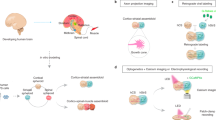
Abstract
The functional complexity and anatomical organization of the nervous system are established during regional patterning of its embryonic precursor—the neural tube. Human pluripotent stem (hPS) cell-based models have emerged as valuable complements to animal models for studying neural development. Here we present the design and implementation of a microfluidic gradient device for modeling human neural tube formation and regional patterning with hPS cells. The microfluidic device enables the formation of tubular or spherical colonies of hPS cells at prescribed locations within microfluidic channels, allowing the cell colonies to form lumenal structures while being exposed to well-controlled chemical gradients for rostral–caudal and/or dorsal–ventral patterning, resulting in the formation of a microfluidic neural tube-like structure (μNTLS) or a forebrain-like structure (μFBLS). The μNTLS recapitulates important hallmarks of early human neural development, including well-defined lumenal morphologies, spatially organized regional marker expression, emergence of secondary signaling centers and the development of neural crest cells. The dorsal–ventral patterned μFBLS further recapitulates spatially segregated dorsal and ventral regions, as well as the layered segregation of early neurons from neural progenitors, mimicking human forebrain pallium and subpallium development. Both the μNTLS and μFBLS are compatible with long-term culture, live imaging, immunofluorescence staining and single-cell sequencing, serving as robust systems for studying human neurodevelopment and disease. This protocol can be implemented by a researcher with polydimethylsiloxane soft lithography and cell culture experience and takes ~8–41 d to complete, depending on the types of neural structure to model and their developmental stages, with an option for prolonged culture to promote neuronal maturation.
Key points
-
This protocol describes the fabrication and implementation of a microfluidic gradient device-based culture system to generate spatially patterned, human neural tube- and forebrain-like structures. This device enables the controllable formations of orthogonal chemical gradients, which are imposed on the human pluripotent stem cell colonies.
-
This allows the recapitulation of several key aspects of neural patterning in both brain and spinal cord along both rostral–caudal and dorsal–ventral axes in three dimensions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the development of this protocol are included in the figures or in the supporting primary research article18.
References
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Meinhardt, A. et al. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep. 3, 987–999 (2014).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 (2017).
Haremaki, T. et al. Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nat. Biotechnol. 37, 1198–1208 (2019).
Zheng, Y. et al. Dorsal–ventral patterned neural cyst from human pluripotent stem cells in a neurogenic niche. Sci. Adv. 5, eaax5933 (2019).
Velasco, S. et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527 (2019).
Rifes, P. et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 38, 1265–1273 (2020).
Karzbrun, E. et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268–272 (2021).
Faustino Martins, J.-M. et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell 26, 172–186.e176 (2020).
Storey, K. G. et al. Early posterior neural tissue is induced by FGF in the chick embryo. Development 125, 473–484 (1998).
Niederreither, K., Subbarayan, V., Dollé, P. & Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444–448 (1999).
Nordström, U., Jessell, T. M. & Edlund, T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 5, 525–532 (2002).
Liem, K. F., Tremml, G. & Jessell, T. M. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127–138 (1997).
Muroyama, Y., Fujihara, M., Ikeya, M., Kondoh, H. & Takada, S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 16, 548–553 (2002).
Dessaud, E. et al. Interpretation of the Sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717–720 (2007).
Wilson, L., Gale, E., Chambers, D. & Maden, M. Retinoic acid and the control of dorsoventral patterning in the avian spinal cord. Dev. Biol. 269, 433–446 (2004).
Xue, X. et al. A patterned human neural tube model using microfluidic gradients. Nature 628, 391–399 (2024).
Lee, J.-H. et al. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat. Biomed. Eng. 6, 435–448 (2022).
Qian, X. et al. Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 13, 565–580 (2018).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Giandomenico, S. L., Sutcliffe, M. & Lancaster, M. A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 16, 579–602 (2021).
Andrews, M. G. & Kriegstein, A. R. Challenges of organoid research. Annu. Rev. Neurosci. 45, 23–39 (2022).
Qian, X., Song, H. & Ming, G. L. Brain organoids: advances, applications and challenges. Development 146, dev.166074 (2019).
Miura, Y. et al. Engineering brain assembloids to interrogate human neural circuits. Nat. Protoc. 17, 15–35 (2022).
Sloan, S. A., Andersen, J., Pașca, A. M., Birey, F. & Pașca, S. P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 13, 2062–2085 (2018).
Paşca, S. P. Assembling human brain organoids. Science 363, 126–127 (2019).
Onesto, M. M., Kim, J.-i. & Pasca, S. P. Assembloid models of cell–cell interaction to study tissue and disease biology. Cell Stem Cell 31, 1563–1573 (2024).
Andersen, J. et al. Generation of functional human 3D cortico–motor assembloids. Cell 183, 1913–1929.e1926 (2020).
Xue, X., Liu, Y. & Fu, J. Bioengineering embryo models. Nat. Rev. Bioeng. https://doi.org/10.1038/s44222-024-00241-x (2024).
Sun, S., Xue, X. & Fu, J. Modeling development using microfluidics: bridging gaps to foster fundamental and translational research. Cur. Opin. Genet. Dev. 82, 102097 (2023).
Pavon, N. et al. Patterning ganglionic eminences in developing human brain organoids using a morphogen-gradient-inducing device. Cell Rep. Methods 4, 100689 (2024).
Liu, Y. et al. A human pluripotent stem cell-based somitogenesis model using microfluidics. Cell Stem Cell 31, 1113–1126 (2024).
Saade, M. & Martí, E. Early spinal cord development: from neural tube formation to neurogenesis. Nat. Rev. Neurosci. 26, 195–213 (2025).
Müller, F. & O’Rahilly, R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat. Embryol. 176, 413–430 (1987).
Serra, D. et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 569, 66–72 (2019).
Yang, M. T., Fu, J., Wang, Y. K., Desai, R. A. & Chen, C. S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat. Protoc. 6, 187–213 (2011).
Acknowledgements
This work is supported by the Michigan–Cambridge Collaboration Initiative (J.F.), the University of Michigan Mcubed Fund (J.F.), the 21st Century Jobs Trust Fund received through the Michigan Strategic Fund from the State of Michigan (grant no. CASE-315037; J.F.), a University of Michigan Mid-career Biosciences Faculty Achievement Recognition Award (J.F.), the National Science Foundation of the United States (I-Corps grant no. 2112458, CBET grant no. 1901718 and EFMA grant no. 2422149 to J.F.), the NIH of the United States (grant nos. R21 NS113518, R21 NS127983, R01 GM143297 and R01 NS129850 to J.F.) and the startup funding from Cincinnati Children’s Research Foundation (X.X.).
Author information
Authors and Affiliations
Contributions
X.X. and J.F. conceived and initiated the projects; X.X. designed, performed and quantified the experiments. O.M.R., S.S., J.B. and A.T. assisted with the experiments. O.M.R. and J.B. independently repeated the experiments. A.T. contributed to the literature search. J.F. supervised the study. All authors edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The University of Michigan, Ann Arbor, has filed a patent application describing microfluidic devices and methods for the development of neural tube-like tissues and neural spheroids (PCT/US2021/058090), with J.F. and X.X. as co-inventors. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Giorgia Quadrato, Guohao Dai, Peter Serles and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key reference
Xue, X. et al. Nature 628, 391–399 (2024): https://doi.org/10.1038/s41586-024-07204-7
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Data 1
An AutoCAD file for the photomask used for microfabrication of the microfluidic device to generate R–C patterned μNTLS. The transparent areas in the AutoCAD file will be etched away during the DRIE process.
Supplementary Data 2
An AutoCAD file for the photomask used for microfabrication of the microfluidic device to generate R–C and D–V patterned μNTLS and D–V patterned μFBLS. The transparent areas in the AutoCAD file will be etched away during the DRIE process.
Supplementary Data 3
An AutoCAD file for the photomask used for microfabrication of the stamp to generate R–C patterned μNTLS. The transparent areas in the AutoCAD file will be etched away during the DRIE process.
Supplementary Data 4
An AutoCAD file for the photomask used for microfabrication of the stamp to generate R–C and D–V patterned μNTLS. The transparent areas in the AutoCAD file will be etched away during the DRIE process.
Supplementary Data 5
An AutoCAD file for the photomask used for microfabrication of the stamp to generate D–V patterned μFBLS. The transparent areas in the AutoCAD file will be etched away during the DRIE process.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, X., Rahman, O.M., Sun, S. et al. Generation of spatially patterned human neural tube-like structures using microfluidic gradient devices. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01266-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41596-025-01266-1
This article is cited by
-
Microfluidic gradients create a stem cell model of the human central nervous system
Nature Protocols (2025)
-
Organoid: a promising solution to current challenges in cancer immunotherapy
npj Biomedical Innovations (2025)