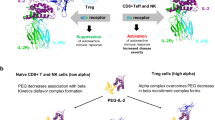
Abstract
The preferential activation of regulatory T (Treg) cells by interleukin-2 (IL-2), which selectively binds to the trimeric IL-2 receptor (IL-2R) on Treg cells, makes this cytokine a promising therapeutic for the treatment of autoimmune diseases. However, IL-2 has a narrow therapeutic window and a short half-life. Here, we show that the pharmacokinetics and half-life of IL-2 can be substantially improved by orthogonally conjugating the cytokine to poly(ethylene glycol) (PEG) moieties via a copper-free click reaction through the incorporation of azide-bearing amino acids at defined sites. Subcutaneous injection of a PEGylated IL-2 that optimally induced sustained Treg-cell activation and expansion over a wide range of doses through highly selective binding to trimeric IL-2R led to enhanced therapeutic efficacy in mouse models of lupus, collagen-induced arthritis and graft-versus-host disease without compromising the immune defences of the host against viral infection. Site-specific PEGylation could be used more generally to engineer cytokines with improved therapeutic performance for the treatment of autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. The quaternary structure of IL-2 associated with the trimeric receptor is available from publicly available datasets (http://www1.rcsb.org).
References
Klatzmann, D. & Abbas, A. K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 15, 283–294 (2015).
Abbas, A. K., Trotta, E., Simeonov, R. D., Marson, A. & Bluestone, J. A. Revisiting IL-2: biology and therapeutic prospects. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aat1482 (2018).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12, 180–190 (2012).
He, J. et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 79, 141–149 (2020).
Rosenzwajg, M. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 78, 209–217 (2019).
Mizui, M. & Tsokos, G. C. Low-dose IL-2 in the treatment of lupus. Curr. Rheumatol. Rep. 18, 68 (2016).
Kim, N. et al. Therapeutic potential of low-dose IL-2 in a chronic GVHD patient by in vivo expansion of regulatory T cells. Cytokine 78, 22–26 (2016).
He, J. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016).
Dwyer, C. J., Ward, N. C., Pugliese, A. & Malek, T. R. Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr. Diab. Rep. 16, 46 (2016).
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Pol, J. G., Caudana, P., Paillet, J., Piaggio, E. & Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. https://doi.org/10.1084/jem.20191247 (2020).
Lotze, M. T. et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 135, 2865–2875 (1985).
Boyman, O., Kovar, M., Rubinstein, M. P., Surh, C. D. & Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311, 1924–1927 (2006).
Levin, A. M. et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 484, 529–533 (2012).
Mitra, S. et al. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 42, 826–838 (2015).
Ward, N. C. et al. IL-2/CD25: a long-acting fusion protein that promotes immune tolerance by selectively targeting the IL-2 receptor on regulatory T cells. J. Immunol. 201, 2579–2592 (2018).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018).
Spangler, J. B. et al. Engineering a single-agent cytokine/antibody fusion that selectively expands regulatory T cells for autoimmune disease therapy. J. Immunol. 201, 2094–2106 (2018).
Trotta, E. et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 24, 1005–1014 (2018).
Rao, B. M., Girvin, A. T., Ciardelli, T., Lauffenburger, D. A. & Wittrup, K. D. Interleukin-2 mutants with enhanced alpha-receptor subunit binding affinity. Protein Eng. 16, 1081–1087 (2003).
Carmenate, T. et al. Human IL-2 mutein with higher antitumor efficacy than wild type IL-2. J. Immunol. 190, 6230–6238 (2013).
Liu, D. V., Maier, L. M., Hafler, D. A. & Wittrup, K. D. Engineered interleukin-2 antagonists for the inhibition of regulatory T cells. J. Immunother. 32, 887–894 (2009).
Carmenate, T. et al. Blocking IL-2 signal in vivo with an IL-2 antagonist reduces tumor growth through the control of regulatory T cells. J. Immunol. 200, 3475–3484 (2018).
Karakus, U. et al. Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abb9283 (2020).
Lee, J. Y. et al. TCB2, a new anti-human interleukin-2 antibody, facilitates heterodimeric IL-2 receptor signaling and improves anti-tumor immunity. Oncoimmunology 9, 1681869 (2020).
Arenas-Ramirez, N. et al. Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci. Transl. Med. 8, 367ra166 (2016).
Spangler, J. B. et al. Antibodies to interleukin-2 elicit selective T cell subset potentiation through distinct conformational mechanisms. Immunity 42, 815–825 (2015).
Sahin, D. et al. An IL-2-grafted antibody immunotherapy with potent efficacy against metastatic cancer. Nat. Commun. 11, 6440 (2020).
Peterson, L. B. et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J. Autoimmun. 95, 1–14 (2018).
Pasut, G. & Veronese, F. M. State of the art in PEGylation: the great versatility achieved after forty years of research. J. Control. Release 161, 461–472 (2012).
Charych, D. H. et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 22, 680–690 (2016).
Tian, F. et al. A general approach to site-specific antibody drug conjugates. Proc. Natl Acad. Sci. USA 111, 1766–1771 (2014).
Cho, H. et al. Optimized clinical performance of growth hormone with an expanded genetic code. Proc. Natl Acad. Sci. USA 108, 9060–9065 (2011).
Zhang, B. et al. Development of next generation of therapeutic IFN-α2b via genetic code expansion. Acta Biomater. 19, 100–111 (2015).
Wu, L. et al. Precise and combinatorial PEGylation generates a low-immunogenic and stable form of human growth hormone. J. Control. Release 249, 84–93 (2017).
Mbua, N. E., Guo, J., Wolfert, M. A., Steet, R. & Boons, G. J. Strain-promoted alkyne-azide cycloadditions (SPAAC) reveal new features of glycoconjugate biosynthesis. ChemBioChem 12, 1912–1921 (2011).
Nguyen, D. P. et al. Genetic encoding and labeling of aliphatic azides and alkynes in recombinant proteins via a pyrrolysyl-tRNA synthetase/tRNACUA pair and click chemistry. J. Am. Chem. Soc. 131, 8720–8721 (2009).
Stauber, D. J., Debler, E. W., Horton, P. A., Smith, K. A. & Wilson, I. A. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc. Natl Acad. Sci. USA 103, 2788–2793 (2006).
Wang, X., Rickert, M. & Garcia, K. C. Structure of the quaternary complex of interleukin-2 with its α, β, and γc receptors. Science 310, 1159–1163 (2005).
Yu, A. et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 64, 2172–2183 (2015).
Miksa, M., Komura, H., Wu, R., Shah, K. G. & Wang, P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J. Immunol. Methods 342, 71–77 (2009).
Rao, B. M., Driver, I., Lauffenburger, D. A. & Wittrup, K. D. High-affinity CD25-binding IL-2 mutants potently stimulate persistent T cell growth. Biochemistry 44, 10696–10701 (2005).
Dominguez-Villar, M. & Hafler, D. A. Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673 (2018).
Brand, D. D., Latham, K. A. & Rosloniec, E. F. Collagen-induced arthritis. Nat. Protoc. 2, 1269–1275 (2007).
Krieg, C., Letourneau, S., Pantaleo, G. & Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl Acad. Sci. USA 107, 11906–11911 (2010).
Ito, T. et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28, 870–880 (2008).
Gesbert, F., Sauvonnet, N. & Dautry-Varsat, A. Clathrin-independent endocytosis and signalling of interleukin 2 receptors IL-2R endocytosis and signalling. Curr. Top. Microbiol. Immunol. 286, 119–148 (2004).
Li, Y. et al. Regulatory T cells control toxicity in a humanized model of IL-2 therapy. Nat. Commun. 8, 1762 (2017).
Arce Vargas, F. et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 46, 577–586 (2017).
Hofmann, K., Clauder, A. K. & Manz, R. A. Targeting B cells and plasma cells in autoimmune diseases. Front. Immunol. 9, 835 (2018).
Mizui, M. Natural and modified IL-2 for the treatment of cancer and autoimmune diseases. Clin. Immunol. 206, 63–70 (2019).
Verhoef, J. J., Carpenter, J. F., Anchordoquy, T. J. & Schellekens, H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov. Today 19, 1945–1952 (2014).
Garay, R. P., El-Gewely, R., Armstrong, J. K., Garratty, G. & Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 9, 1319–1323 (2012).
Tao, X., Fan, F., Hoffmann, V., Longo, N. S. & Lipsky, P. E. Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. Arthritis Res. Ther. 8, R24 (2006).
Wang, H. et al. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat. Immunol. 2, 632–637 (2001).
Deng, G. M., Zheng, L., Chan, F. K. & Lenardo, M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat. Med. 11, 1066–1072 (2005).
Wang, J. et al. Klebsiella pneumoniae alleviates influenza-induced acute lung injury via limiting NK cell expansion. J. Immunol. 193, 1133–1141 (2014).
Acknowledgements
We thank Z. Tian (University of Science and Technology of China, Hefei) for gifting the YT cell line; Y. Hou for her support with flow cytometry technology and D. Liu (Peking University) for her participation in mass spectrometry experiments; X. Wu from the X.Z. laboratory and H. Li from the Tsokos laboratory for their discussions; and all of the donors who participated in the study. This study was supported by grants from the National Key Research and Development Program of China (no. 2019ZX09739), National Natural Science Foundation of China (nos 81788101, 81803419, 81630044, 81802121, 21805311), Chinese Academy of Medical Science Innovation Fund for Medical Sciences (nos CIFMS2016-12M-1-003, 2017-12M-1-008, 2017-I2M-3-011, 2016-12M-1-008) and Capital’s Funds for Health Improvement and Research (no. 2020-2-4019).
Author information
Authors and Affiliations
Contributions
B.Z., Y.W., J.S., D.Z. and X.Z. designed the study. B.Z., Y.W., J.S., Y.H., J.H., Y.Y. and Y.S. performed the experiments in vitro and in vivo and analysed the data. D.J. and F.Y. performed experiments of chemical synthesis. S.L. contributed to the crystal structure analysis and site selection. W.M. and B.C. performed experiments with influenza virus. P.L., L.W., W.S., M.W. and X.L. contributed to the collection and assembly of data and polished the language. L. Zhao, H.C., Y.F., H.L., L. Zhang and G.C.T. provided comments. B.Z., D.Z., G.C.T. and Z.X. wrote and reviewed the manuscript. All of the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Onur Boyman, David Klatzmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the binding of representative PEGylated variants to dimeric or trimeric IL-2Rs, assessed by surface plasmon resonance (SPR), for identification of IL-2 PEGylates showing the highest level of selectivity towards trimeric IL-2Rs.
Fc-tagged extracellular domains of the IL-2R subunits were immobilized on a CM5 chip at an equimolar ratio. Three representative concentrations of each sample in a series of 3-fold dilutions for trimeric (3.3, 10 and 30 nM) or dimeric (33.3, 100 and 300 nM) IL-2R complexes are presented. All kinetic parameters are provided in S Table 2. Representative results from one of two independent experiments are shown. See also Fig. 1.
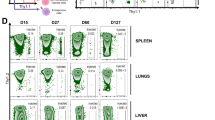
Extended Data Fig. 2 Validation of Treg-selective activation, as reflected by increased expression of CD25 and FoxP3 by PEGylated IL-2s in a xeno-GvHD mouse model.
The MFI bars graphs (top) and flow cytometry histograms (bottom) of induced CD25 and Foxp3 on Tregs and CD8 + T cells in response to IL-2 and its PEGylated variants at a dose of 2 µg, with PBS as the background. The data are presented as the mean ± SEM, n = 3 biologically independent mice per group. Statistical analysis was performed using one-way ANOVA (Dunnett’s multiple-comparison test compared with IL-2 treated group). Representative results from one of three independent experiments are shown. See also Fig. 4a, b.
Extended Data Fig. 3 Dual-31/51-20K-mediated mitigation of kidney lesions in MRL/lpr mice.
The ability of dual-31/51-20 K to reduce kidney lesions, as reflected by H&E staining of the 0.5 µg treatment groups, with the mononuclear cells infiltrating into the cortical tubulointerstitial/perivascular areas and the scores of glomerular and perivascular lesions shown by arrows and bar graphs, respectively. The data are presented as the mean ± SEM, n = 4 biologically independent mice per group. Statistical analysis was performed using one-way ANOVA (Dunnett’s multiple-comparison test compared with PBS treated group). Representative results from one of two experiments are shown. See also Fig. 5b, c.
Extended Data Fig. 4 The effects of IL-2 versus dual-31/51-20 K on activation of pulmonary NK cells.
Bar graphs show the expression of activated markers, including NKG2D, NKp46, and CD69, on pulmonary NK cells of mice with indicated treatment. Data are presented as the mean ± SEM, n = 4 biologically independent mice per group. Statistical analysis was performed using one-way ANOVA (Dunnett’s multiple-comparison test compared with + virus/PBS-treated group). The experiment was repeated three times with similar results, and one of three representative results is shown. See also Fig. 7c.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Zhang, B., Sun, J., Wang, Y. et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat Biomed Eng 5, 1288–1305 (2021). https://doi.org/10.1038/s41551-021-00797-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-021-00797-8
This article is cited by
-
Long-acting IL-2 release from pressure-fused biomineral tablets promotes antitumor immune response
Nature Cancer (2025)
-
Generation of tolerogenic antigen-presenting cells in vivo via the delivery of mRNA encoding PDL1 within lipid nanoparticles
Nature Biomedical Engineering (2025)
-
RNA nanotherapeutics with fibrosis overexpression and retention for MASH treatment
Nature Communications (2024)
-
The immunology of systemic lupus erythematosus
Nature Immunology (2024)
-
The JAK-STAT pathway: from structural biology to cytokine engineering
Signal Transduction and Targeted Therapy (2024)