Abstract
Few prospective studies have evaluated the relation between fat-soluble vitamins and glioma risk. Using three cohorts—UK Biobank (UKB), Nurses’ Health Study (NHS), and Health Professionals Follow-Up Study (HPFS), we investigated associations of pre-diagnostic concentrations of fat-soluble vitamins D, A, and E with incident glioma. In 346,785 participants (444 cases) in UKB, associations with vitamin D (25-hydroxyvitamin D [25(OH)D]) were evaluated by Cox proportional hazards regression. In NHS (52 cases, 104 controls) and HPFS (32 cases, 64 controls), associations with 25(OH)D, vitamin A (retinol), and vitamin E (α- and γ-tocopherol) were assessed using conditional logistic regression. Our results suggested plasma concentrations of 25(OH)D and retinol were not associated with glioma risk. Comparing the highest to lowest tertile, the multivariable hazard ratio (MVHR) for 25(OH)D was 0.87 (95% confidence interval [CI] 0.68–1.11) in UKB and the multivariable risk ratio (MVRR) was 0.97 (95% CI 0.51–1.85) in NHS and HPFS. In NHS and HPFS, the MVRR for the same comparison for retinol was 1.16 (95% CI 0.56–2.38). Nonsignificant associations were observed for α-tocopherol (MVRRtertile3vs1 = 0.61, 95% CI 0.29–1.32) and γ-tocopherol (MVRR tertile3vs1 = 1.30, 95% CI 0.63–2.69) that became stronger in 4-year lagged analyses. Further investigation is warranted on a potential association between α- and γ-tocopherol and glioma risk.
Similar content being viewed by others
Introduction
Gliomas are the most common malignant brain tumors in adults and arise from glial tissue1. Patients with these tumors generally have poor prognoses and survival rates2. In particular, glioblastoma (GBM), which accounts for more than half of all gliomas (~ 60%), has a 5-year relative survival of approximately 7%2.
The brain is comprised of a high content of polyunsaturated fatty acids (PUFAs) with lipids making up 50% of the total mass of neural membranes3, 4. Fat-soluble vitamins including vitamins D, A, and E, have been linked with other cancers5,6,7 and could play a role in the etiology of glioma. Vitamin D has receptors throughout the brain and has demonstrated anti-inflammatory and immunomodulation roles8. Circulating 25-hydroxyvitamin D (25(OH)D), the accepted measure of vitamin D status, captures exposure to all sources of vitamin D, including sunlight, diet, and supplements9, 10. Vitamin A (retinol) participates in numerous, diverse biological events to maintain tissue homeostasis11 and has been shown to inhibit the proliferation and migration of cells in primary cultures of human glioma12. Vitamin E (α- and γ-tocopherol) plays a pivotal role in preventing lipid peroxidation in brain cell membranes13, suggesting potential for a chemo-preventive role in glioma.
In the current study, we examined pre-diagnostic circulating concentrations of 25(OH)D, retinol, and α- and γ-tocopherol in relation to glioma development in three cohorts—UK Biobank (UKB), Nurses’ Health Study (NHS), and Health Professionals Follow-Up Study (HPFS).
Materials and methods
Study population and design
UK Biobank
UKB is a population-based cohort of 502,536 participants living in the United Kingdom (UK), who were aged 40–69 years at recruitment in 2006 to 2010. Participants were identified from National Health Service patient registries and completed questionnaires that included information on demographics, lifestyle, diet, and health and medical history/conditions. At study enrollment, the majority of participants also provided a 45 mL blood sample. Blood samples were collected and transported overnight at 4 °C to a central laboratory, where they were processed and stored as aliquots at − 80 °C14 until analyzed. Participants provided written consent at recruitment, and data were downloaded from UK Biobank Resource under approved protocol 16944.
Nurses’ Health Study and Health Professionals Follow-Up Study
NHS, established in 1976, included 121,701 female nurses aged 30–55 years15. HPFS began in 1986 and enrolled 51,529 male health professionals aged 40–75 years16. All participants completed baseline questionnaires on demographics, lifestyle factors, and medical and other health-related information. Both cohorts have been followed up biennially, with follow-up compliance exceeding 90%17. Blood samples were returned by 32,826 NHS participants from 1989–1990 and by 18,018 HPFS participants from 1993 to 1995, through overnight mail. On arrival at a centralized laboratory, the samples were centrifuged to separate plasma from the buffy coat and red cells and were stored in liquid nitrogen freezers until analyzed. Over 95 percent of samples arrived within 26 h of phlebotomy18, 19. Among participants with available blood samples, two controls were randomly selected for each glioma case via incidence-density sampling among participants who were still alive and free of cancer at the date of the case diagnosis20. Controls were individually matched to the cases on fasting status (fasting vs. non-fasting), year of birth (± 1 year; 2 years was used for 10 matched sets), month of blood collection (± 1 month), and race (white vs. non-white).
The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, as well as participating registries (as required).
Identification of glioma cases
In UKB, participants diagnosed with cancer were identified through linkage to the UK National Health Service Central Register. Glioma cases were those with primary intracranial gliomas (ICD10: C71) and included both GBMs (9440–9441) and lower grade glioma subtypes (non-GBM) (9380–9382, 9400–9401, 9411, 9442, 9450–9451).
In NHS and HPFS, glioma cases were identified initially through self-report with additional cases confirmed by vital status or medical review post-death. Written consent for medical record review was collected from participants or next of kin. Data on tumor subtype (GBM and non-GBM as described above for UKB) was extracted directly from medical records for all cases.
Laboratory assays
In UKB, serum circulating 25(OH)D was measured in all baseline blood samples by UKB investigators using the DiaSorin Liaison XL immunoassay (http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf). The coefficients of variation (CV) for 25(OH)D among high, medium and low sample quality control concentrations were 5%, 5% and 6%, respectively. Data for retinol (vitamin A) and α- and γ-tocopherol (vitamin E) were not available in UKB.
In NHS and HPFS, plasma circulating 25(OH)D2 and 25(OH)D3, retinol, and α- and γ-tocopherol were measured by liquid chromatography-tandem mass spectrometry at Bevital AS (http://bevital.no, Bergen, Norway)21. We calculated total plasma circulating 25(OH)D by summing plasma 25(OH)D2 and 25(OH)D3. Based on blinded quality control samples, in NHS and HPFS, mean intra-assay CVs were 7%, 6% for 25(OH)D, 4%, 4% for retinol, 10%, 10% for α-tocopherol, and 9%, 6% for γ-tocopherol, respectively. As retinol and α- and γ-tocopherol concentrations are correlated with total cholesterol22, we considered associations with adjustment for total cholesterol concentrations, which were directly measured by the investigators using an enzymatic assay (Thermo Scientific, Waltham, Massachusetts, United States). CVs for cholesterol were 11% in NHS and 4% in HPFS.
Statistical analysis
Statistical analyses were performed independently for UKB and NHS/HPFS, as different study designs were implemented and only 25(OH)D was measured in all three studies. In addition, 25(OH)D was measured using different methodologies in UKB compared to NHS and HPFS, and 25(OH)D concentrations have been shown to differ across assay methods23. In UKB, we excluded participants with a history of cancer at baseline, those genetically related, and those without a 25(OH)D measurement, leaving 346,812 participants and 444 incident glioma cases in the final analysis. In NHS and HPFS, we included 84 cases (52 from NHS and 32 from HPFS) and 168 controls (104 from NHS and 64 from HPFS) in the final analysis.
To adjust for seasonal variation in each study, 25(OH)D was regressed on the week of blood draw in the full cohort in the UKB and the control participants in NHS/HPFS using sine–cosine functions24. In each study, a season-standardized value was calculated for each participant by adding their residual (the difference between their observed concentration and predicted concentration from the regression model) to the predicted average 25(OH)D concentration over the entire year from the regression model in that study24. The 25(OH)D concentrations were categorized in two ways—tertiles using cutoffs from the full cohort for UKB and from control participants in NHS/HPFS, and cut-points based on the Institutes of Medicine (IOM) guidelines for bone health: < 50 nmol/L (deficiency/insufficiency), 50 to < 75 nmol/L (sufficiency), and ≥ 75 nmol/L (above sufficiency)25. Tests for linear trend were obtained by assigning the median value of a category and then modeling those values as a continuous variable.
In UKB, Cox proportional hazards regression was used to calculate multivariable hazard ratios (MVHRs) and 95% confidence intervals (CIs) for the associations between 25(OH)D and glioma risk. Follow-up consisted of the time from enrollment to cancer diagnosis, death, or last linkage to the National Health Service (October 31, 2015), whichever came first. Multivariable models adjusted for the matching factors used in NHS and HPFS, including age (continuous), fasting status (fasting vs. non-fasting), race (white, non-white), month of blood draw (continuous), and sex (men, women), as well as body mass index (BMI, continuous) and smoking status (never, past, current smoker). Analyses were performed for all glioma cases and separately for GBM and non-GBM subtypes. In the GBM analysis, non-GBM cases were censored at the date of diagnosis and vice versa. Analyses were performed using the “SURVIVAL” package in R version 3.5.0 (Vienna, Austria).
NHS and HPFS, unless otherwise specified, were analyzed as a combined dataset using conditional logistic regression to estimate multivariable risk ratios (MVRR) for the associations between 25(OH)D, retinol, and α- and γ-tocopherol and glioma risk. The distribution in the control participants was used to assign tertile cutpoints for each vitamin. In addition to being conditioned on matching factors, multivariable models were additionally adjusted for BMI (continuous) and smoking status (never, past, current smoker) reported on the questionnaire completed prior to blood draw. For retinol and α- and γ-tocopherol, we also adjusted for plasma total cholesterol (continuous). Analyses were performed for all glioma and by subtype. Among the controls, Spearman correlation coefficients (r) were calculated between the four fat-soluble vitamins and cholesterol in the control participants. Analyses were performed using the SAS statistical package, version 9.4 for UNIX (SAS Institute, Cary, NC).
In all three cohorts, sensitivity analyses were conducted by sex, excluding current smokers, and excluding the cases diagnosed within four years of blood collection. All statistical tests were two-sided with P values < 0.05 considered statistically significant. All methods were carried out in accordance with relevant guidelines and regulations.
Results
In UKB, average follow-up time was 6.7 (SD: 0.9) years; 444 glioma cases (330 GBM and 114 non-GBM) were identfied. In NHS and HPFS combined, 84 glioma cases (54 GBM and 30 non-GBM) cases were diagnosed, on average, 8.6 (SD: 4.3) years after blood collection. The mean (SD) age at blood collection was 55.7 (8.1) years in UKB, and 60.2 (7.9) years in NHS and HPFS. The NHS and HPFS had a higher proportion of multivitamin users than the UKB (38.1% vs. 17.5%). Only a small proportion of participants were current smokers (UKB: 10.2%, NHS and HPFS: 7.1%); the proportion of never smokers was slightly higher in the UKB (56.1%) than NHS and HPFS (52.4%) (Table 1). Irrespective of source cohort, women and men shared similar characteristics except participants in NHS were younger than those in HPFS (Supplementary Table S1). In the UKB, participants who were diagnosed with glioma during follow-up tended to be older at study enrollment and were less likely to be women compared to the overall study population. By design, in the NHS and HPFS, age and sex were comparable in glioma cases and controls. In the UKB, glioma cases were less likely to be never smokers at study enrollment compared to the entire study population. In NHS and HPFS, glioma cases were also less likely to be never smokers than controls.
25(OH)D
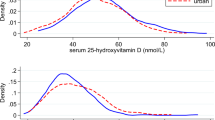
Mean (SD) season-standardized 25(OH)D concentrations were 48.3 (21.0) nmol/L for the UKB cohort and 68.3 (20.1) nmol/L for the controls in the NHS and HPFS studies combined (note: the 25(OH)D concentrations were assessed using different assays and may yield non-comparable results26). For UKB, participants in the highest compared with the lowest tertile of 25(OH)D had a nonsignificant 13% lower risk of glioma (MVHR = 0.87, 95% CI 0.68–1.11) and 6% lower risk of GBM (MVHR = 0.94, 95% CI 0.71–1.25). In NHS and HPFS, the MVRR for the same comparison was 0.97 (95% CI 0.51–1.85) for glioma (Table 2). Likewise, no significant associations were observed in UKB or NHS/HPFS when 25(OH)D was modeled using categories based on IOM guidelines for bone health or continuously (Table 2). Results were similar for women and men (Supplementary Table S2), and after excluding current smokers or the cases diagnosed within four years of blood collection (Supplementary Table S3).
Retinol and α- and γ-tocopherol
In NHS and HPFS, among the controls, the mean (SD) concentration was 2.2 (0.5) μmol/L for retinol and 197 (32) mg/dL for cholesterol. We observed a modest correlation among the controls between retinol and total cholesterol (Spearman r: 0.42 in NHS and 0.39 in HPFS) (Supplementary Table S4). Overall, we observed no significant associations for circulating retinol. The MVRRs were 1.16 (95% CI 0.56–2.38) comparing the highest vs lowest tertile and 1.05 (95% CI 0.50–2.21) per SD increment (0.5 μmol/L) in retinol concentration (Table 3). We observed similar results for women and men (Supplementary Table S5), and after excluding current smokers and the matched pairs where the case was diagnosed within four years of blood collection (Supplementary Table S6).
In NHS and HPFS, among the controls, the mean (SD) concentration was 44.9 (19.4) μmol/L for α-tocopherol and 5.8 (3.0) μmol/L for γ-tocopherol. For the controls, we observed a stronger correlation between α-tocopherol and total cholesterol (Spearman r: 0.55 in NHS and 0.41 in HPFS) than between γ-tocopherol and total cholesterol (0.24 in NHS and 0.31 in HPFS) (Supplementary Table S4). Compared with the lowest tertile, participants in the highest tertile of α-tocopherol had a 39% lower glioma risk (MVRR = 0.61, 95% CI 0.29–1.32), whereas those in the highest tertile of γ-tocopherol had a 30% elevated glioma risk (MVRR = 1.30, 95% CI 0.63–2.69), although neither association was statistically significant. Results from the continuous analyses generally aligned with the tertile results, with the findings for γ-tocopherol being marginally significant (per SD increment [3.0 μmol/L]: MVRR = 1.31, 95% CI 0.99–2.00) (Table 3). Associations were similar when mutually adjusting α- and γ- tocopherol (data not shown). When cross-classifying by the median concentration of the two tocopherols, we found a 40% reduced risk in the higher α- and lower γ-tocopherol group compared with the lower α- and lower γ-tocopherol group (Supplementary Table S7). Results were similar for women and men (Supplementary Table S5) and after excluding current smokers (Supplementary Table S6). Stronger associations were observed after excluding cases (and their matched controls) diagnosed within the first four years after blood collection (MVRRtertile 3vs.1: 0.46 for α-tocopherol and 1.42 for γ-tocopherol) though estimates remained nonsignificant (Supplementary Table S6). Given the association between γ-tocopherol and GBM was different from that for total glioma, we analyzed its association with non-GBM and found a twofold higher risk per SD increment in γ-tocopherol (MVRR = 2.10, 95% CI 1.12–3.93, n = 30 cases).
Discussion
In three prospective cohorts (UKB from the UK, and NHS and HPFS from the US), statistically significant associations were not observed between circulating 25(OH)D and risk of glioma. In two of these cohorts, NHS and HPFS, associations with retinol and α-and γ-tocopherol were also investigated and were not significantly associated with glioma risk. However, the associations for α-tocopherol and γ-tocopherol were in opposite directions (inverse for α-tocopherol and positive for γ-tocopherol).
Most studies investigating potential roles of fat-soluble vitamins in glioma development have examined associations based on self-reported dietary intake. However, fat-soluble vitamin status in particular27, can be affected by numerous factors including bioavailability and specific intrinsic and extrinsic factors, such as food content, health, and genetic characteristics of the population in question28, 29. Moreover, dietary assessment of fat-soluble vitamin status can be confounded by other methodological issues. For instance, depending on the time of year, sun-induced vitamin D synthesis in the skin may be another major source of vitamin D. In addition, vitamin E intake typically does not reflect actual levels of various tocopherol isoforms in the body because γ-tocopherol is disproportionately metabolized and excreted30. Hence, dietary assessment studies could yield different nutrient-disease associations than studies investigating circulating levels.
There is a paucity of studies investigating vitamin D and glioma risk, and results are inconsistent. In an ecological study, the prevalence of brain cancer was elevated in the southern region of the US31. Although this geographical variation could be related to different distributions of genetic and occupational risk factors, it is plausible that geographic differences related to sunlight exposure and vitamin D synthesis could also contribute to the differences in glioma prevalence by region32. A nested case–control study from the Janus Serum Bank in Norway33 found no overall association between vitamin D status and glioma risk (ORquintile5vs.1 = 1.04, 95% CI 0.73, 1.47). Although the authors reported nonsignificant associations in opposite directions in younger (n = 210 cases) and older (n = 185 cases) male participants, the same pattern was not observed in the current study (data not shown). Consistent with a recent Mendelian randomization study that found no evidence for a causal relationship between 25(OH)D and glioma risk34, the current analysis, which used directly measured 25(OH)D, also found an overall null association between 25(OH)D and glioma risk.
Vitamin A has been shown to inhibit proliferation and induce differentiation in various cell types mainly through binding to the nuclear retinoic acid receptors (α, β, γ), which are transcriptional and homeostatic regulators whose functions often compromised early in neoplastic transformation11, 12. Therefore, vitamin A has been hypothesized to lower risk of glioma. Intake studies of vitamin A have shown inconsistent results. A previous meta-analysis of eight case–control studies suggested a significant inverse association between vitamin A intake and glioma risk35. However, there was statistically significant heterogeneity in the study-specific results, as well as the potential for dietary recall and selection bias. A null association between serum retinol and brain cancer risk was observed in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study (HRquintile5vs.1 = 1.03, 95% CI 0.47, 2.25, n = 78 cases)6. In NHS and HPFS, we also observed a weak, nonsignificant association for retinol and glioma risk. Failure to identify an association in our study, as well as the ATBC study, may be attributed to small sample size or the tight regulation of circulating retinol36.
In the present study, we observed a nonsignificant inverse association between circulating α-tocopherol, but a nonsignificant positive association for γ-tocopherol, with risk of glioma. These findings are in-line with some37, 38, but not all prior studies39. In a prospective nested case–control study from ATBC Study with 64 glioma cases, circulating α-tocopherol was inversely associated with glioma risk (for a one SD increment, OR 0.65, 95% CI 0.44–0.96); γ-tocopherol was not investigated38. Further, a case–control study of 34 GBM cases also reported that prediagnostic α-tocopherol, but not γ-tocopherol, was inversely associated with glioma risk37. However, in the Janus Serum Bank study of 110 GBM cases, high α- and γ-tocopherol concentrations were both positively associated with risk of GBM, and the associations were stronger based on samples collected at least 10 years prior to diagnosis39. Although we were unable to apply such a long lag due to our limited sample size, we noted a stronger though still nonsignificant positive association for γ-tocopherol in a 4-year lagged analysis, supporting the possibility of a potentially adverse influence of γ-tocopherol on glioma risk. Results from vitamin E dietary intake studies are not consistent with those utilizing biomarkers. A meta-analysis of 12 studies including 3180 glioma cases found that intake of vitamin E from foods or foods and supplements was not associated with risk of glioma40. Dietary recall or selection bias issues may have contributed to the different findings, as the meta-analysis mainly included retrospective studies; only one of the studies was prospective in design. Among vitamin E intervention trials41,42,43,44,45,46,47,48,49,50,51,52, only one study has reported results for cancers of the central nervous system with equivalent numbers of cases observed in the treatment (11 cases) and placebo (8 cases) arm; glioma was not reported separately48.
Strengths of the current study include the prospective design and availability of pre-diagnostic blood samples, which reduced the possibility of established glioma affecting the measured concentrations of circulating fat-soluble vitamins. In addition, the entire UKB cohort had circulating 25(OH)D measurements, allowing for robust evaluation of potential associations with glioma risk. Although results cannot be directly compared in the UKB and NHS/HPFS studies due to the use of different 25(OH)D assessment methodologies, that could lead to differences in reported 25(OH)D concentrations26, we note that neither study supported a role for vitamin D in glioma development. The primary limitations of this study include the small sample size, particularly for the analyses of retinol and α- and γ-tocopherol (84 cases), and use of a single blood sample for each participant. However, in plasma samples collected at two time points 1–2 years apart in NHS, we have shown high reproducibility for 25OH-D (intraclass correlation coefficients [ICC] = 0.71), retinol (ICC = 0.87), and α-tocopherol (ICC = 0.86); γ-tocopherol was not assessed in that study53. In addition, the population was mainly Caucasian with limited ethnic diversity. The non-statistically significant inverse association observed for α-tocopherol and non-statistically significant positive association observed for γ-tocopherol require further examination in larger, more ethnically heterogeneous cohorts.
In conclusion, our results suggest circulating 25(OH)D (vitamin D) and retinol (vitamin A) are not associated with glioma risk. Further investigation is however warranted to evaluate the potential associations we observed between vitamin E isoforms, α- and γ-tocopherol, and glioma risk.
Data availability
The work is based on the UK Biobank Resource under application number 16944. For the NHS and HPFS cohort data, we provide access through an NIH approved data enclave. Instructions for how to access the data are publicly available through their respective cohort websites.
References
Ostrom, Q. T. et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 21, 1357–1375 (2019).
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 21, v1–v100. https://doi.org/10.1093/neuonc/noz150 (2019).
Bourre, J. Diet, brain lipids, and brain functions: Polyunsaturated fatty acids, mainly omega-3 fatty acids. In Handbook of Neurochemistry and Molecular Neurobiology (ed. Lajtha, A.) 409–441 (Springer, 2009).
Owada, Y. Fatty acid binding protein: Localization and functional significance in the brain. Tohoku J. Exp. Med. 214, 213–220 (2008).
Grant, W. B. Review of recent advances in understanding the role of vitamin D in reducing cancer risk: Breast, colorectal, prostate, and overall cancer. Anticancer Res. 40, 491–499 (2020).
Hada, M., Mondul, A. M., Weinstein, S. J. & Albanes, D. Serum retinol and risk of overall and site-specific cancer in the ATBC study. Am. J. Epidemiol. 189, 532–542 (2020).
Abraham, A., Kattoor, A. J., Saldeen, T. & Mehta, J. L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 59, 2831–2838 (2019).
de Abreu, D. F., Eyles, D. & Feron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34, S265–S277 (2009).
Hollis, B. W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 135, 317–322 (2005).
Hollis, B. W. & Wagner, C. L. Normal serum vitamin D levels. N. Engl. J. Med. 352, 515–516. https://doi.org/10.1056/nejm200502033520521 (2005).
Connolly, R. M., Nguyen, N. K. & Sukumar, S. Molecular pathways: Current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin. Cancer Res. 19, 1651–1659. https://doi.org/10.1158/1078-0432.Ccr-12-3175 (2013).
Bouterfa, H. et al. Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery 46, 419–430. https://doi.org/10.1097/00006123-200002000-00029 (2000).
Mocchegiani, E. et al. Vitamin E–gene interactions in aging and inflammatory age-related diseases: Implications for treatment. A systematic review. Ageing Res. Rev. 14, 81–101 (2014).
Elliott, P. & Peakman, T. C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 37, 234–244. https://doi.org/10.1093/ije/dym276 (2008).
Colditz, G. A. & Hankinson, S. E. The Nurses’ Health study: Lifestyle and health among women. Nat. Rev. Cancer 5, 388–396. https://doi.org/10.1038/nrc1608 (2005).
Wolpin, B. M. et al. ABO blood group and the risk of pancreatic cancer. J. Natl. Cancer Inst. 101, 424–431. https://doi.org/10.1093/jnci/djp020 (2009).
Smith-Warner, S. A. et al. Methods for pooling results of epidemiologic studies: The pooling project of prospective studies of diet and cancer. Am. J. Epidemiol. 163, 1053–1064. https://doi.org/10.1093/aje/kwj127 (2006).
Hankinson, S. E. et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J. Natl. Cancer Inst. 87, 1297–1302 (1995).
Wei, E. K. et al. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol. Prev. Biomark. 15, 750–755 (2006).
Rothmann, K., Greenland, S. & Lash, T. Modern Epidemiology (Lippincott-Raven, 1998).
Midttun, Ø. & Ueland, P. M. Determination of vitamins A, D and E in a small volume of human plasma by a high-throughput method based on liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 25, 1942–1948 (2011).
Huff, T. & Jialal, I. Physiology, Cholesterol (2019).
Sempos, C. T., Vesper, H. W., Phinney, K. W., Thienpont, L. M. & Coates, P. M. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand. J. Clin. Lab. Investig. Suppl. 243, 32–40. https://doi.org/10.3109/00365513.2012.681935 (2012).
Bliss, C. I. Periodic Regression in Biology and Climatology (Connecticut Agricultural Experiment Station, 1958).
Bogazzi, F. et al. Vitamin D status may contribute to serum insulin-like growth factor I concentrations in healthy subjects. J. Endocrinol. Investig. 34, e200–e203 (2011).
Altieri, B. et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 74, 1–17 (2020).
Picó, C., Serra, F., Rodríguez, A. M., Keijer, J. & Palou, A. Biomarkers of nutrition and health: New tools for new approaches. Nutrients 11, 1092. https://doi.org/10.3390/nu11051092 (2019).
Moran, N. E., Mohn, E. S., Hason, N., Erdman, J. W. Jr. & Johnson, E. J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 9, 465–492. https://doi.org/10.1093/advances/nmy025 (2018).
Haskell, M. J. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion—Evidence in humans. Am. J. Clin. Nutr. 96, 1193S-1203S (2012).
Brigelius-Flohe, R. & Traber, M. G. Vitamin E: Function and metabolism. FASEB J. 13, 1145–1155 (1999).
Fang, Z., Kulldorff, M. & Gregorio, D. I. Brain cancer mortality in the United States, 1986 to 1995: A geographic analysis. Neuro Oncol. 6, 179–187. https://doi.org/10.1215/s1152851703000450 (2004).
Jacques, P. F. et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am. J. Clin. Nutr. 66, 929–936. https://doi.org/10.1093/ajcn/66.4.929 (1997).
Zigmont, V. et al. Association between prediagnostic serum 25-hydroxyvitamin D concentration and glioma. Nutr. Cancer 67, 1120–1130 (2015).
Takahashi, H. et al. Mendelian randomisation study of the relationship between vitamin D and risk of glioma. Sci. Rep. 8, 1–8 (2018).
Lv, W., Zhong, X., Xu, L. & Han, W. Association between dietary vitamin A intake and the risk of glioma: Evidence from a meta-analysis. Nutrients 7, 8897–8904. https://doi.org/10.3390/nu7115438 (2015).
Thurnham, D. I. & Northrop-Clewes, C. A. Optimal nutrition: Vitamin A and the carotenoids. Proc. Nutr. Soc. 58, 449–457 (1999).
Schwartzbaum, J. A. & Cornwell, D. G. Oxidant stress and glioblastoma multiforme risk: Serum antioxidants, γ-glutamyl transpeptidase, and ferritin. Nutr. Cancer 38, 40–49 (2000).
Huang, J. et al. A prospective study of serum metabolites and glioma risk. Oncotarget 8, 70366–70377. https://doi.org/10.18632/oncotarget.19705 (2017).
Björkblom, B. et al. Metabolomic screening of pre-diagnostic serum samples identifies association between α- and γ-tocopherols and glioblastoma risk. Oncotarget 7, 37043–37053. https://doi.org/10.18632/oncotarget.9242 (2016).
Qin, S., Wang, M., Zhang, T. & Zhang, S. Vitamin E intake is not associated with glioma risk: Evidence from a meta-analysis. Neuroepidemiology 43, 253–258. https://doi.org/10.1159/000369345 (2014).
Virtamo, J. et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: A postintervention follow-up. JAMA 290, 476–485 (2003).
Virtamo, J. et al. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-Year postintervention follow-up of the alpha-tocopherol, beta-carotene cancer prevention study. Int. J. Cancer 135, 178–185 (2014).
Klein, E. A. et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 306, 1549–1556. https://doi.org/10.1001/jama.2011.1437 (2011).
Lee, I. M. et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 294, 56–65. https://doi.org/10.1001/jama.294.1.56 (2005).
Serebriiskii, I. G. et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat. Commun. 10, 1–12 (2019).
Wang, L. et al. Vitamin E and C supplementation and risk of cancer in men: Posttrial follow-up in the Physicians’ Health Study II randomized trial. Am. J. Clin. Nutr. 100, 915–923. https://doi.org/10.3945/ajcn.114.085480 (2014).
Li, J. Y. et al. Nutrition intervention trials in Linxian, China: Multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J. Natl. Cancer Inst. 85, 1492–1498. https://doi.org/10.1093/jnci/85.18.1492 (1993).
Heart Protection Study Collaborative Group. MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 360, 23–33. https://doi.org/10.1016/s0140-6736(02)09328-5 (2002).
Hercberg, S. et al. The SU.VI.MAX study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 164, 2335–2342. https://doi.org/10.1001/archinte.164.21.2335 (2004).
Hercberg, S. et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: A postintervention follow-up in the SU.VI.MAX Study. Int. J. Cancer 127, 1875–1881. https://doi.org/10.1002/ijc.25201 (2010).
GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 354, 447–455 (1999).
de Gaetano, G. Low-dose aspirin and vitamin E in people at cardiovascular risk: A randomised trial in general practice Collaborative Group of the Primary Prevention Project. Lancet 357, 89–95. https://doi.org/10.1016/s0140-6736(00)03539-x (2001).
Midttun, Ø. et al. Most blood biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within-person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J. Nutr. 144, 784–790 (2014).
Acknowledgements
This project was supported in part by the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292), the Nutrition Round Table of the Harvard T.H. Chan School of Public Health, and National Institutes of Health (NIH) PO1 CA87969, U01 CA167552, UM1 CA186107, UM1 CA176726, UM1 CA167552, F30 CA235791. We also would like to thank the participants and staff of the Nurses’ Health Study and Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: K.M.E. and S.S.W. Acquisition or analysis of data: Y.Y., J.H.C., D.J.C., M.J.S., M.W., Ø.M., A.M., P.M.U., J.F., K.M.E. and S.S.W. Drafted manuscript: Y.Y. and J.H.C. Provided critical input on interpretation and final manuscript: Y.Y., J.H.C., D.J.C., M.J.S., M.W., Ø.M., A.M., P.M.U., J.F., K.M.E. and S.S.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yue, Y., Creed, J.H., Cote, D.J. et al. Pre-diagnostic circulating concentrations of fat-soluble vitamins and risk of glioma in three cohort studies. Sci Rep 11, 9318 (2021). https://doi.org/10.1038/s41598-021-88485-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-88485-0
This article is cited by
-
Antioxidants in brain tumors: current therapeutic significance and future prospects
Molecular Cancer (2022)