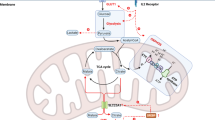
Abstract
Natural killer (NK) cells play an important role in antitumor and viral resistance. However, the mechanism of impaired NK cell function in microgravity remains unclear. Cholesterol metabolism, a new research hotspot, plays a critical role in NK cells function. This study found that simulated microgravity downregulate NK cell membrane cholesterol levels by disrupting cholesterol biosynthesis and transport to the membrane, leading to the obstruction of activated immune synapse formation, which inhibit the release of NK cell cytotoxic particles and ultimately decreasing NK cell immune function. Most importantly, this study identified a new target for regulating NK cell function, LEPR (leptin receptor). LEPR affected NK cell membrane cholesterol levels by influencing the CAMKK–SREBP1–HMGCR cholesterol endogenous synthesis pathway and regulating the expression of NPC1 and NPC2 genes, which ultimately influencing NK cell cytotoxic function. The study is significant for understanding the mechanism of NK cell activity in microgravity and offers new targets for clinical immunotherapy of NK cells.
Similar content being viewed by others
Introduction
Natural killer (NK) cells, as an important component of innate immunity and a key hub linking adaptive immunity, play a significant role in eliminating intracellular pathogens, viral infections, and tumor cells1,2. It has been widely reported in the literature that the microgravity environment in space significantly inhibits the cytotoxic function of NK cells, which then markedly decreases human immune function and increases the susceptibility of astronauts to pathogenic microorganisms3,4,5,6,7. Research has also revealed a significant correlation between the efficacy of clinical treatments for major diseases, such as tumors and viral infections, and the strength or weakness of NK cell function8. Therefore, elucidating the mechanism underlying impaired NK cell function in microgravity environments, developing new targets for more effective immune regulation, and establishing effective protective measures to safeguard the health of astronauts and the development of human spaceflight are crucial. Simultaneously, the discovery of NK cell function regulation mechanism offers new methods and ideas for the immunotherapy effects of related diseases in the terrestrial population.
NK cell function is largely regulated by the balance between inhibitory and stimulatory receptors expressed on their surface. Upon activation, NK cells kill target cells primarily by secreting lethal effector molecules (granzymes and perforins)9. Therefore, these indicators are important markers for characterizing NK cell activation and the function of killing target cells. Currently, researches on the microgravity response mechanism of NK cells also mainly focused on exploring the effects of microgravity on the expression of relevant receptors and the secretion level of cytotoxic granules10,11,12. However, the underlying mechanism remains largely unknown and needs to be further analyzed.
Research has revealed that NK cells rely on the structure of lipid raft-based activated immune synapses as a platform for killing target cells, such as viral infections. This involves the release of cytotoxic particles into the target cells, subsequently triggering their lysis or apoptosis13. As a key component of lipid rafts, cholesterol plays a significant role in the activation and function of NK cells14,15,16,17. Studies have demonstrated that the use of statins interferes with lipid raft formation by decreasing the synthesis of endogenous cholesterol within cells, which significantly inhibits the formation of activated immune synapses and subsequent cytotoxicity in NK cells18. Qin et al. observed elevated plasma membrane cholesterol levels in splenic NK cells in mice fed a high-cholesterol diet as well as an increased formation of lipid rafts, which further facilitated receptor localization and signal transduction during NK cell activation19. However, it remains unreported whether the inhibition of NK cell function in microgravity environments is related to microgravity-induced regulation of cholesterol metabolism, which subsequently affects lipid rafts and activated immune synapse formation. Our research found that simulated microgravity significantly inhibited the cytotoxic function of NK cells along with a significant reduction in cellular cholesterol levels. It is hypothesized that cholesterol metabolism is closely related to the impaired immune function of NK cells in simulated microgravity.
Meanwhile, LEPR, a receptor for leptin (a key metabolic hormone), is expressed in the central nervous system and peripheral systems, including immune tissues20,21. LEPR can combine with leptin and act on the metabolic regulation of the hypothalamus to inhibit adipose synthesis, which is a hot topic in the field of obesity research22,23,24. Surprisingly, in our study, through transcriptomic analysis of NK cells before and after treatment with the activator interleukin (IL)-2, we inadvertently revealed that a significant down-regulation of the expression of LEPR molecules in NK cells, which was accompanied by a significant up-regulation of cytotoxicity. Meanwhile, simulated microgravity inhibited NK cell function while LEPR was significantly upregulated. This seems to indicate a potentially significant negative correlation between LEPR and NK cell function, and it may be closely related to the impaired immune function of NK cells in microgravity environment. Our manuscript should be the first focus on LEPR and NK cell function, although Tian et al. ever reported that leptin receptor-deficient mice had reduced percentages and total numbers of NK cells in the liver, spleen, lungs, and peripheral blood, with significant effects on NK cell development, during their research on leptin25. However, these studies have focused primarily on the effects of leptin on NK cells, without paying attention to the relationship between LEPR itself and NK cell function.
Furthermore, whether LEPR plays a role in regulating cellular cholesterol metabolism is also unclear. It has been shown that LEPR can regulate downstream signaling pathways such as AMPK and JAK-STAT24,26. AMPK has been reported to regulate the SREBP1–HMGCR signaling pathway, which then regulates cellular cholesterol metabolism27,28. This suggests that LEPR may be closely related to cholesterol metabolism. However, the relationship between NK cell function, LEPR, and their specific relationship with cholesterol metabolism in normal or microgravity remains unclear.
Therefore, in the present study, we first investigated the relationship between simulated microgravity and NK cell function from the perspective of cholesterol metabolism and further used LEPR as an entry point to reveal the specific mechanism through which simulated microgravity regulates cholesterol metabolism in NK cells, ultimately leading to impaired NK cell function. To the best of our knowledge, this is the first report studying the effect of cholesterol metabolism on the inhibition of NK cell function under simulated microgravity, as well as the first attention to elucidate the relationship of LEPR and cholesterol metabolism and especially the role of LEPR in NK cell function, which may open up an interesting new area of research. Overall, an in-depth understanding of the immunoregulatory mechanism of NK cells, coupled with the screening of key targets, is significant in the development of targeted intervention strategies for weightlessness-induced immunosuppression as well as the development of immunoprotective drugs for the well-being of astronauts and terrestrial populations. Additionally, it substantially contributes to investigating functional regulatory mechanisms within other immune cells, providing new directions and ideas.
Results
Effects of different treatment time in simulated microgravity on the morphology, diameter, viability, and killing activity of NK cells
In this study, the simulated microgravity treatment time was initially varied (0, 24, and 48 h) to investigate its effect on the morphology, diameter, viability, and killing activity of NK92 cells. The most appropriate treatment time for subsequent studies was determined. As depicted in Fig. 1, subjecting the NK cells to simulated microgravity treatment for 24 h had little effect on their morphology (Fig. 1A), diameter (from 15.44 ± 0.82 μm to 14.42 ± 1.11 μm) (Fig. 1B), and viability (Fig. 1C). However, their ability to kill target cells was considerably reduced, and their killing rate decreased significantly from 87.41% ± 2.10% to 57.86% ± 6.47% (P < 0.01) (Fig. 1E). Microscopic observation revealed that the toxicity of NK cells decreased conspicuously under the SMG (Fig. 1D). However, after 48 h of treatment, the size of cells in the control and simulated microgravity groups decreased obviously and the cell diameter was reduced from 7.33 ± 0.45 μm to 6.73 ± 0.44 μm. This diameter was evidently lower than the normal range, and the survival rate and killing activity of the cells were also significantly reduced. This finding could be attributed to the inadequate supply of nutrients necessary for the cells during 48 h of simulated microgravity. Therefore, considering the effects of this treatment on the phenotype and function of NK cells, the simulated microgravity treatment time in the follow-up studies was set as 24 h.
A NK cell morphology observed by microscopy. Scale bar = 50 μm. Cells indicated by arrows in the figure represent typical live cells. B NK cell diameter, cells with more regular morphology were selected for measurement. C Viability of NK cells, expressed as the ratio of the number of live cells after microgravity treatment to the number of live cells before treatment. D The killing of K562 cells by NK cells treated with microgravity for 24 h was observed under microscope. E Changes of NK cell toxicity after microgravity treatment for different time. (“ns” indicates no significant difference, P > 0.05, “*”, “**” and “***” indicate significant differences at the level of P < 0.05, P < 0.01 and P < 0.001, respectively.).
Study on the role of cholesterol in the inhibition of NK cell function under simulated microgravity
When killing target cells, NK cells must use activated immune synapses based on lipid rafts as a platform to release killer particles into target cells. Cholesterol, which is an important component of lipid rafts, is involved in the activation and function of NK cells. Therefore, in this study, its role in the function of NK cells was investigated after treating them with 10 μg/mL of Avasimibe (ACAT1 inhibitor, which can increase cell membrane cholesterol level). The results revealed that the membrane cholesterol level of the cells (Fig. 2A) was significantly increased (P < 0.05), and the killing activity (Fig. 2B) was significantly upregulated (P < 0.01) in NK cells after treatment with Avasimibe. These findings indicated that the change in cholesterol level was closely related to the function of NK cells. At present, whether the impaired function of NK cells in the microgravity environment is correlated with the change in cholesterol level is not understood. Therefore, the effect of simulated microgravity on the levels of total and cell membrane cholesterol and the distribution of cell membrane cholesterol in NK cells was assessed. The results compared with the control group are presented in Fig. 2C, D. After 24 h of simulated microgravity treatment, the total and cell membrane cholesterol levels of NK cells decreased significantly from 14.36 ± 0.06 μg/mg protein to 10.92 ± 0.05 μg/mg protein (P < 0.001) and from 1.06 ± 0.05 μg/mg protein to 0.68 ± 0.02 μg/mg protein (P < 0.001), respectively. Moreover, the results of membrane cholesterol staining in Fig. 2E signified that the cholesterol level of the NK cell membrane decreased significantly after simulated microgravity treatment and was loosely distributed. The corresponding quantitative results (Fig. 2F) showed that the fluorescence intensity of membrane lipids was dramatically reduced (P < 0.001). These findings establish that the function of NK cells is closely related to their cholesterol level. Simulated microgravity treatment can significantly reduce the total and cell membrane cholesterol levels of NK cells and affect their distribution status. This observation laid the foundation for the theory that simulated microgravity mediates cholesterol metabolism and thus affects the immune function of NK cells.
A Effect of Avasimibe on NK cell membrane cholesterol. B Effect of Avasimibe on NK cell killing activity. C Effect of simulated microgravity on total cholesterol of NK cells. D Effect of simulated microgravity on membrane cholesterol of NK cells. E Filipin staining of membrane cholesterol of NK cells, and the parts shown by the arrows in the figure are the more densely and loosely distributed cholesterol parts on the cell membrane. Scale bar = 20 μm. F Staining quantification results. (“*”, “**” and “***” indicate significant differences at the P < 0.05, P < 0.01 and P < 0.001 levels, respectively.).
Study on the mechanism of simulated microgravity-mediated cholesterol metabolism in the inhibition of NK cell function
The above results demonstrated the role of cholesterol in the function of NK cells, and the SMG was observed to considerably downregulate the cell membrane cholesterol level of NK cells. Therefore, the mechanism of reduction of cholesterol level in NK cell membranes was investigated. Cholesterol metabolism includes the processes of cholesterol biosynthesis, uptake, transport, esterification, and excretion. Collectively, these processes maintain the balance of intracellular cholesterol homeostasis with the synergistic effect of related molecules29. Of these, SREBP is the cholesterol regulatory element binding protein, hydroxymethylglutaryl CoA reductase (HMGCR) is the rate-limiting enzyme in the de novo synthesis of cholesterol and controls endogenous synthesis, low-density lipoprotein receptor (LDLR) promotes cellular uptake of exogenous cholesterol, and NPC1 and NPC2 chiefly transfer intracellular free cholesterol to the cell membrane or other parts and perform biological functions such as forming lipid rafts and synthesizing steroid hormones. ACAT1 catalyzes the linkage of free cholesterol with fatty acids to produce cholesteryl esters, which facilitate cholesterol storage in the cell. ABCG1 is a crucial gene related to cholesterol excretion and can reduce the level of free cholesterol in the cell, thereby avoiding the toxicity of its excessive accumulation29.
Therefore, the expressions of genes related to cholesterol metabolism were investigated. The results of expression at the mRNA level are presented in Fig. 3A. After simulated microgravity treatment, the expressions of SREBP1, SREBP2, HMGCR, NPC1, NPC2, and ACAT1 were significantly downregulated. There was no obvious change in the expression of LDLR, but the expression of ABCG1 was remarkably upregulated. The protein expression results are shown in Fig. 3B, C, and they agreed with the mRNA expression results. SREBP1, SREBP2, and HMGCR were downregulated 1.58 ± 0.05 (P < 0.001), 1.82 ± 0.04 (P < 0.001), and 1.29 ± 0.04 folds (P < 0.001), respectively. The expressions of NPC1 and NPC2 were downregulated 1.1 ± 0.05 (P < 0.001) and 1.04 ± 0.04 folds (P < 0.001), respectively. There was no significant change in LDLR expression. Inconsistent with the mRNA expression, the protein expression of ABCG1 was significantly downregulated 0.64 ± 0.03 folds (P < 0.001) and that of ACAT1 was obviously upregulated 0.19 ± 0.02 folds (P < 0.01). This effect may be related to the regulation of some other regulatory molecules, such as certain miRNAs, and the specific reason remains to be determined. Nonetheless, on the whole, under the comprehensive regulation of multiple pathways of cholesterol metabolism, simulated microgravity can significantly reduce the endogenous cholesterol synthesis of cells by disrupting the SREBP1/2-HMGCR signaling pathway. Furthermore, simulated microgravity inhibited the transfer and aggregation of cholesterol to the membrane and ultimately reduced its level in the membrane significantly.
A Changes in mRNA level expression of cholesterol metabolism genes. B Changes in protein level expression of cholesterol metabolism genes. C Quantitative results of protein expression. D Staining of lipid rafts, the cells shown in the figure with arrows represent the typical cells with obvious changes in lipid rafts. Scale bar = 20 μm. E Quantitative results of (E(a)) lipid rafts and (E(b)) activated immune synapses staining. F Activated immune synapses staining. G Changes in mRNA expression of NK cell killing related genes. H Changes in protein expression of NK cell killing related genes. I Quantitative results of protein expression. (“*”, “**” and “***” respectively represent significant differences at P < 0.05, P < 0.01 and P < 0.001 levels).
Cholesterol and sphingolipids are enriched in the plasma membrane and form signaling platforms called lipid rafts, which are essential for NK cell activation and function. Therefore, the change in cholesterol level in the NK cell membrane may hinder the formation of lipid rafts. As confirmed in Fig. 3D, simulated microgravity treatment significantly reduced the lipid raft content of NK cells. The red fluorescence signal was obviously weakened (Fig. 3D), and the fluorescence intensity was decreased by 0.39 ± 0.05 folds (P < 0.001) (Fig. 3E(a)). This finding suggests that simulated microgravity treatment, possibly by mediating cholesterol metabolism, significantly reduced the membrane cholesterol level of NK cells and further inhibited the formation of lipid rafts.
In addition, in the resting state of NK cells, cell surface receptors were predominantly distributed in the nonlipid raft portion of the cell membrane. However, when activated by stimulation, the total expression of activating receptors was elevated. And the receptors were shifted from the nonlipid raft portion of the cytosolic membrane to the lipid raft portion. By recruiting these receptors and their associated molecules, a dense and stable cell membrane signaling microdomain (immune synapse) is created, which promotes the co-involvement of various activating receptors. Thus, downstream signaling pathways are activated, NK cells are promoted to exert their effector potential, and their tumor-killing ability is enhanced25. Hence, the alterations in the receptors on the surface of NK cells and the subsequent changes in the formation of activated immune synapses were examined.
The expression results of killing-related molecules at the mRNA level are illustrated in Fig. 3G. After simulated microgravity treatment, the expressions of the activating receptors NKG2D, NKp46, and NKp30 were significantly downregulated in the NK cells. On the contrary, the inhibitory receptors NKG2A and CD94 were conspicuously upregulated. At the protein expression level (Fig. 3H, I), which was in line with the mRNA expression level, the activating receptors NKG2D and NKp46 were downregulated 1.61 ± 0.03 (P < 0.01) and 2.25 ± 0.03 (P < 0.01) folds, respectively. The inhibitory receptors NKG2A and CD94 were prominently upregulated 1.23 ± 0.07 (P < 0.01) and 0.21 ± 0.01 (P < 0.05) folds. Overall, simulated microgravity significantly inhibited the expressions of activating receptors and upregulated the expressions of inhibitory receptors, which resulted in the dominance of inhibitory signals. These changes affected the subsequent formation of activated immune synapses, which was verified in Fig. 3E(b) and Fig. 3F. The activated immune synapses in the control group were compact and dense. However, after 24 h of simulated microgravity treatment, the formation of activated immune synapses was obviously impaired (Fig. 3F) and the fluorescence intensity (Fig. 3E(b)) was decreased by 0.35 ± 0.05 folds (P < 0.01).
Upon activation, the NK cells secrete cytotoxic substances (perforins, granzymes, etc.) and various cytokines, including IFN-γ, into the target cells via activated immune synapses to kill them. Of these, perforin oligomerizes on the target cell membrane and creates transmembrane pores with the transmembrane structural domain MACPF. Granzyme is released through these pores and initiates the apoptotic pathway in the target cell, thereby leading to cell death30. IFN-γ plays a key role in cellular immunity and exerts its immunomodulatory role by enhancing antigen processing and presentation, increasing leukocyte transport, and influencing cell proliferation and apoptosis31. These NK cell-killing molecules were explored, which revealed that the mRNA expressions (Fig. 3G) of perforin, GZMB, and IFN-γ were significantly downregulated after simulated microgravity treatment. At the protein expression level (Fig. 3H, I), perforin was significantly upregulated by 2.70 ± 0.03 folds (P < 0.001), which would probably benefit the entry of killing particles such as granzyme into the target cells. However, since the protein expression of the killing effector molecules GZMB and IFN-γ were significantly decreased 0.42 ± 0.01 (P < 0.01), and 1.47 ± 0.02 folds (P < 0.01), respectively. The amount of killing particles that might enter the target cells was still significantly reduced, which ultimately resulted in a significant reduction in the ability of NK cells to kill target cells.
In summary, simulated microgravity inhibits endogenous cholesterol synthesis and the transport of free cholesterol to the membrane. Hence, the cholesterol level in the NK cell membrane is significantly reduced and its aggregation state is affected, thus inhibiting the formation of lipid rafts. Simultaneously, the expressions of activating receptors are downregulated and those of inhibitory receptors are upregulated. These effects lead to the dominance of inhibitory signals and result in the impaired formation of subsequent activated immune synapses. The release of the killer molecules GZMB, and IFN-γ (P < 0.01 and P < 0.01, respectively) is thus inhibited, and ultimately, the tumor-killing ability of NK cells is significantly reduced.
Study on the key targets of regulating NK cell cholesterol metabolism in simulated microgravity
Effect of LEPR expression change on the killing function of NK cells
Interestingly, our study on the function of NK cells using IL2 revealed that the killing function of NK cells was significantly enhanced 2.2 ± 0.10 folds (P < 0.001) after treatment with the NK cell activator IL-2 (Fig. 4A). On the contrary, the transcriptome results hinted that the expression level of LEPR was obviously downregulated (Fig. 4B). Upon further verifying its expression, the mRNA expression level of LEPR was observed to be markedly downregulated 0.37 ± 0.04 folds (P < 0.001) (Fig. 4C). This finding implies the presence of a correlation between LEPR and NK cell function. Therefore, in this study, the expression levels of LEPR before and after simulated microgravity treatment were examined. The results indicated (Fig. 4D, E(a), Fig. 4F) that the expression level of LEPR was significantly upregulated 6.52 ± 1.07 folds (P < 0.001), whereas the killing activity of NK cells was significantly reduced after simulated microgravity treatment. This finding alludes that the expression level of LEPR is closely related to the function of NK cells.
A Effect of IL2 on NK cell killing function. B Transcriptome changes in NK cells after IL2 treatment. C mRNA level expression of the LEPR gene after IL-2 treatment of NK cells. D mRNA level expression of the LEPR gene after simulated microgravity treatment. E Change in LEPR protein expression after simulated microgravity (E(a)) and leptin (E(b)) treatment. (F) Quantification of LEPR protein expression after simulated microgravity treatment. (G) Change of NK cell killing activity after leptin treatment in simulated microgravity. (H) Changes in the mRNA level of LEPR gene after treatment with leptin in simulated microgravity, the expression changes of the drug-added groups are relative to their respective non-drug-added groups. (I) Quantification of LEPR gene protein expression after treatment with leptin in simulated microgravity. (“*”, “**” and “***” respectively indicate significant differences at the P < 0.05, P < 0.01 and P < 0.001 levels).
Therefore, LEPR ligand–leptin molecules were added to treat NK cells in normal and SMGs to affect LEPR-related signaling pathways. The results showed that the killing activity of NK cells was significantly improved in both control and simulated microgravity groups (Fig. 4G). This activity was significantly increased from 24.23% ± 2.63% to 29.37% ± 0.65% (P < 0.05) and from 8.30% ± 2.61% to 21.70% ± 0.71% (P < 0.01). Simultaneously, the expression level of the LEPR gene was significantly downregulated by 1.68 ± 0.19 and 1.43 ± 0.11 folds (P < 0.001) (Fig. 4E(b), Fig. 4H, I). These results not only confirm that LEPR is closely linked to the function of NK cells in the normal environment but also that the impaired function of NK cells in the microgravity environment is associated with the change in LEPR expression.
Effects of the downregulation of LEPR expression on total and cell membrane cholesterol levels, lipid rafts, and activated immune synapses in NK cells
Leptin and its receptor LEPR have been reported to play a vital role in regulating fetal growth, angiogenesis, and immune cell metabolism26,32. For instance, a study by Hongo et al. noted that when bound to the macrophage surface receptor LEPR, leptin inhibits macrophage cholesterol efflux and accelerates the accumulation of cholesterol ester, thus promoting the formation of atherosclerosis33. Furthermore, LEPR has been shown to regulate gene transcription by transducing activation signals into cells via the AMPK signaling pathway, which has also been reported to regulate the expression of HMGCR24,27. This finding implies that LEPR plays a key role in regulating cellular cholesterol metabolism. Therefore, the effect of LEPR on cholesterol levels in NK cells after the downregulation of its expression with leptin treatment was investigated. The results, as illustrated in Fig. 5, indicated that total and membrane cholesterol levels of NK cells were significantly increased after the downregulation of LEPR expression in both normal gravity and SMGs. Of these, the total cholesterol of NK cells was increased from 11.93 ± 0.15 μg/mg to 14.37 ± 0.32 μg/mL in the normal gravity group (P < 0.001) and from 8.83 ± 0.31 μg/mL to 10.78 ± 0.10 μg/mL in the simulated microgravity group (P < 0.001) (Fig. 5A). Correspondingly, the free cholesterol of NK cells was elevated from 1.53 ± 0.02 μg/mL to 1.86 ± 0.03 μg/mL (P < 0.001) and from 0.83 ± 0.06 μg/mL to 1.35 ± 0.04 μg/mL (P < 0.001) (Fig. 5B), respectively. Considering the vital role of cholesterol in the function of NK cells, these observations imply that the alteration in LEPR expression, both in normal gravity and SMGs, may affect the killing activity of these cells by influencing their cholesterol metabolism. Hence, the formation of lipid rafts and activated immune synapses in these cells after the downregulation of LEPR in the microgravity environment was also investigated.
A Effect of LEPR on total cholesterol of NK cells. B Effect of LEPR on membrane cholesterol of NK cells. C Staining of lipid rafts, the cells shown in the figure with arrows represent the typical cells with obvious changes in lipid rafts. D Quantification of (D(a)) lipid rafts and (D(b)) activated immune synapses staining. E Staining of activated immune synapses. The site shown by the arrows in the figure is the activated immune synapse formed by NK cells and target cells (K562 cells). Scale bar = 20 μm. (“**” and “***” respectively indicate significant differences at the P < 0.01 and P < 0.001 levels).
The results are shown in Fig. 5C. After the downregulation of LEPR expression in the SMG, the lipid raft content of NK cells was significantly increased, the red fluorescent signal was significantly enhanced, and fluorescence intensity was elevated 0.26 ± 0.05 folds (P < 0.01) (Fig. 5D(a)). Simultaneously, the activated immune synapses became thick and compact (Fig. 5E), and the quantitative fluorescence value increased by 0.16 ± 0.03 folds (P < 0.01) (Fig. 5D(b)). These results suggest that the downregulation of LEPR expression can significantly increase the cholesterol level in the NK cell membrane, thus promoting the formation of lipid rafts and activated immune synapses, ultimately improving the killing activity of NK cells significantly. This finding indicates that LEPR is one of the key targets in simulating microgravity to regulate NK cell cholesterol metabolism, thereby leading to impaired cell function. However, the specific mechanism by which LEPR regulates NK cell cholesterol metabolism is yet to be elucidated.
Study of the specific mechanism by which LEPR regulates cholesterol metabolism in NK cells
As mentioned above, LEPR can regulate the AMPK signaling pathway, which can subsequently regulate the downstream SREBP1-HMGCR signaling pathway. Therefore, the effects of LEPR expression changes on the expression of the CAMKK molecule, a key member of the AMPK family, and certain key genes in the cholesterol metabolism pathway, such as SREBP1, were studied at mRNA and protein levels. Furthermore, the possible signaling pathways of LEPR regulating cholesterol metabolism were explored. The results, as depicted in Fig. 6, showed that alterations in the protein level were consistent with those at the mRNA level. In other words, the downregulation of LEPR expression in NK cells significantly downregulated the expression level of CAMKK under the SMG (0.44 ± 0.08 folds, P < 0.01). Moreover, SREBP1, HMGCR, NPC1, and NPC2 were upregulated 0.55 ± 0.06 (P < 0.001), 0.60 ± 0.02 (P < 0.001), 1.26 ± 0.02 (P < 0.001), and 0.24 ± 0.03 folds (P < 0.01), respectively. The same trend was observed in the normal gravity environment too.
A Changes in mRNA expression level of cholesterol metabolism genes in NK cells. B Changes in protein expression level of cholesterol metabolism genes in NK cells. C Quantification of protein expression. (“*”, “**” and “***” indicate significant differences at P < 0.05, P < 0.01 and P < 0.001 levels, respectively.).
This finding suggests that the downregulation of LEPR upregulates the endogenous cholesterol synthesis pathway of SREBP1-HMGCR via CAMKK. Simultaneously, it regulates the process of cholesterol transport to the cell membrane mediated by NPC1 and NPC2 in other ways. This regulation promotes cholesterol transport to the membrane, significantly increases the cholesterol level in the membrane, enhances the formation of lipid rafts and activated immune synapses in NK cells. Finally, the killing function of NK cells is augmented. This mechanism can be applied in both microgravity and normal environments.
Discussion
Immunosuppression due to the space environment and immunocompromise in oncology patients in the ground population has been a major concern that has put astronauts and the ground population at risk for infections and the development of malignant diseases34,35,36,37. Presently, the research on the functional inhibition mechanism of NK cells mainly focuses on the expression of activated receptors or the secretion of related killer particles, albeit a more in-depth mechanism analysis is lacking38. As cholesterol is responsible for the key roles in the plasma membrane such as regulating membrane fluidity, permeability, and signaling, the cholesterol metabolism pathways are prevalent in certain cell types, such as immune cells39. It has been shown that changes in the T-cell cholesterol content affect the structure of lipid rafts, and disordered lipid rafts can produce more unstable immune synapses and TCR signaling40,41,42. Moreover, it has been reported that the normal functioning of NK cells may be closely related to cholesterol metabolism18,19. In conclusion, the relationship between cholesterol metabolism and immune cell functions has become one of the major research hotspots in recent years. However, whether the impaired NK cell functions in a microgravity environment are related to cholesterol metabolism remains unclear, which is one of the main research questions for the present study.
The results revealed that simulated-microgravity treatment inhibited the endogenous cholesterol synthesis signaling pathway SREBP1/2-HMGCR of NK cells, thereby reducing endogenous cholesterol synthesis in the cells, and simultaneously significantly downregulated the expression of NPC1 and NPC2, which inhibited the cholesterol transfer to the membrane and together significantly reduced the level of cholesterol in the membrane of NK cells and further hindered the formation of lipid rafts. As a signaling scaffold for NK cell activation, lipid rafts, when NK cells are stimulated, migrate to the lipid raft site by recruiting activation receptors and then form a stable cell membrane signaling microdomain-activated immune synapse, which, in turn, promotes the release of killing particles, thereby achieving the purpose of killing the target cells43. Therefore, we further investigated the effects of simulated microgravity on the expression of NK cell surface receptors and the formation of lipid rafts as well as activated immune synapses. Consistent with Li and our previous studies10,11, this study found that simulated microgravity significantly inhibited the expression of NK cell-activating receptor NKG2D. In addition, we found that the expression of the NK cell-activating receptor NKp46 was markedly downregulated and the expression of the inhibitory receptor NKG2A and CD94 were notably upregulated. Meanwhile, the impaired formation of lipid rafts and the downregulation of the expression of activating receptors further hindered the formation of activated immune synapses, which may have affected the release of NK cell-killing-effector molecules. This finding was verified in our study, wherein the expression of NK cell-effector molecules Perforin, GZMB, and IFN-γ were all significantly downregulated, ultimately decreasing the ability of NK cells to kill the target cells.
These results suggest that simulated microgravity can indeed inhibit NK cell function by regulating the NK cell-cholesterol metabolism. Then new question arises, how does microgravity regulate cholesterol metabolism? Surprisingly, in our analysis of NK cell transcriptome before and after treatment with IL-2, we inadvertently discovered a significant downregulation of the leptin receptor-LEPR molecule (Fig. 4B). Further validation experiments also confirmed that LEPR mRNA expression was indeed significantly reduced (Fig. 4C). Meanwhile, simulated microgravity inhibited NK cell function while LEPR was significantly upregulated (Fig. 4D and Fig. 4E). These findings indicate the potential significantly correlation between LEPR and NK cell functions. Actually, in previous research, most studies involving LEPR content were related to the ligand–leptin, and leptin and its combining receptor LEPR are primarily the key molecules associated with obesity research. However, researches have also discovered that, after leptin binds with the LEPR, it not only regulates energy homeostasis and metabolic processes but also plays a crucial role in regulating the development of immune cells44,45. For example, past studies on mononuclear macrophages, and neutrophils have found that leptin can bind to LEPR to promote the activation, proliferation, and release of cytokines from the abovementioned immune cells46,47. Specially, the study on leptin function conducted by Tian et al., which utilized LEPR gene knockout mice, found that the percentage, total number, and cytotoxicity of NK cells were decreased25. However, these studies have focused primarily on the effects of leptin on immune cells, but overlooked the relationship of LEPR itself to the function of these immune cells. As such, the specific relationship between LEPR and NK cell functions and furthermore with cholesterol metabolism remains unclear, whether under normal conditions, or in a SMG environment. Our study found that the upregulation of the LEPR expression in SMG could significantly inhibit the killing function of NK cells and markedly reduce the membrane cholesterol level of NK cells. Further in-depth investigation of the mechanism revealed that the downregulation of the LEPR expression in both microgravity and normal environments may upregulate the SREBP1-HMGCR cholesterol endogenous synthesis pathway through the regulation of CAMKK, and that the downregulation also promotes the facilitation of NPC1- and NPC2-mediated cholesterol transport to the cell membrane, which ultimately led to a significant increase in the membrane cholesterol level, and subsequently promote the formation of subsequent NK cell lipid rafts and activated immune synapses, thereby ultimately improving the killing function of NK cells. However, it remains unclear as to how LEPR affects the expression of membrane transporter genes NPC1 and NPC2 and whether microgravity regulates the cholesterol metabolism in NK cells through other molecules or signaling pathways, warranting further clarification. In conclusion, the discovery of the LEPR molecule provides a new key therapeutic target for the immunosuppression of NK cells in a microgravity environment; therefore, the development and screening of related drugs are important directions for future research.
In addition, it is noteworthy that using this traditional simulated microgravity method may not meet the demands of long-duration treatments due to the occurrence of nutritional deficiencies. Therefore, the development of new types of instruments is essential. For example, there is a current approach using microvessels where it is able to optimize nutrient and gas exchange and therefore better simulate the microgravity conditions48. Compared with the traditional simulated microgravity method, microvessels can maintain cell integrity and good cell activity over a longer period of time, while ensuring gas exchange during cell culture and reducing the risk of nutrient depletion. Therefore, the application of microvascular approach for space biology research is a very promising direction. However, there are still some problems with this technique, such as the fact that only a small number of cells can be cultured, which makes it unsuitable for experiments that require a large number of cells. Therefore, it is essential to further develop more appropriate instruments for simulating microgravity to meet diverse requirements.
Furthermore, it is also worth noting that 2-D and 3-Dculture models may also differ in terms of how microgravity affects immune cell function. Traditional 2-D environments do not better simulate the in vivo environment, but their main advantage over 3-D environments are easier environmental control cell observation, measurement, and eventual manipulation49,50. 3-D culture models span the gap between 2-D cultures and animal models by better mimicking key features of the native microenvironment, but the additional dimension of 3-D cultures results in differences in cellular responses due to the spatial organization of cell surface receptors and physical constraints on the cells50. Currently, there are a large number of reports on the application of 3-D culture to the study of the effects of microgravity on other cell’s and on T-cells51,52,53, there is still a lack of studies applying it to other immune cells. Therefore, the application of 3-D in vitro culture systems, which are closer to the in vivo environment, in space biology research has also become a direction for future research.
Additionally, it is important to note that this study utilizes NK92 cell line, whereas some reports employ primary NK cells, which may lead to the inconsistencies in findings. For example, the expression changes of certain surface receptors such as NKG2A and NKp30 on NK cells are not consistent across studies. In fact, primary cells are closer to the true in vivo conditions; however, there is a greater individual variability among primary cells, which may not yield stable experimental results. Therefore, this necessitates a larger sample size. Consequently, using cell lines can provide more stable results in comparison. Ideally, it is best to use both cell lines and primary cells simultaneously whenever possible.
Methods
Cell culture
NK92 cells (passaged cell line) were cultured in a X-VIVO medium supplemented with 12.5% fetal equine serum (FES), 10% fetal bovine serum (FBS), 100 μg/mL penicillin and streptomycin, and 500 IU/mL IL-2; the cells were incubated at 37 °C in a 5% CO2 incubator; K562 cells (human myeloid leukemia cells) were cultured in a RPMI-1640 medium containing 10% FBS and 100 μg/mL penicillin and streptomycin, and the cells were incubated at 37 °C under 5% CO2 incubator.
NK cells exposed to SMG
Consistent with past reports10,11, we used the 2D-RWV developed by the China Astronaut Research and Training Center to simulate microgravity conditions. The NK92 cells were re-suspended in a complete IL-2-free medium, RPMI-1640, and inoculated in a rotating cup to eliminate the bubbles by filling the medium to avoid shear forces. The rotary cups were then placed on a rotameter, rotated at 30 revolutions per minute, and incubated continuously for 0, 24, and 48 h at an atmospheric temperature of 37 °C under 5% CO2.
NK cell viability and morphological characterization
The NK cells after microgravity treatment were enumerated using a hemocytometer plate and the cell viability was expressed as a percentage of the number of living cells after microgravity treatment to the number of living cells before treatment. The treated NK cells were observed under an optical microscope and record their morphologic size and their diameters.
NK cell cytotoxicity assay
The cytotoxicity of NK cells was evaluated by measuring the killing rate of target cells (K562 cells). The treated NK cells were washed thrice with sterile PBS. Then, 30 × 104 NK cells were placed in a 96-well plate well and mixed with 10 × 104 K562 cells in a ratio of 30:10 effector cells to target cells. NK control wells (effector cell control) contained 30 × 104 NK cells and K562 control wells (target cell control) contained 10 × 104 K562 cells. After incubating the 96-well plate at 37 °C under a 5% CO2 atmosphere for 4 h, the killing pictures were recorded by using an optical microscope. Then, 10 μL of CCK-8 reagent was added to each well, the well plates were incubated for 2 h, and the OD450 value was recorded, followed by calculation of the killing rate of NK cells to K562 cells using the following Eq. (1):
Where, ODe + t represents the OD450 value of the well for NK cells plus K562 cells, ODe is the value of the NK cell control group, and ODt represents the value for K562 control cells.
Study on the role of cholesterol in the inhibition of NK cell function under simulated microgravity
We determined whether cholesterol plays a role in simulated microgravity inhibition of NK cell functions by measuring the levels of total cholesterol and cell membrane cholesterol in NK cells after SMG treatment.
Effects of Avasimibe treatment on NK cell functions
First, NK cells were treated with 2.5 μg/mL of Avasimibe (ACAT1 inhibitor) under normal gravity for 24 h, and the membrane cholesterol content of NK cells was measured as described below. Meanwhile, K562 cells were used as target cells to detect the changes in the NK killing activity according to the abovementioned method so as to preliminarily determine whether cholesterol plays a role in the functioning of NK cells.
Determination of total cell cholesterol and cell membrane cholesterol content
According to the manufacturer’s instructions, the total cholesterol and cell membrane cholesterol contents of NK cells after simulated microgravity treatment were measured by using a cholesterol quantitative kit (Thermo, USA). Briefly, the treated cells were washed thrice with cold PBS and divided into two equal portions; to one portion, cholesterol oxidase was added to oxidize the plasma membrane cholesterol in a 37 °C water bath for 30 min. Subsequently, both portions were treated with extraction solvent (chloroform:methanol = 2:1) to extract the total cellular or intracellular cholesterol, and the cholesterol content was determined by using the Amplex Red Cholesterol Assay Kit. The cell membrane cholesterol content was calculated by subtracting the intracellular cholesterol content (samples containing cholesterol oxidase) from the total cholesterol content (samples without cholesterol oxidase). The value was normalized to the total cellular protein content (the same amount of cells was used for different treatment groups), as determined by using the BCA protein assay kit (Fdbio Science, Hangzhou, China).
Filipin staining of cell membrane cholesterol
The simulated microgravity-treated cells were fixed with 4% paraformaldehyde (PFA) at 4 °C for 15 min and then stained with a final concentration of 50 μg/mL Fillipin III solution at 4 °C for 30 min away from light, followed by washing thrice with PBS and then photographing under a fluorescence microscope (Zeiss, Germany). Three replicates were prepared for each group and at least 10 fields of view were photographed for each sample. Finally, these 30 fields of view were selected to characterize the cell membrane cholesterol level by quantitative analysis of fluorescence intensity using Image J software.
Study of the mechanism of cholesterol in simulated microgravity inhibition of NK cell functions
The changes in genes related to cholesterol metabolism, lipid raft formation, kill-related receptors, and subsequent activated immune synapses were further investigated to clarify the mechanism of cholesterol in simulated microgravity inhibition of NK cell functions.
QPCR was used to detect the expression of NK cell-killing-related molecules and cholesterol metabolism-related genes
Real-time fluorescence quantitative PCR (RT-qPCR) was used to detect the changes in the expression of NK cell-activating receptors, inhibitory receptors, and genes related to killer particles and cholesterol metabolism at the mRNA level after 24 h of simulated microgravity treatment. The primers used in this study are listed in Table 1. Total RNA from NK cells was extracted by using an RNA extraction kit (Omega, USA). RNA reverse-transcription kit (Vazyme, Nanjing, China) was then used to reverse transcribe the mRNA into cDNA. RT-PCR reaction was performed in a 96-well plate on a CFX96-PCR instrument with SYBR as the green fluorescent group. A program of 95 °C, 30 s; 95 °C, 10 s; 60 °C, 30 s was set to perform 40 amplification cycles. At the end of the cycle, the specificity of the reaction was verified based on the melting curve. The relative expression of the target gene, gene A, was calculated by using the Eq. (2):
Ct refers to the number of amplification when the fluorescence signal reaches the set threshold value during the process of qPCR.
Western blotting to detect the expression of NK cell-killing-related molecules and cholesterol metabolism-related genes
Western blotting was performed to detect the changes in the expression of activating receptors, inhibitory receptors, and genes related to killing granules and cholesterol metabolism at the protein level in NK cells after simulated microgravity treatment. The total protein was extracted by lysing-treated NK cells using RIPA lysate buffer, and the BCA method (Fdbio Science) was used to measure the total protein content. The protein samples were denatured and then electrophoresed in an SDS gel. Subsequently, the gels with separated proteins were transferred onto PVDF membranes, and the protein bands were detected with antibodies against the target proteins. After chemiluminescence treatment, the gel image was photographed and recorded.
Lipid raft staining of NK cells
Cholera toxin B (CTB), which can specifically bind with ganglioside GM1 (rich in fat rafts), was used to dye the lipid rafts. Briefly, polylysine-treated coverslips were washed thrice with PBS, and 2 × 105 cells were inoculated into a 6-well plate. After the cells climbed the plate, they were fixed with 4% paraformaldehyde at room temperature for 20 min. Subsequently, CTB staining solution was added at a final concentration of 1 µg/mL and incubated for 10 min at 4 °C in the dark, and the cover glass was washed twice with PBS. Another 1 mL of DAPI staining solution was added, incubated for 10 min at room temperature in the dark, cleaned twice with PBS, and finally photographed with a fluorescence microscope. Subsequently, quantitative analysis was performed as described in section for Filipin staining of cell membrane cholesterol.
Activated immune synapse staining of NK cells
Rhodamine-labeled ghost pen cyclic peptide was employed for staining the cytoskeleton in order to indirectly reflect the position and status of synapses, and DAPI staining and green fluorescent protein labeling were performed to differentiate the NK92 cells from the target cells. Briefly, treated NK92 cells were mixed with K562 cells in a ratio of 2:1, inoculated into a 6-well plate, and co-cultured for 2 h. The cells were fixed in 4% paraformaldehyde for 20 min at room temperature, infiltrated with PBS-0.5% TX for 10 min, and sealed with Abdil at room temperature for 10 min. Ghost pen cyclic peptide-rhodamine (Abdil 1:40 dilution 200 U/mL) was added and the cells were refrigerated at 4 °C overnight, followed by thrice washing with PBS-0.1% TX, re-stained with DAPI for 10 min at room temperature, and photographed for observation using a fluorescence microscope. Subsequently, quantitative analysis was performed as described in section for Filipin staining of cell membrane cholesterol.
Study of the key targets of simulated microgravity-regulating cholesterol metabolism in NK cells
Effect of LEPR on NK cell-killing function
The correlation between LEPR and NK cell functions was preliminarily determined by combining the changes in the NK cell functions and the LEPR expression before and after IL-2 treatment. Specifically, NK cells was treated with 500 IU IL-2 (NK cell-activating factor, which can upregulate the NK cell killing function) for 24 h. After collecting and washing the cells, a portion of the cells was used to detect NK cell killing function, and the remaining portion of the cells was used for transcriptome sequencing to detect changes in LEPR expression and then the change in LEPR expression was further verified through qPCR. Then, the changes in the LEPR expression and cytotoxicity of NK cells after simulated-microgravity treatment were also detected by qPCR and Western blotting, and the NK cell cytotoxicity assay, as described earlier.
Subsequently, LEPR ligand-leptin was added to the simulated microgravity treatment process in order to regulate the LEPR-related signals of NK cells, the cell-killing activity and LEPR expression of NK cells were detected so as to further clarify the role of LEPR in the functions of NK cells. Briefly, the cells were resuspended in 1640 complete medium, and NK cells were inoculated into cell culture bottles at the density of 5 × 106 cells, followed by incubation at 37 °C under a 5% CO2 for 1 day to synchronize the NK cells. For the IL-2 treatment, the control group and the 500-IU IL-2 treatment group were set up. For leptin treatment, different treatment groups were set as follows: control group without leptin (Control), control group with leptin (Control + Leptin), simulated-microgravity treatment without leptin (SMG), and simulated-microgravity treatment with leptin (SMG + leptin), and the treatment was conducted for 24 h. After 24 h, the NK cells were collected and washed thrice with PBS, and the killing activity of NK cells and changes in the LEPR expression were detected as per the abovementioned steps.
Role of LEPR in regulating cholesterol metabolism in NK cells
The contents of total and free cholesterol and the formation of lipid rafts and activated immune synapses of NK cells after the abovementioned group treatment (Control, Control + Leptin, SMG, and SMG + Leptin) were detected as per the abovementioned steps so as to clarify the role of LEPR expression changes in regulating the cholesterol metabolism of NK cells.
Study of the mechanism of LEPR regulating NK cell cholesterol metabolism
The expression of CAMKK molecules, an important member of the AMPK family, and certain key genes of cholesterol metabolism such as SREBP1 and their potential upstream regulatory genes in NK cells treated with the above groupings were examined at the mRNA and protein levels by real-time fluorescence qPCR and Western blotting, respectively, so as to clarify the possible signaling pathways of LEPR regulation in cholesterol metabolism.
Statistical analysis
All experiments were repeated thrice and the results were expressed as mean ± standard deviation. Significance analyses between the data were performed by one-way analysis of variance (ANOVA) using GraphPad Prism 9.5 software, and comparisons between the two groups were analyzed by t-tests, with P < 0.05, P < 0.01, and P < 0.001 indicating that the differences between the data were statistically significance.
Data availability
All data presented in the study are presented in the manuscript. The source data that supports the findings of this study are available from the corresponding author upon request.
References
Frutoso, M. & Mortier, E. NK cell hyporesponsiveness: more is not always better. Int. J. Mol. Sci. 20, 4514 (2019).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Komtantinova, I. V. et al. Natural killer cells altafr mission. Acfa Asrmmwica 36, 713–718 (1995).
Meshkov, D. & Rykova, M. The natural cytotoxicity in cosmonauts on board space stations. Acta Astronaut. 36, 719–726 (1995).
Mylabathula, P. L. et al. Simulated microgravity ‘disarms’ human natural killer cells and suppresses cytotoxic activity against tumor target cells. Brain Behav. Immun. 66, e31 (2017).
Bigley, A. B. et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 126, 842–853 (2019).
Gregg, R. K. et al. Interactions of natural killer cells and pancreatic cancer cells in simulated microgravity. J. Immunol. 204, 88.6 (2020).
Shin, E. et al. Understanding NK cell biology for harnessing NK cell therapies: targeting cancer and beyond. Front. Immunol. 14, 1192907 (2023).
Caligiuri, M. A. Human natural killer cells. Blood 112, 461–469 (2008).
Shao, D. et al. Mechanisms of the effect of simulated microgravity on the cytotoxicity of NK cells following the DNA methylation of NKG2D and the expression of DAP10. Microgravity Sci. Technol. 33, 6 (2021).
Li, Q. et al. Effects of simulated microgravity on primary human NK cells. Astrobiology 13, 703–714 (2013).
Mylabathula, P. L. et al. Simulated microgravity disarms human NK-cells and inhibits anti-tumor cytotoxicity in vitro. Acta Astronaut. 174, 32–40 (2020).
Orange, J. S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 8, 713–725 (2008).
Dupré, L. et al. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity 17, 157–166 (2002).
Kusumi, A. et al. Defining raft domains in the plasma membrane. Traffic 21, 106–137 (2020).
King, R. J., Singh, P. K. & Mehla, K. The cholesterol pathway: impact on immunity and cancer. Trends Immunol. 43, 78–92 (2022).
Aguilar-Ballester, M., Herrero-Cervera, A., Vinué, Á., Martínez-Hervás, S. & González-Navarro, H. Impact of cholesterol metabolism in immune cell function and atherosclerosis. Nutrients 12, 2021 (2020).
Hillyard, D. Z. et al. Statins inhibit NK cell cytotoxicity by membrane raft depletion rather than inhibition of isoprenylation. Atherosclerosis 191, 319–325 (2007).
Qin, W.-H. et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology 158, 1713–1727 (2020).
Liu, J., Yang, X., Yu, S. & Zheng, R. The leptin signaling. in Neural Regulation of Metabolism Vol. 1090 (eds Wu, Q. & Zheng, R.) 123–144 (Springer, 2018).
Procaccini, C., Jirillo, E. & Matarese, G. Leptin as an immunomodulator. Mol. Asp. Med. 33, 35–45 (2012).
Wasim, M., Awan, F. R., Najam, S. S., Khan, A. R. & Khan, H. N. Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem. Genet. 54, 565–572 (2016).
Li, M. et al. Association of single nucleotide polymorphisms in LEP, LEPR, and PPARG with humoral immune response to influenza vaccine. Front. Genet. 12, 725538 (2021).
Guo, Z., Yang, H., Zhang, J.-R., Zeng, W. & Hu, X. Leptin receptor signaling sustains metabolic fitness of alveolar macrophages to attenuate pulmonary inflammation. Sci. Adv. 8, eabo3064 (2022).
Tian, Z., Sun, R., Wei, H. & Gao, B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 298, 297–302 (2002).
Pereira, S., Cline, D. L., Glavas, M. M., Covey, S. D. & Kieffer, T. J. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr. Rev. 42, 1–28 (2021).
Stockebrand, M., Sauter, K., Neu, A., Isbrandt, D. & Choe, C. Differential regulation of AMPK activation in leptin‐and creatine‐deficient mice. FASEB J. 27, 4147–4156 (2013).
Ke, R., Xu, Q., Li, C., Luo, L. & Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 42, 384–392 (2018).
Xu, H., Zhou, S., Tang, Q., Xia, H. & Bi, F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim. Biophys. Acta Rev. Cancer 1874, 188394 (2020).
Sankar, J., Arora, S., Joshi, G. & Kumar, R. Pore-forming proteins and their role in cancer and inflammation: mechanistic insights and plausible druggable targets. Chem. Biol. Interact. 366, 110127 (2022).
Kak, G., Raza, M. & Tiwari, B. K. Interferon-gamma (IFN-γ): exploring its implications in infectious diseases. Biomol. Concepts 9, 64–79 (2018).
Obradovic, M. et al. Leptin and obesity: role and clinical implication. Front. Endocrinol. 12, 585887 (2021).
Hongo, S. et al. Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am. J. Physiol. Endocrinol. Metab. 297, E474–E482 (2009).
Williams, D., Kuipers, A., Mukai, C. & Thirsk, R. Acclimation during space flight: effects on human physiology. Mil. Med. Res. 180, 1317–1323 (2009).
Sonnenfeld, G. Space flight, microgravity, stress, and immune responses. Adv. Space Res. 23, 1945–1953 (1999).
Zayzafoon, M., Meyers, V. E. & McDonald, J. M. Microgravity: the immune response and bone. Immunol. Rev. 208, 267–280 (2005).
Domaica, C. I., Sierra, J. M., Zwirner, N. W. & Fuertes, M. B. Immunomodulation of NK cell activity. in Cell Reprogramming for Immunotherapy Vol. 2097 (eds Katz, S. G. & Rabinovich, P. M.) 125–136 (Springer US, 2020).
Lv, H. et al. Microgravity and immune cells. J. R. Soc. Interface 20, 20220869 (2023).
de Boer, J. F., Kuipers, F. & Groen, A. K. Cholesterol transport revisited: a new turbo mechanism to drive cholesterol excretion. Trends Endocrinol. Metab. 29, 123–133 (2018).
Fessler, M. B. The intracellular cholesterol landscape: dynamic integrator of the immune response. Trends Immunol. 37, 819–830 (2016).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Tabas, I. & Lichtman, A. H. Monocyte-macrophages and T cells in atherosclerosis. Immunity 47, 621–634 (2017).
Roda-Navarro, P. Assembly and function of the natural killer cell immune synapse. Front. Biosci. 14, 621–633 (2009).
Kiernan, K. & MacIver, N. J. The role of the adipokine leptin in immune cell function in health and disease. Front. Immunol. 11, 622468 (2021).
Maya-Monteiro, C. M. et al. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J. Biol. Chem. 283, 2203–2210 (2008).
Procaccini, C. et al. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr. Pharm. Des. 16, 1902–1912 (2010).
Abella, V. et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 13, 100–109 (2017).
ElGindi, M., Ibrahim, I. H., Sapudom, J., Garcia-Sabate, A. & Teo, J. C. M. Engineered microvessel for cell culture in simulated microgravity. Int. J. Mol. Sci. 22, 6331 (2021).
Duval, K. et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology 32, 266–277 (2017).
ElGindi, M. et al. May the force be with you (or not): the immune system under microgravity. Cells 10, 1941 (2021).
Dietrichs, D. et al. Three-dimensional growth of prostate cancer cells exposed to simulated microgravity. Front. Cell Dev. Biol. 10, 841017 (2022).
Ma, C., Duan, X. & Lei, X. 3D cell culture model: from ground experiment to microgravity study. Front. Bioeng. Biotechnol. 11, 1136583 (2023).
ElGindi, M. et al. 3D microenvironment attenuates simulated microgravity-mediated changes in T cell transcriptome. Cell. Mol. Life Sci. 79, 508 (2022).
Acknowledgements
This study is supported by the National Natural Science Foundation of China (12172302 and 32072324), the Shaanxi Provincial Key R&D Program (2023-YBNY-122 and 2021ZDLSF01-06), the Xianyang Key R&D Program (L2023-ZDYF-SF-002), and the Natural Science Foundation of Guangdong Province (2024A1515011179).
Author information
Authors and Affiliations
Contributions
Hongfang Lv, Huan Yang: Data curation, Formal analysis, Validation, Investigation, Methodology, Writing—original draft; Xiaojia Guo, Jing Li, Yuanyuan Xie: Writing—review and editing; Chunmei Jiang and Junling Shi: Conceptualization, Supervision, Project administration, Writing—review and editing; Qingsheng Huang: Conceptualization, Supervision, Project administration, Funding acquisition,Writing—review and editing; Dongyan Shao: Conceptualization, Resources, Supervision, Funding acquisition, Methodology, Project administration, Writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, H., Yang, H., Guo, X. et al. Mechanism of LEPR-mediated cholesterol metabolism involved in NK cell function suppression under simulated microgravity. npj Microgravity 11, 83 (2025). https://doi.org/10.1038/s41526-025-00473-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41526-025-00473-0