Abstract
The intricate topology of vascular networks and the complex functions of vessel-rich tissues are challenging to reconstruct in vitro. Here we report the development of: in vitro pathological models of erectile dysfunction and Peyronie’s disease; a model of the penis that includes the glans and the corpus spongiosum with urethral structures; and an implantable model of the corpus cavernosum, whose complex vascular network is critical for erectile function, via the vein-occlusion effect. Specifically, we 3D printed a hydrogel-based corpus cavernosum incorporating a strain-limiting tunica albuginea that can be engorged with blood through vein occlusion. In corpus cavernosum defects in rabbits and pigs, implantation of the 3D-printed tissue seeded with endothelial cells restored normal erectile function on electrical stimulation of the cavernous nerves as well as spontaneous erectile function within a few weeks of implantation, which allowed the animals to mate and reproduce. Our findings support the further development of 3D-printed blood-vessel-rich functional organs for transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The RNA-sequencing data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE286369. The data generated during the study, including source data for the figures, are available from figshare at https://doi.org/10.6084/m9.figshare.28218347 (ref. 48).
References
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Weaver, J. D. et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 3, e1700184 (2017).
Ghesquiere, B., Wong, B. W., Kuchnio, A. & Carmeliet, P. Metabolism of stromal and immune cells in health and disease. Nature 511, 167–176 (2014).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
O’Connor, C., Brady, E., Zheng, Y., Moore, E. & Stevens, K. R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 7, 702–716 (2022).
Andersson, K. E. Pharmacology of penile erection. Pharmacol. Rev. 53, 417–450 (2001).
Yafi, F. A. et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2, 16003 (2016).
Chen, K. L., Eberli, D., Yoo, J. J. & Atala, A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc. Natl Acad. Sci. USA 107, 3346–3350 (2010).
Goldstein, I. et al. Oral sildenafil in the treatment of erectile dysfunction. N. Engl. J. Med. 338, 1397–1404 (1998).
Burnett, A. L., Lowenstein, C. J., Bredt, D. S., Chang, T. S. & Snyder, S. H. Nitric oxide: a physiologic mediator of penile erection. Science 257, 401–403 (1992).
Udelson, D., L’Esperance, J., Morales, A. M., Patel, R. & Goldstein, I. The mechanics of corporal veno-occlusion in penile erection: a theory on the effect of stretch-associated luminal constrictability on outflow resistance. Int. J. Impot. Res. 12, 315–327 (2000).
Bosch, R. J. et al. Penile detumescence: characterization of three phases. J. Urol. 146, 867–871 (1991).
Chai, M. et al. Bionic artificial penile tunica albuginea. Matter 6, 626–641 (2023).
Gundogdu, G. et al. Evaluation of bi-layer silk fibroin grafts for penile tunica albuginea repair in a rabbit corporoplasty model. Front. Bioeng. Biotechnol. 9, 791119 (2021).
Gelbard, M. K., Dorey, F. & James, K. The natural history of Peyronie’s disease. J. Urol. 144, 1376–1379 (1990).
Kadioglu, A. et al. A retrospective review of 307 men with Peyronie’s disease. J. Urol. 168, 1075–1079 (2002).
Dibenedetti, D. B., Nguyen, D., Zografos, L., Ziemiecki, R. & Zhou, X. A population-based study of Peyronie’s disease: prevalence and treatment patterns in the United States. Adv. Urol. 2011, 282503 (2011).
Chen, J., Gefen, A., Greenstein, A., Matzkin, H. & Elad, D. Predicting penile size during erection. Int. J. Impot. Res. 12, 328–333 (2000).
Lee, A. et al. 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 (2019).
Grigoryan, B. et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 (2019).
Kuang, X. et al. Grayscale digital light processing 3D printing for highly functionally graded materials. Sci. Adv. 5, eaav5790 (2019).
Wetterauer, U. in Sexology (eds Eicher, W. et al.) 115–126 (Springer, 1988).
Jang, K. I. et al. Soft network composite materials with deterministic and bio-inspired designs. Nat. Commun. 6, 6566 (2015).
Pritchard, R. H., Huang, Y. Y. & Terentjev, E. M. Mechanics of biological networks: from the cell cytoskeleton to connective tissue. Soft Matter 10, 1864–1884 (2014).
Onck, P. R., Koeman, T., van Dillen, T. & van der Giessen, E. Alternative explanation of stiffening in cross-linked semiflexible networks. Phys. Rev. Lett. 95, 178102 (2005).
Meyers, M. A., McKittrick, J. & Chen, P. Y. Structural biological materials: critical mechanics-materials connections. Science 339, 773–779 (2013).
Veale, D., Miles, S., Bramley, S., Muir, G. & Hodsoll, J. Am I normal? A systematic review and construction of nomograms for flaccid and erect penis length and circumference in up to 15,521 men. BJU Int. 115, 978–986 (2015).
Chen, X. B., Li, R. X., Yang, H. N. & Dai, J. C. A comprehensive, prospective study of penile dimensions in Chinese men of multiple ethnicities. Int. J. Impot. Res. 26, 172–176 (2014).
de Meyer, J. M. & Thibo, P. The correlation among cavernous pressure, penile rigidity and resistance index. J. Urol. 160, 63–66 (1998).
Mudau, M., Genis, A., Lochner, A. & Strijdom, H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc. J. Afr. 23, 222–231 (2012).
de Meyer, J. M. & Thibo, P. The effect of re-dosing of vasodilators on the intracavernosal pressure and on the penile rigidity. Eur. Urol. 33, 293–296 (1998).
Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science 356, eaaf3627 (2017).
Yuk, H. et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019).
An, G. et al. Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells. Nat. Commun. 11, 2687 (2020).
Zhao, X. et al. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 5, 108–118 (2016).
Chiappone, A. et al. 3D printed PEG-based hybrid nanocomposites obtained by sol-gel technique. ACS Appl. Mater. Interfaces 8, 5627–5633 (2016).
Kim, S. H. et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 9, 1620 (2018).
Wu, Y., Chee, A. J. Y., Golzar, H., Yu, A. C. H. & Tang, X. Embedded 3D printing of ultrasound-compatible arterial phantoms with biomimetic elasticity. Adv. Funct. Mater. 32, 2110153 (2022).
Bakarich, S. E., Panhuis, M. I. H., Beirne, S., Wallace, G. G. & Spinks, G. M. Extrusion printing of ionic-covalent entanglement hydrogels with high toughness. J. Mater. Chem. B 1, 4939–4946 (2013).
Tavafoghi, M. et al. Engineering tough, injectable, naturally derived, bioadhesive composite hydrogels. Adv. Healthc. Mater. 9, 1901722 (2020).
Ge, Q. et al. 3D printing of highly stretchable hydrogel with diverse UV curable polymers. Sci. Adv. 7, eaba4261 (2021).
Kim, D. H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Wang, S. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Jones, T. J., Jambon-Puillet, E., Marthelot, J. & Brun, P. T. Bubble casting soft robotics. Nature 599, 229–233 (2021).
Kwon, T. G., Yoo, J. J. & Atala, A. Autologous penile corpora cavernosa replacement using tissue engineering techniques. J. Urol. 168, 1754–1758 (2002).
Fandel, T. M. et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur. Urol. 61, 201–210 (2012).
Ferretti, L. et al. Tissue engineering for penile surgery: comparative study of noncellular and cell-seeded synthetic grafts for tunica albuginea replacement. J. Sex. Med. 9, 625–631 (2012).
Wang, Z. & Liu, X. Data for 3D-printed perfused models of the penis for the study of penile physiology and for restoring erectile function in rabbits and pigs. figshare https://doi.org/10.6084/m9.figshare.28218347 (2025).
Acknowledgements
We thank Q. Zhang for providing technical assistance and M. Chai for participating in helpful discussions. Y.W. discloses support for the research described in this study from the National Natural Science Foundation of China (grant number T2288101). X.S. discloses support for the publication of this study from the National Natural Science Foundation Joint Fund of China (grant number U22A20157). X.L. discloses support for the publication of this study from the National Natural Science Foundation of China (grant no. 32301136 to X.L.).
Author information
Authors and Affiliations
Contributions
K.W.L., Y.W. and X.S. conceived the project. Z.W., X.L., T.Y. and Z.Z. designed and performed most of the experiments. D.S., K.W., Y.K. and S.O. conducted experimental investigations and analysed the data. Z.W. and X.L. wrote the original draft. K.W.L., Y.W. and X.S. supervised the work and revised the paper. All authors reviewed and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Andres Garcia, Petra de Graaf, Ji Liu, Y. Shrike Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The mechanical tensile properties and 3D printing results of the hydrogels prepared in this study.
a, The stress‒strain curve of the hydrogel. b-c, Ashby chart of the mechanical properties for various 3D-printed hydrogels, tensile strength versus Young’s modulus (b) and elongation at break versus Young’s modulus (c). Hydrogels include the hydrogel prepared in this work (red area), gelatin methacryloyl (GelMA)35, polyethylene glycol diacrylate (PEGDA)36, glycidyl methacrylated silk fibroin (Sil-GMA)37, glycidyl methacrylated poly (vinyl alcohol) (PVAGMA)38, polyacrylamide (PAAm)/Alginate39, methacrylate-modified alginate (AlgMA)/GelMA40, and polyacrylic acid (PAA)/PEGDA41. d, Various 3D printing models of the hydrogel, including a fox (i), axial vessel and helix (ii) and a Hilbert microchannel (iii).
Extended Data Fig. 2 Numerical simulation of the BCC models.
a-b, Displacement maps (a) and von Mises stress maps (b) show that the V.1.0 BCC model can achieve venous occlusion during erection. The height and diameter of the flaccid model in the numerical simulation are 9.6 mm and 16 mm, respectively. c, Time‒volume expansion curve, showing that the V.2.0 BCC model exhibits faster erectile deformation than the V.1.0 BCC model.
Extended Data Fig. 3 Schematic diagram of the structural design of the BCC models.
a, Procedural derivation of the two versions of the BCC models from the structural units. b, Statistics for the numbers of cavernous sinuses in the different versions of the BCC models. c, Statistics for the hollow rates of the different versions of BCC models.
Extended Data Fig. 4 Perfusion device for all the models (including the BCC, BCC with the wrapped biomimetic tunica albuginea, ED, and PD models).
The fixation frame ensures that the model remains stable during perfusion.
Extended Data Fig. 5 Local deformation to damage and flow measurement.
a, During perfusion (average flow, 90 mL/min), the BCC model exhibited notable local deformation without the constraint imposed by the biomimetic tunica albuginea. As a result, the BCC model suffers from local damage (scale bar: 9 mm). b, The outflow rate changes over time during in vitro erection perfusion in the BCC model wrapped with biomimetic tunica albuginea. The decrease in outlet flow caused by vein occlusion can be clearly observed. The blue shaded area indicates the venous occlusion state during BCC perfusion erection. c, Inflow rate of the ED model under high and low perfusion flow rates.
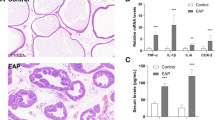
Extended Data Fig. 6 Implantation of the hydrogel model into the porcine corpus cavernosum.
a, Surgical procedure for implanting the model into the corpus cavernosum defect site (scale bar: 10 mm). b-c, Routine blood test results for the samples obtained from pigs implanted with the hydrogel model, including the cell count index (b) and the cell percentage index (c) (scale bar: 10 mm).
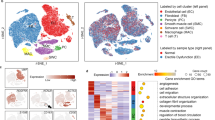
Extended Data Fig. 7 RNA sequencing.
a, Enriched Gene Ontology (GO) terms in the upregulated proliferation-related genes of the porcine penis corpus cavernosum tissues from the implantation group versus the normal group (n = 3 pigs). The statistical analysis was performed using one-side hypergeometric test. b, Heatmap showing the differences in the expression of proliferation-related genes and inflammation-related genes among the three different groups (normal, defect and implantation groups). The color key from red to blue represents high to low gene expression levels, respectively (n = 3 pigs). Statistical analysis was performed using two-sided One-way ANOVA followed by Tukey’s multiple comparison test. Data are presented as mean ± SD (n = 3 pigs).
Supplementary information
Supplementary Information
Supplementary methods, table, figures, references and video captions.
Supplementary Video 1
BCC model perfusion.
Supplementary Video 2
BCC model perfusion with bionic tunica albuginea.
Supplementary Video 3
ED model perfusion.
Supplementary Video 4
PD model perfusion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Liu, X., Ye, T. et al. 3D-printed perfused models of the penis for the study of penile physiology and for restoring erectile function in rabbits and pigs. Nat. Biomed. Eng 9, 1276–1289 (2025). https://doi.org/10.1038/s41551-025-01367-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01367-y
This article is cited by
-
2025 in review
Nature Biomedical Engineering (2025)
-
Biomimetic model to study penile dysfunctions
Nature Biomedical Engineering (2025)