Abstract
Singapore has advanced in precision medicine, which is largely based on genetic testing and sequencing, yet its safeguard against genetic discrimination (GD) is limited to a non-binding insurance moratorium, with no protections in employment. This study examined the prevalence of self-reported GD and factors influencing willingness to undergo genetic testing in Singapore. A cross-sectional survey assessed experiences of GD, awareness of protections and testing willingness. Twenty percent reported GD in insurance and 9% in employment. The majority identified existing safeguards incorrectly. Sixty-four percent expressed willingness to undergo medically indicated genetic testing. Willingness was positively associated with education, trust in healthcare and perceived fair treatment and negatively associated with age, parental status, deterministic thinking and cultural-religious beliefs. The results highlight that, though policymakers aim to mitigate GD in Singapore, enhanced legal protections and public education are needed to support equitable access to genetic testing.
Similar content being viewed by others
Introduction
The concept of genetic discrimination (GD) has received increasing social and academic attention since its emergence in the 1970s1. UNESCO’s Declaration on the Human Genome and Human Rights (1997) posits that people shall be free from discrimination based on genetic characteristics that infringes ‘human rights, fundamental freedoms and human dignity’2. GD may arise in different aspects of life, including insurance, employment, healthcare, romantic relationships, marriage and family and its salience may differ across societies3,4. Importantly, not all differential treatment constitutes discrimination in the ethical sense. According to one reasonable ethical standpoint, discrimination occurs when individuals are treated differently despite having no meaningful moral distinctions that would justify such different treatment. A classic case would be not hiring someone for a job purely based on their sex or race. It is important to prevent discrimination like this because it is an injustice.
Despite Asia leading in the development of genetic technologies, its adoption of policies to address GD has progressed slowly. To date, only two countries in East Asia—South Korea and Japan—have enacted specific laws targeting GD. The first was the South Korean Bioethics and Safety Act of 2005. It has a clause that prohibits the use of genetic information in insurance underwriting and imposes criminal sanctions for violations, which can deter some instances of GD in insurance. While this law marked a significant step forward, it lacks a detailed implementation framework or explicit rules on which types of insurance are covered or excluded. Moreover, it does not prohibit insurers from merely ‘requesting’ for clients’ genetic information. Given that the law allows Direct-to-Consumer (DTC) testing, insurers can still treat clients differently based on such results5. The second GD law in Asia was Japan’s Genomic Medicine Promotion Act, which was enacted much later in 2023. It provides extensive protections for genomic information obtained through research and medical services5. In addition to this law, the Life Insurance Association of Japan has also maintained self-imposed restrictions on collection and voluntary submission of all genetic information including family medical history for underwriting purposes6. Genetic testing uptake has risen significantly in Japan since 2019 when national insurance coverage for the Cancer Gene Panel Test was introduced7. Nevertheless, concerns about discrimination by insurance companies persist, prompting Japan’s Ministry of Finance to warn insurers against requesting genetic tests or requiring genetic testing from clients8,9.
Research on GD has so far been conducted mostly in Europe and Northern America, with limited studies in Asia (as well as other regions of the world). Countries like Japan have a history of draconian eugenic programmes4, which may make people reluctant to reveal their genetic information and, in turn, negatively affect the accuracy of statistics for genetic conditions like Huntington’s disease or variations in the BRCA gene10,11. With existing genetic research being predominantly based on Eurocentric data12, people in Asia may be disadvantaged. There is an urgent need for studies focusing on genetically diverse populations to improve global health predictions. A potential barrier to genetic testing in Asia is the social stigma associated with genetic conditions and the accompanying GD, which is a pattern observed in countries such as China, Japan, South Korea and Singapore3,4,10,13.
Given this context, research on GD and people’s willingness to undergo genetic testing among the Asian population is much needed. This study focuses on Singapore, a country known for its ethnic and cultural diversity and often seen as a bridge between the ‘East’ and the ‘West’6,14. It aims to address the knowledge gap in Asian perspectives in GD research and offer insights into how GD may be shaped by Singapore’s distinct social context.
The next section describes the Singaporean legal landscape surrounding GD, the research questions and the study methods. In the following two sections we present the empirical results of this study and discuss the practical and policy significance of the results.
Results
Participant demographics
Descriptive statistics of participant demographics are summarised in Table 1. While the HOPS panel is designed to broadly reflect the general population in Singapore, the 1000 participants of this survey generally had higher education qualifications and were less ethnically diverse than the national average. Fifty-three percent of the surveyed participants held a bachelor’s degree or higher, while the national average (among those above 25 years old) is 37%. Eighty-seven percent of participants self-identified as Chinese, 7% Indian, 4% Malay and 2% other ethnic groups, whereas the national average is 74%, 9%, 14% and 3% respectively15.
Prevalence and lived experience of GD
As shown in Table 2, the most common forms of reported GD in the insurance setting were higher premium charges and rejection of insurance applications. A total of 726 (72.6% of the total) participants indicated that insurance companies never learnt about their genetic information. Furthermore, 206 (20.6% of the total) indicated experiencing at least one form of reported GD by insurance companies. For example, a participant reported being charged a higher insurance premium and expressed reluctance to undergo genetic tests despite potential benefits. This participant also indicated in the free text response ‘I am just a carrier, so it won’t affect my work’. Though we do not know what condition this participant is a carrier of, the response still suggests that differential treatment based on one’s genetic information in insurance exists, and the mere knowledge of a client’s carrier status suffices to prompt the insurer to charge a higher premium.
As shown in Table 3, when it comes to employment, the most common form of reported GD was denied job applications. In total, 57 (5.7% of the total) participants indicated at least one form of reported GD by employers. 836 (83.6% of the total) participants reported that employers never learnt about their genetic information.
Table 4 presents results on the prevalence of perceived GD within family and romantic relationships. Over half (55–58%) did not disclose their genetic information. Among those who disclosed their genetic information, ~12–14% of participants experienced perceived GD that negatively impacted their romantic relationships, while 8–9% experienced perceived GD within their family relationships or acquaintances. For example, one participant (female, age 65) revealed that she was a Down syndrome translocation carrier and only her family knew about it. She indicated that her family relationships worsened after revealing her genetic information to her family.
Table 5 illustrates perceived GD by healthcare professionals, courts, landlords, banks and schools. Compared to perceived GD in employment, insurance, or personal relationships, perceived GD in these sectors was less prevalent, with only 3–8% of participants reporting negative experiences from disclosing their genetic information. Notably, 66 participants indicated receiving better treatment from healthcare professionals after disclosing their genetic information. Upon cross-referencing with free-text responses, we inferred that such reports most plausibly reflect cases where healthcare professionals accommodated patients’ medical needs rather than providing preferential treatment. For instance, one participant stated, ‘as a [patient with a genetic condition] … I would tell them [healthcare professionals] my tendency to faint at blood loss and they have always made accommodations for me (male, age 32).’ While not all participants provided free-text responses, it is reasonable to interpret these findings as healthcare professionals responding to medical conditions rather than conferring advantages based on genetic status.
Knowledge of existing protections and misconceptions
Our findings suggest substantial misconceptions about GD protection in Singapore. Across all questions measuring participants’ awareness of the existing GD protections (and the lack thereof) in Singapore shown in Table 6, fewer than half of the participants answered correctly.
When it comes to insurance, only 49% of the participants correctly answered that medical and life insurers cannot require applicants to take a genetic test. Misunderstanding was also high regarding genetic test results from research, with only 46% answering correctly and the majority (54%) either providing incorrect answers or being unsure. Only 20% and 18% correctly answered that health and life insurers, respectively, are allowed to ask for diagnostic genetic test results, indicating a widespread lack of awareness about the nuances of insurance regulations regarding genetic data.
Additionally, participants may have been confused about the conditions under which they are required to disclose genetic information for insurance. Specifically, they may not clearly understand the double-key model in the moratorium (which permits the use of predictive genetic test results only when specific financial and test-related conditions are met as shown in Fig. 1), leading to incorrect responses. The complexity of these conditions may not be well understood by the public, contributing to the uncertainty and misconceptions observed in our findings.
*At the time of revising this manuscript, the Moratorium was updated. The revised moratorium protects results of any genetic tests (predictive or diagnostic) conducted under the national Familial Hypercholesterolaemia (FH) genetic testing programme from being used in insurance underwriting55.
Regarding employment, participants’ misconception about protection against GD was even more prevalent (as shown in Table 6). Only 15–18% correctly answered that treating workers differently based on their genetic information in employment currently does not violate either the fair employment practices or the workplace fairness law. This suggests that most participants had a false sense of security that they are protected against GD in the workplace when they are, in fact, not protected.
Demographic variables and willingness to undergo genetic testing
When participants were asked about willingness to undergo genetic testing ordered by a physician for medical reasons with cost fully covered by Medisave, 64% expressed willingness to undergo testing, 20% remained neutral and 16% were unwilling.
To examine the correlation between willingness to undergo testing and demographic variables, as well as the four potential correlating factors explained at the end of section ‘Introduction’, we conducted ordinal regressions. A total of ten ordinal regressions were performed, with willingness to undergo testing as the dependent variable. Six of these regressions included demographic variables (age, education, race, religion, housing type and marital status) as independent variables, while the remaining four included four potential correlating factors. Two demographic variables, gender and parental status (having children) were binary; therefore, we used the Mann-Whitney U test to assess differences in willingness to undergo testing between these groups. The analysis was performed using the package MASS in R (version 4.3.3)16,17.
As shown in Table 7 and Table 8, age, education level and parental status were the only demographic factors that showed statistically significant correlation with willingness to undergo genetic testing. Specifically, older individuals exhibited lower willingness to undergo testing, while individuals without children and those with higher levels of education exhibited greater willingness.
Given that both age and parental status showed statistically significant correlations with willingness to undergo genetic testing, there is a need to check whether the greater willingness to undergo testing by those without children could be due to their younger age. We did another ordinal regression Testing Willingness ~ Having Child(ren) + Age, the result of which is shown in Table 9. Both parental status and age are statistically significant predictors of testing willingness, suggesting that each has an independent association with willingness.
As shown in Table 10, all four potential correlating factors measured in this study showed statistically significant correlations with willingness to undergo genetic testing. Specifically, trust in Singapore’s healthcare system and perception of fair treatment were positively correlated with willingness to undergo genetic testing. In contrast, deterministic thinking about genetics and cultural-religious beliefs against genetic testing were negatively correlated with willingness to test.
Discussion
Our results indicate that ~20% of participants encountered at least one form of reported GD from insurance companies, while around 6% experienced at least one form of reported GD in employment. In Japan, a national survey by Muto et al. found only a 3% prevalence of (reported) GD in 2017 and 20224. Due to the limited academic attention on GD in Asia, we found no other comparable population-based data from the region in English, Chinese, or Japanese.
Notably, previous studies on individuals at risk of Huntington’s disease—arguably a group more vulnerable to GD—showed a similar level of prevalence of reported and perceived GD as our study. For example, Erwin et al.18 found that 25.9% of individuals at risk of Huntington’s experienced GD in insurance, 6.5% in employment and 32.9% in personal relationships. Since those studies were conducted on a sample of people more vulnerable to GD and our study was conducted on a general population sample, the similarity in the prevalence again indicates that the prevalence found in this study may be higher than usual.
We believe that it is not plausible to attribute the high prevalence of reported and perceived GD in our study to survey design. Our questions were modelled after Muto et al. whose instrument also used multiple-choice formats to assess specific discriminatory experiences19,20. Both studies also defined genetic information broadly to include family medical history. The similarity in survey structure lends confidence that our results are not an artefact of design but reflective of the prevalence of reported and perceived GD among the general population.
Indeed, the lack of robust protections against GD in Singapore as well as the lack of transparency in and public scrutiny over the insurance sector are conducive to GD, particularly in the insurance sector. As previously mentioned, the exceptions within the moratorium on using genetic information for insurance underwriting unwittingly create opportunities for discrimination. In comparison, there has been strong public advocacy for statutory safeguards against GD in Japan over the past decade21. This period of heightened public and political engagement may have served as a deterrent against discriminatory practices in Japan, encouraging greater compliance even in the absence of strict legal enforcement. Moreover, the persistence of reported GD in insurance despite the moratorium found in this study mirrors the findings of another Australian study, where participants with adverse genetic test results also reported being denied insurance even though there is an insurance moratorium similar to that in Singapore22. After that study, Australia declared that it will ban the use of adverse genetic test results in life insurance23, suggesting its government’s recognition of the inadequacy of only relying on a moratorium to prevent GD.
One risk of measuring reported and perceived GD through a survey is the possibility of underreporting. Cultural norms may influence the disclosure of the experience of GD. Singapore is influenced by Confucian values that prioritise social harmony, which may cause some to avoid expressing their grievances24,25,26. The Japanese may also have a lower willingness to confront opposing views and to express their feelings27, which may also have led to underreporting in Muto et al.’s survey. Some forms of GD may have been deemed normal and are therefore unrecognised. For example, in our study, one participant noted, ‘I am just a carrier, so it won’t affect my work. Got my children to be tested and advised them accordingly.’ This individual also reported being charged a higher insurance premium and expressed reluctance to undergo further genetic testing. This participant did not frame the experience as undesirable. This raises the suspicion that some people may rationalise or downplay the GD they experience, which can lead to underreporting.
This study is, to our knowledge, the first to assess the prevalence of perceived GD in settings such as education, housing, the judiciary and banking using a general population sample. Existing studies in these areas focused primarily on individuals with known or suspected genetic conditions18,28,29,30, while our study focused on the general population. Our findings offer a baseline for understanding (perceived) GD beyond insurance and employment in Singapore, paving the road for subsequent research and policy in this area. We found that the prevalence of perceived GD in this area was relatively low. When interacting with schools and medical professionals, people reported being treated better after disclosure of genetic information, which may suggest that their needs were accommodated.
Beyond institutional settings, 3–6% of participants perceived GD in their families or romantic relationships after disclosing genetic information—much higher than the 0.1–0.3% found by Uchiyama et al. in Japan19. This discrepancy points to potential differences in how disclosure of genetic information affects personal relationships in Singapore compared to other societies. Future research could explore how cultural expectations around health, heredity and marriage shape these outcomes.
As genetic information becomes increasingly important for various aspects of medical decisions, there has also been increasing research interest in public willingness to undergo genetic testing. 64% of participants in this study indicated a willingness to undergo genetic testing when ordered by a physician for medical reasons and assuming the cost was covered by Medisave. This is lower than the results of a Qatari (71%) and an international (82%) study31,32. However, these figures are not strictly comparable due to differences in how questions were framed. For instance, the Qatari study framed the testing as part of a nation-wide initiative, while the international study explicitly highlighted the potential benefits of the test. Our study, in contrast, referred to testing ‘for medical reasons’ and made the cost implications explicit. These differences may partly explain the comparatively lower willingness in our findings. Future studies could standardise question phrasing or develop validated instruments to improve comparability across studies.
Our analysis revealed that willingness to undergo genetic testing is negatively associated with age and positively associated with education level. Additionally, individuals without children showed higher willingness to undergo testing. These findings are in line with previous studies finding that younger individuals demonstrated greater interest in cancer genetic testing, possibly due to lower fatalism33. Willingness to undergo genetic testing for hypertension has also been found to correlate with a higher level of education34. However, a literature review of genetic testing decisions found inconsistent associations between willingness and demographic variables across different studies with various genetic conditions35. This suggests that socio-cultural and historical contexts may influence such correlations, and a universal pattern may not be evident globally.
As noted in Table 10, four psychosocial factors were statistically significantly correlated with willingness to undergo genetic testing: trust in Singapore’s healthcare system, perceived fair treatment, cultural-religious beliefs against testing and deterministic views of genetics. The first two were positively associated with willingness, while the latter two showed negative correlations. Previous reports by clinicians in Singapore mention cultural, religious or personal beliefs as factors against genetic testing uptake13. These findings suggest that enhancing public trust, addressing misconceptions and engaging with cultural or spiritual concerns may increase willingness to test. Future research should investigate the causal direction of these relationships. If any of these factors are shown to directly influence willingness, targeted policies and outreach efforts could be developed to support informed and equitable access to genetic testing.
Since this study found a high prevalence of reported GD, particularly in insurance and possibly employment, the first practical implication of our findings is that the current moratorium has not eliminated GD in Singapore. To the extent policymakers are interested in reducing instances of GD, stronger legal protections are required36,37. Indeed, similar legislations are already present in other countries. For example, GINA prohibits GD in employment and health insurance. Canada’s Genetic Non-Discrimination Act (GNDA, 2017) prohibits the compelled use of genetic test results—including in life insurance—except in cases of voluntary disclosure with written consent38. These laws take a pragmatic approach, aiming to reduce public fears and promote broader participation in genetic testing39. In contrast, Japan’s legal framework, which includes life insurance industry self-regulation, adopts a more justice-based approach, prohibiting the use of all genetic information—including family history—in underwriting decisions40.
A spectrum of types of potential policy offering varying levels of protection against GD is shown in Table 11 and Table 12. The tables categorise policies into weak, moderate and strong protections in life insurance and employment contexts. They also outline the advantages and disadvantages of protection at each level. Note that we discuss these types of policy very broadly. In actual policies, what genetic information can be protected is contingent on the details of the policy (e.g. treating family medical history as genetic information is more feasible if the policy only applies to insurance than if it also applies to areas like social services). We do not consider such nuanced details here.
It is worth noting that there is limited evidence that pragmatic approaches have succeeded in promoting testing uptake, since public understanding of legal protections in some jurisdictions with anti-GD laws such as the US remains limited. Nevertheless, such laws may be effective in preventing some cases of unjust or unethical discrimination even with low public awareness. For example, a decade after the passing of GINA, respondents reported ‘relatively low subjective knowledge’ of GINA (M = 3.10 out of 7)41. Among those who claimed high knowledge, most misunderstood what types of insurance are covered under GINA41. When it comes to GNDA, an analysis of contents of life-insurance application forms reveals that the law had only a modest effect on insurers’ practice. This is because most companies use vague language that may inadvertently prompt applicants to disclose their genetic information42. To address this issue, public education on the potential advantages of taking insurance prior to genetic or other medical testing may be helpful. Governmental monitoring of insurance companies’ risk-loading practices may be needed to ensure that any increased premium is commensurate with the level of risk. Employers may be scrutinised to ensure that their use of genetic information in employment is relevant to public safety.
A more stringent regulatory approach, such as a total ban on the use of genetic information in insurance and employment, though entailing substantial bureaucratic burden, has its advantages. It may alleviate public reluctance to undergo genetic testing. From a distributive justice perspective, treating people differently based on genetic information may compound existing social inequalities and affect vulnerable individuals disproportionately. In a diverse society like Singapore, protections against the above consequences are important for ensuring that no one is unfairly disadvantaged by their genetic makeup and maintaining social harmony. Moreover, regardless of the actual prevalence of GD, a strict anti-GD law combined with adequate public education provides a strong assurance that undergoing genetic tests will not disadvantage people in their insurance application or work, which may increase the public willingness to undergo such tests. It may also enhance public trust in the government and, in turn, willingness to undergo testing in public healthcare institutions. This discussion does not suggest that such a total ban is definitively the best policy choice, but only serves to point out its advantages over strategies such as a partial ban explained above.
The second implication concerns efforts to promote equitable access to genetic testing. While 64% of participants were willing to undergo testing when cost was covered, a significant 36% were either unsure or unwilling. This suggests that cost is not the only barrier to genetic testing—concerns over privacy, psychological distress, or distrust in how genetic data may be used could be contributing factors. Our data further show that older and less well-educated individuals, as well as parents, were less likely to express willingness to test. Addressing this gap will require targeted outreach that not only educates but also reassures these populations. Public awareness campaigns and professional training could be strengthened to clarify how genetic data are protected and how testing may benefit individuals. Future qualitative research could explore in greater depth the motivations and concerns behind these attitudes, enabling more informed policy and programme design.
A few limitations of this study are worth noting. First, the survey was only available in English, which may have excluded non-English speakers. Second, as explained earlier, there is an under-representation of non-Chinese participants. Both may limit the generalisability of our findings in Singapore. Third, the survey did not specifically ask participants the type of genetic tests they had undergone or the type of insurance they had applied for. This means we are unable to verify whether the GD they reported comply with the current moratorium. Despite these limitations, this study still provides an important foundation for understanding GD in Singapore and highlights key areas for policy intervention and further research.
In conclusion, this study addresses critical gaps in understanding and addressing GD in Singapore. We found that the prevalence of reported GD in insurance and employment in Singapore was notably higher than documented in a comparable study in Asia. We also found a lack of public awareness of existing safeguards against GD. Enhanced statutory protections, combined with transparent insurance practices and public education, may be needed to further reduce prevalence of GD. Furthermore, initiatives to address deterministic thinking about genetics and cultural-religious beliefs against testing may increase public willingness to undergo genetic testing. As Singapore advances its precision medicine strategy, ensuring ethical and equitable practices through comprehensive regulation and informed public engagement is paramount for integrating genomic medicine into healthcare and society effectively.
Methods
Legal landscape and research questions
Like many other Asian countries, Singapore has not introduced legislation prohibiting GD. In 2005, the Bioethics Advisory Committee published non-legally binding guidelines on genetic testing and research, and recommended against unfair discrimination, particularly in insurance7. Subsequent regulations have acknowledged the confidentiality of genetic data but not explicitly prohibited GD. For example, the Code of Practice on the Provision of Clinical Genetic Testing was introduced in 2018 to establish standards for genetic testing in healthcare institutions, but it lacks specific protections against GD8. Similarly, the Personal Data Protection Act (PDPA) recognises genetic data as personal information. Data protection laws, however, often permit the sharing or disclosure of personal data with individual consent, which could be provided via contract9. So, laws like the PDPA could still permit mandatory disclosure of genetic information as a condition of signing an insurance or employment contract, which means using privacy protections to pre-empt discrimination43 may not work under the current legal framework. It is then unsurprising that countries like Singapore, Australia and elsewhere have proposed statutory prohibitions against GD in addition to existing data protection laws.
Meanwhile, Singapore has made significant advancements in precision medicine. The country’s 10-year National Precision Medicine Strategy was launched in 2017, aiming to build the world’s largest genetic databank for multi-ethnic Asian populations44. With this growing focus on genetic research, there is a pressing need for legislation prohibiting GD. Without such protections, people may be reluctant to take genetic tests or disclose their genetic history, fearing it may negatively impact their employment prospects, insurance claims and other aspects of their lives. Recognising these concerns, the Ministry of Health of Singapore announced its intention to develop new laws to regulate the use of genetic and genomic data, in 2024, particularly in areas such as insurance and employment45. It is within this evolving landscape that we conducted our study on GD as the country advances in genetic research and precision medicine.
Currently, the Moratorium on Genetic Testing and Insurance46 offers some protection against GD in Singapore. The moratorium was implemented by the Ministry of Health and Life Insurance Association in 2021. It prohibits the use of genetic test results for both biomedical research and direct-to-consumer genetic tests in insurance underwriting. It also prohibits the use of predictive genetic test results for medical insurance underwriting. However, for life insurance and other types of insurance, the insurer can ask for and use results of predictive tests, if two specific financial and test-related conditions are met. This ‘double-key model’ is shown in Fig. 1. The moratorium allows the use of diagnostic genetic tests conducted for clinical care for both medical and life insurance. A visual summary of the protections offered by the moratorium is shown in Fig. 2.
By allowing the use of voluntarily disclosed genetic information in insurance underwriting, GD could occur even with the moratorium. This is because insurers are not required to be transparent on how they set premiums or determine whether to insure any individual based on their genetic information47, which means they can adopt unfair pricing models that could disadvantage individuals with certain genetic profiles.
There are no specific laws in Singapore that protect individuals from GD in employment. The latest Workplace Fairness Act (WFA), passed in November of 2024, prohibits discrimination in employment based on five personal characteristics: (a) age; (b) nationality; (c) sex, marital status, pregnancy status and caregiving responsibilities; (d) race, religion and language; and (e) disability and mental health conditions48. It does not mention genetic information or family disease history. This means employers in Singapore may potentially use genetic information in hiring or employment decisions, a concern that has been raised by Bylstra et al.49.
There is a lack of research on willingness to undergo genetic testing in the Singaporean context. Cheung et al. investigated the level of genetic literacy among the Singapore public and found it to be average, similar to that of Qatar50. Given there is a possible link between genetic literacy and willingness to undergo genetic testing31, Cheung et al.’s findings may be used indirectly to evaluate the level of willingness to undergo genetic testing in Singapore. However, there is no direct empirical data for this in Singapore. Given the rapid development of genomic initiatives and the risks of GD, more targeted research is needed to assess Singaporeans’ attitudes toward genetic testing.
This study focuses on GD and factors influencing willingness to undergo genetic testing in Singapore. For GD, we aim to measure the prevalence of perceived and reported GD across multiple aspects of life, including insurance, employment, family and romantic relationships, education, the legal system, healthcare, housing and banking. We understand that in insurance and employment, the impact of GD can be reflected in concrete, observable outcomes such as increased premiums or being fired. In contrast, in other aspects of life, the impact of GD may manifest in more subjective outcomes such as receiving poorer treatment from other people. Thus, subsequently we refer to GD in insurance and employment contexts as measured in this study as reported GD, and refer to GD in non-insurance, non-employment contexts as perceived GD. However, we suspend judgement about whether and how GD measured in this study fits the philosophical definition of ‘discrimination’, as we are using the term in a broad, practical sense rather than adhering to the precise distinctions made in literature on moral philosophy. Importantly, we were not able to verify whether these reports or perceptions reflected genuine GD.
For willingness to undergo genetic testing, we aim to investigate the key factors that correlate with such willingness. Understanding these factors could help to shape policies and genetic testing promotion programmes to address public concerns. Specifically, we aim to examine the following potential correlates of willingness to test: trust in the local healthcare system (‘I trust the healthcare system in Singapore’), perception of fair treatment (‘I am treated fairly in Singapore’), cultural-religious beliefs against genetic testing (‘Planning my future based on genetic test results interferes with nature’s plan’), and deterministic thinking about genetics (‘If I carry a cancer gene, that means I will definitely get cancer’). While Cheung et al. measured some of these factors, they did not analyse the relationship between those factors and the willingness to undergo testing. Studies from the U.S. and Italy36,51 suggest that trust in the healthcare system and religious beliefs may influence testing attitudes, and it is worthwhile to examine if this holds in Singapore. Additionally, previous research linked perceived unfair treatment and deterministic beliefs about genetics with willingness to undergo testing in other countries32. We thus included this factor into the potential correlates of willingness to undergo testing to be investigated in this study. We seek to answer the following research questions:
-
What is the prevalence of reported and perceived GD in Singapore across insurance, employment, family and romantic relationships, education, courts, healthcare, housing and banking?
-
What is the level of public awareness of existing legal protections against GD in insurance and employment?
-
Which demographic groups exhibit greater willingness to undergo genetic testing?
-
How does willingness to undergo genetic testing correlate with trust in the local healthcare system, perception of fair treatment, cultural-religious beliefs and deterministic thinking about genetics?
Participant recruitment and ethics approval
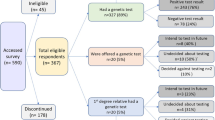
Between December 6 and 11, 2024, an anonymous, cross-sectional online survey was conducted using the Health Opinion Panel Singapore (HOPS). The panel was primarily recruited from existing databases maintained at the Saw Swee Hock School of Public Health, National University of Singapore and through postal invitations mailed to households selected from a sampling frame of deidentified household addresses provided by the Singapore Department of Statistics. The eligibility criteria for enroling in this panel include being a Singapore citizen or permanent resident; age 21 years and above; ability to read and understand English; having an internet-connected device (phone/computer) and personal email account; and having capacity to consent to taking the surveys distributed on this panel. The survey was administered via the Qualtrics TX platform, with email invitations sent to all panel members. At the point of the survey, there were 2527 panel members. Those who completed the survey received a S$30 supermarket voucher as a token for their participation. The survey closed upon reaching 1000 responses, 6 days after distribution. Informed consent was obtained through participant completion of the questionnaire, and the study received approval from the Institutional Review Board of the authors’ university (LH-18-011). The research complies with all relevant ethical regulations in Singapore, and the Declaration of Helsinki.
Survey development and validation
To develop a survey appropriate for the Singaporean context, a literature review was conducted to identify existing research on public, patient and physician attitudes toward genetic testing and GD in the Asia-Pacific region. Articles published between 2005 and 2024 were retrieved from PubMed and Google Scholar, using search terms including ‘attitudes,’ ‘awareness,’ ‘genetic testing,’ ‘genetic information,’ and ‘genetic discrimination.’ A total of 15 articles met the inclusion criteria and were reviewed to inform the survey question design. Those studies assessed genetic literacy, public awareness of legislation and lived experiences of GD in various domains, including insurance, employment and healthcare. Findings from those studies revealed common concerns about GD and misconceptions regarding genetic testing, which were used to guide the formulation of survey questions.
Among the studies that we reviewed when formulating the questionnaire, two studies served as key reference points: one by Cheung et al.50 on public perceptions of genetic testing in Singapore, and the other by Uchiyama et al.19 on experiences of GD in Japan19,50. To further refine the questionnaire, consultations were conducted with clinicians managing hereditary genetic conditions. Informal interviews were held with three endocrine physicians from Khoo Teck Puat Hospital Singapore, who provided clinical insights into patient hesitancy regarding genetic testing due to concerns about GD. They also explained their impression that public and professional awareness of existing protections against GD is limited, and that ethnic minorities in Singapore may have greater reservations about genetic testing, citing concerns over potential misuse of genetic data in employment and insurance. The questionnaire was also reviewed by bioethicists, genetic counsellors and patient support groups to ensure clarity, feasibility and cultural appropriateness for the Singaporean population. The full list of questions is provided in Supplementary Material. Below we provide further details on the questionnaire.
We provided the definitions of ‘genetic information’ at the start of the questionnaire after questions on whether the participants have heard of the terms ‘genetic testing’ and ‘genetic information’ and what kinds of testing they have heard of. Shadowing the definition provided by the U.S. Genetic Information Nondiscrimination Act (GINA), we defined genetic information as individual or family genetic test results, family medical history, past requests for genetic services and foetal or embryonic genetic details in assisted reproduction. This is also consistent with the definition of genetic information in Uchiyama et al.’s study19. To suit the local context, the question eliciting participants’ willingness to undergo genetic testing, measured on a 7-point Likert scale, was phrased as ‘how willing are you to undergo a clinical genetic test that is offered by your doctor for medical reasons, where the cost is fully covered by Medisave’. Medisave is a national medical savings scheme in Singapore. By law, all working Singaporeans and permanent residents must contribute a portion of their income to their MediSave accounts and their employers contribute a similar amount (i.e. it is co-funded by individuals and their employers)52,53. Four more 7-point Likert scale questions were also developed to measure the four potential correlating factors, as shown in Table 10. Open-ended responses were also collected to provide additional insights into individual experiences of reported or perceived GD, or the lack thereof, though the question was optional.
Statistical analysis
Ordinal regression models were used to examine associations between willingness to test and demographic and the four psychosocial variables in Table 10. Binary demographic predictors (e.g. gender, parental status) were analysed using Mann-Whitney U tests. Analyses were conducted using R version 4.3.3 and the MASS package. Significance was determined at the 95% confidence level.
Data availability
The data supporting the findings of this study are provided within the manuscript. Additional data are available from the corresponding author upon reasonable request.
References
Joly, Y. & Dalpe, G. Genetic discrimination still casts a large shadow in 2022. Eur. J. Hum. Genet. 30, 1320–1322 (2022).
United Nations Educational, Scientific and Cultural Organization. Universal Declaration on the Human Genome and Human Rights. OHCHR https://www.ohchr.org/en/instruments-mechanisms/instruments/universal-declaration-human-genome-and-human-rights (1997).
Kim, H. et al. Genetic discrimination: introducing the Asian perspective to the debate. NPJ Genom. Med. 6, 54 (2021).
Muto, K., Nagai, A., Ri, I., Takashima, K. & Yoshida, S. Is legislation to prevent genetic discrimination necessary in Japan? An overview of the current policies and public attitudes. J. Hum. Genet. 68, 579–585 (2023).
Yang, J. H. & Kim, S. Y. Legal and regulatory issues in genetic information discrimination: focusing on overseas regulatory trends and domestic implications. Korean Soc. Law Med. 18, 237–264 (2017).
Wee, C. J. W.-L. Capitalism and ethnicity: creating ‘local’ culture in Singapore. Inter-Asia Cult. Stud. 1, 129–143 (2000).
Bioethics Advisory Committee. Genetic Testing and Genetic Research. https://www.bioethics-singapore.gov.sg/publications/reports/genetic-testing-genetic-research/ (2005).
Ministry of Health. Updates to code of practice on the standards for the provision of clinical genetic/genomic testing services and clinical laboratory genetic/genomic testing services. Ministry of Health https://isomer-user-content.by.gov.sg/3/897325a8-c148-4f0b-ab00-f56a12fff4f3/1-moh-cir-no-234_2020_16dec20_genetic-testing.pdf (2020).
Personal Data Protection Commission Singapore. Advisory Guidelines on Key Concepts in the Personal Data Protection Act. https://www.pdpc.gov.sg/-/media/files/pdpc/pdf-files/advisory-guidelines/ag-on-key-concepts/advisory-guidelines-on-key-concepts-in-the-pdpa-17-may-2022.pdf (2022).
Papanna, B., Lazzari, C. & Rabottini, M. Huntington’s disease prevalence in Asia: a systematic review and meta-analysis. Riv. Psichiatr. https://doi.org/10.1708/4205.41943 (2024).
Gervas, P. et al. A systematic review of the prevalence of germline BRCA mutations in North Asia Breast Cancer Patients. Asian Pac. J. Cancer Prev. 25, 1891–1902 (2024).
Sirugo, G., Williams, S. M. & Tishkoff, S. A. The missing diversity in human genetic studies. Cell 177, 26–31 (2019).
Pinto, D., Jong, M. C. D. & Parameswaran, R. Challenges in genetic screening for inherited endocrinopathy affecting the thyroid, parathyroid and adrenal glands in Singapore. Ann. Acad. Med. Singap. 53, 252–263 (2024).
Kumar, P. Bridging East and West educational divides in Singapore. Comp. Educ. 49, 72–87 (2013).
Ministry of Trade & Industry. Population Trends 2024. https://www.singstat.gov.sg/-/media/files/publications/population/population2024.ashx (2024).
R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. (2012).
Ripley, B. et al. Support Functions and Datasets for Venables and Ripleyas MASS. Version 7.3–65 (2025)..
Erwin, C. et al. Perception, experience, and response to genetic discrimination in Huntington disease: the international RESPOND-HD study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1081–1093 (2010).
Uchiyama, M., Nagai, A. & Muto, K. Survey on the perception of germline genome editing among the general public in Japan. J. Hum. Genet 63, 745–748 (2018).
Muto, K. et al. ‘Shakai ni okeru kojin iden jōhō riyō no jittai to genomu riterashī ni kansuru chōsa kenkyū’[A Study on the Actual Use of Personal Genetic Information in Society and Genomic Literacy]. MHLW Grants System. https://mhlw-grants.niph.go.jp/project/25825 (2017).
Uchijo, Y. A new law for promoting genome medicine enacted: Definite provisions for preventing genetic discrimination and mandating the government to formulate a national plan. ScienceJapan https://sj.jst.go.jp/stories/2023/s0825-01p.html (2023).
Tiller, J. et al. Community concerns about genetic discrimination in life insurance persist in Australia: a survey of consumers offered genetic testing. Eur. J. Hum. Genet 32, 286–294 (2024).
Worthington, E. & Branley, A. Genetic testing to be banned from life insurance, income protection, disability cover. ABC News. https://www.abc.net.au/news/2024-09-10/life-insurers-banned-from-using-genetic-tests-to-deny-cover/104333828 (2024).
Lim, L. L. The influences of harmony motives and implicit beliefs on conflict styles of the collectivist. Int. J. Psychol. 44, 401–409 (2009).
Tan, C. & Tan, C. S. Fostering social cohesion and cultural sustainability: character and citizenship education in Singapore. Diaspora Indig. Minor. Educ. 8, 191–206 (2014).
Saito, T. & Ohbuchi, K. Who suffers pluralistic ignorance of conflict avoidance among Japanese? Individual differences in the value of social harmony. Int. J. Confl. Manag. 24, 112–125 (2013).
Niikura, R. Assertiveness among Japanese, Malaysian, Filipino, and U.S. white-collar workers. J. Soc. Psychol. 139, 690–699 (1999).
Bombard, Y. et al. Perceptions of genetic discrimination among people at risk for Huntington’s disease: a cross sectional survey. BMJ 338, b2175 (2009).
Gopalakrishnan, R. et al. Should I Let Them Know I Have This?: multifaceted genetic discrimination and limited awareness of legal protections among individuals with hereditary cancer syndromes. Public Health Genom. 27, 240–254 (2024).
Taylor, S., Treloar, S., Barlow-Stewart, K., Stranger, M. & Otlowski, M. Investigating genetic discrimination in Australia: a large-scale survey of clinical genetics clients. Clin. Genet. 74, 20–30 (2008).
Abdul Rahim, H. F. et al. Willingness to participate in genome testing: a survey of public attitudes from Qatar. J. Hum. Genet 65, 1067–1073 (2020).
Likhanov, M. et al. Attitudes towards genetic testing: the role of genetic literacy, motivated cognition, and socio-demographic characteristics. PLoS ONE 18, e0293187 (2023).
Aizuddin, A. N. et al. Genetic testing for cancer risk: is the community willing to pay for it?. IJERPH 18, 8752 (2021).
Takeshima, T., Okayama, M., Ae, R., Harada, M. & Kajii, E. Influence of family history on the willingness of outpatients to undergo genetic testing for salt-sensitive hypertension: a cross-sectional study. BMJ Open 7, e016322 (2017).
Sweeny, K., Ghane, A., Legg, A. M., Huynh, H. P. & Andrews, S. E. Predictors of genetic testing decisions: a systematic review and critique of the literature. J. Genet. Couns. 23, 263–288 (2014).
Armstrong, K. et al. The influence of health care policies and health care system distrust on willingness to undergo genetic testing. Med. Care 50, 381–387 (2012).
Burnett-Hartman, A. N. et al. Return of research-related genetic test results and genetic discrimination concerns: facilitators and barriers of genetic research participation in diverse groups. Public Health Genom. 23, 59–68 (2020).
Cowan, J. S., Kagedan, B. L., Graham, G. E., Heim-Myers, B. & Bombard, Y. Health care implications of the Genetic Non-Discrimination Act. Can. Fam. Physician 68, 643–646 (2022).
McGuire, A. L. & Majumder, M. A. Two cheers for GINA?. Genome Med. 1, 6 (2009).
Amano, S. Genomu Iryou Suishinhou ni Motozuku Kihon Keikaku no Kentou ni Kakaru Wākingu Gurūpu Teishutsu Shiryō[Working Group Submission Materials on the Basic Plan Based on the Genome Medical Promotion Act]. https://www.mhlw.go.jp/content/10808000/001278395.pdf (2024).
Lenartz, A. et al. The persistent lack of knowledge and misunderstanding of the Genetic Information Nondiscrimination Act (GINA) more than a decade after passage. Genet. Med. 23, 2324–2334 (2021).
Fernando, A., Kondrup, E., Cheung, K., Uberoi, D. & Joly, Y. Still using genetic data? A comparative review of Canadian life insurance application forms before and after the GNDA. Facets 9, 1–10 (2024).
Schneider, A. Analogous wrongs: privacy invasions and discrimination. Oxf. J. Leg. Stud. 45, 245–271 (2025).
Wong, E. et al. The Singapore national precision medicine strategy. Nat. Genet. 55, 178–186 (2023).
Abdullah, Z. Precision medicine to be covered by MediShield Life; new law mooted to govern genetic test use | The Straits Times. https://www.straitstimes.com/singapore/health/precision-medicine-added-to-medishield-life-coverage-new-law-mooted-to-govern-genetic-test-use? (2024).
Ministry of Health Singapore. Moratorium on Genetic Testing and Insurance. Ministry of Health Singapore https://www.moh.gov.sg/others/resources-and-statistics/moratorium-on-genetic-testing-and-insurance/ (2021).
Life Insurance Association Singapore. Lia Guide to Medical Underwriting for Life Insurance 2024. Life Insurance Association Singapore https://www.lia.org.sg/tools-and-resources/consumer-guides/2024/lia-guide-to-medical-underwriting-for-life-insurance-2024/ (2024).
Ministry of Manpower. Passing Of Workplace Fairness Bill Marks Next Step In Building Fair and Harmonious Workplaces. Ministry of Manpower Singapore https://www.mom.gov.sg/newsroom/press-releases/2025/passing-of-workplace-fairness-bill-marks-next-step-in-building-fair-and-harmonious-workplaces (2025).
Bylstra, Y. et al. Implementation of genomics in medical practice to deliver precision medicine for an Asian population. npj Genom. Med. 4, 1–7 (2019).
Cheung, R., Jolly, S., Vimal, M., Kim, H. L. & McGonigle, I. Who’s afraid of genetic tests?: an assessment of Singapore’s public attitudes and changes in attitudes after taking a genetic test. BMC Med. Ethics 23, 5 (2022).
Pivetti, M. & Melotti, G. Prenatal genetic testing: an investigation of determining factors affecting the decision-making process. J. Genet. Couns. 22, 76–89 (2013).
Ministry of Health. MediSave. MediSave https://www.moh.gov.sg/managing-expenses/schemes-and-subsidies/medisave/ (2024).
CPF Board. CPFB | How much CPF contributions to pay. https://www.cpf.gov.sg/employer/employer-obligations/how-much-cpf-contributions-to-pay (2024).
Life Insurance Assosiation. Moratorium on Genetic Testing and Insurance. Singlife https://singlife.com/en/search?q=Moratorium (2025).
Ministry of Health & Life Insurance Association. Amended and Restated Moratorium on Genetic Testing and Insurance. https://isomer-user-content.by.gov.sg/3/6ce3a0a4-f9da-47cc-8ac9-7817ceafda54/moh-lia-moratorium-on-genetic-testing-and-insurance.pdf (2025).
Acknowledgements
This research is supported by Ministry of Health Singapore under the programme titled Clinical Ethics Network and Research Ethics Support (CENTRES) (MH 24:63/10-1), and by the Social Science Research Council Singapore (administered by the Ministry of Education, Singapore) under its Social Sciences Research Thematic Grant (SSRC2023-SSRTG-006). We would like to thank Dr. Rajeev Parameswaran, Dr. Anil Rao and Dr. Zhimin Lin at Khoo Teck Puat Hospital, Singapore, for generously sharing their insights into the experiences of patients and their families affected by hereditary cancer in Singapore. We would like to thank A/P Saumya Shekhar Jamuar at KK Women’s and Children’s Hospital, Singapore, A/P Konstantina Griva at the National Technological University, Singapore, Ms Yasmin Bylstra at SingHealth Duke-NUS Institute of Precision Medicine, Singapore. Their input was invaluable for shaping the survey design and contextualising our findings on genetic discrimination and testing uptake. We also thank A/P Michael Dunn, A/P Brian Earp and Prof. Richard Huxtable for their feedback on the survey design.
Author information
Authors and Affiliations
Contributions
S.T. Writing—original draft, Writing—review and editing, Visualisation, Validation, Project administration, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. L.T. Writing—original draft, Writing—review and editing, Visualisation, Validation, Project administration, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. L.T. contributed equally with ST to this manuscript. T.H.J. Visualisation, Validation, Project administration, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. A.J.N.B. Writing—review and editing, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. I.T. Writing—review, Project administration, Methodology, Investigation, Formal analysis, Data curation. S.M. Writing—review and editing, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation, Funding Acquisition. J.S. Writing—review and editing, Validation, Supervision, Project administration, Methodology, Conceptualisation, Funding Acquisition. O.S. Writing—review and editing, Supervision, Methodology, Investigation, Formal analysis, Funding Acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takahashi, S., Lan, T., Toh, H.J. et al. Addressing genetic discrimination for stronger legal protections and enhanced public awareness. npj Genom. Med. 11, 5 (2026). https://doi.org/10.1038/s41525-025-00542-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41525-025-00542-z