Abstract
The mesopelagic zone, between 100 m and 1,000 m depth, is a crucial layer in which carbon preliminary coming down from the surface is transformed before a portion makes it into the deep ocean. While eddies and their fronts influence surface productivity and carbon export, their effects deeper in the water column remain poorly understood. Here we show the importance and contribution of dark carbon fixation—the conversion of inorganic into organic carbon by prokaryotes—across five contrasting hydrological features in the North Atlantic, using isotopic tracers and quantification of chemoautotrophy genes. The approach allows simultaneous assessment of dark carbon fixation and heterotrophic activity of prokaryotes living suspended in seawater and attached to gravitationally settling particles. Our results highlight that heterotrophic prokaryotes attached to sinking particles contribute up to 21% of the total organic carbon required to sustain prokaryotic metabolism under the influence of eddy fronts. By contrast, dark carbon fixation by suspended prokaryotes can contribute up to half of the total carbon input to the mesopelagic zone in the cyclonic eddy. Our findings challenge the idea that carbon cycling in the mid-depth ocean is uniform and highlight the need to integrate microbial fractions and physical heterogeneity into ocean carbon models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated and used in this study are available via SEANOE (Sea Scientific Open Data Edition) at https://doi.org/10.17882/103735. The dataset, titled ‘Biogeochemical datasets collected during the APERO cruise’, is structured by methodological approach and includes all biogeochemical, microbial and environmental measurements used in the present study. No external or publicly available datasets were used in this study.
Code availability
The code used for data processing and figure generation is not custom developed and consists only of standard scripts. It is available from the corresponding author upon request. No publicly archived version is provided.
References
Passow, U. & Weber, T. in Treatise on Geochemistry 3rd edn (eds Anbar, A. & Weis, D.) 333–369 (Elsevier, 2025); https://doi.org/10.1016/B978-0-323-99762-1.00031-0
Henson, S. A. et al. A reduced estimate of the strength of the ocean’s biological carbon pump. Geophys. Res. Lett. 38, L04606 (2011).
Boyd, P. W. & Trull, T. W. Understanding the export of biogenic particles in oceanic waters: is there consensus?. Prog. Oceanogr. 72, 276–312 (2007).
Boyd, P. W., Claustre, H., Levy, M., Siegel, D. A. & Weber, T. Multi-faceted particle pumps drive carbon sequestration in the ocean. Nature 568, 327–335 (2019).
Siegel, D. A., DeVries, T., Cetinić, I. & Bisson, K. M. Quantifying the ocean’s biological pump and its carbon cycle impacts on global scales. Annu. Rev. Mar. Sci. 15, 329–356 (2023).
Martin, J. H., Knauer, G. A., Karl, D. M. & Broenkow, W. W. VERTEX: carbon cycling in the northeast Pacific. Deep Sea Res. A 34, 267–285 (1987).
Azam, F. et al. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263 (1983).
Kwon, E. Y., Primeau, F. & Sarmiento, J. L. The impact of remineralization depth on the air–sea carbon balance. Nat. Geosci. 2, 630–635 (2009).
Maier-Reimer, E., Mikolajewicz, U. & Winguth, A. Future ocean uptake of CO2: interaction between ocean circulation and biology. Clim. Dyn. 12, 711–722 (1996).
Iversen, M. H. Carbon export in the ocean: a biologist’s perspective. Annu. Rev. Mar. Sci. 15, 357–381 (2023).
Bressac, M. et al. Decoding drivers of carbon flux attenuation in the oceanic biological pump. Nature 633, 587–593 (2024).
Chelton, D. B., Schlax, M. G., Samelson, R. M. & de Szoeke, R. A. Global observations of large oceanic eddies. Geophys. Res. Lett. 34, L15606 (2007).
Harrison, C. S., Long, M. C., Lovenduski, N. S. & Moore, J. K. Mesoscale effects on carbon export: a global perspective. Glob. Biogeochem. Cycles 32, 680–703 (2018).
Levy, M. & Martin, A. P. The influence of mesoscale and submesoscale heterogeneity on ocean biogeochemical reactions. Glob. Biogeochem. Cycles 27, 1139–1150 (2013).
Herndl, G. J. & Reinthaler, T. Microbial control of the dark end of the biological pump. Nat. Geosci. 6, 718–724 (2013).
Reinthaler, T., van Aken, H. M. & Herndl, G. J. Major contribution of autotrophy to microbial carbon cycling in the deep North Atlantic’s interior. Deep Sea Res. 2 57, 1572–1580 (2010).
Baltar, F. et al. Significance of non-sinking particulate organic carbon and dark CO2 fixation to heterotrophic carbon demand in the mesopelagic northeast Atlantic. Geophys. Res. Lett. 37, L09602 (2010).
Hansman, R. L. et al. The radiocarbon signature of microorganisms in the mesopelagic ocean. Proc. Natl Acad. Sci. USA 106, 6513–6518 (2009).
Zhao, Z. et al. Metaproteomic analysis decodes trophic interactions of microorganisms in the dark ocean. Nat. Commun. 15, 6411 (2024).
Ricci, F. & Greening, C. Chemosynthesis: a neglected foundation of marine ecology and biogeochemistry. Trends Microbiol. 32, 631–639 (2024).
Burd, A. B. et al. Assessing the apparent imbalance between geochemical and biochemical indicators of meso- and bathypelagic biological activity: what the @$♯! is wrong with present calculations of carbon budgets?. Deep Sea Res. 2 57, 1557–1571 (2010).
Steinberg, D. K. et al. Bacterial vs. zooplankton control of sinking particle flux in the ocean’s twilight zone. Limnol. Oceanogr. 53, 1327–1338 (2008).
Berg, I. A. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 77, 1925–1936 (2011).
Hügler, M. & Sievert, S. M. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Annu. Rev. Mar. Sci. 3, 261–289 (2011).
Herndl, G. J. et al. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl. Environ. Microbiol. 71, 2303–2309 (2005).
Yakimov, M. M., Cono, V. L. & Denaro, R. A first insight into the occurrence and expression of functional amoA and accA genes of autotrophic and ammonia-oxidizing bathypelagic Crenarchaeota of Tyrrhenian Sea. Deep Sea Res. Part 2 56, 748–754 (2009).
Braun, A. et al. Reviews and syntheses: heterotrophic fixation of inorganic carbon – Significant but invisible flux in environmental carbon cycling. Biogeosciences 18, 3689–3700 (2021).
Baumas, C. et al. Reconstructing the ocean’s mesopelagic zone carbon budget: sensitivity and estimation of parameters associated with prokaryotic remineralization. Biogeosciences 20, 4165–4182 (2023).
Giering, S. L. C. et al. Reconciliation of the carbon budget in the ocean’s twilight zone. Nature 507, 480–483 (2014).
Karl, D. M., Knauer, G. A., Martin, J. H. & Ward, B. B. Bacterial chemolithotrophy in the ocean is associated with sinking particles. Nature 309, 54–56 (1984).
Acinas, S. G. et al. Deep ocean metagenomes provide insight into the metabolic architecture of bathypelagic microbial communities. Commun. Biol. 4, 604 (2021).
Baumas, C. M. J. et al. Mesopelagic microbial carbon production correlates with diversity across different marine particle fractions. ISME J. 15, 1695–1708 (2021).
Zhao, Z., Baltar, F. & Herndl, G. J. Linking extracellular enzymes to phylogeny indicates a predominantly particle-associated lifestyle of deep-sea prokaryotes. Sci. Adv. 6, eaaz4354 (2020).
Grossart, H.-P. Ecological consequences of bacterioplankton lifestyles: changes in concepts are needed. Environ. Microbiol. Rep. 2, 706–714 (2010).
Zhou, W. et al. High dark carbon fixation in the tropical South China Sea. Cont. Shelf Res. 146, 82–88 (2017).
Baltar, F., Arístegui, J., Gasol, J. M., Lekunberri, I. & Herndl, G. J. Mesoscale eddies: hotspots of prokaryotic activity and differential community structure in the ocean. ISME J. 4, 975–988 (2010).
Zhou, N. et al. Responses of nutrient biogeochemistry to a mesoscale eddy detached from the Kuroshio loop current. J. Geophys. Res. Oceans 130, e2024JC021792 (2025).
Lee-Sánchez, E., Camacho-Ibar, V. F., Velásquez-Aristizábal, J. A., Valencia-Gasti, J. A. & Samperio-Ramos, G. Impacts of mesoscale eddies on the nitrate distribution in the deep-water region of the Gulf of Mexico. J. Mar. Syst. 229, 103721 (2022).
Devresse, Q., Becker, K. W., Bendinger, A., Hahn, J. & Engel, A. Eddy-enhanced primary production sustains heterotrophic microbial activities in the Eastern Tropical North Atlantic. Biogeosciences 19, 5199–5219 (2022).
Jaffe, A. L., Salcedo, R. S. R. & Dekas, A. E. Abundant and metabolically flexible bacterial lineages underlie a vast potential for rubisco-mediated carbon fixation in the dark ocean. Genome Biol. 26, 167 (2025).
Alfreider, A. et al. CO2 assimilation strategies in stratified lakes: diversity and distribution patterns of chemolithoautotrophs. Environ. Microbiol. 19, 2754–2768 (2017).
Klawonn, I., Bonaglia, S., Brüchert, V. & Ploug, H. Aerobic and anaerobic nitrogen transformation processes in N2-fixing cyanobacterial aggregates. ISME J. 9, 1456–1466 (2015).
De Corte, D. et al. Microbes mediating the sulfur cycle in the Atlantic Ocean and their link to chemolithoautotrophy. Environ. Microbiol. 23, 7152–7167 (2021).
Könneke, M. et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl Acad. Sci. USA 111, 8239–8244 (2014).
Kache, S., Bartl, I., Wäge-Recchioni, J. & Voss, M. Influence of organic particle addition on nitrification rates and ammonium oxidiser abundances in Baltic seawater. Mar. Ecol. Prog. Ser. 674, 59–72 (2021).
Pachiadaki, M. G. et al. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation. Science 358, 1046–1051 (2017).
Dekas, A. E. et al. Characterizing chemoautotrophy and heterotrophy in marine Archaea and Bacteria with single-cell multi-isotope nanoSIP. Front. Microbiol. 10, 2682 (2019).
Thibault, H., Ménard, F., Abitbol-Spangaro, J., Poggiale, J.-C. & Martini, S. Modeling the contribution of micronekton diel vertical migrations to carbon export in the mesopelagic zone. Biogeosciences 22, 2181–2200 (2025).
Burd, B. J. & Thomson, R. E. A review of zooplankton and deep carbon fixation contributions to carbon cycling in the dark ocean. J. Mar. Syst. 236, 103800 (2022).
Biggs, T. E. G., Huisman, J. & Brussaard, C. P. D. Viral lysis modifies seasonal phytoplankton dynamics and carbon flow in the Southern Ocean. ISME J. 15, 3615–3622 (2021).
Memery, L. et al. Biogeochemical datasets collected during the APERO Cruise. SEANOE https://doi.org/10.17882/103735 (2024).
Lampitt, R. S., Billett, D. S. M. & Martin, A. P. The sustained observatory over the Porcupine Abyssal Plain (PAP): insights from time series observations and process studies. Deep Sea Res. 2 57, 1267–1271 (2010).
Rypina, I. I., Pratt, L. J. & Lozier, M. S. Near-surface transport pathways in the North Atlantic Ocean: looking for throughput from the Subtropical to the Subpolar Gyre. J. Phys. Oceanogr. 41, 911–925 (2011).
Planquette, H. & Sherrell, R. M. Sampling for particulate trace element determination using water sampling bottles: methodology and comparison to in situ pumps. Limnol. Oceanogr. Methods 10, 367–388 (2012).
Kirchman, D. L. in Handbook of Methods in Aquatic Microbial Ecology (eds Kemp, P. F. et al.) 509–512 (CRC Press, 1993).
Giering, S. L. C. & Evans, C. Overestimation of prokaryotic production by leucine incorporation—and how to avoid it. Limnol. Oceanogr. 67, 726–738 (2022).
Smith, D. C. & Azam, F. A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine. Mar. Microb. Food Webs 6, 107–114 (1992).
González-Sánchez, J. M. et al. What happens to biomass burning-emitted particles in the ocean? A laboratory experimental approach based on their tracers. Sci. Total Environ. 907, 167770 (2024).
Alfreider, A., Vogt, C., Hoffmann, D. & Babel, W. Diversity of ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes from groundwater and aquifer microorganisms on JSTOR. Microb. Ecol. 45, 317–328 (2003).
Selesi, D., Schmid, M. & Hartmann, A. Diversity of green-like and red-like ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes (cbbL) in differently managed agricultural aoils. Appl. Environ. Microbiol. 71, 175–184 (2005).
Watson, G. M. F. & Tabita, F. R. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a molecule for phylogenetic and enzymological investigation. FEMS Microbiol. Lett. 146, 13–22 (1997).
Berg, I. A., Kockelkorn, D., Buckel, W. & Fuchs, G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 318, 1782–1786 (2007).
Karner, M. B., DeLong, E. F. & Karl, D. M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409, 507–510 (2001).
Gaby, J. C. & Buckley, D. H. The use of degenerate primers in qPCR analysis of functional genes can cause dramatic quantification bias as revealed by investigation of nifH primer performance. Microb. Ecol. 74, 701–708 (2017).
Smith, C. J. & Osborn, A. M. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol. Ecol. 67, 6–20 (2009).
Paris, E. R. et al. Single-cell analysis in hypersaline brines predicts a water-activity limit of microbial anabolic activity. Sci. Adv. 9, eadj3594 (2023).
Polerecky, L. et al. Look@NanoSIMS—a tool for the analysis of nanoSIMS data in environmental microbiology. Environ. Microbiol. 14, 1009–1023 (2012).
Parada, A. E. et al. Constraining the composition and quantity of organic matter used by abundant marine Thaumarchaeota. Environ. Microbiol. 25, 689–704 (2023).
del Giorgio, P. A. & Cole, J. J. Bacterial growth efficiency in natural aquatics systems. Annu. Rev. Ecol. Syst. 29, 503–541 (1998).
Copernicus Marine Service. North Atlantic Ocean Colour Plankton, Reflectance, Transparency and Optics MY L3 daily observations https://doi.org/10.48670/moi-00286 (2023).
Copernicus Marine Service. Atlantic Ocean Colour (Copernicus-GlobColour), Bio-Geo-Chemical, L4 (daily interpolated) from Satellite Observations (1997–ongoing) https://doi.org/10.48670/moi-00289 (2023).
Picheral, M. et al. The Underwater Vision Profiler 5: an advanced instrument for high spatial resolution studies of particle size spectra and zooplankton. Limnol. Oceanogr. Methods https://doi.org/10.4319/lom.2010.8.462 (2010).
Acknowledgements
This Article contributes to the APERO project funded by the National Research Agency under the grant APERO (grant no. ANR ANR-21-CE01-0027) and by the French LEFE-Cyber programme (CNRS, INSU). The authors thank the captain and crew of N/O ‘Le Pourquoi Pas’ (Flotte Océanographique Française) for their help during the APERO cruise (https://doi.org/10.17600/1800066). We thank the ‘Moyen Commun’ SAM and Radioactivity from the Mediterranean Institute of Oceanography (MIO) as well as the ‘parc national d’instrumentation océanographique’ (PNIO) of the ‘division technique de l’Institut national des sciences de l’Univers du CNRS (DT INSU) for their technical expertise and facilities. This work was also supported by the Pure Ocean Fund via the Microcean project granted to P.L.C. and by ANR-JC ARMORIC-ANR-21-CE01-0005 granted to F.L.M. This work was funded by the European Union under grant agreement no. 101083922 (OceanICU) and UK Research. The views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or European Research Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
P.L.C. and C.T. conceived this work; P.L.C., U.C., F.V.W., B.A.A., M. Garel, C.T., S.G., C.M.J.B., M.P.P., M. Gesson, F.L.M., E.C., T.M., O.C., N.B., E.d.S.L. and O.G. conducted fieldwork and/or laboratory analysis; C.T., U.C., F.V.W., H.-P.G., A.E.D., P.B., F.A., B.Z., L.G. and L.M. advised on interpretations. P.L.C. wrote the manuscript, with all authors contributing to its revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: James Super, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Hydrological conditions and surface Chlorophyll a during the APERO cruise.
(A) Conservative temperature (ϴ) and absolute salinity (SA) diagram of the five Process Studies Stations (PSS1 to PSS5 in yellow, light blue, green, brown and purple, respectively). NEACW: Northeast Atlantic Central Water; NEABW: Northeast Atlantic Bottom Water; MW: Mediterranean Water; SAIW: Subarctic Intermediate Water; LSW: Labrador Sea Water; Polar-like SW: Surface Water; (B) Time series of remote sensed Chlorophyll a (Chl.a) surface concentrations averaged over the area sampled by the PSS (squared area from minima/maxima of the drifting mooring line positions) from April 1 to August 31, 2023, and timings of the APERO cruise (thin black line) and PSSs (thick red line). Chl.a data are L3 (dark-green crosses) and L4 (green line) CMEMS North Atlantic 1 km resolution GlobColour products. Data from refs. 70,71.
Extended Data Fig. 2 Biogeochemical characteristics of the mesopelagic zone (100 - 1000 m depths).
Particulate organic carbon (POC), dissolved organic carbon (DOC), nitrates plus nitrites (NOx) and ammonium (NH4) concentrations sampled with Niskin bottles (panels A, C, D and E) and sinking POC fluxes measured by the sediment traps over 4 days (panel B). These measurements were done on the five Process Studies Stations (PSSs) during the APERO cruise.
Extended Data Fig. 3 Illustration of the sampling methods employed during the APERO cruise to study prokaryotic fraction.
“Suspended” prokaryotes (that is, free-living and attached to suspended particles) and “sinking” prokaryotes (that is, attached to sinking particles) were sampled by Niskin and sediment traps respectively. The investigation of prokaryotic communities included PHP (3H-leucine) and DCF (14C-bicarbonate) measurements, prokaryotic cell abundance and gene quantification (qPCR) of chemoautotrophy in all PSSs. A single cell nanoSIMS analysis (13C-bicarbonate and 15N-ammonium) was added in PSS3 at 500 m. CTD rosette illustration reproduced with permission from ref. 72.
Extended Data Fig. 4 Boxplots of prokaryotic activities in the mesopelagic zone.
Rates of dark carbon fixation (yellow) and prokaryotic heterotrophic production (blue) were measured at the five PSSs (expressed in µmol C m⁻³ d⁻¹). Upper panels represent the “suspended” fraction (free-living prokaryotes and those attached to suspended particles), whereas lower panels represent the “sinking” fraction (prokaryotes attached to sinking particles). Each boxplot integrates measurements at 100, 200, 500, and 1000 m, with each depth analyzed in triplicate technical incubations (n = 12 data points per PSS). Technical replicates correspond to independent incubations of subsamples from the same Niskin or sediment trap collection. Boxplots (A), (B), (D), and (E) compare activities between PSSs, while (C) and (F) compare activities between fractions. Boxplots indicate the interquartile range (boxes), median (central line), and whiskers extending to 1.5×IQR. Statistical differences were assessed using Kruskal-Wallis multiple tests followed by Dunn post-hoc tests (* = p < 0.05) and the Wilcoxon-Mann-Whitney test (**** = p < 0.0001). Upper and lower panels cannot be compared directly, as the sampled volumes differ between Niskin bottles and sediment traps.
Extended Data Fig. 5 Normalized prokaryotic activity rates.
Rates normalized by (A) the prokaryotic cell abundance and (B) the organic carbon content. Each boxplot includes data pooled from all sampled stations and depths (n = 20). Overlayed boxplots indicate the interquartile range (boxes), median (central line), and whiskers extending to 1.5×IQR. Statistically significant differences are indicated with asterisks (two-sided Wilcoxon-Mann-Whitney statistical: **** p ≤ 0.0001; ** p < 0.01; * p < 0.05; Non-significative-NS).
Extended Data Fig. 6 Chemolithoautotrophic gene quantification.
Each boxplot includes data pooled from all sampled stations and depths (n = 20). Overlayed boxplots indicate the interquartile range (boxes), median (central line), and whiskers extending to 1.5×IQR. Statistically significant differences are indicated with asterisks (two-sided Wilcoxon-Mann-Whitney statistical test: **** p ≤ 0.0001; *** p < 0.001; * p < 0.05). (A) Ratio of total chemolithoautotrophic communities (gene copy numbers calculated as the sum of the marker genes accA, cbbL and cbbM) in the entire prokaryotic community (sum of bacterial and archaeal 16S gene copy numbers). qPCR gene ratio for AOA (ratio of amoA gene / 16S prokaryotes gene) and AOB (ratio of amoB gene / 16S prokaryotes gene) in (B) the “Suspended” fraction (that is, free-living and prokaryotes attached to suspended particles) and in (C) the “Sinking” fraction (that is, prokaryotes attached to sinking particles).
Extended Data Fig. 7 Chemolithoautotrophic fixation pathways.
Boxplot of qPCR gene ratio of the “suspended” fraction (that is, free-living and prokaryotes attached to suspended particles) and the “sinking” fraction (that is, prokaryotes attached to sinking particles). Each data point represents a ratio measured in the five PSSs at depths of 100, 200, 500, and 1000 m. Overlayed boxplots indicate the interquartile range (boxes), median (central line), and whiskers extending to 1.5×IQR. Statistically significant differences are indicated with asterisks (two-sided Wilcoxon-Mann-Whitney statistical test, **** p ≤ 0.0001; *** p < 0.001; ** p < 0.01). (A) Ratio of accA gene / 16S prokaryotes gene, proxy of chemolithoautotrophic prokaryotes using the 3-hydroxypropionate/4-hydroxybutyrate (3-HP/4-HB) pathway. (B) Ratio of cbbL gene / 16S prokaryotes gene, proxy of chemoautotrophic prokaryotes using Form I Rubisco of the Calvin-Benson-Bassham (CBB) cycle. (C) Ratio of cbbM gene / 16S prokaryotes gene, proxy of chemolithoautotrophic prokaryotes using Form II Rubisco of the CBB cycle. (D) Ratio of amoA (AOA + AOB) genes / 16S prokaryotes gene, proxy of ammonia oxidizers. (E) Ratio of total chemolithoautotrophic communities (gene copy numbers calculated as the sum of the marker genes accA, cbbL and cbbM) in the entire prokaryotic community (sum of bacterial and archaeal 16S gene copy numbers).
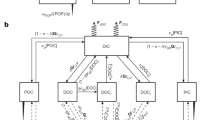
Extended Data Fig. 8 Mesopelagic carbon budgets of the five stations (PSSs) resulting from the APERO cruise.
The input of carbon to the mesopelagic (ΔPOC plus dark carbon fixation fluxes) were compared to the prokaryotic carbon demand integrated between depths of 100 and 1000 m depths, expressed in mg C m−2 d−1. The legend is detailed above, with the “suspended” fraction that includes free-living and prokaryotes attached to suspended particles, and the “sinking” fraction that corresponds to prokaryotes attached to sinking particles. We applied a PGEsinking of 0.24 and PGESuspended of 0.08 as in29. At PSS2 the lack of DCFSuspended values prevented integration. DCFsinking is <1% and therefore not visible.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Coq, P., Christaki, U., Van Wambeke, F. et al. Distinct contributions of suspended and sinking prokaryotes to mesopelagic carbon budget. Nat. Geosci. (2026). https://doi.org/10.1038/s41561-025-01888-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41561-025-01888-w