Abstract
Cardiac regenerative therapy has recently progressed by reprogramming somatic cells into induced pluripotent stem cells (iPSCs) and advanced by large-scale differentiation-derived cardiomyocytes (hiPSC-CMs). However, repairing damaged cardiac tissues with hiPSC-CMs remains limited due to immune rejection, cardiac arrhythmias, and concerns over tumor formation after hiPSC-CM transplantation. Despite efforts in profiling epigenomic changes during cardiac differentiation, regulatory mechanisms underlying 5-methylcytosine (m5C) deposition in RNA m5C epitranscriptomic landscape during hiPSC-to-cardiomyocyte differentiation remain unclear. Herein, bisulfite RNA-sequencing analysis was conducted in human pluripotent stem cells (hPSCs) from three independent cellular origins, and their derived cardiomyocytes (hPSC-CM), metabolic-maturation of derived cardiomyocytes (hPSC-CM-lac) and biochemical-enhanced derived cardiomyocytes (hPSC-CM-TDI). Integrated analysis of differentially methylated RNA m5C profiles and transcriptome-wide expression facilitated the identification of m5C sites coupled to the cardiomyocyte differentiation and RNA-dependent regulatory mechanisms of stem cell pluripotency. The RNA m5C profiles in this dataset allow the evaluations of the m5C level and distribution of specific m5C loci and facilitate understanding of the m5C epitranscriptomic landscape in biological functions of hPSC-CM beyond in vivo transplantation barriers.
Similar content being viewed by others
Background & Summary
Heart disease is a leading cause of death globally, taking over 20 million lives in 20211. Myocardial ischemia caused by obstructed coronary blood flow into the heart increases the risks of a heart attack, which might result in heart failure and death2. Since myocardial cells in adult mammals exhibit limited regenerative capacity3, replacing fibrotic scar tissues with external cardiomyocytes might functionally restore myocardial contractility. Human pluripotent stem cells (hPSC)-derived cardiomyocytes (hPSC-CMs), i.e., human embryonic stem cells (hESC)-derived cardiomyocytes (hESC-CMs) and human induced pluripotent stem cells (hiPSC)-derived cardiomyocytes (hiPSC-CMs), have been proven to possess therapeutic potential to regenerate myocardium, to improve cardiac output, and to reduce myocardial fibrosis in several studies including heart failure models in immunocompromised rats with hiPSC-CMs4 and with hESC-CMs5; in swine with hiPSC-CMs6; in human ischemic cardiomyopathy clinical case report with hiPSC-CMs7; as well as our previous work in non-human primates which demonstrated the capacity of hESC-CMs to regenerate large-scale myocardium and to displace host fibrotic myocardium8. However, this clinical application of hPSC-CMs remains premature primarily due to immaturity, post-transplant arrhythmias, teratoma, and immunoreaction. Recent reports showed increased maturity of hPSC-CM9,10, decreased immune rejection, cardiac arrhythmias, and teratocarcinoma formation11,12,13,14 after transplantation. Understanding the efficiency in the differentiation process driven by Wnt-signals remains critical for generating mature cardiomyocytes on a large scale and enabling the capacities of hPSC-CMs to overcome barriers of maturity, functions, and immunogenicity.
Developmental regulatory mechanisms of cardiomyocyte differentiation of hPSCs may be orchestrated by DNA methylations15,16 and chromatin remodeling17,18 of the genome structure and by functional non-coding RNAs19,20,21 which overall converged in gene expression patterns that direct the cardiomyocyte commitment of hPSCs22,23. Epitranscriptomic post-transcriptional modifications of RNA species by N6-methyladenosine (m6A), 5-methylcytosine (m5C), and N1-methyladenosine (m1A) mechanisms represent distinct layers of complexity utilized via RNA modification in germ cells and somatic cells to rewire DNA memory and to template the translation into peptides. RNA base modifications can alter the secondary structure, the association of RNA binding proteins in embryonic development24,25, cardiovascular diseases26, immune maturation27, and disease pathogenesis. m6A deposition participates in the cardiac differentiation of PSCs to the mesodermal stage28, and the m6A writer METTL3 and eraser ALKBH5 enzymes regulate cardiac regeneration and proliferation29,30,31. The binding of m6A reader protein YTHDC2 with the m6A-marked HERV-H RNA concertedly recruited TET1 demethylase to the LTR7-spanning HERV-H in the genome of hESC to inhibit the HERV-H mediated neural differentiation of the hESC32. However, the m5C-dependent context and mechanism in regulating myocardial differentiation and functions remain largely unknown.
RNA 5-methylcytosine (m5C) modification presents in nearly all types of RNA molecules, including ribosomal RNA, messenger RNA, transfer RNA, long non-coding RNA, PIWI-interacting piRNA, and vault RNA, accentuating its diverse biological regulations33. Embryonic development via dynamic mRNA m5C landscape alteration has been reported during oocyte differentiation to ESC in humans24 and mechanistically regulated by the m5C reader protein YBX1 in stabilizing RNA as a critical protective mechanism of zebrafish embryogenesis34. The m5C eraser TET1 converts the 5-methylcytosine to hydroxymethylcytosine and maintains the pluripotency of mouse ESC by stabilizing RNA transcripts35. However, the functional role and mechanism of RNA:m5C in myocardial differentiation of hPSCs still needs to be fully illustrated. Detection of RNA 5-methylcytosine can be achieved from several orthogonal methodologies, including the liquid chromatography followed by tandem mass spectrometry (LC-MS/MS), anti-m5C enrichment-based RNA immunoprecipitation followed by sequencing (Me-RIP-seq), mapping of m5C at individual-nucleotide resolution using Crosslinking and immunoprecipitation followed by sequencing (miCLIP-seq), nanopore sequencing36, and the transcriptome-wide RNA bisulfite sequencing (bsRNA-seq), which provides the transcription-wide coverage and m5C methylation level of RNA at the single-nucleotide resolution.
In this study, the RNA m5C epitranscriptome landscape mapping of three sources of hPSCs and the derived hPSC-CMs using the three induction protocols were analyzed by standard bsRNA-seq workflow (Fig. 1). To differentiate hPSCs into cardiomyocytes (hPSC-CM), the 14-day induction protocol was conducted with adaptation from Wu et al.37 by using Wnt-pathway molecules. Samples in technical triplicates were prepared on day 0 and day 14 time points, and the quality control steps for experimental stages hPSC and hPSC-CM were measured to ensure quality assurance. The hPSC-CM with metabolite and biochemical enhancement was conducted at 11-day induction to allow the maturation of cardiomyocytes38,39. This dataset has potential uses in providing valuable m5C epitranscriptomic landscape measurement regarding hPSCs and derived hPSC-CMs to clarify RNA m5C regulatory mechanisms in hPSCs’ cell-fate commitment and pluripotency in myocardial differentiation and maturation.
The diagram of the sample preparation and the analysis of the reads. The experimental workflow presents the cardiac differentiation protocol of human pluripotent stem cells by changing the appropriate medium and adding indicated small molecules at a specific timeframe. The bsRNA-seq library preparation workflow includes sample total RNA extraction, poly(A) enrichment, bisulfite conversion, and building sequencing library. The analysis workflow (below) presents the sequencing reads filtering, alignment, and m5C identification.
Methods
hPSCs culture
Human embryonic stem cell (hESC), the Rockefeller University Embryonic Stem cell line 2 (RUES2), and hiPSCs (SC81103) were obtained from the Human Disease iPSC Service Consortium (Institute of Biomedical Sciences, Academia Sinica, Taiwan). The human amniotic fluid stem cell-derived iPSCs (hAFSC-iPSCs, a.k.a. NCKUi001-A) are obtained from the Core Lab in the Center of Cell Therapy, National Cheng Kung University Hospital. All hPSCs, including hiPSCs and hESCs, were cultured on a Matrigel matrix (Corning; 354230) pre-coated cell culture dish in Stemflex medium (Thermo; A3349401). To allow proper cell attachment, ROCK inhibitor Y27632 (Cayman; 10005583) at the concentration of 10 µM was supplemented in the culture medium in the cell suspension state, which is removed after cell adhesion. The cells were subsequently incubated at 37° C in a 5% CO2 atmosphere. After reaching ~90% confluency, cells were detached by incubation in the Accutase cell dissociation reagent (Innovative Cell Technologies; AT-104) in a 37 ° C CO2 incubator for 5 minutes. The resulting cell suspension was collected and centrifuged at 300 g for 5 minutes to remove the supernatant. The cells were cultured at a 1:12 ratio for passages. All suspended hPSCs were attached to Matrigel pre-coated dishes using a culture medium containing ROCK inhibitor Y27632 (10 µM; Cayman; 10005583).
Differentiation of hPSCs into cardiomyocytes
These three hPSCs were differentiated into cardiomyocytes under the same induction process, using an adapted 14-day protocol through Wnt signaling regulation37. Briefly, 1.4 × 106 hPSCs were seeded on a Matrigel pre-coated 3.5 cm cell culture dish with Stemflex medium containing 10 µM ROCK inhibitor Y27632 at 37° C in a 5% CO2 incubator overnight. On the following day (defined as day 0 of cardiac differentiation), the medium was changed to the RPMI-1640, containing B27 minus insulin supplement (Thermo; 1895201) and Wnt activator Chir99021 (5 µM; Stemcell Technology; 72054). On day 2, the medium was changed to RPMI-1640, which contained B27 minus an insulin supplement. On day 3, the medium consisted of RPMI-1640 containing B27 minus insulin supplement and Wnt inhibitor IWR-1 (5 µM; Sigma; I0161). On day 5, RPMI-1640 medium containing B27 minus insulin supplement; on day 7, the addition of insulin (B27 supplement; Thermo; 17504044) in the medium induces cardiomyocyte formation, and this same differentiation medium was changed every two days to allow differentiation process until day 11. On day 11 of cardiomyocyte differentiation, cells were divided into three culture conditions. (1) Cells were cultured in RPMI-1640 medium supplemented with B27 and refreshed the medium every two days. (2) For metabolite enhancement, the medium was replaced with glucose-free RPMI-1640 (Thermo. Cat: 11879020), supplemented with 1X Non-Essential Amino Acids Solution (Thermo. Cat:11140050), Glutamax, and 1 M Lactate (Sigma. Cat: L7022), and cultured for four days, with medium changes every two days. (3) For maturation induction, the medium was replaced with RPMI-1640 supplemented with B27, TDI cocktail with 10 ng/ml Thyroxine (Sigma. Cat: 699594), 10 mM Dexamethasone (Sigma. Cat: D1756), and 100 µg/ml IGF (Stemcell Technology. Cat: 78022), and cultured for seven days, with medium changes every two days. Only cardiomyocytes with spontaneous contractility and ≥ 70% expression of cardiac-specific marker–cardiac troponin T (cTnT) were used for the following experiments40 (Fig. 1).
Bisulfite mRNA library preparation and sequencing
The total RNA was purified by Direct-zol RNA MiniPrep (Zymo, Irvine, CA) with DNase I treatment, and the mRNA enrichment protocol followed our previous reports41,42. Briefly, the mRNA fraction was enriched by using oligo (dT)25 magnetic beads to capture poly(A) RNA samples from total RNA ranging from 12 to 46 µg with Dynabeads mRNA DIRECT purification kit (Thermo Fisher Scientific, Waltham, MA). The second round of purification was conducted by adding lysis/binding buffer to the purified mRNA sample and following the manufacturer’s instructions. The concentration and purity were determined by Qubit Fluorometer high sensitivity RNA assay (Thermo Fisher Scientific, Waltham, MA, USA) and Bioanalyzer Agilent RNA 6000 Pico assay (Agilent, Santa Clara, CA), respectively (Table S1; Fig. S1A). Afterward, the bisulfite conversion of the enriched mRNA samples ranging from 140 to 750 ng was used with an EZ RNA methylation kit (Zymo) with bisulfite incubation reaction using three cycles of 70° C 10 minutes and 64° C 45 minutes at a final step of 4° C. The spike-in firefly luciferase mRNA (Promega, Madison, WI) was added before the bisulfite conversion step as an internal standard in each enriched mRNA sample at the ratio of 1: 10000 quantities (w/w) except for the hAFSC-iPSC-2 and the hESC(RUES2)-2 selectively used a 1:100 (w/w) ratio to assess the bisulfate conversion efficiency more deeply at a larger scale. The quantities of each bisulfite-treated mRNA and the fragment size distribution were measured by Qubit high-sensitivity RNA assay and Agilent Bioanalyzer RNA 6000 Pico assay, respectively (Table S1; Fig. S1A). Bisulfite-treated mRNA was used to build the bsRNA libraries (their range distributed from 1.2 to 30.0 ng) using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA). The resulting bsRNA-seq libraries were verified with the Qubit high-sensitivity DNA assay and Bioanalyzer high-sensitivity DNA assay for quantity assessment and quality validation (Table S1; Fig. S1A). Libraries of the hPSC and hPSC-CM that passed quality control were multiplexed at 4 nM and sequenced on an Illumina NextSeq500 platform with 150 bp pair-end runs, and those of the hPSC-CM_lac and hPSC-CM_TDI which passed the quality control were multiplexed at 8 nM and sequenced on an Illumina Illumina NovaSeq X Plus platform with 150 bp pair-end runs.
Sequencing quality validation, reads alignment, and the identification of m5C sites
The sequencing fastq files were uploaded to the Galaxy web platform to merge the raw fastq files of the same sample into one R1 and one R2 fastq file by the Concatenate datasets tool if needed43, then proceeded to remove low-quality reads (Q < 20) and adapter contaminations by the Trim Galore! tool via the Galaxy platform or by the cutadapt tool. In the Trim Galore! tool or cutadapt, the argument for read 1 ‘-j 1 -e 0.1 -q 20 -O 1 -a AGATCGGAAGAGCACACGTCTGAACTCCAGTCAC’ and read 2 ‘-j 1 -e 0.1 -q 20 -O 1 -a AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATCTCGGTGGTCGCCGTATCATT’ were used to remove adaptor sequence and reads end’s base with quality Phred score less than 20. Trimmed and good quality reads were then ready to be aligned to the human reference genome GRCh38 (Release-100 from Ensembl website) with gene transfer format (GTF) by meRanGh align, which applies splice-aware HISAT2 algorithm, and with arguments of maximum mismatch ratio set at 0.1 (‘-mmr 0.1’) in the meRanTK tool kits44. The generated SAM file were proceeded to conduct m5C site identification with meRanCall arguments ‘-rl 150 -md 5 -ei 0.1 -cr 99 -fdr 0.05 -mcov 10 -mr 0.05’ to filter m5C sites that meet the criteria of 10 reads coverage minimum, methylation ratio at least 0.05 and with false discovery rate 5%. A total of 28,886 to 270,190 m5C sites were identified with the parameter, reaching an average of 99.82% total conversion rate when aligning to the human reference genome (Table 1, left). The spike-in control sequence reached an average of 99.85% total conversion rate aligning to unmethylated Firefly luciferase mRNA sequence, and with a large-scale of the spike-in in hAFSC-iPSC-2 and hESC(RUES2)-2 samples did not affect the bisulfite conversion efficiency (Table 1, right panel; Fig. S2; Table S2).
Data transformation and gene expression analysis
This data descriptor could be valuable for downstream analysis, including the data format transformation, estimation of mRNA expression, and visualization of genome-wide m5C distribution in the mRNA. The m5C gene annotation and gene biotype were retrieved from the reference genome Gene Transfer Format (GTF) file. With the BAM file generated from meRanGh and reference genome Gene Transfer Format (GTF) file (Homo_sapiens.GRCh38.100.gtf), the read counts per genomic features could be calculated by utilizing the python package HTSeq-Count in each sample. Using the bsRNA-seq fastq reads (GSE24056945), the output table of HTSeq-Count can proceed to R package DESeq2 to generate normalized gene expression and differentially expressed transcripts spanning m5C read regions. (Fig. 2A). Analysis into differentially expressed transcripts essential for differentiating into muscle structure, sarcomere, and muscle development pathways should be possible in this dataset, which included three hPSCs and their differentiated cardiomyocyte counterparts (Fig. 2B).
The transcriptome difference between hPSCs and the derived cardiomyocyte samples. (A) Exemplary demonstration of volcano plots in differential expressed genes hiPSCs(1103) to hiPSC(1103)-CM, hAFSC-iPSC to hAFSC-iPSC-CM, hESC(RUES2) to hESC-CM(RUES2-CM), hiPSC(1103)-CM-lac to hiPSC(1103)-CM-TDI, hAFSC-iPSC-CM-lac to hAFSC-iPSC-CM-TDI, and RUES2-CM-lac to RUES2-CM-TDI. Colored dots present genes with absolute log2(fold change) over 1 and p.adjust below 0.05. (red: upregulated; blue: downregulated). (B) Exemplary demonstration of a Venn diagram which presented the unique (bold line circled), intersected, common differentially expressed genes with p.adjust below 0.05 in hiPSCs verse hiPSC-CMs (top) and hiPSC-CM-lac verse hiPSC-CM-TDI (bottom). (C,D) The (C) sample-to-sample distance and (D) the Principal Component Analysis (PCA) plot of 38 bsRNA-seq libraries generated by DESeq2.
Data Records
All the bisulfite RNA sequencing datasets were deposited in the NCBI Sequence Read Archive (SRA) with the accession number SRP45448646. The processed data files were deposited in the NCBI Gene Expression Omnibus (GEO) database with accession number GSE24056945, including (1) differential gene expression between groups (datafile: DESeq2_DEGs.xlsx), (2) raw HTseq-count output file of each sample (datafile: HTseqCount_raw.txt), and (3) frequent common m5C sites among three biological replicates in each group (datafile: m5C_loci.xlsx).
Technical Validation
Cardiomyogenic differentiation
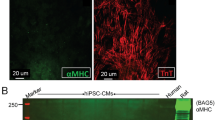
The flow cytometry was conducted to examine cellular marker expression before and after the differentiation process towards cardiomyocytes. All three hPSCs exhibited a consistent expression level of pluripotency-associated markers ( > 80%) before initiating the cardiomyocyte differentiation protocol, including Stage-specific embryonic antigen-4 (SSEA4), octamer-binding transcription factor 4 (OCT4), and Nanog. After a 14-day myocardial differentiation protocol, the hPSC-derived cardiomyocytes (hPSC-CMs) displayed minimal to negligible expression of these pluripotency markers (Fig. 3A). The differentiated cardiomyocytes showed a substantial upregulation in the expression of cardiac-specific markers cardiac troponin T (cTnT) while no expression in hPSCs (Fig. 3B). These measurements indicated a successful transition from pluripotent stem cells to the cardiac lineage.
The marker expression of hPSCs and derived cardiomyocytes. The histograms present flow cytometry analysis of cellular expression of pluripotency genes–SSEA4, NANOG, and OCT4; and cardiomyocyte genes–cardiac TnT in three sources of (A) hPSCs (blue) and (B) derived cardiomyocytes (red). Isotype antibody was used as a control (grey).
Quality assessment of raw sequences and sequence alignment
The trimmed reads without adaptor sequence nor low-quality reads were quality-checked with FastQC to ensure the mean quality score of reads. The reads phred quality score was above 30 (Fig. S1B) and further aligned to the human reference genome (Release-100 from Ensembl website) between 65–80% rates (Table 1). Using derivative arguments of DESeq2 generated the principal component analysis and the sample-sample distance of these 38 samples, which showed a relatively consistent between hPSC, hPSC-CM, hPSC-CM-lac, and hPSC-CM-TDI samples (Fig. 2C,D).
Usage Note
The underlying mechanisms involved in the reprogramming as well as differentiating process are still unclear regarding regulations mediated by RNA post-transcriptional modification; hence, these bisulfite RNA seq data can be used to explore altered m5C RNAs or differentially expressed genes participate in the hPSC to CM transition. The main limitation of using bisulfite treatment to identify m5C is that it cannot distinguish from other cytosine modifications, according to the report from Schaefer et al.47. Researchers are suggested to validate the modification identity by using different complementary methodologies such as MeRIP-seq before proceeding to further experiments.
Code availability
The following software or packages were used for data analysis:
Cutadapt (version 1.15)
HISAT2 (version 2.0.4)
meRanTK (version 1.2.1b)
FastQC (version 0.11.5)
MultiQC (version 1.15)
Samtools (version 1.7)
HTseq-count (version 0.6.1p1)
R (version 4.1.2)
DESeq2 (version 1.34.0)
ClusterProfiler (version 4.2.2)
Venn Diagram: https://bioinformatics.psb.ugent.be/webtools/Venn/
The custom code performed in R used for differential gene expression analysis has been published in the following repository: https://github.com/NCKU-PHH-Lab/DEseq2.git.
References
Lindstrom, M. et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990-2021. Journal of the American College of Cardiology 80, 2372–2425, https://doi.org/10.1016/j.jacc.2022.11.001 (2022).
Liu, M., López de Juan Abad, B. & Cheng, K. Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies. Adv Drug Deliv Rev 173, 504–519, https://doi.org/10.1016/j.addr.2021.03.021 (2021).
Eschenhagen, T. et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 136, 680–686, https://doi.org/10.1161/circulationaha.117.029343 (2017).
Kawaguchi, S. et al. Intramyocardial Transplantation of Human iPS Cell–Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals. JACC: Basic to Translational Science 6, 239–254, https://doi.org/10.1016/j.jacbts.2020.11.017 (2021).
Moon, S.-H. et al. The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials 34, 4013–4026, https://doi.org/10.1016/j.biomaterials.2013.02.022 (2013).
Romagnuolo, R. et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Reports 12, 967–981, https://doi.org/10.1016/j.stemcr.2019.04.005 (2019).
Miyagawa, S. et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front Cardiovasc Med 9, 950829, https://doi.org/10.3389/fcvm.2022.950829 (2022).
Liu, Y. W. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 36, 597–605, https://doi.org/10.1038/nbt.4162 (2018).
Ahmed, R. E., Anzai, T., Chanthra, N. & Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front Cell Dev Biol 8, 178, https://doi.org/10.3389/fcell.2020.00178 (2020).
Tohyama, S. et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Reports 9, 1406–1414, https://doi.org/10.1016/j.stemcr.2017.08.025 (2017).
Yoshida, S. et al. Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation. Sci Rep 10, 4593, https://doi.org/10.1038/s41598-020-58126-z (2020).
Nakamura, K. et al. Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes. Stem Cell Reports 16, 2473–2487, https://doi.org/10.1016/j.stemcr.2021.08.005 (2021).
Martin, R. M. et al. Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nature Communications 11, 2713, https://doi.org/10.1038/s41467-020-16455-7 (2020).
Silver, S. E., Barrs, R. W. & Mei, Y. Transplantation of Human Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Regenerative Therapy. Front Cardiovasc Med 8, 707890, https://doi.org/10.3389/fcvm.2021.707890 (2021).
Hoff, K. et al. DNA methylation profiling allows for characterization of atrial and ventricular cardiac tissues and hiPSC-CMs. Clinical Epigenetics 11, 89, https://doi.org/10.1186/s13148-019-0679-0 (2019).
Lan, Y. et al. Stage-specific regulation of DNA methylation by TET enzymes during human cardiac differentiation. Cell Rep 37, 110095, https://doi.org/10.1016/j.celrep.2021.110095 (2021).
Lee, S., Lee, J. W. & Lee, S. K. UTX, a histone H3-lysine 27 demethylase, acts as a critical switch to activate the cardiac developmental program. Dev Cell 22, 25–37, https://doi.org/10.1016/j.devcel.2011.11.009 (2012).
Cattaneo, P. et al. DOT1L-mediated H3K79me2 modification critically regulates gene expression during cardiomyocyte differentiation. Cell Death Differ 23, 555–564, https://doi.org/10.1038/cdd.2014.199 (2016).
Hoelscher, S. C. et al. miR-128a Acts as a Regulator in Cardiac Development by Modulating Differentiation of Cardiac Progenitor Cell Populations. Int J Mol Sci 21 https://doi.org/10.3390/ijms21031158 (2020).
Kay, M. et al. The conserved long non-coding RNA CARMA regulates cardiomyocyte differentiation. Cardiovasc Res 118, 2339–2353, https://doi.org/10.1093/cvr/cvab281 (2022).
Taliani, V. et al. The long noncoding RNA Charme supervises cardiomyocyte maturation by controlling cell differentiation programs in the developing heart. Elife 12, https://doi.org/10.7554/eLife.81360 (2023).
Zare, A. et al. Epigenetic Modification Factors and microRNAs Network Associated with Differentiation of Embryonic Stem Cells and Induced Pluripotent Stem Cells toward Cardiomyocytes: A Review. Life (Basel) 13, https://doi.org/10.3390/life13020569 (2023).
Fujita, J., Tohyama, S., Kishino, Y., Okada, M. & Morita, Y. Concise Review: Genetic and Epigenetic Regulation of Cardiac Differentiation from Human Pluripotent Stem Cells. Stem Cells 37, 992–1002, https://doi.org/10.1002/stem.3027 (2019).
Liu, J. et al. Developmental mRNA m5C landscape and regulatory innovations of massive m5C modification of maternal mRNAs in animals. Nature Communications 13, 2484, https://doi.org/10.1038/s41467-022-30210-0 (2022).
Liu, H., Zheng, J. & Liao, A. The regulation and potential roles of m6A modifications in early embryonic development and immune tolerance at the maternal-fetal interface. Front Immunol 13, 988130, https://doi.org/10.3389/fimmu.2022.988130 (2022).
Zhou, W. et al. RNA Methylations in Cardiovascular Diseases, Molecular Structure, Biological Functions and Regulatory Roles in Cardiovascular Diseases. Front Pharmacol 12, 722728, https://doi.org/10.3389/fphar.2021.722728 (2021).
Cui, L. et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduction and Targeted Therapy 7, 334, https://doi.org/10.1038/s41392-022-01175-9 (2022).
Dong, S. et al. Stage-specific requirement for m(6)A RNA methylation during cardiac differentiation of pluripotent stem cells. Differentiation 133, 77–87, https://doi.org/10.1016/j.diff.2023.07.001 (2023).
Dorn, L. E. et al. The N(6)-Methyladenosine mRNA Methylase METTL3 Controls Cardiac Homeostasis and Hypertrophy. Circulation 139, 533–545, https://doi.org/10.1161/circulationaha.118.036146 (2019).
Gong, R. et al. Loss of m(6)A methyltransferase METTL3 promotes heart regeneration and repair after myocardial injury. Pharmacol Res 174, 105845, https://doi.org/10.1016/j.phrs.2021.105845 (2021).
Han, Z. et al. ALKBH5-mediated m(6)A mRNA methylation governs human embryonic stem cell cardiac commitment. Mol Ther Nucleic Acids 26, 22–33, https://doi.org/10.1016/j.omtn.2021.05.019 (2021).
Sun, T. et al. Crosstalk between RNA m(6)A and DNA methylation regulates transposable element chromatin activation and cell fate in human pluripotent stem cells. Nat Genet 55, 1324–1335, https://doi.org/10.1038/s41588-023-01452-5 (2023).
Trixl, L. & Lusser, A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip Rev RNA 10, e1510, https://doi.org/10.1002/wrna.1510 (2019).
Yang, Y. et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol Cell 75, 1188–1202.e1111, https://doi.org/10.1016/j.molcel.2019.06.033 (2019).
Lan, J. et al. Functional role of Tet-mediated RNA hydroxymethylcytosine in mouse ES cells and during differentiation. Nat Commun 11, 4956, https://doi.org/10.1038/s41467-020-18729-6 (2020).
Guo, G. et al. Advances in mRNA 5-methylcytosine modifications: Detection, effectors, biological functions, and clinical relevance. Mol Ther Nucleic Acids 26, 575–593, https://doi.org/10.1016/j.omtn.2021.08.020 (2021).
Feaster, T. K. et al. Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res 117, 995–1000, https://doi.org/10.1161/circresaha.115.307580 (2015).
Tohyama, S. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137, https://doi.org/10.1016/j.stem.2012.09.013 (2013).
Huang, C. Y. et al. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J Mol Cell Cardiol 138, 1–11, https://doi.org/10.1016/j.yjmcc.2019.10.001 (2020).
Fang, Y. H. et al. Efficient Cardiac Differentiation of Human Amniotic Fluid-Derived Stem Cells into Induced Pluripotent Stem Cells and Their Potential Immune Privilege. Int J Mol Sci 21, https://doi.org/10.3390/ijms21072359 (2020).
Chen, S.-Y. et al. RNA bisulfite sequencing reveals NSUN2-mediated suppression of epithelial differentiation in pancreatic cancer. Oncogene 41, 3162–3176, https://doi.org/10.1038/s41388-022-02325-7 (2022).
Chen, S.-Y. & Huang, P.-H. Enrichment of mRNA and Bisulfite-mRNA Library Preparation for Next-Generation Sequencing. J Vis Exp, https://doi.org/10.3791/65352 (2023).
Afgan, E. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res 44, W3–w10, https://doi.org/10.1093/nar/gkw343 (2016).
Rieder, D., Amort, T., Kugler, E., Lusser, A. & Trajanoski, Z. meRanTK: methylated RNA analysis ToolKit. Bioinformatics 32, 782–785, https://doi.org/10.1093/bioinformatics/btv647 (2016).
Chen, S.-Y. et al. NCBI GEO https://identifiers.org/geo/GSE240569 (2023).
Chen, S.-Y. et al. NCBI Sequence Read Archive https://identifiers.org/ncbi/insdc.sra:SRP454486 (2023).
Schaefer, M., Pollex, T., Hanna, K. & Lyko, F. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res 37, e12, https://doi.org/10.1093/nar/gkn954 (2009).
Acknowledgements
The authors acknowledge the technical services from core facilities: Research Center of Clinical Medicine of National Cheng Kung University Hospital; Reagents, Cell lines, and technical support from the National RNAi Core Facility at Academia Sinica in Taiwan for providing shRNA reagents, services; and the Human Disease iPSC Service Consortium for iPSC generation. Sequencing runs, computational analyses, and data mining were performed using the Center for Bioinformatics and Digital Health system at the National Cheng Kung University, supported by the Ministry of Science and Technology, Taiwan. JHW received a Summer Research Project Grant (National Cheng Kung University College of Medicine, #NCKUMCS2023057). SYC received a Ph.D. Program Fellowship from the National Science and Technology Council (NSTC#A1102-U015). This work is supported by grant support from the NCKUH (NCKUH-10909028; NCKUH-11009024; and NCKUH-11109014) to YWL and in part from the Ministry of Science and Technology, Taiwan (MOST110-2628-B-006-026 to YWL, MOST111-2321-B006-013 to YSS, and the National Science and Technology Council NSTC112-2314-B-006-107 and NSTC113-2314-B-006-054- to PHH).
Author information
Authors and Affiliations
Contributions
Investigation: bsRNA-seq library construction, mapping of reads to genome and transcriptome, and m5C site analysis: SYC.; Investigation: cellular experiments, preparation of hPSC and hPSC-differentiated cardiomyocyte samples: YSF and CYH; Bioinformatics: JHW and SYC; Formal analysis and interpretation: SYC, PHH, YSF, YWL; Concept and experiment design: PHH and YWL; Writing of initial draft: SYC and YSF; Writing: SYC and PHH with proofreading and inputs from all authors. Supervision: YSS, PHH, and YWL. All authors read and approve the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, SY., Fang, YH., Huang, CY. et al. Transcriptome-wide RNA 5-methylcytosine profiles of human iPSCs and iPSC-derived cardiomyocytes. Sci Data 11, 1378 (2024). https://doi.org/10.1038/s41597-024-04209-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-024-04209-9