Abstract
GATA binding protein 3 (GATA3) is essential for normal development of the mammary gland and associated with ER-positive breast cancer. Loss of GATA3 has been associated with epithelial-mesenchymal transition (EMT) in experimental studies. We investigated tumoral GATA3 in a cohort of postmenopausal patients with lymph-node negative breast cancer, randomized to adjuvant tamoxifen or control. Nuclear GATA3 expression was assessed with immunohistochemistry and GATA3 gene expression with Agilent microarrays. High GATA3 nuclear expression was associated with a lower rate of distant recurrence in ER-positive breast cancer (HR = 0.60, 95% CI 0.39–0.93). Low gene expression of GATA3 was associated with limited long-term benefit from adjuvant tamoxifen (interaction: p = 0.033). GATA3 gene expression was associated with the epithelial markers CDH1 (E-cadherin) and FOXA1, whereas negatively associated with several mesenchymal markers. Low expression of CDH1 was associated with marginal tamoxifen benefit (HR = 0.80 (0.43–1.49)), whereas patients with higher expression showed a significant benefit (HR = 0.33 (0.20–0.55), interaction: p = 0.029). In ER-positive breast cancer, diminished expression of GATA3 is associated with markers of EMT and poor long-term benefit from tamoxifen.
Similar content being viewed by others
Introduction
Breast cancer commonly arises in luminal cells of the mammary gland expressing the estrogen receptor (ER). The expression of ER in the tumor is a cornerstone for the selection of adjuvant treatment. Patients with ER-positive breast cancer receiving endocrine therapy have initially a good prognosis, but studies with long-term follow-up have shown that there is a continuous risk of late relapse of the disease for these patients1,2,3. The endocrine treatment lasts for five or even ten years and there is a need for better prediction of overtreatment as well as of development of treatment resistance and late relapse.
The range of target genes regulated by ER is dependent on the phosphorylation of ER at different sites and cofactors that bind to DNA in the proximity of ER-binding sites4. GATA binding protein 3 (GATA3) and forkhead box A1 (FOXA1) are two proteins forming a strong transcriptional network together with ER. This network is required for correct development of the mammary gland5,6. ER-positive tumors often express GATA3, but a low GATA3 expression has been associated with worse prognosis7,8. However, whether GATA3 is an independent prognostic factor, and whether it predicts the benefit from tamoxifen, has yet not been settled. The importance of phosphorylated ER (pER) for the efficacy of tamoxifen has been previously studied by others and by us9,10,11,12. The receptor is phosphorylated at different sites by intracellular signaling molecules including MAP-kinase (pERser118), S6K1 (pERser167) and, PKA and PAK1 (pERser305)11,12,13,14.
Loss of functional GATA3 can arise due to mutations in the gene, which have been found in 10–15% of ER-positive breast cancer15. Mutations seldom silence the GATA3 gene- or protein expression but could alter its function. Without functional GATA3, or perhaps with too much of a function, the transcriptional landscape will change. Experimental studies suggest that loss of GATA3 expression is associated with epithelial-mesenchymal transition (EMT), which in turn might facilitate dissemination of tumor cells and the establishment of metastases16. To the best of our knowledge, the relationship between GATA3 and EMT factors in human breast tumors has not been thoroughly investigated.
The functionality of GATA3 can be disrupted by gene mutations or could be reduced by decreased gene expression or increased protein degradation. Here, we investigate the tumoral expression of GATA3 in a cohort of postmenopausal patients with lymph-node negative breast cancer, randomized to adjuvant tamoxifen or no systemic treatment. With gene expression data from the same cohort, we analyze several genes involved in EMT and cell adhesion in relation to GATA3 in ER-positive breast cancer. Furthermore, we examine the long-term prognosis and benefit from adjuvant tamoxifen in association to GATA3 and related factors.
Methods
Patient cohort
In a trial conducted by the Stockholm Breast Cancer Study Group, postmenopausal breast cancer patients with a negative lymph node status and tumor size not exceeding three cm were randomized to adjuvant tamoxifen, 40 mg daily for two years or no tamoxifen, the Stockholm tamoxifen trial STO-317. The trial recruited patients regardless of ER status. Patient entry to the study was from November 1976 to May 1990. In 1983, tamoxifen-treated recurrence-free patients were randomized, if consenting, to three more years of tamoxifen or no further treatment with tamoxifen. The follow-up period lasted at most to 30 years with a median of 22.6 years of follow-up.
Figure 1 shows a flowchart of the study cohort. For 912 of the 1780 patients in the trial, formalin-fixed paraffin-embedded tumor tissue was available and used to construct tissue micro arrays (TMAs) with three tissue cores from each tumor. Previously, data for samples on the TMA were compared with data of the original cohort of 1780 patients9. The results showed no bias with respect to tumor size, ER status, or treatment arm. The STO‐3 trial was approved by the ethics committee at Karolinska Institutet (KI) in Stockholm, Sweden, and participants provided oral consent (KI 76–51). Further, the ethics committee at KI approved retrospective studies on archived tumor tissue for the present cohort, with the purpose to evaluate prognostic and treatment predicting factors (KI 97–451 with amendments 030201 and 2017 2066-32). Further need for patient consent was waived by the ethics committee.
Biomarkers previously investigated in this cohort
Immunohistochemistry of ER, progesterone receptor (PgR) and HER2 was previously assessed18,19. Further, for ER phosphorylated at two different sites (pER), pERs167 and pERs305, data were available9,10. The antibodies used for ER, PgR and HER2 were, respectively, the CONFIRM™ mouse anti-ER antibody (clone 6F11) and the CONFIRM™ mouse anti-PR antibody (clone 16) from Ventana Medical Systems, and the DAKO AO0485 polyclonal rabbit antibody according to the guidelines provided by the manufacturer. The antibodies for pER were a rabbit polyclonal pERαser305 primary antibody (Bethyl Laboratories, Montgomery, TX) and anti-pERs167 from Cell Signaling Technologies (Danvers, MA).
GATA3 protein staining
The GATA3 protein was investigated with immunohistochemistry on TMA sections. Several studies comparing antibody sensitivity revealed the L50-823 as the most sensitive GATA3 antibody compared to another commonly used antibody, HG3-31, reviewed in Kandalaft et al.20. The PT-link station was used for deparaffinization and antigen retrieval in a low-pH buffer (K800521-2, EnVision FLEX Target Retrieval Solution, DakoCytomation, Glostrup, Denmark), starting at 65 °C, gradually increasing and ending at 96 °C for 20 min and cooled down to 65 °C. Inactivation of endogenous peroxidase in 3% hydrogen peroxide in water was followed by blocking in serum-free protein block for 10 min (DPB-125, Spring Bioscience, Freemont, CA). TMA sections were incubated in a moisturized chamber at 4 °C during 24 h with the anti-GATA3 mouse monoclonal antibody diluted 1:500 (L50-823, Merck KGaA, Darmstadt, Germany). Secondary anti-mouse antibody (K4000, DakoCytomation Envision+ HRP system, Agilent Technologies, CA) was applied for 30 min at room temperature and protein staining was developed with 3,3’-diaminobenzidine chromogen and substrate buffer, dilution 1:50, for 8 min (K3467, DakoCytomation, Agilent Technologies, CA) and counterstained with hematoxylin for 1 min. All washing steps were in phosphate buffered saline including 0.5% bovine serum albumin. The tissue was dehydrated, and cover glass was mounted with Pertex (00871, Histolab, Askim, Sweden). Slides were visualized using the Aperio CS2 brightfield digital scanner at ×400 magnification and analyzed with the ImageScope software (Leica biosystems, Buffalo Grove, IL).
GATA3 protein grading
Of the 912 tumors, 749 were successfully stained and graded for GATA3 protein expression. Nuclear staining intensity was graded in three steps: negative (0), weak (1) and strong (2). Frequency of positive tumor nuclei were scored as follows; 0% (0), 1–10% (1), 11–50% (2), 51–89% (3) and ≥90% (4). For statistical analysis, the nuclear staining was divided in low and high, with a cut-off defining the group with high expression as strong nuclear staining in >50% of tumor cells. Primary grading was performed by two independent observers (J.S. and J.B.), blinded to clinical data, and secondary grading was performed jointly by the two observers to reach consensus.
Gene expression analysis
Messenger RNA was extracted from FFPE breast tumor tissue and 652 samples were available for microarray gene-expression analysis using custom-designed arrays, containing 32.1 K probes, detecting about 21.5 K unique genes (Agilent Technologies, CA)21. The Prediction Analysis of Microarray 50 (PAM50) intrinsic subtype analysis classifier was used as described by Parker et al.22.
We selected a number of genes encoding proteins known to interact with GATA3 or EMT. FOXA1 is a strong transcriptional partner of GATA3 and NOTCH3 might upregulate GATA3. Several factors related to EMT are known from the literature. Among them, we selected E-cadherin (CDH1), an epithelial marker frequently lost in EMT. On the other hand, N-cadherin (CDH2) and the intermediate filament protein vimentin (VIM) are expressed in mesenchymal cells. In addition, we selected alpha-smooth muscle actin (ACTA2) that is associated with the TGF-β pathway, which in turn can drive the EMT process. Finally, the EMT-activating transcription factors Snail (SNAI1) and Twist-related protein 1 (TWIST1) were included in the list of genes analyzed.
Expression levels of GATA3 and other genes were analyzed by tertiles (T1-T3) in the association analyses. In the survival analyses, the lowest tertile (T1) was used as cut-off for GATA3, CDH1 and FOXA1, as the low expression was expected to stand out from the group as more aggressive. For the EMT-related genes, the cut-off was set at the highest tertile (T3), as a high expression of these genes was expected to stand out from the remaining group as more aggressive. In the multiple regression analysis, the gene-expression levels were analyzed as continuous variables.
Statistics
Statistical analyses were performed using Statistica 14 (TIBCO Software Inc.). For comparisons of GATA3 protein expression with other characteristics, the Pearson χ2 test was applied for 2 × 2 tables. For associations of GATA3 gene expression in three categories with other factors, the Spearman rank order correlation was applied. Distribution of GATA3 in the PAM50 molecular signatures was compared with the Kruskal–Wallis test. Multiple linear regression analysis was used to investigate how each mesenchymal marker could be predicted by GATA3, FOXA1 and NOTCH3. Distant recurrence-free interval (DRFI) time distributions were compared, and plots were drawn with the Kaplan–Meier method, visualizing time from randomization to first event of distant metastasis. Hazard ratios (HRs) of distant metastasis were estimated using the Cox proportional hazards model. Cox models were furthermore applied in multivariable analysis and in interaction analysis exploring the expression of genes as potential predictive factors for tamoxifen treatment benefit. A p-value of less than 0.05 was considered significant.
Results
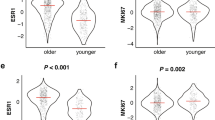
Overall, 70% of the tumors exhibited high nuclear GATA3 expression and GATA3 was strongly associated with ER status. Among ER-positive tumors, high nuclear and mRNA GATA3 expression, was seen in 84% and 41% of the cases, respectively. Corresponding numbers for ER-negative tumors were 19% and 5%, respectively (p < 0.0001 for both). Accordingly, the PAM50 molecular subtypes differed in relation to GATA3, for both nuclear and mRNA expression, with Luminal A showing the highest levels and the Basal subtype the lowest (Fig. 2). Therefore, we restricted the analyses in the following to patients with ER-positive breast cancer and the tertiles for GATA3 were from now on based on this subgroup.
Associations of GATA3 with clinicopathological variables and pER
Nuclear GATA3 was more frequently expressed at high levels in small and PgR-positive tumors (Table 1). HER2 positivity was significantly associated with low GATA3 gene expression levels and a similar trend was seen for GATA3 protein expression (Tables 1 and 2). Both protein and gene expression levels of GATA3 were associated with ER phosphorylated at serine-305 (pERs305), whereas the association of GATA3 with pERs167 was less clear.
There was a significant correlation between GATA3 mRNA and protein levels (Table 1), but this relationship showed differences dependent on pER. For tumors with a positive status of pERs167, nuclear GATA3 was frequently highly expressed also at low GATA3 mRNA levels, resulting in no correlation between gene and protein expression levels.
On the other hand, the subgroup of pERs167- negative tumors showed a significant gene to protein correlation (Fig. 3). In contrast, the status of pERs305 did not markedly affect the correlation between gene and protein expression levels.
Associations of GATA3 with epithelial and mesenchymal biomarkers
Based on previous experimental research showing that the loss of GATA3 may contribute to EMT, we next analyzed the relationship between GATA3 and the expression of several genes associated with this process. Comparing gene-expression levels, GATA3 was negatively correlated with all the mesenchymal biomarkers investigated, including ACTA2, CDH2, SNAI1, TWIST1 and VIM (Table 3). For GATA3 protein levels, the same was true for three of the biomarkers. Furthermore, the gene expression of GATA3 was positively associated with CDH1, FOXA1, and NOTCH3.
Since the transcription factors GATA3 and FOXA1 have been suggested to inhibit EMT, and NOTCH3 could be a contributing factor, we performed multiple regression analysis to investigate these factors as independent predictors of CDH1 and the mesenchymal markers, respectively (Table 4). GATA3 turned out to be the variable that most consistently correlated with the markers investigated.
Distant recurrence-free interval in relation to GATA3
Patients with high tumoral nuclear GATA3 expression had a longer DRFI than those with low GATA3 levels (HR = 0.60, 95% CI 0.39–0.93, p = 0.023, Fig. 4a). When adjusting for treatment and other tumor characteristics, including tumor size, PgR and HER2, the statistical significance for GATA3 was lost (HR = 0.81, 95% CI 0.50–1.31, p = 0.39), whereas tumor size (>20 mm vs ≤20 mm; HR = 2.16, 95% CI 1.39–3.36, p = 0.00067) and tamoxifen (HR = 0.45, 95% CI 0.30–0.67, p < 0.0001) were significant. We did not find a significant association of GATA3 gene expression levels with DRFI (T2-T3 vs T1; HR = 1.06, 95% CI 0.71–1.57, p = 0.78, Fig. 4b).
The benefit from adjuvant tamoxifen in relation to GATA3 and related factors
Given that GATA3 and ER interact, the benefit from tamoxifen could potentially be dependent on expression levels of GATA3. Whereas patients with ER-positive tumors with intermediate to high GATA3 mRNA levels showed significant benefit from tamoxifen (HR = 0.39 (0.24–0.64), p = 0.00014), those with levels in the bottom tertile did not as evidently benefit (HR = 0.61 (0.31–1.17), p = 0.14) (Fig. 5). The difference was more pronounced when considering the long-term prognosis. For patients still alive and without a distant recurrence after five years, there was no further benefit from tamoxifen in the group with low GATA3 (HR = 1.10 (0.46–2.61), p = 0.83), in contrast to the group with higher levels (HR = 0.35 (0.19–0.64), p = 0.00064). A test for interaction between GATA3 and tamoxifen for this period was significant (p = 0.033). The efficacy of tamoxifen did not significantly differ for patients with low or high tumoral nuclear GATA3 protein expression (HR = 0.53 (0.23–1.23) and HR = 0.52 (0.34–0.79), respectively), however the number of patients in the former group was small.
The GATA3-associated genes CDH1 and FOXA1 in addition tended to predict the efficacy of tamoxifen. Patients with CDH1 levels in the bottom tertile experienced no evident benefit from tamoxifen (HR = 0.80 (0.43–1.49), p = 0.49), whereas those with higher levels did (HR = 0.33 (0.20–0.55), p = 0.000021), and the interaction was significant (p = 0.029, Fig. 6a, b). Although a similar interaction between FOXA1 and tamoxifen did not reach statistical significance (p = 0.22), the benefit from the treatment was more evident in the group with higher levels as compared with the group with low levels (HR = 0.41 (0.26–0.63), p = 0.000075) and (HR = 0.71 (0.32–1.58), p = 0.40), respectively, (Fig. 6c, d). Moreover, the EMT biomarkers CDH2 and VIM tended to predict less tamoxifen benefit when expressed at higher levels as compared to low levels (Fig. 6E–H). For the remaining EMT markers, the benefit from tamoxifen was similar comparing groups with low/intermediate versus high levels (tests for interaction, all p > 0.7).
DRFI for the tamoxifen and controls groups in patients with low (a) and medium/high tumor CDH1 (b), in patients with low (c) and medium/high tumor FOXA1 (d), in patients with low (e) and medium/high tumor CDH2 (f), in patients with low (g) and medium/high tumor VIM (h). Hazard ratio (HR), tamoxifen (TAM), tertile (T).
Discussion
GATA3, known as a marker used to identify mammary or urothelial origin of metastases from unknown primary tumors, is widely expressed in breast tumors and has been suggested as a potential prognostic and/or treatment predictive biomarker23,24. A quantitative decrease or functional loss of GATA3 seems to interfere with the characteristics of the tumor25. In line with previously published data, we see a distinct nuclear expression of GATA3 in more than 80% of ER-positive tumors as compared to in only about 20% of the ER-negative tumors7,26,27. GATA3 positivity was largely associated with a PAM50 Luminal subtype, supporting its functional role as co-regulator of the ER and its association with differentiation.
The ER protein is phosphorylated at distinct sites when regulated. The tight connection between ER and GATA3 suggests that the two proteins may regulate each other. We found a significant association between pERs305 and high GATA3 expression. We suggest that this might be related to PKA, which, besides its involvement in ERs305 phosphorylation11, has been shown to interact with GATA328. Overall, there was a strong association of GATA3 gene expression with GATA3 protein expression. Interestingly, in tumors phosphorylated at ERs167, GATA3 gene- and protein expression did not correlate. Hypothetically, a stabilization of the GATA3 protein could be affected by intracellular signaling proteins associated with ER phosphorylation, supported by the finding that MAPK controls GATA3 protein stability by a post-transcriptional mechanism29. Furthermore, S6K1, that phosphorylates ER at serine 167, interacts with ER in a positive feedback loop also involving GATA330.
We found low GATA3 protein levels to be significantly associated with increased risk of distant recurrence when compared to high GATA3 protein levels in the group of patients with ER-positive tumors. Mehra et al. reported already in 2005 that detection of GATA3 with immunohistochemistry could predict outcome of breast cancer, also when adjusting for other prognostic factors8. Several studies have found similar results7,27,31, although the independent prognostic value was absent in one of the studies when the analysis was restricted to ER-positive breast cancer31, similar to our results. Moreover, GATA3 was not independently prognostic in a huge study comprising more than 3000 patients32. We did not see a prognostic value of GATA3 gene-expression levels in the present cohort. This contrasts with some other gene-expression studies with microarray data, reviewed by Fang et al, showing that GATA3 is prognostic33. Taken together, GATA3 appears as a favorable prognostic factor, but the question of its importance as an independent factor needs further elucidation, considering the choice of cut-off for positivity as well as the influence of breast cancer therapy.
GATA3 is highly correlated with ER and thus a potential predictive marker of benefit from hormonal therapy. Using a breast cancer model with parental and tamoxifen-resistant MCF7 cells, endocrine resistance was associated with downregulation of luminal/epithelial differentiation markers and upregulation of basal/mesenchymal invasive markers34. One important factor for the transcriptional landscape was GATA3. Further studies have indicated that GATA3 counteracts EMT. The protein complex GATA3/G9A/MTA3 represses ZEB2, and other genes involved in EMT, leading to suppression of metastasis from human breast cancer cells in mice. In turn, ZEB2 repressed the expression of G9A and MTA335. Moreover, ectopic expression of GATA3 in GATA3-negative triple-negative breast cancer cells led to increased CDH1 expression and decreased expression of some mesenchymal markers16. A similar transcriptional change could also be related to mutations of GATA336. Here, the results give support for the experimental findings of an inverse relationship between GATA3 and EMT in a large series of ER-positive tumors. GATA3 expression correlated with high expression of E-cadherin and FOXA1 and low expressions of all five mesenchymal markers investigated.
Besides a prognostic value, one could ask whether GATA3 is a treatment predictive factor. In a minor series of 28 ER-positive cases of breast cancer, lack of GATA3 expression was associated with unresponsiveness to hormonal therapy37. Furthermore, GATA3 mRNA expression was associated with longer progression-free survival in patients with ER-positive breast cancer treated with first-line tamoxifen for recurrent disease38. To the best of our knowledge, there are no previous reports on the relationship of GATA3 with the efficacy of adjuvant endocrine therapy based on a randomized trial. In the present study, we were able to show that a substantial benefit from tamoxifen was restricted to patients with intermediate/high GATA3 mRNA expression, most evident when focusing on late relapse. We found similar patterns for FOXA1 and E-cadherin, both of which are closely related to GATA3, whereas CDH2 (N-cadherin) and VIM (vimentin) tended to show opposite associations. When the transcriptional landscape of ER is altered upon loss of GATA3, one might speculate that the anti-tumoral effects of tamoxifen is diminished.
One strength with this study is that it is based on a randomized clinical trial and long-term follow-up. The study limitations include that the cohort is confined to postmenopausal patients with lymph-node negative disease, and it is not known if the results related to tamoxifen benefit can be translated to the use of aromatase inhibitors. Another limitation is that we lack data on GATA3 mutations for this cohort. GATA3 is the third most mutated gene in luminal breast cancer. In part, the results of the present study might be applicable to tumors with GATA3 mutation as such mutations affect gene transcription patterns and EMT36,39,40.
In conclusion, GATA3 expression is associated with ER-positive breast cancer and particularly with the Luminal A subtype. Diminished expression of GATA3 in ER-positive tumors is associated with changes of gene expression resembling EMT. Both such changes and GATA3 expression itself were related to the efficacy of adjuvant tamoxifen therapy.
Data availability
Restrictions apply to the availability of these data according to GDPR. Data were obtained from the STO Trialist Group and are available from the authors with the permission from the STO Trialist Group.
References
Pan, H. et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 377, 1836–1846 (2017).
Dar, H. et al. Assessment of 25-Year Survival of Women With Estrogen Receptor-Positive/ERBB2-Negative Breast Cancer Treated With and Without Tamoxifen Therapy: A Secondary Analysis of Data From the Stockholm Tamoxifen Randomized Clinical Trial. JAMA Netw. Open 4, e2114904 (2021).
Johansson, A. et al. Twenty-Year Benefit From Adjuvant Goserelin and Tamoxifen in Premenopausal Patients With Breast Cancer in a Controlled Randomized Clinical Trial. J. Clin. Oncol. 40, 4071–4082 (2022).
Faus, H. & Haendler, B. Post-translational modifications of steroid receptors. Biomed. Pharmacother. 60, 520–528 (2006).
Kouros-Mehr, H., Slorach, E. M., Sternlicht, M. D. & Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127, 1041–1055 (2006).
Chou, J., Provot, S. & Werb, Z. GATA3 in development and cancer differentiation: cells GATA have it! J. Cell Physiol. 222, 42–49 (2010).
Yoon, N. K. et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum. Pathol. 41, 1794–1801 (2010).
Mehra, R. et al. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res. 65, 11259–11264 (2005).
Bostner, J., Skoog, L., Fornander, T., Nordenskjold, B. & Stal, O. Estrogen receptor-alpha phosphorylation at serine 305, nuclear p21-activated kinase 1 expression, and response to tamoxifen in postmenopausal breast cancer. Clin. Cancer Res. 16, 1624–1633 (2010).
Bostner, J. et al. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res. Treat. 137, 397–406 (2013).
Kok, M. et al. PKA-induced phosphorylation of ERalpha at serine 305 and high PAK1 levels is associated with sensitivity to tamoxifen in ER-positive breast cancer. Breast Cancer Res. Treat. 125, 1–12 (2011).
Rayala, S. K., Molli, P. R. & Kumar, R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 66, 5985–5988 (2006).
Kato, S. et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270, 1491–1494 (1995).
Yamnik, R. L. et al. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J. Biol. Chem. 284, 6361–6369 (2009).
Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Yan, W., Cao, Q. J., Arenas, R. B., Bentley, B. & Shao, R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J. Biol. Chem. 285, 14042–14051 (2010).
Rutqvist, L. E., Johansson, H. & Stockholm Breast Cancer Study, G. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 46, 133–145 (2007).
Khoshnoud, M. R. et al. Immunohistochemistry compared to cytosol assays for determination of estrogen receptor and prediction of the long-term effect of adjuvant tamoxifen. Breast Cancer Res. Treat. 126, 421–430 (2011).
Jerevall, P. L. et al. Predictive relevance of HOXB13 protein expression for tamoxifen benefit in breast cancer. Breast Cancer Res. 12, R53 (2010).
Kandalaft, P. L., Simon, R. A., Isacson, C. & Gown, A. M. Comparative Sensitivities and Specificities of Antibodies to Breast Markers GCDFP-15, Mammaglobin A, and Different Clones of Antibodies to GATA-3: A Study of 338 Tumors Using Whole Sections. Appl. Immunohistochem. Mol. Morphol. 24, 609–614 (2016).
Esserman, L. J. et al. Use of Molecular Tools to Identify Patients With Indolent Breast Cancers With Ultralow Risk Over 2 Decades. JAMA Oncol. 3, 1503–1510, (2017).
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009).
Khazaeli Najafabadi, M. et al. Role of GATA3 in tumor diagnosis: A review. Pathol. Res. Pr. 226, 153611 (2021).
Bonacho, T., Rodrigues, F. & Liberal, J. Immunohistochemistry for diagnosis and prognosis of breast cancer: a review. Biotech. Histochem. 95, 71–91 (2020).
Kouros-Mehr, H. et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13, 141–152 (2008).
Gulbahce, H. E. et al. Significance of GATA-3 expression in outcomes of patients with breast cancer who received systemic chemotherapy and/or hormonal therapy and clinicopathologic features of GATA-3-positive tumors. Hum. Pathol. 44, 2427–2431 (2013).
Querzoli, P. et al. GATA3 as an Adjunct Prognostic Factor in Breast Cancer Patients with Less Aggressive Disease: A Study with a Review of the Literature. Diagnostics 11, https://doi.org/10.3390/diagnostics11040604 (2021).
Bouchard, M. F., Taniguchi, H. & Viger, R. S. Protein kinase A-dependent synergism between GATA factors and the nuclear receptor, liver receptor homolog-1, regulates human aromatase (CYP19) PII promoter activity in breast cancer cells. Endocrinology 146, 4905–4916 (2005).
Yamashita, M. et al. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J. Biol. Chem. 280, 29409–29419 (2005).
Maruani, D. M. et al. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene 31, 5073–5080 (2012).
Jacquemier, J. et al. Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res. 11, R23 (2009).
Voduc, D., Cheang, M. & Nielsen, T. GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol. Biomark. Prev. 17, 365–373 (2008).
Fang, S. H., Chen, Y. & Weigel, R. J. GATA-3 as a marker of hormone response in breast cancer. J. Surg. Res. 157, 290–295 (2009).
Bi, M. et al. Enhancer reprogramming driven by high-order assemblies of transcription factors promotes phenotypic plasticity and breast cancer endocrine resistance. Nat. Cell Biol. 22, 701–715 (2020).
Si, W. et al. Dysfunction of the Reciprocal Feedback Loop between GATA3- and ZEB2-Nucleated Repression Programs Contributes to Breast Cancer Metastasis. Cancer Cell 27, 822–836 (2015).
Takaku, M., Grimm, S. A., De Kumar, B., Bennett, B. D. & Wade, P. A. Cancer-specific mutation of GATA3 disrupts the transcriptional regulatory network governed by Estrogen Receptor alpha, FOXA1 and GATA3. Nucleic Acids Res. 48, 4756–4768 (2020).
Parikh, P., Palazzo, J. P., Rose, L. J., Daskalakis, C. & Weigel, R. J. GATA-3 expression as a predictor of hormone response in breast cancer. J. Am. Coll. Surg. 200, 705–710 (2005).
Liu, J. et al. GATA3 mRNA expression, but not mutation, associates with longer progression-free survival in ER-positive breast cancer patients treated with first-line tamoxifen for recurrent disease. Cancer Lett. 376, 104–109 (2016).
Saotome, M., Poduval, D. B., Nair, R., Cooper, M. & Takaku, M. GATA3 Truncation Mutants Alter EMT Related Gene Expression via Partial Motif Recognition in Luminal Breast Cancer Cells. Front. Genet 13, 820532 (2022).
Cohen, H. et al. Shift in GATA3 functions, and GATA3 mutations, control progression and clinical presentation in breast cancer. Breast Cancer Res. 16, 464 (2014).
Acknowledgements
This study was funded by the Swedish Cancer Society (Cancerfonden, grant No. 190268 to O.S. and grant No. 222081 and 220552SIA to L.S.L., ALF medicine (grant number Lio-795201, O.S. and grant number FoUI-974882 to L.S.L.), Onkologiska Klinikernas i Linköpings forskningsfond, 2019 to J.S., Swedish Research Council (Vetenskapsrådet, grant number 2020-02466 to L.S.L.). The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Funding
Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Contributions
Conception and design: J.S., O.S. Data acquisition: J.S., J.B., B.N., T.F., L.S.L. Formal analysis: J.S., J.B., O.S. Data interpretation: all authors. Funding acquisition: J.S., O.S., L.S.L. Writing—original draft: J.S., O.S. Writing—review & editing: all authors. The final version was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sandström, J., Bomanson, J., Pérez-Tenorio, G. et al. GATA3 and markers of epithelial-mesenchymal transition predict long-term benefit from tamoxifen in ER-positive breast cancer. npj Breast Cancer 10, 78 (2024). https://doi.org/10.1038/s41523-024-00688-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-024-00688-6