into routine care, ultimately benefiting individuals affected by lipedema. Emphasizing translational practice ensures that the latest advancements directly influence patient outcomes and treatment strategies, facilitating a more responsive and patient-centered healthcare approach.
Discussion
This is the first Delphi study undertaken to systematically evaluate and report the level of agreement on best-practice recommendations for patients with lipedema. The study represents a foundational milestone in developing consensus among international experts about lipedema disease characteristics, diagnostic approaches, and treatment modalities. This marks a significant initial step toward enhancing clarity and guiding future policy development and research on lipedema.
The international approach also allowed for a range of perspectives to be considered, so that patients benefit from shared experiences that improve clinical care in different health care systems. Unlike other consensus documents or best-practice guidelines, these statements, pertaining to the definition and management of lipedema were developed through a transparent process that involved a broad panel of international experts and representatives from patient organizations (Fig. 1). The use of the Delphi methodology enhances the strength and credibility of these statements, as it is a validated tool for developing best-practice guidance based on collective expert opinion when research is limited195.
Considering the level of available evidence and the rapid pace of innovation in the field, the authors anticipate a limited lifespan for the statements presented in their current form. Therefore, the LWA is committed to maintaining this document as a “living document” that will require periodic review, expansion, and potential revision as new evidence emerges. In addition, although experts from 19 countries participated, significant populations affected by lipedema—such as those in Asia and Africa—remain underrepresented in this work. It is the authors hope that the global community grows in awareness of lipedema and future participation in best-practice guidelines.
At this current time, this document also aims to advance lipedema-related research by (1) underscoring the need for clear diagnostic criteria, (2) emphasizing the importance of standardized reporting, and (3) serving as a resource to engage large, traditional health research funding bodies in recognizing lipedema as a women’s health disparity that requires attention. Considering the limited evidence in several areas, a potential next step to enhance the quality of evidence could involve identifying priority research areas, based on findings from the Delphi study with expert focus groups in relevant fields. However, the authors hope that the current consensus will provide an evolving framework to guide future clinical and research advances in the field. In addition, standardized reporting was identified as essential for improving data comparability and reproducibility. To this end, a case report form (CRF) could be developed specifically for use in lipedema research studies.
Through three voting rounds, consensus was reached on 59 statements regarding the definition and management of lipedema. Overall, a high level of agreement was achieved for the majority of aspects, with 36 statements achieving 90–100% consensus, 17 statements achieving 80–90% consensus, and 6 statements achieving 70% consensus (Table 1). However, it became evident that many of the statements were supported by a low level of evidence, highlighting the urgent need for high-quality research on lipedema.
To conduct such research, the diagnostic criteria or disease definition must be clear and standardized across research groups. During the on-site meeting in Potsdam and through feedback collected from the anonymous Delphi rounds, significant discrepancies in assessments were identified in certain aspects of the definition and pathophysiology of lipedema.
A key point of contention is the characterization of “pain” as a cardinal symptom of lipedema. A better understanding of the distinctly altered sensory sensitivity in patients with lipedema may help resolve any existing definitional differences and contribute to a more standardized definition of lipedema. Clarity on the experience of pain in individuals with lipedema would inform more effective treatment approaches.
Another aspect that generated significant variability in assessments and was the focus of extensive discussion was the possible presence of (protein-bound) extracellular fluid in lipedema, the eponymous “edema” in lipedema. Among all the statements, in the final round of the Delphi study, this was one of the top areas where participants identified a greater need for further research to enable a definitive conclusion. The authors believe that obtaining higher-quality evidence about edema in lipedema would be instrumental in standardizing diagnostic criteria across different geographic regions and disciplines, ultimately leading to a more unified understanding of the disease “lipedema”.
This document has several strengths. The founding members of the LWA are recognized experts in the management of lipedema, and participants in the Delphi survey have extensive experience (Table 3). The diverse professional background of the participants—including conservative and surgical healthcare providers, researchers, and patient representatives—highlights the significant applicability of this project (Table 4). Furthermore, the geographical representation of participants from 19 countries across five continents (Table 2) reinforces the document’s assertion of universal applicability. The anonymized methodology aimed to reduce the potential for introducing bias and enhance the validity of the consensus process. However, to preserve anonymity throughout the Delphi process, participant interactions were restricted, which limited opportunities for discussion and refinement of statements, aside from the feedback that was available to the project team.
In terms of limitations of the present study, it is of critical importance to note that the statements made are predominantly based on limited evidence. Additional constraints arise from the methodology employed. By definition, the use of a modified Delphi approach and reliance on expert opinions may introduce potential biases in the interpretation of the literature review, particularly since a substantial number of available publications were authored by participants in the Delphi process. Furthermore, the response rates in the initial two rounds of the Delphi survey—48 and 49 out of 103 LWA founding members—although balanced in terms of geographical distribution and professional background, limit the representativeness of the findings (Tables 2–4). The in-person meeting after Round 2 during the dedicated session at the Lipedema World Congress in Potsdam on October 7, 2023, significantly increased the project’s visibility and attention. This led to a higher number of participants in the third Delphi round, ensuring the final results are well-founded and reliable.
Methods
The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. Ethics committee approval was not required, as no individual health data were used. Study procedures and reporting followed the DELPHISTAR reporting guidelines for Delphi studies in health research196. A completed DELPHISTAR checklist is provided in the Supplementary Information. No further consulting in regard to the method took place.
Project team selection
This project was led by the Lipedema World Alliance (LWA), a non-for-profit organization composed of healthcare professionals, researchers, and patient association representatives dedicated to addressing lipedema globally. Experts invited by the LWA Board of Directors to join the Delphi-Consensus Project Team were selected based on their expertise and scholarly contributions in the field. Special attention was given to ensuring a diverse perspective of participants, considering demographic factors (age, sex, country), specialty, and level of experience.
Participant selection
All 103 LWA founding members were invited to participate in the Delphi process via email. Founding Members included (1) physicians/medical doctors, healthcare professionals, and researchers dedicated to lipedema and related pathologies, as well as (2) national and regional organizations registered as not-for-profit and dedicated to fighting lipedema and/or related pathologies, comprising patients and/or family members and/or caregivers and/or professionals. The geographical distribution of the founding members reflects the project’s wide-reaching, transnational, and intercontinental scope.
Modified Delphi process
Delphi statements were developed by the project team around 8 domains regarding lipedema: (1) definition and leading symptoms, (2) pathophysiology, (3) epidemiology, (4) comorbidities and concomitant diseases, (5) impact on quality of life and symptom burden, (6) diagnostic approach, (7) treatment modalities, and (8) future directions.
A three-round Delphi process design was used (Fig. 1). Rounds 1 and 2 were survey-based via an online platform (SurveyMonkey Inc., San Mateo, CA, USA). Round 3 involved an on-site meeting held as part of the Lipedema World Congress on October 7th 2023 in Potsdam, Germany and a final follow-up survey. Although the administrator (PK) was aware of the identities of all respondents for tracking purposes, no identifying information was shared with the Delphi-Consensus Project Team or LWA Board members. For the online voting, all participants remained anonymous to each other throughout the Delphi study. Each stage of the Delphi process was systematically reported to and approved by the LWA Board of Directors.
During each Delphi round, statements were presented to the participant via an online survey. For each statement, participants evaluated 5 criteria: (i) the clarity of the statement, (ii) the degree of agreement with the statement, (iii) the recommendation for inclusion in the final document, (iv) the level of evidence supporting the statement, and (v) whether additional evidence is required to make a conclusive evaluation of the statement. Statement clarity and the need for additional evidence were assessed by yes/no response. Agreement with each statement and recommendation for inclusion were evaluated using a five-point Likert scale, as follows: (1) strongly disagree, (2) disagree, (3) neither agree nor disagree, (4) agree, and (5) strongly agree. Evaluation of the strength of evidence for each statement was similarly conducted using a five-point Likert scale: (1) very low quality, (2) low quality, (3) neither high nor low quality, (4) high quality, and (5) very high quality. The degree of agreement on each criterion was calculated as the percentage of votes with a score of ≥ 4 on the five-point Likert scale or by the percentage of yes responses.
Consensus, or sufficient agreement, for a given statement was defined a priori as greater than 70% of expert agreement on criterion (ii), along with more than 70% of participants recommending inclusion in the document on criterion (iii)197,198,199,200. Criteria (i), (iv), and (v) are not relevant to the consensus threshold but serve to contextualize each statement. Statements that did not meet the established consensus threshold in the subsequent Delphi rounds were excluded from the final document but were included in the supplementary material to reflect the expert’s preferences199,201. In each round of voting, participants were also encouraged to provide comments to explain their respective voting scores, and responses were anonymized. The LWA Delphi-Consensus Project Team reviewed the voting results and comments after each round, refining the statements for subsequent rounds of voting. If the comments implied that language-related misunderstandings contributed to insufficient agreement or that specific phrasing could be modified to achieve a higher level of consensus, the respective statement was adjusted accordingly and re-submitted for voting in the subsequent Delphi round (round 1: #17, #21, #23, #24, #27, #29, #30, #33, #35, #40, #41, #42, #54, #55, #56; round 2: #15, #24, #25, #35, #38).
Delphi evaluation round 1 consisted of 62 statements. All statements were developed by the LWA Delphi-Consensus Project Team and reviewed by the entire LWA board of directors. The online survey evaluation on the statements were distributed between September 9th and September 22nd 2023.
Round 2 included the aggregate results from round 1 and adjusted a total of 29 statements (#5, #7, #10, #13, #14, #15, #19, #20, #21, #24, #25, #26, #28, #32, #33, #34, #35, #36, #37, #39, #41, #43, #44, #47, #50, #51, #52, #57, #59) according to the comments from round 1. Five statements were removed from further evaluation due to an insufficient level of agreement and/or recommendation for inclusion in the final document. Two additional statements (#48, #62) were incorporated based on feedback received, resulting in a total of 59 statements. Online distribution took place between October 1st 2023 and October 6th 2023. Participants were provided with a summary of comments when necessary to understand changes from the previous version.
After the second Delphi evaluation round all LWA founding members were invited to participate in an on-site panel discussion during a dedicated session at the Lipedema World Congress in Potsdam on October 7th 2023. Online participation was also facilitated. During the on-site meeting, results showing the percentage of votes scoring ≥ 4 on the five-point Likert scale or the percentage of yes responses per statement were shared and related comments were reviewed. Statements that reached consensus in the previous e-Delphi rounds were endorsed with minimal discussion. Statements that did not reach consensus in the previous e-Delphi rounds were discussed, and, when appropriate, reformulated or removed altogether from further evaluation rounds.
Finally, to enhance clarity for the final round (Round 3) of the survey, input from participants of the in-person meeting, along with open-ended feedback from previous Delphi rounds, were both utilized to draft corresponding “expert panel comments” for each of the remaining statements. In addition, 26 statements were revised based on a combination of the discussions held in Potsdam and comments from the online surveys (Statements #2, #3, #4, #6, #10, #16, #18, #19, #24, #31, #33, #36, #39, #40, #41, #42, #44, #46, #47, #49, #51, #52, #53, #54, #55, #57). All remaining 59 statements were included in the final survey after appropriate revision. The online distribution of the survey occurred between April 15th 2024, and July 25th 2024. After the third and final round of the Delphi process, no further modifications were made to the statements, apart from any grammatical changes to implement people-first language. Revisions following careful consideration of all feedback were limited exclusively to the “expert panel comments”.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon request without restrictions.
References
Allen, E. V. & Hines, E. A. Lipedema of the legs: a syndrome characterized by fat legs and orthostatic edema. Ann. Intern. Med. 34, 1243–1250 (1940).
Fife, C. E., Maus, E. A. & Carter, M. J. Lipedema: a frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv. Skin Wound Care 23, 81–92 (2010).
Deutsche Gesellschaft für Phlebologie e.V. S1-Leitlinie Lipödem (AWMF Registernummer 037-012. www.awmf.org/leitlinien/detail/ll/037-012.html (2015).
Halk, A. & Damstra, R. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebology 32, 152–159 (2017).
Coppel, T., UK Best Practice Guidelines: The Management of Lipoedema. (2017).
Spanish Association of Lymphedema and Lipedema (AEL). Consensus Document on Lipedema 20 18 - Includes current status of Lipedema 2019. https://aelinfedema.org/wp-content/uploads/2018/03/Consenso-Lipedema-2018.pdf (2023).
Bertsch, T., Erbacher, G. & Elwell, R. Lipoedema: a paradigm shift and consensus. J. Wound Care 29, 1–51 (2020).
Herbst, K. L. et al. Standard of care for lipedema in the United States. Phlebology 36, 779–796 (2021).
Deutsche Gesellschaft für Phlebologie u. Lymphologie e.V. (DGPL). S2k-Leitlinie Lipödem (AWMF Registernummer 037-012). www.awmf.org/leitlinien/detail/ll/037-012.html (2024).
Faerber, G. et al. S2k guideline lipedema. J. Dtsch. Dermatol. Ges. 22, 1303–1315 (2024).
Daftuar, F. et al. Lipedema Research Roadmap. (2023).
Bernell, S. & Howard, S. W. Use your words carefully: what is a chronic disease?. Front. Public Health 4, 159 (2016).
Ghods, M., Georgiou, I., Schmidt, J. & Kruppa, P. Disease progression and comorbidities in lipedema patients – a 10-year retrospective analysis. Dermatol. Ther. 33, e14534 (2021).
Bauer, A. T. et al. New insights on lipedema: the enigmatic disease of the peripheral fat. Plast. Reconstr. Surg. 144, 1475–1484 (2019).
Fetzer, A. & S. Fetzer. Lipoedema UK Big Survey Research Report. https://lipoedema.co.uk/wp-content/uploads/2024/05/LATEST-LUK000-UK-Big-Survey16-Mar24-web.pdf (2014).
Aday, A. W. et al. National survey of patient symptoms and therapies among 707 women with a lipedema phenotype in the United States. Vasc. Med. 29, 36–41 (2024).
Rank, B. K. & Wong, G. S. C. LIPOEDEMA. Aust. N. Zeal. J. Surg. 35, 165–169 (1966).
Foldi, E. & Foldi, M. Textbook of lymphology. (2003).
Herbst, K., Mirkovskaya, L., Bharhagava, A., Chava, Y. & Te, C. Lipedema fat and signs and symptoms of illness, increase with advancing stage. Arch. Med. 7, 1–8 (2015).
Poojari, A., Dev, K. & Rabiee, A. Lipedema: Insights into Morphology, Pathophysiology, and Challenges. Biomedicines 10, 3081 (2022).
Chakraborty, A. et al. Indications of Peripheral Pain, Dermal Hypersensitivity, and Neurogenic Inflammation in Patients with Lipedema. Int. J. Mol. Sci. 23, 10313 (2022).
Aksoy, H., Karadag, A. S. & Wollina, U. Cause and management of lipedema-associated pain. Dermatol. Ther. 34, e14364 (2021).
Gensior, M. H. L. & Cornely, M. Pain in lipoedema, fat in lipoedema and its consequences: results of a patient survey based on a pain questionnaire. Handchir. Mikrochir. Plast Chir. 51, 249–254 (2019).
Szolnoky, G. et al. Lymphedema treatment decreases pain intensity in lipedema. Lymphology 44, 178–182 (2011).
Dinnendahl, R., Tschimmel, D., Löw, V., Cornely, M. & Hucho, T. Non-obese lipedema patients show a distinctly altered quantitative sensory testing profile with high diagnostic potential. Pain Rep. 9, e1155 (2024).
Taylor, S. et al. Whole-leg chemical-shift encoded MRI analysis reveals differential subcutaneous adipose tissue accumulation in lipedema. https://doi.org/10.1117/12.2611240 (2022).
Cellina, M. et al. Non-contrast MR Lymphography of lipedema of the lower extremities. Magn. Reson. Imaging 71, 115–124 (2020).
Dimakakos, P. B. et al. MRI and ultrasonographic findings in the investigation of lymphedema and lipedema. Int. Surg. 82, 411–416 (1997).
Crescenzi, R. et al. Upper and lower extremity measurement of tissue sodium and fat content in patients with lipedema. Obesity 28, 907–915 (2020).
Iker, E., Mayfield, C. K., Gould, D. J. & Patel, K. M. Characterizing lower extremity lymphedema and lipedema with cutaneous ultrasonography and an objective computer-assisted measurement of dermal echogenicity. Lymphat. Res. Biol. 17, 525–530 (2019).
Buso, G. et al. Body composition assessment by dual-energy X-ray absorptiometry: a useful tool for the diagnosis of lipedema Obes. Facts 28, 762–773 (2022).
Ishaq, M. et al. Key signaling networks are dysregulated in patients with the adipose tissue disorder, lipedema. Int. J. Obes. 46, 502–514 (2021).
Schulte, E., Schumacher, U. & Schünke, M. PROMETHEUS Allgemeine Anatomie und Bewegungssystem: LernAtlas der Anatomie. (2018).
Brenner, E. Basics of the lymphatic system. Phlebologie 52, 87–99 (2023).
Buck, D. W. 2nd & Herbst, K. L. Lipedema: a relatively common disease with extremely common misconceptions. Plast Reconstr. Surg. Glob. Open 4, e1043 (2016).
Raja, S. N. et al. The revised International Association for the study of pain definition of pain: concepts, challenges, and compromises. Pain 161, 1976–1982 (2020).
Hucho, T. Lipedema pain-the neglected symptom. Dermatologie 74, 575–579 (2023).
Nankam, P. A. N., Cornely, M., Klöting, N. & Blüher, M. Is subcutaneous adipose tissue expansion in people living with lipedema healthier and reflected by circulating parameters? Front. Endocrinol. 13, https://doi.org/10.3389/fendo.2022.1000094 (2022).
Kruppa, P. et al. Lipedema stage affects adipocyte hypertrophy, subcutaneous adipose tissue inflammation and interstitial fibrosis. Front. Immunol. 14, 1223264 (2023).
Luta, X. et al. Clinical characteristics, comorbidities, and correlation with advanced lipedema stages: A retrospective study from a Swiss referral centre. PLoS ONE 20, e0319099 (2025).
The diagnosis and treatment of peripheral lymphedema 2020 Consensus document of the International Society of lymphology. Lymphology 53, 3–19 (2020).
Forner-Cordero, I., Perez-Pomares, M. V., Forner, A., Ponce-Garrido, A. B. & Munoz-Langa, J. Prevalence of clinical manifestations and orthopedic alterations in patients with lipedema: A prospective cohort study. Lymphology 54, 170–181 (2021).
Szolnoky, G. et al. Measurement of capillary fragility: a useful tool to differentiate lipedema from obesity?. Lymphology 50, 203–209 (2017).
Kaposi, M. Pathologie und Therapie der Hauterkrankungen. (1887).
Stemmer, R. Ein klinisches Zeichen zur Früh- und Differentialdiagnose des Lymphödems. Vasa 5, 261–262 (1976).
Kruppa, P. et al. Lipedema—pathogenesis, diagnosis and treatment options. Dtsch. Arztebl. Int. 117, 396–403 (2020).
Ahima, R. S. & Flier, J. S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 11, 327–332 (2000).
Suga, H. et al. Adipose tissue remodeling in lipedema: adipocyte death and concurrent regeneration. J. Cutan. Pathol. 36, 1293–1298 (2009).
AL-Ghadban, S. et al. Dilated blood and lymphatic microvessels, angiogenesis, increased macrophages, and aipocyte hypertrophy in lipedema thigh skin and fat tissue. J. Obes. 2019, 10 (2019).
Wolf, S. et al. A distinct cytokine profile and stromal vascular fraction metabolic status without significant changes in the lipid composition characterizes lipedema. Int. J. Mol. Sci. 22, 3313 (2021).
Felmerer, G. et al. Adipose tissue hypertrophy, an aberrant biochemical profile and distinct gene expression in lipedema. J. Surg. Res. 253, 294–303 (2020).
Koyama, H., Tanaka, T. & Imaeda, K. Suspected case of lipoedema in Japanese woman with a characteristic histology in skin biopsy. BMJ Case Rep. 2017, bcr-2017-221049 (2017).
Felmerer, G. et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 10, 10947 (2020).
Kaiserling, E., Zur Histologie des Lipödems, in Lipödem und Cellulitis sowie andere Erkrankungen des Fettgewebes (2001).
Crescenzi, R. et al. Tissue sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema. Obesity 26, 310–317 (2018).
Straub, L. G. et al. Defining lipedema’s molecular hallmarks by multi-omics approach for disease prediction in women. Metabolism 168, 156191 (2025).
Wolf, S. et al. A distinct M2 macrophage infiltrate and transcriptomic profile decisively influence adipocyte differentiation in lipedema. Front. Immunol. 13, 1004609 (2022).
Szel, E., Kemeny, L., Groma, G. & Szolnoky, G. Pathophysiological dilemmas of lipedema. Med. Hypotheses 83, 599–606 (2014).
Katzer, K., Hill, J. L., McIver, K. B. & Foster, M. T. Lipedema and the potential role of estrogen in excessive adipose tissue accumulation. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms222111720 (2021).
Al-Ghadban, S., Isern, S. U., Herbst, K. L. & Bunnell, B. A. The expression of adipogenic marker is significantly increased in estrogen-treated lipedema adipocytes differentiated from adipose stem cells in vitro. Biomedicines 12, https://doi.org/10.3390/biomedicines12051042 (2024).
Gavin, K. M., Cooper, E. E. & Hickner, R. C. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism 62, 1180–1188 (2013).
Al-Ghadban, S., Teeler, M.L. & Bunnell, B.A. Estrogen as a contributing factor to the development of lipedema. in Physiology and Disorders of Adipose Tissue (2021).
Allen, M., Schwartz, M. & Herbst, K. L. Interstitial fluid in lipedema and control skin. Womens Health Rep. 1, 480–487 (2020).
Esmer, M. & Schingale, F. J. Intracellular and extracellular water balance in patients with lipedema. Lymphat. Res. Biol. 21, 501–503 (2023).
Crescenzi, R. et al. Lipedema and Dercum’s Disease: A New Application of Bioimpedance. Lymphat. Res. Biol. 17, 671–679 (2019).
Crescenzi, R. et al. Subcutaneous adipose tissue edema in lipedema revealed by noninvasive 3T MR lymphangiography. J. Magn. Reson. Imaging 57, 598–608 (2023).
Rasmussen, J. C., Aldrich, M. B., Fife, C. E., Herbst, K. L. & Sevick-Muraca, E. M. Lymphatic function and anatomy in early stages of lipedema. Obesity 30, 1391–1400 (2022).
Alim, S. C. & Suryana, K. Lipedema subsequent with lymphedema and obesity in male patient: a rare case. Int. J. Res. Med. Sci. 10, 4 (2022).
Bertlich, M., Jakob, M., Bertlich, I., Schift, R. & Bertlich, R. Lipedema in a male patient: report of a rare case - management and review of the literature. GMS Interdiscip. Plast Reconstr. Surg. DGPW 10, Doc11 (2021).
Cadart, O. livier et al. Lipedema and klinefelter syndrome in two morbidly obese patients. Androgens Clin. Res. Ther. 2, 134–140 (2021).
Pereira de Godoy, L. M., Guerreiro Godoy, M. F. & Pereira de Godoy, J. M. Lipedema in male progressing to subclinical and clinical systemic lymphedema. J. Med. Cases 13, 249–252 (2022).
Chen, S. G., Hsu, S. D., Chen, T. M. & Wang, H. J. Painful fat syndrome in a male patient. Br. J. Plast Surg. 57, 282–286 (2004).
Forner-Cordero, I., Szolnoky, G., Forner-Cordero, A. & Kemeny, L. Lipedema: an overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome - systematic review. Clin. Obes. 2, 86–95 (2012).
Kruppa, P., Georgiou, I., Schmidt, J., Infanger, M. & Ghods, M. A 10-Year Retrospective before-and-after study of lipedema surgery: patient-reported lipedema-associated symptom improvement after multistage liposuction. Plast Reconstr. Surg. 149, 529e–541e (2022).
Herbst, K. L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 33, 155–172 (2012).
Harwood, C. A., Bull, R. H., Evans, J. & Mortimer, P. S. Lymphatic and venous function in lipoedema. Br. J. Dermatol. 134, 1–6 (1996).
Child, A. H. et al. Lipedema: an inherited condition. Am. J. Med. Genet. A 152a, 970–976 (2010).
Paolacci, S. et al. Genetics of lipedema: new perspectives on genetic research and molecular diagnoses. Eur. Rev. Med. Pharmacol. Sci. 23, 5581–5594 (2019).
Michelini, S. et al. A multi-gene panel to identify lipedema-predisposing genetic variants by a next-generation sequencing strategy. J. Pers. Med. 12, https://doi.org/10.3390/jpm12020268 (2022).
Michelini, S. et al. Aldo-keto reductase 1C1 (AKR1C1) as the first mutated gene in a family with nonsyndromic primary lipedema. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21176264 (2020).
Klimentidis, Y. C. et al. Genome-wide association study of a lipedema phenotype among women in the UK Biobank identifies multiple genetic risk factors. Eur. J. Hum. Genet. 31, 338–344 (2023).
Gualtieri, P. et al. The role of MTHFR polymorphisms in the risk of lipedema. Eur. Rev. Med. Pharmacol. Sci. 27, 1625–1632 (2023).
Di Renzo, L. et al. The role of IL-6 gene polymorphisms in the risk of lipedema. Eur. Rev. Med. Pharmacol. Sci. 24, 3236–3244 (2020).
Bano, G. et al. Pit-1 mutation and lipoedema in a family. Exp. Clin. Endocrinol. Diabetes 118, 377–380 (2010).
Wold, L. E., Hines, E. A. Jr. & Allen, E. V. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann. Intern. Med. 34, 1243–1250 (1951).
Forner-Cordero, I., Navarro-Monsoliu, R., Langa, J. & Rel-Monzó, P. Early or late diagnosis of lymphedema in our lymphedema unit. Eur. J. Lymphology Relat. Probl. 16, (2006).
Herpertz, U. Krankheitsspektrum des Lipödems an einer Lymphologischen Fachklinik - Erscheinungsformen, Mischbilder und Behandlungsmöglichkeiten. Vasomed 9, 301–307 (1997).
Meier-Vollrath, I., Schneider, W. & Schmeller, W. Lipödem: verbesserte lebensqualität durch therapiekombination. Dtsch. Ärzteblatt. 102, 1061 (2005).
Schook, C. C. et al. Differential diagnosis of lower extremity enlargement in pediatric patients referred with a diagnosis of lymphedema. Plast. Reconstr. Surg. 127, 1571–1581 (2011).
Amato, A. C. M., Amato, F. C. M., Amato, J. L. S. & Benitti, D. A. Lipedema prevalence and risk factors in Brazil. J. Vasc. Bras. 21, e20210198 (2022).
Marshall, M. & Schwahn-Schreiber, C. Prävalenz des Lipödems bei berufstätigen Frauen in Deutschland. Phlebologie 40, 127–134 (2011).
Fink, J. M. et al. Leg volume in patients with lipoedema following bariatric surgery. Visc. Med. 37, 206–211 (2021).
Angst, F., Benz, T., Lehmann, S., Sandor, P. & Wagner, S. Common and contrasting characteristics of the chronic soft-tissue pain conditions fibromyalgia and lipedema. J. Pain. Res. 14, 2931–2941 (2021).
Dudek, J. E., Bialaszek, W., Ostaszewski, P. & Smidt, T. Depression and appearance-related distress in functioning with lipedema. Psychol. Health Med. 23, 846–853 (2018).
Brenner, E., Forner-Cordero, I., Faerber, G., Rapprich, S. & Cornely, M. Body mass index vs. waist-to-height-ratio in patients with lipohyperplasia dolorosa (vulgo lipedema). J. Dtsch. Dermatol. Ges. 21, 1179–1185 (2023).
Ernst, A. M. et al. Lipedema Research-Quo Vadis? J. Pers. Med. 13, https://doi.org/10.3390/jpm13010098 (2022).
Forner-Cordero, I., Forner-Cordero, A. & Szolnoky, G. Update in the management of lipedema. Int. Angiol. 40, 345–357 (2021).
Cornely, M. Lipohyperplasia dolorosa (LiDo): Renaming, prima vista Diagnose, Koinzidenz, Palpation und Resektion. Akt. Dermatol. 49, 107–119 (2023).
Michelini, S. et al. Endothelial cell alterations in capillaries of adipose tissue from patients affected by lipedema. Obesity 33, 695–708 (2025).
Cornely, M. E. et al. Persistent lipedema pain in patients after bariatric surgery: a case series of 13 patients. Surg. Obes. Relat. Dis. 18, 628–633 (2022).
Brenner, E. & Cornely, M. Der anthropometrische parameter waist-to-height-ratio bei der lipohyperplasia dolorosa vulgo Lipödem. Lymphol. Forsch. Prax. 26, 6–14 (2022).
Reich-Schupke, S., Altmeyer, P. & Stucker, M. Thick legs - not always lipedema. J. Dtsch. Dermatol.Ges. 11, 225–233 (2013).
Busetto, L. et al. A new framework for the diagnosis, staging and management of obesity in adults. Nat. Med. 30, 2395–2399 (2024).
Jeziorek, M. et al. The benefits of low-carbohydrate, high-Fat (LCHF) diet on body composition, leg volume, and pain in women with lipedema. J. Obes. 2023, 5826630 (2023).
Lundanes, J. et al. Effect of a low-carbohydrate diet on pain and quality of life in female patients with lipedema: a randomized controlled trial. Obesity 32, 1071–1082 (2024).
Bast, J. H., Ahmed, L. & Engdahl, R. Lipedema in patients after bariatric surgery. Surg. Obes. Relat. Dis. 12, 1131–1132 (2016).
Pouwels, S., Huisman, S., Smelt, H. J. M., Said, M. & Smulders, J. F. Lipoedema in patients after bariatric surgery: report of two cases and review of literature. Clin. Obes. 8, 147–150 (2018).
Pouwels, S., Smelt, H. J., Said, M., Smulders, J. F. & Hoogbergen, M. M. Mobility problems and weight regain by misdiagnosed lipoedema after bariatric surgery: illustrating the medical and legal aspects. Cureus 11, e5388 (2019).
Greene, A. K. & Sudduth, C. L. Lower extremity lymphatic function predicted by body mass index: a lymphoscintigraphic study of obesity and lipedema. Int. J. Obes. 45, 369–373 (2021).
Buso, G. et al. Indocyanine green lymphography as novel tool to assess lymphatics in patients with lipedema. Microvasc. Res. 140, 104298 (2022).
Marsch, W. C. The lymphatic system and the skin. Classification, clinical aspects und histology. Hautarzt 56, 277–293 (2005).
Forner-Cordero, I., Olivan-Sasot, P., Ruiz-Llorca, C. & Munoz-Langa, J. Lymphoscintigraphic findings in patients with lipedema. Rev. Esp. Med. Nucl. Imagen. Mol. 37, 341–348 (2018).
Vanderpump, M. P. The epidemiology of thyroid disease. Br. Med. Bull. 99, 39–51 (2011).
Sanyal, D. & Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 20, 554–557 (2016).
Jagtman, B. A., Kuiper, J. P. & Brakkee, A. J. Measurements of skin elasticity in patients with lipedema of the Moncorps “rusticanus” type]. Phlebologie 37, 315–319 (1984).
Szolnoky, G., Nemes, A., Gavaller, H., Forster, T. & Kemeny, L. Lipedema is associated with increased aortic stiffness. Lymphology 45, 71–79 (2012).
Torre, Y. S., Wadeea, R., Rosas, V. & Herbst, K. L. Lipedema: friend and foe. Horm. Mol. Biol. Clin. Investig. 33, 1–10 (2018).
Beltran, K. & Herbst, K. L. Differentiating lipedema and Dercum’s disease. Int. J. Obes.41, 240–245 (2017).
van Esch-Smeenge, J., Damstra, R. J. & Hendrickx, A. A. Muscle strength and functional exercise capacity in patients with lipoedema and obesity: a comparative study. J. Lymphoedema 12, 27–31 (2017).
Morgan, S. et al. A family-based study of inherited genetic risk in lipedema. Lymphat. Res. Biol. 22, 106–111 (2024).
Dudek, J. E., Bialaszek, W. & Ostaszewski, P. Quality of life in women with lipoedema: a contextual behavioral approach. Qual. Life Res. 25, 401–408 (2016).
Dudek, J. E., Białaszek, W. & Gabriel, M. Quality of life, its factors, and sociodemographic characteristics of Polish women with lipedema. BMC Women’s Health 21, 27 (2021).
Alwardat, N. et al. The effect of lipedema on health-related quality of life and psychological status: a narrative review of the literature. Eat. Weight Disord. 25, 851–856 (2020).
Kloosterman, L. M., Hendrickx, A., Scafoglieri, A., Jager-Wittenaar, H. & Dekker, R. Functioning of people with lipoedema according to all domains of the international classification of functioning, disability and health: a scoping review. Int. J. Environ. Res. Public Health, 20, https://doi.org/10.3390/ijerph20031989 (2023).
Falck, J., Rolander, B., Nygårdh, A., Jonasson, L. L. & Mårtensson, J. Women with lipoedema: a national survey on their health, health-related quality of life, and sense of coherence. BMC Womens Health 22, 457 (2022).
West-Leuer, B. Lipohyperplasia dolorosa: An unreasonable burden and challenge for the psychological well-being of affected patients]. Dermatologie 74, 580–587 (2023).
Goodliffe, J. M., Ormerod, J. O., Beale, A. & Ramcharitar, S. An under-diagnosed cause of leg swelling. BMJ Case Rep. https://doi.org/10.1136/bcr-2013-009538 (2013).
Szolnoky, G. Differential Diagnosis: Lipedema, in Lymphedema: A Concise Compendium of Theory and Practice(2018).
Amato, A. C. M., Saucedo, D. Z., Santos, K. D. S. & Benitti, D. A. Ultrasound criteria for lipedema diagnosis. Phlebology 36, 651–658 (2021).
Cestari, M. Lipedema: Usefulness of 3D ultrasound diagnostics. Lymphat. Res. Biol. 21, 485–487 (2023).
Hirsch, T., Schleinitz, J., Marshall, M. & Faerber, G. Ist die Differenzialdiagnostik des Lipödems mittels hochauflösender Sonografie möglich?. Phlebologie 47, 182–187 (2018).
Organization, W. H. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 894, 1–253 (2000).
Czerwińska, M., Gruszecki, M., Rumiński, J. & Hansdorfer-Korzon, R. Examining the characteristic features of lipedema and the usefulness of BMI and WHtR in clinical evaluation. BMC Womens Health 25, 292 (2025).
Tewari, A., Kumar, G., Maheshwari, A., Tewari, V. & Tewari, J. Comparative evaluation of waist-to-height ratio and BMI in predicting adverse cardiovascular outcome in people with diabetes: a systematic review. Cureus 15, e38801 (2023).
Schneider, H. J. et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J. Clin. Endocrinol. Metab. 95, 1777–1785 (2010).
Strößenreuther, R., Das Lipödem, in Lipödem und Cellulitis sowie andere Erkrankungen des Fettgewebes. (2001).
Brenner, E. Lipohyperplasia dolorosa – neu betrachtet. Phlebologie 52, 266–286 (2023).
Meier-Vollrath, I. & Schmeller, W. [Lipoedema-current status, new perspectives]. J. Dtsch. Dermatol. Ges. 2, 181–186 (2004).
Herpertz, U. Das Lipödem. Zeitschrift. fur. Lymphol. 19, 1–11 (1995).
Yeung, C. H. et al. Integrated multidisciplinary care for the management of chronic conditions in adults: an overview of reviews and an example of using indirect evidence to inform clinical practice recommendations in the field of rare diseases. Haemophilia 22, 41–50 (2016).
Faerber, G. Ernährungstherapie bei Lipödem und Adipositas – Ergebnisse eines leitliniengerechten Therapiekonzepts. Vasomed 29, 176–177 (2017).
Keith, L. et al. Ketogenic diet as a potential intervention for lipedema. Med. Hypotheses 146, 110435 (2021).
Szolnoky, G. et al. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology 41, 161–166 (2008).
Szolnoky, G., Borsos, B., Barsony, K., Balogh, M. & Kemeny, L. Complete decongestive physiotherapy with and without pneumatic compression for treatment of lipedema: a pilot study. Lymphology 41, 40–44 (2008).
Czerwińska, M., Teodorczyk, J., Spychała, D. & Hansdorfer-Korzon, R. The usefulness of the application of compression therapy among lipedema patients-pilot study. Int. J. Environ. Res. Public Health 20, https://doi.org/10.3390/ijerph20020914 (2023).
Dionne, A., Goulet, S., Leone, M. & Comtois, A. S. Aquatic exercise training outcomes on functional capacity, quality of life, and lower limb lymphedema: pilot study. J. Altern. Complement Med. 24, 1007–1009 (2018).
Burger, R. et al. Wirkung von Aqua-Cycling als Bewegungstherapie bei der Diagnose Lipödem. Phlebologie 48, 182–186 (2019).
Donahue, P. M. C. et al. Physical therapy in women with early stage lipedema: potential impact of multimodal manual therapy, compression, exercise, and education interventions. Lymphat. Res. Biol. 20, 382–390 (2022).
Atan, T. & Bahar-Özdemir, Y. The effects of complete decongestive therapy or intermittent pneumatic compression therapy or exercise only in the treatment of severe lipedema: a randomized controlled trial. Lymphat Res. Biol. 19, 86–95 (2021).
Cannataro, R. et al. Ketogenic diet and microRNAs linked to antioxidant biochemical homeostasis. Antioxidants 8, 269 (2019).
Cannataro, R. & Cione, E. Lipedema and nutrition: what’s the link?. Acta Sci. Nutr. Health 4, 86–89 (2020).
Amato, A. C. M. Is lipedema a unique entity? EC Clin. Med. Cases Rep. 2, 1–7 (2020).
Amato, A. C. M. & Benitti, D. A. Lipedema can be treated non-surgically: a report of 5 cases. Am. J. Case Rep. 22, e934406 (2021).
Di Renzo, L. et al. Potential effects of a modified mediterranean diet on body composition in lipoedema. Nutrients 13, 358 (2021).
Faerber, G. Antiinflammatorische Ernährung, was ist das und was bringt sie uns beim Lipödem? Vasomed 29, 228–230 (2017).
Faerber, G. Obesity and chronic inflammation in phlebological and lymphatic diseases. Phlebologie 47, 55–65 (2018).
Jeziorek, M., Szuba, A., Kujawa, K. & Regulska-Ilow, B. The effect of a low-carbohydrate, high-fat diet versus moderate-carbohydrate and fat diet on body composition in patients with lipedema. Diabetes Metab. Syndr. Obes. 15, 2545–2561 (2022).
Lundanes, J. et al. Changes in cytokines and fibrotic growth factors after low-carbohydrate or low-fat low-energy diets in females with lipedema. Curr. Dev. Nutr. 9, 104571 (2025).
Blüher, M. et al. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp. Clin. Endocrinol. Diabetes 113, 534–537 (2005).
Shoelson, S. E., Herrero, L. & Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180 (2007).
Feinman, R. D. et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31, 1–13 (2015).
Jeziorek, M. et al. The Effect of a Low-Carbohydrate High-Fat Diet on Laboratory Parameters in Women with Lipedema in Comparison to Overweight/Obese Women. Nutrients, 15, https://doi.org/10.3390/nu15112619 (2023).
Sørlie, V. et al. Effect of a ketogenic diet on pain and quality of life in patients with lipedema: The LIPODIET pilot study. Obes. Sci. Pract. 8, 483–493 (2022).
Verde, L. et al. Ketogenic Diet: A Nutritional Therapeutic Tool for Lipedema?. Curr. Obes. Rep. 12, 529–543 (2023).
Cannataro, R. et al. Management of lipedema with ketogenic diet: 22-month follow-up. Life 11, https://doi.org/10.3390/life11121402 (2021).
Apkhanova, T. V. et al. Influence of ketogenic diet and nutraceutical correction in the complex treatment of Lower limbs lipedema. Bull. Rehab. Med. 20, 26–36 (2021).
Rubino, F. et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 13, 221–262 (2025).
Romeijn, J. R. M., de Rooij, M. J. M., Janssen, L. & Martens, H. Exploration of patient characteristics and quality of life in patients with lipoedema using a survey. Dermatol. Ther. 8, 303–311 (2018).
Christoffersen, V. & Tennfjord, M. K. Younger women with lipedema, their experiences with healthcare providers, and the importance of social support and belonging: a qualitative study. Int. J. Environ. Res. Public Health 20, 1925 (2023).
Schwarze, D., Vibrationstraining zur Therapie des Lipödems der Beine, in Physikalische Medizin und Rehabilitation. (2011).
Becker, J., Jung, M., Kleinschmidt, J. & Kleinschmidt, B. Der Einfluss von aqua-cycling auf das Volumen ödematöser Schwellungen bei Lip-/lipolymphödemen im vergleich zur manuellen Lymphdrainage – Eine Pilotstudie. Lymphol. Forsch. Prax. 22, 2–8 (2018).
Aitzetmüller-Klietz, M. L. et al. Understanding the vicious circle of pain, physical activity, and mental health in lipedema patients-A response surface analysis. J. Clin. Med. 12, https://doi.org/10.3390/jcm12165319 (2023).
Annunziata, G. et al. The role of physical exercise as a therapeutic tool to improve lipedema: A consensus atatement from the Italian society of motor and sports sciences (Società Italiana di Scienze Motorie e Sportive, SISMeS) and the Italian society of phlebology (Società Italiana di Flebologia, SIF). Curr. Obes. Rep. 13, 667–679 (2024).
Cifarelli, V. Lipedema: progress, challenges, and the road ahead. Obes. Rev. 26, e13953 (2025).
Bhowmik Bhunia, G. & Ray, U. S. Improvement in muscular strength, body flexibility and balance by yoɡasana and with reduced detraining effects by yoɡa breathing maneuvers: A non-randomized controlled study. J. Ayurveda Integr. Med. 15, 100815 (2023).
Zhang, H., Wang, J., Shuai, T., Li, K. & Nie, Y. Effects of long-term walking exercise on structural progression, symptoms, and extensor muscle strength in patients with mild or at high risk of knee osteoarthritis: data from the osteoarthritis initiative. Am. J. Phys. Med. Rehabil. 103, 603–610 (2024).
Nourollahi, S., Mondry, T. & Herbst, K. Bucher’s broom and selenium improve lipedema: a retrospective case study. Altern. Integ. Med. 2, 1–7 (2013).
Calder, P. C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans. 45, 1105–1115 (2017).
Carracedo, M., Artiach, G., Arnardottir, H. & Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 41, 757–766 (2019).
Cornely, M. E. Surgical lymphology. Therapy option for lymphoedema and lipohyperplasia dolorosa. J. Dtsch. Dermatol. Ges. 21, 147–168 (2023).
Czerwińska, M., Ostrowska, P. & Hansdorfer-Korzon, R. Lipoedema as a social problem. a scoping review. Int. J. Environ. Res. Public Health, 18, 10223 (2021).
Cifarelli, V. et al. Adipose tissue biology and effect of weight loss in women with lipedema. Diabetes 74, 308–319 (2025).
Kaoutzanis, C. et al. Cosmetic liposuction: preoperative risk factors, major complication rates, and safety of combined procedures. Aesthet. Surg. J. 37, 680–694 (2017).
Gensior, M. & Cornely, M. [Complications and their management in the surgical treatment of lipohyperplasia dolorosa]Dermatologie 74, 114–120 (2022).
Cornely, M. Fatter through lipids or water: lipohyperplasia dolorosa versus lymphedema. Am. J. Cosmet. Surg. 31, 189–195 (2014).
Peprah, K. & MacDougall, D. Liposuction for the Treatment of Lipedema: A Review of Clinical Effectiveness and Guidelines. (2019).
Tran, K.& Horton, J. Liposuction for Lipedema: 2022 Update. (2022).
Amato, A. C., Amato, J. L. & Benitti, D. Efficacy of liposuction in the treatment of lipedema: a meta-analysis. Cureus 16, e55260 (2024).
Mortada, H. et al. Safety and effectiveness of liposuction modalities in managing lipedema: systematic review and meta-analysis. Arch. Plast Surg. 51, 510–526 (2024).
Podda, M. et al. A randomised controlled multicentre investigator-blinded clinical trial comparing efficacy and safety of surgery versus complex physical decongestive therapy for lipedema (LIPLEG). Trials 22, 758 (2021).
Skuladottir, H. The National Lipedema Study. https://clinicaltrials.gov/study/NCT05284266 (2022).
Dudley, L. et al. What difference does patient and public involvement make and what are its pathways to impact? qualitative study of patients and researchers from a cohort of randomised clinical Trials. PLoS ONE 10, e0128817 (2015).
Crocker, J. C. et al. Patient and public involvement (PPI) in UK surgical trials: a survey and focus groups with stakeholders to identify practices, views, and experiences. Trials 20, 119 (2019).
Forster, K. Together we can beat lipoedema: collaboratively founding a Lipedema World Alliance. Br. J. Community Nurs. 28, S10–s13 (2023).
Nasa, P., Jain, R. & Juneja, D. Delphi methodology in healthcare research: How to decide its appropriateness. World J. Methodol. 11, 116–129 (2021).
Niederberger, M. et al. Delphi studies in social and health sciences-Recommendations for an interdisciplinary standardized reporting (DELPHISTAR). Results of a Delphi study. PLoS ONE 19, e0304651 (2024).
Spranger, J., Homberg, A., Sonnberger, M. & Niederberger, M. Reporting guidelines for Delphi techniques in health sciences: A methodological review. Z. Evid. Fortbild. Qual. Gesundhwes. 172, 1–11 (2022).
Boulkedid, R., Abdoul, H., Loustau, M., Sibony, O. & Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE 6, e20476 (2011).
Keeney, S., Hasson, F. & McKenna, H. Consulting the oracle: ten lessons from using the Delphi technique in nursing research. J. Adv. Nurs. 53, 205–212 (2006).
Vernon, W. The Delphi technique: A review. Int. J. Therapy Rehabil. 16, 69–76 (2009).
Olsen, A. A., Wolcott, M. D., Haines, S. T., Janke, K. K. & McLaughlin, J. E. How to use the Delphi method to aid in decision making and build consensus in pharmacy education. Curr. Pharm. Teach. Learn. 13, 1376–1385 (2021).
Acknowledgements
We thank the participants involved in this Delphi study. All participants were not paid an honorarium for their contributions to the Delphi study or any ensuing publication activities. The LWA Delphi-Consensus Project Team, assigned by the LWA Board members, consisting of the authors of this paper, takes full responsibility for the contents of this publication. In addition to the author team, the below specialists participated in the voting process of the Delphi methodology, but not in interpretation of the results, and agreed to be mentioned here:Amato, Alexandre; Belgrado, Jean-Paul; Boccardo, Francesco; Brenner, Erich; Burgos de la Obra, Enrique; Cannataro, Roberto; Cestari, Marina; Dimakakos, Evangelos; Drehmer Rieger, Eraci; Dudek, Joanna; Fetzer, Sharie; Forster, Kathryn Jennifer; Giordano, Valeria; Gupta, Puneet; Helmbrecht, Susanne; Hucho, Tim; Kahn, Linda Anne; Keith, Leslyn; Kollecker, Karsten; Lourenço Marques, Manuela; Majewski, Heike; Mazzolai, Lucia; Michelini, Serena; Ott, Johannes; Rapprich, Stefan; Reina Gutierrez, Lourdes; Schrader, Klaus; Schulz, Tim; Seo, Catherine; Szuba, Andrzej; Tofteng Hansen, Lene; Ure, Christian; Warrilow, Mary; Wigg, Jane; Wright, Thomas. We are also grateful to the additional specialists who participated in the Delphi study and chose to remain anonymous. Special thanks are extended to the representatives of patient advocacy organizations, whose invaluable contributions were instrumental in the successful execution of this project. Although no patient representatives were directly involved in the scientific evaluation or manuscript preparation, their feedback greatly enhanced many of the formulated statements. The following patient advocacy organizations participated in the voting process of the Delphi methodology but did not contribute to the interpretation of the results: Brazil: Associação Brasileira de Lipedema (ABRALI). Denmark: Dansk Lymfødem Forening (DALYFO). Germany: Lymphselbsthilfe e.V. Italy: LIO Lipedema Italia APS/ETS – Associazione Italiana Lipedema. Norway: Norsk lymfødem og lipødemforbund (NLLF). Portugal: Associação Nacional de Doentes Linfáticos (andLINFA). United Kingdom: Lipoedema UK. United Kingdom: Talk Lipoedema. USA: Fat Disorders Resource Society. USA: The Lipedema Project. We would also like to extend our gratitude to all healthcare professionals, and researchers affiliated with the LWA who did not participate in the voting process of the Delphi methodology, but have expressed their support and endorsement for this document: Bertelli, Matteo; Bruno, Agostino; Haag, Margareta; Henriques, Margarida; Puigdellivol, Cristina; Rajewski, Diana; Torres Gonçalves, Daniel. Furthermore, we extend our sincere appreciation to organizations affiliated with the LWA who were not directly involved in the Delphi Project but have expressed their support and endorsement for this document: France: Association de la maladie du Lipœdeme France (AMLF). Germany: LY.SEARCH. USA: American Lipedema Association. USA: Lipedema Foundation.
Author information
Authors and Affiliations
Contributions
S.M. and P.K. developed the concept and designed the survey. P.K. collected and analyzed the data and reviewed all comments submitted as part of the three survey rounds. All authors discussed the results and implications of the respective Delphi rounds and commented on statement refinements. P.K. drafted the manuscript. R.C., G.F., I.F.C., M.C., R.S., T.K., J.L.S., K.L.H., M.G., and S.M. edited and commented on the manuscript at all stages. All authors contributed to the full Delphi study and contributed to significant amendments to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Karen L. Herbst discloses her role as an independent contractor for Lympha Press. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bernard Ho, Siren Nymo and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kruppa, P., Crescenzi, R., Faerber, G. et al. Lipedema World Alliance Delphi Consensus-Based Position Paper on the Definition and Management of Lipedema: Results from the 2023 Lipedema World Congress in Potsdam. Nat Commun 17, 427 (2026). https://doi.org/10.1038/s41467-025-68232-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-68232-z