Abstract
We examined the relationship between Short Physical Performance Battery (SPPB) and clinical and laboratory factors and the effect of sarcopenia and sarcopenic obesity (SO) on clinical and laboratory factors for cardiovascular disease (CVD) inpatients. CVD male (n = 318) and female (n = 172) inpatients were recruited. A stepwise multiple-regression analysis was performed to predict total SPPB scores and assess clinical and laboratory factors (physical characteristics, functional and morphological assessments, etc.). Each test outcome were compared among sarcopenia, SO and non-sarcopenic groups. To predict total SPPB scores, the predicted handgrip, Controlling Nutritional Status score, % body fat, anterior mid-thigh muscle thickness, standing height and systolic blood pressure were calculated for males and anterior mid-thigh MTH, BMI, knee extension and fat mass were calculated for females. There were no differences in blood pressure, total SPPB scores and functional assessments between sarcopenia and SO groups for CVD male and female inpatients. In conclusion, the physical performance of CVD inpatients can be predicted by nutritional, functional, clinical and anthropometric variables, regardless the gender and the presence of sarcopenia. Furthermore, the presence of sarcopenia has a negative effect on the clinical and laboratory factors, but there is a difference in impact between sarcopenia and SO regardless the gender.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is a major contributor to the global burden of diseases1,2. In addition, CVD (heart failure and stroke, etc.) often causes disuse muscle atrophy in the acute and subacute phases, which increases the likelihood of wheelchair or bedridden state in the chronic phase3,4,5. Consequently, the progression of muscle atrophy in lower extremity function in CVD inpatients leads to a high need for medical and nursing care.
The Short Physical Performance Battery (SPPB), a brief performance battery based on a timed short distance walk, repeated chair stands, and a set of balance tests, is a validated assessment tool for measuring lower extremity function that is widely used in both clinical and research settings6,7. This means of assessment offers relative ease of use, perceived potential for implementation in clinical practice, and a good relationship with physical activity levels and walking disability in a variety of patients or older adults. In fact, a recent study demonstrated that the SPPB is an effective assessment tool for strength and lower extremity morphological evaluation for middle-aged and older cardiovascular disease patients (mixed inpatients and outpatients)8. It has also been found to predict mortality, hospitalization rate, and a variety of comorbid disease conditions7. However, a previous study of older inpatients reported that approximately 88% were malnourished and approximately 12% were at risk for malnutrition9. In a SPPB study for older inpatients, therefore, it is particularly necessary to carry out a comprehensive analysis (muscle strength, muscle morphology, nutrition status and body composition, etc.).
The assessment of body composition is commonly performed by quantifying fat mass and fat-free mass (e.g., skeletal muscle mass) components. These components are often utilized to assess the risk for adverse health outcomes in a variety of conditions10. In addition, some studies have demonstrated that sarcopenia, the age-related loss of skeletal muscle mass and strength11, and also obesity, an increase in fat mass12, increase the risk of mortality. Recently, sarcopenia is found to often co-occur with an increase in fat mass, termed sarcopenic obesity, which may carry the cumulative risk derived from each of the two individual body composition factors13,14. Accordingly, recent clinical studies have focused on health outcomes in sarcopenic obesity as well as sarcopenia15,16.
Thus, the purpose of this study was to examine the relationship between the SPPB and clinical and laboratory factors and the effect of sarcopenia and sarcopenic obesity on clinical and laboratory factors in CVD inpatients.
Results
The physical characteristics and clinical data
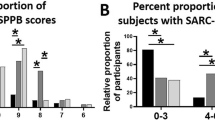
Age and % body fat were greater (P < 0.01) in females than in males, but standing height, body weight, and BMI were greater (p < 0.01) in males than in females (Table 1). SPPB, functional and morphological assessments are shown in Table 2. Some subjects failed to perform the chair stand test (19 males and 17 females) and gait test (1 male and 2 females). In addition, BIA (InBody device) was contraindicated for 11 males and 15 females. Anterior-mid MTH correlated positively with SMI of males (r = 0.685, p < 0.0001) and females (r = 0.608, P < 0.0001) and the whole sample (r = 0.691, p < 0.0001) (Fig. 1). Total SPPB score, functional, and morphological assessments were greater (p < 0.01) in males than in females. Unlike the sarcopenia, the proportion of SO assessments for CVD patients was larger in females than in males (Fig. 2). In both CVD male and female inpatients, body weight, BMI, % body fat, and body fat mass were greater (p < 0.05) in SO than in sarcopenia. There were no differences (p > 0.05) in systolic and diastolic blood pressures, CRP, total SPPB scores and functional assessments between the sarcopenia and the SO groups for CVD male and female inpatients (Table 3).
The relationship between SPPB and clinical and laboratory factors
To predict total SPPB scores, the predicted handgrip, CONUT, % body fat, anterior mid-thigh MTH, standing height and diastolic BP were calculated for males (total SPPB scores = 0.101 × handgrip − 0.199 × CONUT − 0.110 × % body fat + 0.782 × anterior mid-thigh MTH −0.072 × standing height + 0.029 × diastolic BP + 17.836) (n = 318, R2 = 0.523, p < 0.05) and anterior mid-thigh MTH, BMI, knee extension and fat mass were calculated for females (total SPPB scores = 1.902 × anterior mid-thigh MTH −0.627 × BMI + 0.157 × knee extension + 0.223 × fat mass + 10.940) (n = 172, R2 = 0.411, p < 0.05).
Discussion
The main findings of this study were as follows: First, anterior mid-thigh MTH can predict total SPPB scores for both CVD male and female inpatients. Second, non-sarcopenic group was superior for morphological and functional assessments compared with sarcopenia and SO groups, and there were slightly differences between the two groups in assessment for CVD male and female inpatients.
A previous study reported that impaired mobility was reflected by total SPPB scores of less than 10 and those with total SPPB scores of 7–9 were 1.6 to 1.8 times more likely to become disabled17,18. In addition, total SPPB scores of 4–6 were 4.2 to 4.9 times more likely to have disability in the activities of daily living or mobility-related disability at 4 years. Therefore, the SPPB assessment was established as a disability evaluation for older adults, healthy elderly and for some disability conditions6,19,20. In this study, the average score (9.7 for males and 8.2 for females) and the distribution (0–3: 6% and 5%, 4–6: 20% and 20%, 7–9: 31% and 25%, 10–12: 43% and 49% for male and female inpatients, respectively) of total SPPB scores were almost the same as the previous CVD study (score 9.0; distribution 0–3: 0%, 4–6: 17%, 7–9: 25%, 10–12: 50% for mixed male and female inpatients)8. Although a previous study investigated whether SPPB can be validated as an assessment tool for muscle strength and morphology in various physical condition (12 inpatients, 12 outpatients and 12 healthy subjects), a stepwise multiple-regression analysis could be applied to the predictor anterior mid-thigh muscle thickness to predict total SPPB scores for CVD in both studies. Taken together, these results suggested that the total SPPB scores could be evaluated by morphological assessment in knee extensor muscles for middle-aged and older adults regardless of male/female and CVD outpatient/inpatient.
In general, SMI assessed by BIA and DXA is used extensively in sarcopenia criteria as in European Working Group on Sarcopenia in Older People (EWGSOP)21 and AWGS22. However, we could not measure the SMI for 26 inpatients (11 males and 15 females), because individuals with cardiac pacemakers are generally regarded as a contraindication to BIA, DXA and MRI measurements. In contrast, MTH assessed by ultrasound is also used as a muscle mass in clinical medicine and sports science, and there is no contraindication even for individuals with cardiac pacemakers. In addition, there was a high correlation coefficient (r = 0.685, p < 0.0001 for males and r = 0.608, p < 0.0001 for females) between anterior mid-thigh MTH (mainly dependent on quadriceps muscles) and SMI in this study (Fig. 1). Furthermore, previous studies revealed that sarcopenia is muscle specific and greater quadriceps muscle loss was found in older adults23,24. These results indicated that additional research into anterior mid-thigh MTH assessed by ultrasound on sarcopenia criteria is worthy of attention.
There was little relationship between the total SPPB scores and CONUT score in this study. The CONUT score was 3.6 for male and 3.7 for female inpatients and the prevalence of malnutrition (mild [2–4]: 40% and 34%; moderate [5–8]: 26% and 37%; severe [9–12]: 7% and 4% for male and female, respectively) was 73–75%. These results were similar to those in a previously reported studies in inpatients with acute or chronic heart failure (78% for AHF and 60–69% for CHF)25,26. This appears that prognostic value of malnutrition assessed by CONUT score may not be a decisive factor in the assessment of SPPB score for CVD male inpatients.
A previous study reported that SO is associated with all-cause mortality and also with greater CVD mortality, largely because of their association with blood pressure, blood lipids and inflammation27. In this study, there was no difference in blood pressures, inflammation, and functional assessment between the sarcopenia and the SO groups for CVD male and female inpatients. Additionally, the CONUT score and the knee extensor muscle morphology for the SO group were either equal to or surpassed those of the sarcopenia group. Therefore, it is highly likely that the SO group induced greater cardiovascular mortality and all-cause mortality was independent of functional assessment, as well as blood pressure and inflammation, although the mortality for the sarcopenia and the SO groups was not investigated. Additional research into these issues is needed.
The present study has some limitations. First, it was very difficult to evaluate the various assessments within the same hospital admission for the inpatients. Second, SPPB assessment could not be evaluated for severely impaired mobility patients because some subjects failed to perform the chair stand test (19 males and 17 females) and the gait test (1 male and 2 females). Third, some data were missing due to impossible measurement (method discussed above), sudden discharge from hospital, acute deterioration, contraindication and others for the inpatients. Additional research into these issues is needed.
In conclusion, the physical performance of CVD inpatients can be predicted by nutritional, functional, clinical and anthropometric variables, regardless the gender and the presence of sarcopenia. Furthermore, the presence of sarcopenia has a negative effect on the clinical and laboratory factors, but there is a difference in impact between sarcopenia and SO regardless the gender.
Methods
Participants
Four hundred-ninety (aged 22 to 96 years) male (n = 318) and female (n = 172) inpatients with cardiovascular disease volunteered to participate in the study and were selected according to the exclusion criteria (i.e. cerebrovascular disease patients and those undergoing arthroscopic joint surgery) (Table 1). In addition, volunteers who suffered from a chronic disease such as severe orthopedic disorders, or cognitive dysfunction were excluded from the study. All participants had undergone complete chemistry and hematologic evaluation and were informed of the risks associated with involvement in the study and signed an informed consent document before participation. All participants were recruited through oral communications in the Dokkyo Medical University Hospital. The principles of the World Medical Association Declaration of Helsinki and the American College of Sports Medicine Guidelines for Use of Human Subjects were adopted in this study. The study was approved by the Ethics Committee of the Dokkyo Medical University, and informed assent consent were obtained from the participants. All data were analyzed by the same author (a specialist in exercise physiology research for more than 15 years), who was blinded to all of the measurements.
Short physical performance battery
Participants performed the Short Physical Performance Battery (SPPB) according to the National Institute on Aging protocol. The tests were performed in the following sequence: a) standing balance tests, b) gait test (4 m), and c) chair stand test (5 repetitions). The standing balance portion requires participants to maintain, for 10 seconds each, stances with their feet placed side by side, semi-tandem, and in tandem. The scores ranged from 0 to 4 (maximum performance). The gait test measured the time needed to walk 4 m at a typical pace. The chair stand required participants to rise from a steel chair (0.40 m height and 0.30 m depth) with their arms across their chest, five times. Categorical scores (range: 0 to 4) for both the gait and the chair stand tests were based on timed quartiles established previously in a large population. Individuals who were unable to complete either the 4 m gait task or the 5 repetitions chair stand test received a score of 0. The sum of the three components comprised the final SPPB score, with a possible range from 0 to 12. A score of 12 indicated the highest degree of lower extremity function6,28.
Maximum voluntary isometric contraction
Maximum voluntary isometric contraction (MVIC) of the handgrip was determined using a factory-calibrated hand dynamometer (TKK 5401, TAKEI Scientific Instruments Co., Ltd., Tokyo, Japan). All of the subjects were instructed to maintain an upright standing position, arms at their side, holding the dynamometer in the right hand with the arm at a right angle and the elbow held at the side of the body. The size of the dynamometer handle was set so that it felt comfortable to the subject while squeezing the grip. Each subject underwent 2 trials, and the best value of 2 trials was used for analysis.
MVIC of the knee extensors was determined using a digital handheld dynamometer (μTas MT-1, ANIMA Co., Ltd., Tokyo, Japan)29. The dynamometer pad used was 55 × 55 mm, and its front side was curved to fit the shape of the area of the extremity to be measured. Subjects were seated in a hard chair with their knees flexed 90° and their arms on their thighs. The dynamometer was placed perpendicular to the leg just above the malleoli. During all tests, the dynamometer was kept stable by the examiner using both hands and the subject’s leg was fixed by a belt to keep the knee flexed at 90°. Subjects were told to push against the dynamometer by attempting to straighten their leg. They were asked to build force gradually to a maximum voluntary effort. Each subject performed 2 trials with an interval of at least 2 min between the trials. The highest score was adopted for the individual data.
Skeletal muscle index
The multi-frequency bioelectrical impedance analyzer (BIA), InBody S10 Biospace device (Biospacte Co., Ltd., Korea/Model JMW140) was used according to the manufacturer’s guidelines. BIA estimates body composition using the difference of conductivity of the various tissues based on the differences in their biological characteristics. Conductivity is proportional to water content, and more specifically to electrolytes, and it decreases as the cell approaches a perfect spherical shape. Adipose tissue is composed of round shaped cells and contains relatively little water compared to other tissues like muscle; therefore, conductivity isdecreased as body fat increases. In practice, electrodes are placed at 8 precise tactile-points of the body to achieve a multi-segmental frequency analysis. A total of 30 impedance measurements were obtained using 6 different frequencies (1, 5, 50, 250, 500, and 1000 kHz) at the 5 following segments of the body: right and left arms, trunk, right and left legs30. The measurements were carried out while the subjects rested quietly in the supine position, with their elbows extended and relaxed along their trunk. Percent body fat, fat-free mass and skeletal muscle mass were recorded. Also, skeletal muscle index (SMI; AMM/height2, kg/m2) was measured as the sum of lean soft tissue of the two upper limbs and two lower limbs (appendicular muscle mass: AMM).
Muscle thickness
After thigh length measurements using anatomic landmarks, all measurement sites were marked with a marker pen and then mid-thigh (at 50% between the lateral condyle of the femur and the greater trochanter) girth and mid-thigh muscle thickness were measured using a tape measure on the right side of the body31. Ultrasound evaluation of muscle thickness (MTH) was performed by using a real-time linear electronic scanner with a 10.0-MHz scanning head and Ultrasound Probe (L4–12t-RS Probe, GE Healthcare Japan) by using LOGIQ e ultrasound (GE Healthcare Japan). The scanning head was coated with a water-soluble transmission gel to provide acoustic contact without depressing the dermal surface. The subcutaneous adipose tissue-muscle interface and the muscle-bone interface were identified from the ultrasonic image. The perpendicular distance from the adipose tissue-muscle interface to the muscle-bone interface was considered to represent MTH. Briefly, the measurements were carried out while the subjects stood with their elbows extended and relaxed31.
Controlling nutritional status score
The Controlling Nutritional Status (CONUT) score, which is calculated by the serum albumin concentration (range: 0 to 6), the total peripheral lymphocyte count (range: 0 to 3), and the total cholesterol concentration (range: 0 to 3), was developed as a screening tool for early detection of poor nutritional status. The sum of the three components comprised the final CONUT score, with a possible range from 0 to 12. A score of 12 indicated the poorest nutritional status32. Use of the CONUT score has advantages, such as simplicity and cost effectiveness32,33.
Definition of sarcopenia and sarcopenic obesity
In this study, sarcopenia was defined according to the Asian Working Group for Sarcopenia (AWGS)22 criteria (age, ≥65 years; handgrip, <26 kg for males and <18 kg for females; gait speed, ≤0.8 m/sec; SMI, <7.0 kg/m2 for males and <5.7 kg/m2 for females). Sarcopenia obesity (SO) was considered to be the combination of sarcopenia and obesity (% body fat >25% for males and >30% for females)34,35,36,37.
Statistical analyses
Results are expressed as mean ± standard deviation for all variables. All data were analyzed using JMP v.12.0 for Mac (SAS Institute Inc., Tokyo, Japan). Pearson product correlations of total SPPB scores and variable factors were also statistically quantified. When the data were not normally distributed, non-parametric statistical analysis (Wilcoxon signed rank test) was used to identify differences in sarcopenia, SO and non-sarcopenic groups. Since the value of events per variable = 10 seems most prudent for regression analysis38, 17 variable factors were acceptably for both genders (n = 318 and 172 for male and female inpatients, respectively). In addition, the variance inflation factor (VIF) were used to determine the degree of multi-collinearity of the i-th independent variable with other independent variables for all hierarchal regression models39. Multi-collinearity between variables was defined as a VIF ≥ 10, and fat free-mass was excepted for male in this study. Based on the result of VIF, a stepwise multiple-regression analysis (method of increasing and decreasing the variables, criterion was set at p < 0.05) was performed to predict SPPB scores and variable factors (age, standing height, body weight, BMI, % body fat, systolic and diastolic blood pressures, resting heart rate, handgrip, knee extension, fat free-mass (used exclusively for female), skeletal muscle mass, SMI, mid-thigh girth, anterior and posterior mid-thigh muscle thickness and CONUT score). Consequently, the predicted variables, coefficients and intercept coefficients were automatically picked out by the JMP software. Statistical significance was set at p < 0.05.
References
Murray, C. J. et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 1005–1070 (2014).
Ohira, T. & Iso, H. Cardiovascular disease epidemiology in Asia: an overview. Circ J 77, 1646–1652 (2013).
Arpaia, G. et al. Risk of deep venous thrombosis (DVT) in bedridden or wheelchair-bound multiple sclerosis patients: a prospective study. Thromb Res 125, 315–317 (2010).
Naritomi, H. et al. MARVELOUS Study Group. Effects of edaravone on muscle atrophy and locomotor function in patients with ischemic stroke: a randomized controlled pilot study. Drugs R D 10, 155–163 (2010).
Naritomi, H. & Moriwaki, H. Prevention of post-stroke disuse muscle atrophy with a free radical scavenger. Front Neurol Neurosci 32, 139–147 (2013).
Guralnik, J. M. et al. Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49, M85–M94 (1994).
Stookey, A. D. et al. The short physical performance battery as a predictor of functional capacity after stroke. J Stroke Cerebrovasc Dis 23, 130–135 (2014).
Yasuda, T., Fukumura, K. & Nakajima, T. Short Physical Performance Battery for middle-aged and older adult cardiovascular disease patients: implication for strength tests and lower extremity morphological evaluation. J Phys Ther Sci 29, 748–753 (2017).
Wakabayashi, H. & Sashika, H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital-associated deconditioning a prospective cohort study. J Rehabil Med 46, 277–282 (2014).
Siervo, M. & Jebb, S. A. Body composition assessment: theory into practice: introduction of multicompartment models. IEEE Eng Med Biol Mag 29, 48–59 (2010).
Visser, M. et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60, 324–333 (2005).
Flegal, K. M., Graubard, B. I., Williamson, D. F. & Gail, M. H. Excess deaths associated with underweight, overweight, and obesity. JAMA 293, 1861–1867 (2005).
Roubenoff, R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann NY Acad Sci 904, 553–557 (2000).
Roubenoff, R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 12, 887–888 (2004).
Honda, H. et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr 86, 633–638 (2007).
Prado, C. M. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9, 629–635 (2008).
Stuart, M. et al. Community-based adaptive physical activity program for chronic stroke: feasibility, safety, and efficacy of the Empoli model. Neurorehabil. Neural Repair 23, 726–734 (2009).
Guralnik, J. M. et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332, 556–561 (1995).
Westlake, K. P. & Patten, C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil 12, 18 (2009).
Vazzana, R. et al. Trail Making Test predicts physical impairment and mortality in older persons. J Am Geriatr Soc 58, 719–723 (2010).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010).
Chen, L. K. et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15, 95–101 (2014).
Miyatani, M. et al. Site-related differences in muscle loss with aging: a cross sectional survey on the muscle thickness in Japanese men and Age-related muscle loss in Japanese adults 150 women aged 20 to 79 years. Int J Sport Health Sci 1, 34–40 (2003).
Abe, T. et al. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J Sports Sci Med 10, 145–150 (2011).
Iwakami, N. et al. Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int J Cardiol 230, 529–536 (2017).
Narumi, T. et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol 62, 307–313 (2013).
Atkins, J. L. et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc 62, 253–260 (2014).
Bernabeu-Mora, R. et al. The Short Physical Performance Battery is a discriminative tool for identifying patients with COPD at risk of disability. Int J Chron Obstruct Pulmon Dis 10, 2619–2626 (2015).
Watanabe, M. et al. The relationship between bilateral knee muscle strength and gait performance after stroke: the predictive value for gait performance. J Phys Ther Sci 27, 3227–3232 (2015).
Buckinx, F. et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord 16, 60 (2015).
Abe, T., Kawakami, Y., Kondo, M. & Fukunaga, T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol 6, 161–170 (1994).
Ignácio, A. R., Müller, Y. M., Carvalho, M. S. & Nazari, E. M. Distribution of microglial cells in the cerebral hemispheres of embryonic and neonatal chicks. Braz J Med Biol Res 38, 1615–1621 (2005).
Toyokawa, T. et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer 16, 722 (2016).
Rantanen, T. et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55, M168–M173 (2000).
Al Snih, S. et al. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc 50, 1250–1256 (2002).
Wen, C. P. et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 12, 497–506 (2009).
Stenholm, S. et al. Obesity and muscle strength as long-term determinants of all-cause mortality–a 33-year follow-up of the Mini-Finland Health Examination Survey. Int J Obes (Lond) 38, 1126–1132 (2014).
Peduzzi, P., Concato, J., Feinstein, A. R. & Holford, T. R. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48, 1503–1510 (1995).
O’Brien, R. M. A caution regarding rules of thumb for variance inflation factors. Qual Quant 41, 673–690 (2007).
Acknowledgements
This study was supported, in part, by KAKENHI Grant (#16H03203 to TN and #15K01553 to TY) from the Japan Ministry of Education, Culture, Sports, Science, and Technology, the Vehicle Racing Commemorative Foundation (to TN), and Fukuda Foundation for Medical Technology (to TN).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: T.Y., T.N., S.T., and T.I. Performed the experiments: T.N., T.S., N.N., T.A., R.T., Y.M., S.K., and K.M. Analyzed the data: T.Y., T.N., and N.N. Wrote the paper: T.Y., and T.N.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yasuda, T., Nakajima, T., Sawaguchi, T. et al. Short Physical Performance Battery for cardiovascular disease inpatients: implications for critical factors and sarcopenia. Sci Rep 7, 17425 (2017). https://doi.org/10.1038/s41598-017-17814-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17814-z