Abstract
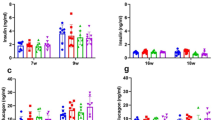
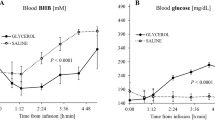
There is a need to optimize closed-loop automated insulin delivery in type 1 diabetes. We assessed the glycemic efficacy and safety of empagliflozin 25 mg d−1 as add-on therapy to insulin delivery with a closed-loop system. We performed a 2 × 2 factorial randomized, placebo-controlled, crossover two-center trial in adults, assessing 4 weeks of closed-loop delivery versus sensor-augmented pump (SAP) therapy and empagliflozin versus placebo. The primary outcome was time spent in the glucose target range (3.9–10.0 mmol l−1). Primary comparisons were empagliflozin versus placebo in each of closed-loop or SAP therapy; the remaining comparisons were conditional on its significance. Twenty-four of 27 randomized participants were included in the final analysis. Compared to placebo, empagliflozin improved time in target range with closed-loop therapy by 7.2% and in SAP therapy by 11.4%. Closed-loop therapy plus empagliflozin improved time in target range compared to SAP therapy plus empagliflozin by 6.1% but by 17.5% for the combination of closed-loop therapy and empagliflozin compared to SAP therapy plus placebo. While no diabetic ketoacidosis or severe hypoglycemia occurred during any intervention, uncomplicated ketosis events were more common on empagliflozin. Empagliflozin 25 mg d−1 added to automated insulin delivery improves glycemic control but increases ketone concentration and ketosis compared to placebo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Code availability
The code used for analysis is available from the corresponding author. The predictive control algorithm cannot be made publicly available because it is proprietary intellectual property. The control algorithm cannot be used in routine practice in the outpatient setting because regulatory approval has not yet been granted.
References
Haidar, A. The artificial pancreas: how closed-loop control is revolutionizing diabetes. IEEE Control Syst. 36, 28–47 (2016).
Brown, S. A. et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med. 381, 1707–1717 (2019).
Thabit, H. et al. Use of an artificial beta cell in type 1 diabetes. N. Engl. J. Med. 373, 2129–2140 (2015).
Collyns, O. J. et al. Improved glycemic outcomes with Medtronic MiniMed Advanced Hybrid Closed-Loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care 44, 969–975 (2021).
Benhamou, P.-Y. et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit. Health 1, e17–e25 (2019).
Bergenstal, R. M. et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 397, 208–219 (2021).
Heerspink, H. J. L., Perkins, B. A., Fitchett, D. H., Husain, M. & Cherney, D. Z. I. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134, 752–772 (2016).
Mudaliar, S. et al. Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes. Diabetes Care 35, 2198–2200 (2012).
Taylor, S. I., Blau, J. E., Rother, K. I. & Beitelshees, A. L. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol. 7, 949–958 (2019).
Lu, J., Tang, L., Meng, H., Zhao, J. & Liang, Y. Effects of sodium-glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: a meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 35, e3169 (2019).
Groop, P.-H. et al. Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials. Lancet Diabetes Endocrinol. 8, 845–854 (2020).
Musso, G., Gambino, R., Cassader, M. & Paschetta, E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 365, l1328 (2019).
Taylor, S. I., Blau, J. E. & Rother, K. I. SGLT2 inhibitors may predispose to ketoacidosis. J. Clin. Endocrinol. Metab. 100, 2849–2852 (2015).
Rosenstock, J. et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 41, 2560–2569 (2018).
Mathieu, C. et al. Benefit/risk profile of dapagliflozin 5 mg in the DEPICT-1 and -2 trials in individuals with type 1 diabetes and body mass index ≥27 kg/m2. Diabetes Obes. Metab. 22, 2151–2160 (2020).
Kaku, K., Isaka, H., Sakatani, T. & Toyoshima, J. Efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized, double-blind, phase 3 trial. Diabetes Obes. Metab. 21, 2284–2293 (2019).
Pieber, T. R. et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes. Metab. 17, 928–935 (2015).
Battelino, T. et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 42, 1593–1603 (2019).
Danne, T. et al. Efficacy and safety of adding sotagliflozin, a dual sodium-glucose co-transporter (SGLT)1 and SGLT2 inhibitor, to optimized insulin therapy in adults with type 1 diabetes and baseline body mass index ≥27 kg/m2. Diabetes Obes. Metab. 23, 854–860 (2021).
Singh, S., Rushakoff, R. J. & Neinstein, A. B. A case report of diabetic ketoacidosis with combined use of a sodium glucose transporter 2 inhibitor and hybrid closed-loop insulin delivery. J. Diabetes Sci. Technol. 13, 605–606 (2019).
Zhang, J. Y., Shang, T., Koliwad, S. K. & Klonoff, D. C. Continuous ketone monitoring: a new paradigm for physiologic monitoring. J. Diabetes Sci. Technol. 15, 775–780 (2021).
Goldenberg, R. M., Gilbert, J. D., Hramiak, I. M., Woo, V. C. & Zinman, B. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA Protocol. Diabetes Obes. Metab. 21, 2192–2202 (2019).
Garg, S. K., Peters, A. L., Buse, J. B. & Danne, T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH Protocol. Diabetes Technol. Ther. 20, 571–575 (2018).
Alva, S., Castorino, K., Cho, H. & Ou, J. Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J. Diabetes Sci. Technol. 15, 768–774 (2021).
Nguyen, K. T. et al. Continuous Ketone Monitoring Consensus Report 2021. J. Diabetes Sci. Technol. https://doi.org/10.1177/19322968211042656 (2021).
Cifuentes, M., Albala, C. & Rojas, C. V. Differences in lipogenesis and lipolysis in obese and non-obese adult human adipocytes. Biol. Res. 41, 197–204 (2008).
Herring, R. A. et al. Metabolic effects of an SGLT2 inhibitor (dapagliflozin) during a period of acute insulin withdrawal and development of ketoacidosis in people with type 1 diabetes. Diabetes Care 43, 2128–2136 (2020).
Schoelwer, M. J. et al. Predictors of time-in-range (70–180 mg/dL) achieved using a closed-loop control system. Diabetes Technol. Ther. 23, 475–481 (2021).
Pasqua, M.-R., Tsoukas, M. A. & Haidar, A. Strategically playing with fire: SGLT inhibitors as possible adjunct to closed-loop insulin therapy. J. Diabetes Sci. Technol. 15, 1232–1242 (2021).
Lind, M. et al. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 371, 1972–1982 (2014).
Biester, T. et al. Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes. Metab. 23, 599–608 (2021).
Haidar, A. et al. Reducing the need for carbohydrate counting in type 1 diabetes using closed-loop automated insulin delivery (artificial pancreas) and empagliflozin: a randomized, controlled, non-inferiority, crossover pilot trial. Diabetes Obes. Metab. 23, 1272–1281 (2021).
Ndefo, U. A., Anidiobi, N. O., Basheer, E. & Eaton, A. T. Empagliflozin (Jardiance): a novel SGLT2 inhibitor for the treatment of type-2 diabetes. P T 40, 364–368 (2015).
Haidar, A., Duval, C., Legault, L. & Rabasa-Lhoret, R. Pharmacokinetics of insulin aspart and glucagon in type 1 diabetes during closed-loop operation. J. Diabetes Sci. Technol. 7, 1507–1512 (2013).
Castle, J. R. et al. Randomized outpatient trial of single- and dual-hormone closed-loop systems that adapt to exercise using wearable sensors. Diabetes Care 41, 1471–1477 (2018).
Haidar, A., Messier, V., Legault, L., Ladouceur, M. & Rabasa-Lhoret, R. Outpatient 60-hour day-and-night glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or sensor-augmented pump therapy in adults with type 1 diabetes: an open-label, randomised, crossover, controlled trial. Diabetes Obes. Metab. 19, 713–720 (2017).
Haidar, A. et al. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 3, 17–26 (2015).
Acknowledgements
This research was supported by the Juvenile Diabetes Research Foundation and by Diabetes Action Canada, a Canadian Institutes for Health Research (CIHR) Strategy for Patient-Oriented Research Network in Chronic Disease (grant no. 4-PAR-2017-327-A-N to A.H. and B.A.P.). B.A.P. holds the Sam and Judy Pencer Family Chair in Diabetes Clinical Research, University of Toronto and acknowledges research program support from the Menkes Fund and the David Wright Fund. A.H. holds the Canada Research Chair in Artificial Pancreas Systems (950-231305). We thank P. Jacob for providing the iPancreas research platform. No funder had any role in study design, data collection or interpretation, or writing of the manuscript. The study investigators had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
A.H., N.C., A.O., L.E.L., J.R., J.-F.Y. and B.A.P. designed the study. A.H., N.C., N.G.-P., A.O., M.A.T., C.M.F., J.-F.Y. and B.A.P. conducted the study. A.H., L.E.L., A.J., M.G. and D.E. carried out the data and statistical analyses. A.H. and B.A.P. are the guarantors of this work and, as such, had full access to the data and take responsibility for the integrity of the data analysis. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.H. received research support/consulting fees from Eli Lilly, Medtronic, AgaMatrix, Tandem, Adocia and Dexcom, and has pending patents in the artificial pancreas area. M.A.T. received research support from AgaMatrix, consulting fees from Sanofi and speaker honoraria from Eli Lilly, Novo Nordisk, Boehringer Ingelheim, Janssen and AstraZeneca. J.-F.Y. received research support from Sanofi, Bayer and Novo Nordisk, and consulting fees and speaker honoraria from Sanofi, Eli Lilly, Novo Nordisk, Boehringer Ingelheim, Janssen, Takeda, Abbott, Merck and AstraZeneca. B.A.P. received speaker honoraria from Abbott, Medtronic, Insulet and Novo Nordisk, research support to his research institute from Boehringer Ingelheim and the Bank of Montreal, and has served as a consultant to Boehringer Ingelheim, Abbott and Novo Nordisk. N.C. has received speaker honoraria from AstraZeneca and consultation fees from Novo Nordisk and Antibody Research Corporation. L.E.L. received support from a CIHR Canada Graduate Scholarship Doctoral Award. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Tadej Battelino, Victor Volovici and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Riikka Jokinen and Jennifer Sargent were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
The proportion of participants achieving glycemic targets in each arm. n = 24 independent participants in each of the four settings.
Extended Data Fig. 2
The median (IQR) profiles of basal insulin delivery during the interventions. n = 24 independent participants in each of the four settings.
Extended Data Fig. 3
The relationship between individual mean fasting ketone level and BMI in each intervention arm.
Extended Data Fig. 4
Brochure provided to participants for the management of ketone levels while on empagliflozin, page 1 of 2.
Extended Data Fig. 5
Brochure provided to participants for the management of ketone levels while on empagliflozin, page 2 of 2.
Supplementary information
Supplementary Information
Supplementary Tables 1–8
Rights and permissions
About this article
Cite this article
Haidar, A., Lovblom, L.E., Cardinez, N. et al. Empagliflozin add-on therapy to closed-loop insulin delivery in type 1 diabetes: a 2 × 2 factorial randomized crossover trial. Nat Med 28, 1269–1276 (2022). https://doi.org/10.1038/s41591-022-01805-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-022-01805-3
This article is cited by
-
Simplified meal announcement study (SMASH) using hybrid closed-loop insulin delivery in youth and young adults with type 1 diabetes: a randomised controlled two-centre crossover trial
Diabetologia (2025)
-
The emergence of obesity in type 1 diabetes
International Journal of Obesity (2024)
-
The Effect of Sodium–Glucose Cotransporter Inhibitors on Renal Function as Adjunctive to Insulin in Adults with Type 1 Diabetes: An Updated Multilevel Meta-analysis of Randomized Controlled Trials
Diabetes Therapy (2024)
-
Higher fibre and lower carbohydrate intake are associated with favourable CGM metrics in a cross-sectional cohort of 470 individuals with type 1 diabetes
Diabetologia (2024)
-
Elevated urinary albumin predicts increased time in range after initiation of SGLT2 inhibitors in individuals with type 1 diabetes on sensor-augmented pump therapy
Diabetology International (2024)