Abstract
Present study aims to investigate the role of AGEs, TGF-β1, BDNF and their receptors on diabetes-induced colon remodeling. Diabetes was induced by a single tail vein injection 40 mg/kg of STZ. The parameters of morphometric and biomechanical properties of colonic segments were obtained from diabetic and normal rats. The expressions of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB were immunohistochemically detected in different layers of the colon. The expressions of AGE, RAGE, TGF-β1 and TGF- β1 receptor were increased whereas BDNF and TrkB were decreased in the diabetic colon (P < 0.05, P < 0.01). AGE, RAGE and TGF-β1 receptor expressions were positively correlated whereas the BDNF expression was negatively correlated with most of the morphometry and biomechanical parameters (P < 0.05, P < 0.01, P < 0.001). AGE, TGF- β1 and BDNF in different layers correlated with their receptors RAGE, TGF- β1 receptor and TrkB respectively. STZ-induced diabetes up-regulated the expression of AGE, RAGE, TGF- β1 and TGF- β1 receptors and down-regulated BDNF and TrkB in different layers of diabetic colon mainly due to hyperglycemia. Such changes maybe important for diabetes-induced colon remodeling, however it is needed to further perform mechanistic experiments in order to study causality or approaches that explain the relevance of the molecular pathways.
Similar content being viewed by others
Introduction
A previous study has demonstrated that experimental diabetes could induce colon morphological and biomechanical remodeling1. Following the development of diabetes, the colonic wall became thicker and the stiffness of the wall increased in a time-dependent manner. Such remodeling may play an important role in diabetic GI complications, including constipation2. However, the molecular pathways of diabetes-induced colon remodeling are not well understood.
Advanced glycation end products (AGEs) are formed physiologically and s accelerated in diabetes3. AGEs can lead to structural and functional changes by direct interaction with target protein or through their receptor (RAGE)4. AGEs and RAGE have been demonstrated to play an important role in diabetic complications including the complications of the gastrointestinal (GI) tract5. TGF-β1 is considered to be a core factor in the development of diabetic nephropathy (DN)6. In recent years, the relationship between TGF-β1 and other diabetic complications has been gradually brought to attention7,8. Some studies have demonstrated that an association exists in the expressions of AGE/RAGE and TGF-β1 during the development of diabetes9,10. Active TGF-β1 signaling is transmitted through TGF-β1 receptor and the activated TGF-β1 receptor propagates intracellular signaling by recruiting and phosphorylating receptor-regulated Smad proteins11. Neurotrophic factors (NTFS) are polypeptides or small proteins that support and enhance the growth, differentiation, and survival of neurons11. Brain derived neurotrophic factor (BDNF) is a member of NTFS12. Tropomyosin receptor kinase B (TrkB) is a receptor for BDNF13. BDNF and TrkB are broadly expressed in the brain and the peripheral nervous system14,15,16. BDNF/TrkB-stimulated intracellular signaling is critical for neuronal survival, morphogenesis, and plasticity17. When BDNF binds to the TrkB receptor, it results in the recruitment of proteins that activate three different signaling pathways: Ras/MAPK-ERK pathway, PI3-K pathway and PLC pathway17. Reduced levels of BDNF have been evidenced in diabetic patients18 and may be associated with diabetic complications19,20. It has been shown that BDNF is highly expressed in the colon21. However, no data on the relation between BDNF/TrkB and diabetic colon remodeling has been reported so far.
In order to investigate whether AGE, RAGE, TGF- β1 and their receptors play a role in the colon remodeling induced by diabetes, the expressions of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB were detected in the colon wall of streptozotocin (STZ)-induced rats. Furthermore, the association between the expression of these proteins and the histomorphometric and biomechanical parameters were analyzed.
Results
General data, morphometry data and biomechanical data
The general data, morphometry data and biomechanical data obtained from our previous publication22 are shown in Table 1. The blood glucose level was about 4-fold higher in the Diabetes group compared with that of the Control group (p < 0.01). The body weight in the Diabetes group was nearly 50% lower than that in the Control Group (p < 0.01). The wet weight per unit length, wet-weight to body weight ratio, no-load wall thickness, and cross-section wall area of the colonic segments were significantly higher in the Diabetes group compared with those of the Control group (p < 0.01). The opening angles were significantly higher in the Diabetes group compared with those in the Control group (p < 0.01). A similar trend was found for the inner and outer residual strains; i.e., the absolute values of the residual strain were significantly higher in the Diabetes group compared with those in the Control group (p < 0.05, p < 0.01). Computation of constant a showed a significant difference between the Diabetes group and the Control group (p < 0.01).
Fractions of AGE, RAGE, TGF-β1, TGF- β1 receptor, BDNF and TrkB between two groups
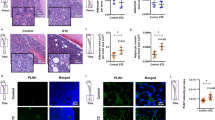
The representative samples of immunohistochemical staining is shown in Fig. 1 and the fraction of AGE, RAGE, TGF-β1, TGF- β1 receptor, BDNF and TrkB in the different layers of the colon between two groups are shown in Fig. 2. Generally, the expression of all proteins was stronger in the muscle layer than other layers. In different layers of colon wall, the expressions of AGE, RAGE, TGF-β1 and TGF- β receptor were stronger whereas BDNF and TrkB expressions were weaker in the Diabetes group than in Control group. Significant difference were found for AGE, RAGE and TrkB for all three layers (P < 0.05, P < 0.01), TGF- β1 in mucosa layer (P < 0.05), and TGF- β1 receptor and BDNF in muscle layer (P < 0.05).
The representative samples of immunohistochemical staining for AGE, RAGE, TGF-β1, TGF- β1 receptor, BDNF and TrkB in the colon wall of two groups. The microscopy with high magnification have been inserted in each single histological photo (arrow) in order to display the localization of markers. The staining of all proteins was stronger in the muscle layer than other layers. In the different layers, the staining of AGE, RAGE, TGF-β1 and TGF- β1 receptor was stronger whereas the staining of BDNF and TrkB was weaker in the Diabetes group than in Control group. Bar = 100 um.
The fraction of AGE, RAGE, TGF-β1, TGF- β1 receptor, BDNF and TrkB in the different layers of the colon between two groups. In the different layers, the fraction of AGE, RAGE, TGF-β1 and TGF- β1 receptor was bigger whereas the fraction of BDNF and TrkB was smaller in the Diabetes group than in Control group. Compared with Control group: *P < 0.05, **P < 0.01.
Correlation analysis results
Single linear correlation analysis
It was shown that the glucose level was associated with the most parameters of morphometry, biomechanical properties and the expressions of all proteins studied in different layers. In relation to morphometry parameters, the most significant correlation was found for the body weight and the wet weight per unit length of the segments to body weight ratio (P < 0,001). In relation to the biomechanical parameters, besides inner residual strain, all other parameters were significantly correlated with the glucose levels (P < 0.05, P < 0.01). In relation to the expressions of all proteins studied, glucose levels were strongly correlated with the AGE and RAGE expressions in different layers (P < 0.05, P < 0.01). The glucose levels were also significantly correlated with TGF-β1 in mucosa layer, TGF- β1 receptor in muscle layer, BDNF in muscle layer and TrkB in mucosa and submucosa layers (P < 0.05). The details of correlation equations and values of R and P are shown in Table 2.
Table 3 shows the correlation between expressions of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB with morphometry and biomechanicl parameters. Only significant correlation results (P < 0.05 or close to 0.05) are shown. AGE and RAGE in different layers were significantly correlated with most morphometry and biomechanical parameters. It is especially interesting that AGE and RAGE in the muscle and submucosa layers were positively correlated with circumferential and longitudinal material constant a (Fig. 3 A&B, P < 0.05, P < 0.01). The expression of TGF-β1 in mucosa and muscle layers was also positively and significantly correlated with circumferential and longitudinal material constant a (Fig. 3 C, P < 0.05). TGF-β1 receptor in different layers correlated with most morphometry parameters and Opening angle. Whereas the BDNF in muscle layer negatively correlated with most of the morphometry and biomechanical parameters including circumferential constant a (Fig. 3D, P < 0.05) and in submucosa layer with longitudinal constant a (Fig. 3D, P < 0.05). TrkB in different layers was found to correlate with wet weight per unit length of the segments, wall thickness, opening angle as well as the residual strain. The details of correlation equations and values of R and P are shown in Table 3.
(A) Correlation between AGE and RAGE in muscle layer and submucosa layer with circumferential constant a; (B) Correlation between AGE and RAGE in muscle layer and submucosa layer with longitudinal constant a; (C) Correlation between TGF-β1 and TGF-β1 receptor in mucosa layer and TGF-β1 muscle layer with circumferential and longitudinal material constant a; (D) Correlation between BDNF in muscle and submucosa layers with longitudinal constant a.
Multiple linear correlation analysis
Interrelation among AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB expressions in different layers are shown in Table 4. AGE, TGF- β1 and BDNF in different layers mostly correlated with their receptors RAGE, TGF- β1 receptor and TrkB (Fig. 4A-C). Similarly, RAGE, TGF- β1 receptor and TrkB in different layers also mostly correlated with AGE, TGF- β1 and BDNF. However, it is interesting to notice that RAGE and TGF- β1 receptor in different layers were strongly correlated with each other (Fig. 4D). The details of correlation equations and values of R, F and P are shown in Table 4.
Discussion
Diabetic GI complications are common in longstanding diabetes23. Poor control of diabetes can affect any segment of the gut including the colon24. Although many studies have demonstrated that multiple factors are involved in diabetic GI complications25, the mechanisms are not well understood. The main findings found in the present study showed that the expression of AGE, RAGE, TGF- β1 and TGF- β1 receptor was significantly higher whereas the expression of BDNF and TrkB was significantly lower in different colon layers in the Diabetes group than in Control group. The glucose level was associated with the most parameters of morphometry, biomechanical properties and the expressions of all proteins studied in different layers. Furthermore, the expressions of these proteins were highly correlated with the most of the histomorphometric and biomechanical remodeling parameters.
AGEs and RAGE accumulated during the development of DM are associated with cardiovascular complications26, retinopathy27, nephropathy28 as well as GI complications29. Chen and co-workers have found that AGE and RAGE are up-regulated in the diabetic rat colon30,31. Furthermore, it has been demonstrated that histomorphological and biomechanical remodeling occurred in the colon of diabetic rat model1,22. However, the mechanism of such remodeling is not clear. A study on the relation between AGEs and vascular wall stiffness has shown that glycation-induced inter-molecular cross-links contribute to diabetic vascular stiffening32. More recently, one study demonstrated that AGEs induced arterial stiffness and aging in a RAGE-dependent manner in mice33. Previously we demonstrated that the most histomorphometric and biomechanical parameters of intestinal wall in the GK diabetic rats are associated with the expression of AGE and RAGE5. In the present study, we showed that the glucose levels were strongly correlated with the AGE and RAGE expressions in different layers. Diabetes-induced morphological and biomechanical remodeling of rat colon were also found to be associated with the glucose level and abnormal expressions of AGE and RAGE in the different layers of colon wall. Hyperglycemia promotes and accelerates AGE formation. The accumulated AGEs affect the tissue structural changes and neuromuscular functions of diabetic GI tract through receptor-dependent and -independent pathways34,35. The former modulates cellular functions through ligation of specific cell surface receptors such as RAGE. The latter alters the extracellular matrix architecture by nonenzymatic glycation and the formation of protein cross-links. Therefore, the abnormal expressions of AGEs and RAGE contribute to diabetes-induced colon remodeling which plays an important role in the GI disorders in diabetes.
Transforming growth factor (TGF)-β1 is a ubiquitously expressed cytokine belonging to a large superfamily of activins/bone morphogenetic proteins36. This mediator plays an active role in the processes of wound healing37 and the synthesis of ECM molecules38. It has been reported that plasma levels of TGF- β 1 are elevated in NIDDM patients and might contribute to the occurrence of diabetic complications39. Indeed, many studies have demonstrated that TGF-β1 strongly contributes to diabetic nephropathy6, diabetic retinopathy40,41 and diabetic neuropathy42. In the present study, we demonstrate that the TGF- β1 and TGF- β1 receptor are up-regulated in the diabetic colon wall. Although no detailed molecular pathway has been demonstrated in the present study, the increasing level of TGF- β1 may activate TGF- β1 receptors through ligand binding and subsequently activate Smad proteins through phosphorylation43. It has been reported that a significant upregulation of TGF-β1, TGF-β receptors and the effectors p-Smad2/3 in the colon mucosa of diabetic rats11. Such deregulation of the TGFβ1 pathway associated with the appearance of myofibroblasts and the accumulation of ECM in the mucosa of diabetic colon. Insulin treatment could attenuate the stimulating effect of diabetes on colon ECM deposition and TGFβ/Smad signaling11. Therefore, their data provide the evidence that TGF-β1/Smad is a key component of intestinal tissue remodeling in diabetes. Furthermore, having studies demonstrated the effects of bone morphogenetic protein (BMP2) on promoting enteric neuronal differentiation in culture through a Smad dependent pathway44. Using an in vivo STZ-induced diabetic model, it has been demonstrated a decrease in the number of myenteric neurons after the onset of diabetes. At same time, BMP/Smad signaling in the myenteric plexus of the diabetic small intestine has also been changed45. Therefore, TGF-β1/Smad may be a key component of intestinal tissue remodeling in diabetes. Data lacks in relation to the association between TGF-β1 and TGF-β1 receptor with gastrointestinal morphological and biomechanical remodeling in diabetes. Fleenor and co-workers reported that arterial stiffening with aging is associated with TGF-β1-related changes in adventitial collagen46. Few studies demonstrated that TGF- β1 increased F-actin levels in single chondrocytes leading to the stiffening of cells47. In the present study we demonstrated that TGF-β1 in muscle layer correlated with circumferential and longitudinal material constant a, whereas TGF-β1 receptor in different layers correlated with most morphological and biomechanical parameters. It is also interesting to note that RAGE and TGF- β1 receptor in muscle layers were strongly correlated each other. It may indicate that either TGF-β1 is an independent contributing factor or TGF-β1 and AGE are co-contributors to the morphological and biomechanical remodeling of colon in diabetes. The detailed molecular pathways for the effects of AGE and TGF-β1 on colonic remodeling in the diabetes need to be explored further.
Brain-derived neurotrophic factor (BDNF) and its receptor TrkB regulate dendritic and axonal growth during development and maintenance of the mature nervous system through different cellular and molecular mechanisms48. Decreased levels of BDNF are associated with neurodegenerative diseases with neuronal loss, such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis and Huntington’s disease17. In relation to diabetes, data from animal experiments and human studies suggested that BDNF may contribute to glucose metabolism and plays a pathogenic role in the development of type 2 diabetes mellitus in human49. Some studies have found that BDNF levels are lower in individuals with type 2 diabetes compared to non-diabetic individuals both in plasma and serum18,19,20,50,51. The abnormal level of BDNF may be associated with diabetic complications18,19,20. One study demonstrated that in the diabetic rat brains, both protein and mRNA levels of BDNF are severely reduced. These results suggest that diabetic neuropathies in the brain are, at least in part, caused by a failure of BDNF synthesis in the brain52. However, data lacks on the expression of BDNF and TrkB in the diabetic GI tract. Lommatzsch et al. has shown that BDNF was highly expressed in the colon, but lacked the expression of both the high- and low-affinity receptors for BDNF, i.e., TrkB and p75NTR21. Liu et al. has shown that the levels of some neurotrophic factors such as glia cell-derived neurotrophic factor (GDNF), neurotrophin 3 and nerve growth factor were down-regulated in the colon of diabetic rats53. It has been reported that the enteric neuropathy was induced in STZ-induced diabetic rats which is partly mediated via a reduction of GDNF and its main downstream signalling pathway PI3K/Akt54. On the other hand, it has been demonstrate that GDNF could increasing the number of NADPH diaphorase–containing neurons in the diabetic intestine through activation of the PI3K/Akt pathway55. In the present study, we found that both BDNF and TrkB were highly expressed in the normal colonic wall, whereas such expression was significantly decreased in the diabetic colonic wall. The expressions of BDNF and TrkB were negatively correlated with some morphometric and biomechanical remodeling parameters. The diabetes-induced down-regulation of BDNF and TrkB expression may be associated with diabetes-induced colon dysfunction. BDNF binds to its high-affinity receptor TrkB, resulting in the activation of three different signal transduction cascades17: 1) Insulin receptor substrate-1 (IRS-1/2), phosphatidylinositol-3-kinase (PI-3K) and protein kinase B (Akt), 2) Shc/Grb2, Ras, Raf, mitogen-activated protein kinase kinases (MEKs) and extracellular signal regulated kinases (ERKs) and 3) Phospholipase C (PLC), inositol (1,4,5)-trisphosphate [Ins(1, 4, 5)P3], diacylglycerol (DAG) and protein kinase C (PKC). Therefore, the down-regulation of BDNF and its receptor TrkB in the diabetic colon found from the present study may through the same molecular pathway to promote the colon remodeling in diabetes. Future studies are needed to investigate how diabetes induces abnormal expressions of BDNF and TrkB in GI tract. Details on the molecular mechanisms underlying the effect of BDNF and TrkB on diabetes induced GI remodeling are needed to be explored as well.
In the present study we found that the expressions of all proteins - AGE, RAGE, TGF-β1, TGF- β1 receptor, BDNF and TrkB are associated with the glucose level and colon remodeling parameters to some degree. Furthermore, the abnormal expression of these proteins is associated with diabetes-induced complications such as diabetic neuropathy in one way or another. Analysis for interrelations between AGE, RAGE, TGF-β1,TGF- β1 receptor, BDNF and TrkB showed that AGE, TGF- β1 and BDNF in different layers mostly correlated with their receptors RAGE,TGF- β1 receptor and TrkB. This seems to be obvious since the effects of AGE TGF- β1 and BDNF are mediated through their corresponding receptors. However, it is interesting to note that RAGE and TGF- β1 receptor in different layers were strongly correlated each other. There are some studies which have investigated the complicated interaction of AGE and TGF-β1 with their receptors in the pathological progression of diabetic nephropathy56,57 and interstitial fibrosis induced by imbalances in extracellular matrix homeostasis58. However, to the best of our knowledge, the interplay among these proteins in relation to diabetes-induced GI disorders has not been reported yet and needs to be explored.
In conclusion, STZ-induced diabetes up-regulated the expression of AGE, RAGE, TGF- β1 and TGF- β1 receptor and down-regulated the expression of BDNF and TrkB in different colon layers of rats mainly due to hyperglycemia. The expressions of AGE and TGF- β1 were highly and positively associated with histomorphometric and biomechanical remodeling parameters of colon, and also highly associated with the expressions of their receptors. Therefore, our results suggest that AGE, RAGE, TGF- β1 and TGF- β1 receptor are likely promoting factors for diabetes-induced colon histomorphological and biomechanical remodeling. AGE and TGF- β1 play their roles through their specific receptors RAGE and TGF- β1 receptors. The expression of BDNF and TrkB were highly and negatively associated with histomorphometric and biomechanical remodeling parameters of colon, however we could not conclude that BDNF and TrkB are inhibiting factors or not for diabetes-induced colon remodeling, because novel contribution of BDNF and TrkB in diabetic intestinal dysfunction is scarce. It is needed to further perform mechanistic experiments aimed to study causality or approaches that explain the relevance of the molecular pathways. In the future, it is necessary to investigate the detailed molecular pathway of the abnormal expressions of these proteins in diabetes and their association with diabetes-induced colon remodeling.
Materials and Methods
Animal model and groups
Twenty male Sprague-Dawley rats weighing 220-250 g were included in this study. Ten rats were made diabetic by a single tail-vein injection of 40 mg/kg STZ (Sigma-Aldrich, China). After 7 days, this dose of STZ resulted in a random blood glucose level (≥16.7 mmol/L) in 9 of 10 rats which were used for the diabetic group (Diabetes). Another ten rats of similar age and body weight from the same vendor were used as the non-diabetic control group (Control). Approval of the protocol and experimental methods were obtained from The Committee of Guang’anmen Hospital, China Academy of Chinese Medical Sciences for Animal Experimentation.
Experimental procedures and sampling
The body weight and blood glucose levels were measured at 2-week intervals after the start of the experiment. The experimental period was 60 d. At the end of the experiment, the rats fasted overnight and were then anesthetized with 4% chloral hydrate (10 mL/kg, ip). Following laparotomy, the whole colon was harvested. After the lumen of the segments was gently cleansed with saline, the length and the wet weight were measured. The middle colonic segment was divided into three parts: A 2-cm-long tissue was cut from the proximal end of the segments and fixed in 10% formalin for immunohistochemistry examination. Then a 1-cm-long part was cut and used for the zero-stress state experiment and the remaining part was used for the distension test. The results of zero-stress state and the distension test were reported in our previous paper22. Therefore, the parameters of morphometric properties, residual strains and stress-strain of the wall in colonic segments were adopted from our previous paper22 and used for correlation analysis for the expressions of different proteins used in the present paper.
Immunohistochemistry staining
Tissue pretreatment
The tissue samples for immunohistochemistry were fixed in 10% phosphate-buffered formalin for 24 h and embedded in paraffin. Five-micron sections were cut perpendicular to the mucosa surface and placed in a water bath at 40 °C. Thereafter, sections were transferred onto pretreated microscopic slides which electrostatically attracted formalin fixed tissue and bond them to the slides. After drying the slides completely at room temperature, they were treated in an oven at 37 °C overnight to enhance the attachment of tissue to the slides. The sections were deparaffinized two times in xylene, 15 min per time, and rehydrated in 100%, 95%, 90%, 80%, 70%, 60% and 50% ethanol two times respectively, 3 sec per time, and were subsequently rinsed 10 min and washed in 0.01 M PBS (pH 7.4).
AGE
After treatment with H2O2 (3% in ethanol, room temperature, 15 min.) and proteinase K (100 µg/ml, 100 µl, 37 °C, 20 min.), the sections were incubated with 5% BSA-PBS buffer (room temperature, 30 min.) in order to block non-specific staining. Afterwards, the sections were incubated with the primary anti-AGE antibody (abcam, 1:100, diluted in 1% BSA-PBS), or normal mouse IgG (250 µg/ml) pre-treated with excessive CML (1:250, diluted in 1% BSA-PBS, negative control) over night at 4 °C. The sections were then washed and incubated with LINK (biotinylated anti-rabbit and anti-mouse immunoglobulin) and STREPTAVIDIN PEROXIDASE (streptavidin conjugated with horseradish peroxidase) respectively at room temperature for 10 min (both are part of reagents of LSAB2 System-HRP, products of Dako Company, Denmark). Then the peroxidase activity was visualized by incubating the sections in substrate working solution containing hydrogen peroxide and 3, 3′-diaminobenzidine tetrahydrochloride at room temperature for 5 min. The sections were rinsed for 10 min, counterstained with Mayer Haematoxylin for 1 min, treated in HCl-ethanol for 3 sec, and dehydrated in 80%, 90%, 95%, 100% ethanol for 3 sec, respectively. Then the slides were immersed in xylene for 15 min two times and mounted.
RAGE
The primary anti- RAGE antibody was produced in rabbits immunized with a synthetic peptide corresponding to a sequence at the N-terminal of human RAGE (Sigma). Only two amino acids are different from the related rat sequence. The sections were boiled in 10 mM citrate buffer (pH 6.0) 18 min for retrieving the antigen. Normal rat lungs were used as positive control since RAGE is highly expressed in the lungs59. The primary antibody was diluted (1:80) with 1% BSA-PBS and normal rabbit serum (diluted 1:60) pre-treated with excessive soluble RAGE was used as negative control. Other processes were similar to the AGE immunostaining.
TGF- β1, TGF- β1 receptor, BDNF and TrkB
The primary antibodies of TGF- β1, TGF- β1 receptor, BDNF and TrkB were obtained from Wuhan Boster Biological Engineering Co., Ltd. They were all diluted (1:100) with 1% BSA-PBS. The second antibody is HRP-Goat Anti-Rabbit IgG and was diluted (1:150) with 1% BSA-PBS. The sections were placed in 3% H2O2 (AR1108) at room temperature for 5-10 minutes to inactivate endogenous enzymes. The sections were then rinsed with distilled water for 3 times. The sections were immersed in 0.01 M citrate buffer (AR0024, PH6.0) or 0.02 M PBS (AR0030, PH7.2-7.6) and heated to boiling using electricity or microwave in order to retrieve the antigen. This process was repeated one or two times for an interval between 5-10 minutes. Then the slides were naturally cooled to room temperature. The sections were incubated with 5% BSA blocking solution (AR0004) (37 °C, 30 minutes) for blocking non-specific staining. Then the excess liquid was shanked off from the slides (don’t wash) and incubated with diluted primary antibody over night at 4 °C or 2 hours at 37 °C. The slides were rinsed with PBS (pH7.2-7.6) for 3 times (each time lasts 5 minutes). Then the slides were incubated with corresponding second antibodies (37 °C, 30 minutes). The slides were rinsed again for 3 times (each time lasts 5 minutes). The slides were then incubated with SABC (37 °C, 30 minutes) and washed for 4 times (each time lasts 5 minutes) with PBS (pH7.2-7.6). The peroxidase activity was subsequently visualized by incubating the sections with DAB visualized kit (AR1022, taking 1 drop from each of the A, B, C reagents and mixing into 1 ml of distilled water) for about 15 minutes at room temperature. The slides were washed with distilled water and counterstained with hematoxylin (AR0005). At last, the slides were dehydrated, transparent and mounted.
Image analysis
AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB are shown to be brown staining, but such color does not appear in the negative control slides, indicating that the staining is specific. To minimize errors, 6 to 10 photographs from different locations of the same layer in each slide were randomly taken, after that, different parts were saved as individual image files. The region of interest (ROI) was defined using Sigmascan Pro 4.0 image analysis software. The color due to 3,3′-diaminobenzidene staining was distinguished in the ROI using intensity thresholds. Finally, the images were exported as binary images. The total area and area fraction of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB positive staining were calculated by a Matlab program (Matlab 6.5, The MathWorks Inc. USA). Then the fraction of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB in mucosa, muscle and submucosa layers were computed as: Fraction of protein expressions = immuno-positive area/total measured area.
Correlation analysis
Single linear correlation analysis
As the hyperglycemia is the most common characteristic of diabetic rats, firstly we made linear regression analysis between blood glucose level with all other parameters including morphometry biomechanics and expressions of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB in different layers of colon wall. In order to analyze the expressions of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB with the parameters of morphmetry and biomechanics, the single linear regression analysis was done on the expressions of AGE, RAGE, TGF- β1, TGF- β1 receptor, BDNF and TrkB in different layers of the colon wall with body weight, wet weight per unit length of the colon, wet weight of colon to body weight ratio, wall thickness, wall cross-sectional area, opening angle, inner residual strain, outer residual strain, circumferential material constant a and longitudinal material constant a. Furthermore, the single linear regression analysis was also performed in order to examine the correlations between the expression of AGE, TGF-β1 and BDNF in different layers of colon wall with the expressions of their corresponding receptors, i.e., RAGE, TGF-β1 receptor and TrkB respectively.
Multiple linear correlation analysis
In order to determine the interrelation among AGE, RAGE, TGF- β, TGF- β receptor, BDNF and TrkB expressions, a multiple linear correlation analysis was done.
Statistical analysis
The data were representative of a normal distribution and accordingly the results were expressed as means ± SEM. Student’s t-test and analysis of variance (ANOVA) were used to detect differences between parameters and groups (Sigmastat 2.0TM). Linear regression analysis was used to demonstrate eventual association between AGE, RAGE, TGF- β, TGF- β receptor, BDNF and TrkB with histomorphometric and biomechanical parameters. The results were regarded as significant when P < 0.05.
Data availability statement
HS and JZ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
Zhao, J., Nakaguchi, T. & Gregersen, H. Biomechanical and histomorphometric colon remodelling in STZ-induced diabetic rats. Digestive diseases and sciences 54, 1636–1642, https://doi.org/10.1007/s10620-008-0540-3 (2009).
Zhao, M., Liao, D. & Zhao, J. Diabetes-induced mechanophysiological changes in the small intestine and colon. World journal of diabetes 8, 249–269, https://doi.org/10.4239/wjd.v8.i6.249 (2017).
Mendez, J. D., Xie, J., Aguilar-Hernandez, M. & Mendez-Valenzuela, V. Trends in advanced glycation end products research in diabetes mellitus and its complications. Molecular and cellular biochemistry 341, 33–41, https://doi.org/10.1007/s11010-010-0434-5 (2010).
Kay, A. M., Simpson, C. L. & Stewart, J. A. Jr. The Role ofAGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. Journal of diabetes research 2016, 6809703, https://doi.org/10.1155/2016/6809703 (2016).
Zhao, J., Chen, P. & Gregersen, H. Morpho-mechanical intestinal remodeling in type 2 diabetic GK rats–is it related to advanced glycation end product formation? Journal of biomechanics 46, 1128–1134, https://doi.org/10.1016/j.jbiomech.2013.01.010 (2013).
Qiao, Y. C. et al. Changes of transforming growth factor beta 1 in patients with type 2 diabetes and diabetic nephropathy: A PRISMA-compliant systematic review and meta-analysis. Medicine 96, e6583, https://doi.org/10.1097/md.0000000000006583 (2017).
Betts-Obregon, B. S. et al. TGFbeta induces BIGH3 expression and human retinal pericyte apoptosis: a novel pathway of diabetic retinopathy. Eye (London, England) 30, 1639–1647, https://doi.org/10.1038/eye.2016.179 (2016).
Liu, L. et al. TGF-beta1 gene polymorphism in association with diabetic retinopathy susceptibility: a systematic review and meta-analysis. PloS one 9, e94160, https://doi.org/10.1371/journal.pone.0094160 (2014).
Xu, X. et al. Blockade of TGF-beta-activated kinase 1 prevents advanced glycation end products-induced inflammatory response in macrophages. Cytokine 78, 62–68, https://doi.org/10.1016/j.cyto.2015.11.023 (2016).
Kim, Y. S. et al. Effects of Allium victorialis leaf extracts and its single compounds on aldose reductase, advanced glycation end products and TGF-beta1 expression in mesangial cells. BMC complementary and alternative medicine 13, 251, https://doi.org/10.1186/1472-6882-13-251 (2013).
D’Arpino, M. C., Fuchs, A. G., Sanchez, S. S. & Honore, S. M. Extracellular matrix remodeling and TGF-beta1/Smad signaling in diabetic colon mucosa. Cell biology international 42, 443–456, https://doi.org/10.1002/cbin.10916 (2018).
Skaper, S. D. Neurotrophic Factors: An Overview. Methods in molecular biology (Clifton, N.J.) 1727, 1–17, https://doi.org/10.1007/978-1-4939-7571-6_1 (2018).
Mitre, M., Mariga, A. & Chao, M. V. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clinical science (London, England: 1979) 131, 13–23, https://doi.org/10.1042/cs20160044 (2017).
Popova, N. K., Ilchibaeva, T. V. & Naumenko, V. S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry. Biokhimiia 82, 308–317, https://doi.org/10.1134/s0006297917030099 (2017).
Yan, Q. et al. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. The Journal of comparative neurology 378, 135–157 (1997).
Yamamoto, M., Sobue, G., Yamamoto, K., Terao, S. & Mitsuma, T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochemical research 21, 929–938 (1996).
Bathina, S. & Das, U. N. Brain-derived neurotrophic factor and its clinical implications. Archives of medical science: AMS 11, 1164–1178, https://doi.org/10.5114/aoms.2015.56342 (2015).
Liu, W. et al. Brain derived neurotrophic factor in newly diagnosed diabetes and prediabetes. Molecular and cellular endocrinology 429, 106–113, https://doi.org/10.1016/j.mce.2016.04.002 (2016).
Ola, M. S., Nawaz, M. I., El-Asrar, A. A., Abouammoh, M. & Alhomida, A. S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cellular and molecular neurobiology 33, 359–367, https://doi.org/10.1007/s10571-012-9901-8 (2013).
Li, B., Lang, N. & Cheng, Z. F. Serum Levels of Brain-Derived Neurotrophic Factor Are Associated with Diabetes Risk, Complications, and Obesity: a Cohort Study from Chinese Patients with Type 2 Diabetes. Molecular neurobiology 53, 5492–5499, https://doi.org/10.1007/s12035-015-9461-2 (2016).
Quan, X. et al. Brain-Derived Neurotrophic Factor Contributes to Colonic Hypermotility in a Chronic Stress Rat Model. Digestive diseases and sciences 60, 2316–2326, https://doi.org/10.1007/s10620-015-3695-8 (2015).
Sha, H., Zhao, Z. D., Liu, J., Zhen, G. F., Chen, Z., Tong, P. M. & Gregersen, X. L. H. Effect of Chang Run Tong on the Biomechanical and Morphometric Remodeling of Colon and Rectum in STZ Induced Diabetic Rats. J Med Biol Eng 33, 149–154 (2013).
Krishnan, B., Babu, S., Walker, J., Walker, A. B. & Pappachan, J. M. Gastrointestinal complications of diabetes mellitus. World journal of diabetes 4, 51–63, https://doi.org/10.4239/wjd.v4.i3.51 (2013).
Piper, M. S. & Saad, R. J. Diabetes Mellitus and the Colon. Current treatment options in gastroenterology 15, 460–474, https://doi.org/10.1007/s11938-017-0151-1 (2017).
Careyva, B. & Stello, B. Diabetes Mellitus: Management of Gastrointestinal Complications. American family physician 94, 980–986 (2016).
Deluyker, D., Evens, L. & Bito, V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: focus on high molecular weight AGEs. Amino acids. https://doi.org/10.1007/s00726-017-2464-8 (2017).
Chen, M., Curtis, T. M. & Stitt, A. W. Advanced glycation end products and diabetic retinopathy. Current medicinal chemistry 20, 3234–3240 (2013).
Kumar Pasupulati, A., Chitra, P. S. & Reddy, G. B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomolecular concepts 7, 293–309, https://doi.org/10.1515/bmc-2016-0021 (2016).
Bhor, V. M. & Sivakami, S. Regional variations in intestinal brush border membrane fluidity and function during diabetes and the role of oxidative stress and non-enzymatic glycation. Molecular and cellular biochemistry 252, 125–132 (2003).
Chen, P., Zhao, J. & Gregersen, H. Up-regulated expression of advanced glycation end-products and their receptor in the small intestine and colon of diabetic rats. Digestive diseases and sciences 57, 48–57, https://doi.org/10.1007/s10620-011-1951-0 (2012).
Chen, P. M., Gregersen, H. & Zhao, J. B. Advanced glycation end-product expression is upregulated in the gastrointestinal tract of type 2 diabetic rats. World journal of diabetes 6, 662–672, https://doi.org/10.4239/wjd.v6.i4.662 (2015).
Sims, T. J., Rasmussen, L. M., Oxlund, H. & Bailey, A. J. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 39, 946–951 (1996).
Grossin, N. et al. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Molecular nutrition & food research 59, 927–938, https://doi.org/10.1002/mnfr.201400643 (2015).
Bierhaus, A. et al. Understanding RAGE, the receptor for advanced glycation end products. Journal of molecular medicine (Berlin, Germany) 83, 876–886, https://doi.org/10.1007/s00109-005-0688-7 (2005).
Ajith, T. A. & Vinodkumar, P. Advanced Glycation End Products: Association with the Pathogenesis of Diseases and the Current Therapeutic Advances. Current clinical pharmacology 11, 118–127 (2016).
Yan, X., Xiong, X. & Chen, Y. G. Feedback regulation of TGF-beta signaling. Acta biochimica et biophysica Sinica, https://doi.org/10.1093/abbs/gmx129 (2017).
Kiritsi, D. & Nystrom, A. The role of TGFbeta in wound healing pathologies. Mechanisms of ageing and development, https://doi.org/10.1016/j.mad.2017.11.004 (2017).
Hocevar, B. A. & Howe, P. H. Analysis of TGF-beta-mediated synthesis of extracellular matrix components. Methods in molecular biology (Clifton, N.J.) 142, 55–65, https://doi.org/10.1385/1-59259-053-5:55 (2000).
Pfeiffer, A., Middelberg-Bisping, K., Drewes, C. & Schatz, H. Elevated plasma levels of transforming growth factor-beta 1 in NIDDM. Diabetes care 19, 1113–1117 (1996).
Zorena, K., Malinowska, E., Raczynska, D., Mysliwiec, M. & Raczynska, K. Serum concentrations of transforming growth factor-Beta 1 in predicting the occurrence of diabetic retinopathy in juvenile patients with type 1 diabetes mellitus. Journal of diabetes research 2013, 614908, https://doi.org/10.1155/2013/614908 (2013).
Paine, S. K. et al. Association of vascular endothelial growth factor, transforming growth factor beta, and interferon gamma gene polymorphisms with proliferative diabetic retinopathy in patients with type 2 diabetes. Molecular vision 18, 2749–2757 (2012).
Hussain, G., Rizvi, S. A., Singhal, S., Zubair, M. & Ahmad, J. Serum levels of TGF-beta1 in patients of diabetic peripheral neuropathy and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes & metabolic syndrome 10, S135–139, https://doi.org/10.1016/j.dsx.2015.10.011 (2016).
Tanigawa, T., Pai, R., Arakawa, T., Higuchi, K. & Tarnawski, A. S. TGF-beta signaling pathway: its role in gastrointestinal pathophysiology and modulation of ulcer healing. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society 56, 3–13 (2005).
Anitha, M. et al. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. American journal of physiology. Gastrointestinal and liver physiology 298, G375–383, https://doi.org/10.1152/ajpgi.00343.2009 (2010).
Honore, S. M., Zelarayan, L. C., Genta, S. B. & Sanchez, S. S. Neuronal loss and abnormal BMP/Smad signaling in the myenteric plexus of diabetic rats. Autonomic neuroscience: basic & clinical 164, 51–61, https://doi.org/10.1016/j.autneu.2011.06.003 (2011).
Fleenor, B. S., Marshall, K. D., Durrant, J. R., Lesniewski, L. A. & Seals, D. R. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. The Journal of physiology 588, 3971–3982, https://doi.org/10.1113/jphysiol.2010.194753 (2010).
Leipzig, N. D., Eleswarapu, S. V. & Athanasiou, K. A. The effects of TGF-beta1 and IGF-I on the biomechanics and cytoskeleton of single chondrocytes. Osteoarthritis and cartilage 14, 1227–1236, https://doi.org/10.1016/j.joca.2006.05.013 (2006).
Gonzalez, A., Moya-Alvarado, G., Gonzalez-Billaut, C. & Bronfman, F. C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton (Hoboken, N.J.) 73, 612–628, https://doi.org/10.1002/cm.21312 (2016).
Fujinami, A. et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clinical biochemistry 41, 812–817, https://doi.org/10.1016/j.clinbiochem.2008.03.003 (2008).
Krabbe, K. S. et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50, 431–438, https://doi.org/10.1007/s00125-006-0537-4 (2007).
He, M. & Wang, J. Decreased serum brain-derived neurotrophic factor in Chinese patients with Type 2 diabetes mellitus. Acta biochimica et biophysica Sinica 46, 426–427, https://doi.org/10.1093/abbs/gmu008 (2014).
Nitta, A. et al. Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicology and teratology 24, 695–701 (2002).
Liu, W., Yue, W. & Wu, R. Effects of diabetes on expression of glial fibrillary acidic protein and neurotrophins in rat colon. Autonomic neuroscience: basic & clinical 154, 79–83, https://doi.org/10.1016/j.autneu.2009.12.003 (2010).
Du, F., Wang, L., Qian, W. & Liu, S. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 21, 1229–e1114, https://doi.org/10.1111/j.1365-2982.2009.01379.x (2009).
Anitha, M. et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. The Journal of clinical investigation 116, 344–356, https://doi.org/10.1172/jci26295 (2006).
Brizzi, M. F. et al. RAGE- and TGF-beta receptor-mediated signals converge on STAT5 and p21waf to control cell-cycle progression of mesangial cells: a possible role in the development and progression of diabetic nephropathy. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 18, 1249–1251, https://doi.org/10.1096/fj.03-1053fje (2004).
Huang, K. P. et al. AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-beta1 expression in male rat glomerular mesangial cells. Endocrinology 156, 268–279, https://doi.org/10.1210/en.2014-1381 (2015).
Serban, A. I. et al. Extracellular matrix is modulated in advanced glycation end products milieu via a RAGE receptor dependent pathway boosted by transforming growth factor-beta1 RAGE. Journal of diabetes 7, 114–124, https://doi.org/10.1111/1753-0407.12154 (2015).
Wada, R. & Yagihashi, S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Annals of the New York Academy of Sciences 1043, 598–604, https://doi.org/10.1196/annals.1338.067 (2005).
Acknowledgements
The studies were financially supported by National Natural Science Foundation of China (No. 81173259/H2708) and Karen Elise Jensen foundation.
Author information
Authors and Affiliations
Contributions
H.S. performed experiments, made immunohistochemical staining, analyzed data and prepared manuscript. X.T. directed to do experiments, revised manuscript and provided part financial support (National Natural Science Foundation of China, No. 81173259/H2708). J.Z. designed and supervised the study, analyzed data, prepared and revised manuscript and figures, and provided part financial support (Karen Elise Jensen foundation).
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sha, H., Tong, X. & Zhao, J. Abnormal expressions of AGEs, TGF-β1, BDNF and their receptors in diabetic rat colon–Associations with colonic morphometric and biomechanical remodeling. Sci Rep 8, 9437 (2018). https://doi.org/10.1038/s41598-018-27787-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-018-27787-2
This article is cited by
-
Protective effect of acetylcysteine, histidine, and their combination against diabetes vascular complications in type-2 diabetic rats via reducing NF-kβ pathway signaling
Journal of Diabetes & Metabolic Disorders (2022)