Abstract
The purpose of this prospective case–control study was to assess whether parameters of retinal and choriocapillaris perfusion are altered in patients with peripheral arterial disease (PAD). Patients with PAD and healthy controls were imaged with swept-source optical coherence tomography angiography (OCT-A). Macula centered 3 × 3 mm OCT-A scans were acquired, binarized and perfusion was evaluated for vessel density (VD) and choriocapillaris non-perfused area. Clinical examination and non-invasive assessment included Fontaine staging, ankle-brachial-pressure-index (ABI) and vascular color-coded Doppler sonography. Fifty-two patients with PAD and 23 healthy controls were included. Superficial retinal VD was reduced in patients compared to controls (difference = − 0.013, p = 0.02), decreased with higher Fontaine stage (p = 0.01) and correlated with ABI (r = 0.42, p < 0.0001, 95% confidence interval [CI] 0.23–0.58). Choriocapillaris non-perfused area was larger in patients compared to controls (difference = 3.64%, p = 0.002, 95% CI 1.38–5.90%) and significantly correlated with ABI (r = − 0.22, p = 0.03, 95% CI − 0.40– − 0.03). Multivariate multiple regression analysis revealed a significant association of all OCT-A parameters with ABI and of deep retinal vessel density and choriocapillaris non-perfused area with Fontaine stage. In this first study of retinal and choroidal perfusion in patients with PAD we found both retinal and choroidal perfusion to be significantly impaired. OCT-A parameters could aid as indirect imaging biomarkers for non-invasive PAD staging and monitoring.
Similar content being viewed by others
Introduction
Over 200 million people worldwide are affected by peripheral arterial disease (PAD), which causes excess morbidity by cardiovascular events like stroke and myocardial infarction1,2. Patients with cardinal symptoms like intermittent claudication have a 2.6 higher 10-year-mortality and even asymptomatic patients’ mortality risk is twice as high compared with unaffected age-matched peers3,4. The only available causal therapy is addressing risk factors like smoking, hypercholesterolemia or hypertension, which is why early PAD diagnosis is important5,6. Current diagnostic imaging techniques include color-coded Doppler sonography, magnet resonance angiography, computed tomography angiography and catheter based intra-arterial digital subtraction angiography7. However, their resolution is limited to non-capillary vessels, they are invasive and have a number of additional limitations8. Thus there is a lack of non-invasive, reliable and objective biomarkers for monitoring of disease progression, both in the clinical setting, as well as for use in clinical studies.
Ocular biomarkers, largely based on measurements of retinal vasculature, have previously been shown to allow for cardiovascular risk stratification and prediction of risk for strokes and heart failure9,10,11,12,13,14. Recently retinal microvascular abnormalities on color fundus photographs were shown to be strongly associated with the incidence of PAD15. However, resolution of fundus photography is low and it is inherently limited as it is a two-dimensional imaging modality imaging a three-dimensional vascular network.
Optical coherence tomography angiography (OCT-A) is a reliable, high-resolution, non-invasive and three-dimensional imaging modality based on optical interference which enables microvascular visualization stratified for specific retinal and choroidal layers16,17. OCT-A images are generated by repeated scans of the same structure detecting motion, i.e. blood flow. A previous study using OCT-A in acute coronary syndrome found both perfusion density and vessel density to be decreased as well as a correlation of these ocular vascular parameters to cardiovascular risk scores12. However, so far no studies have assessed the value of ocular OCT-A as an indirect imaging biomarker in PAD.
In this study we used OCT-A to assess ocular microvascular involvement in PAD as it might provide an easily obtainable and reproducible indirect PAD imaging biomarker in the future.
Methods
Subject recruitment
In this exploratory case–control study we recruited patients with PAD from the outpatient-clinic at the Department of Angiology and healthy age-matched controls at the outpatient-clinic of the Department of Ophthalmology at the University Hospital Bonn, Germany. Approval was obtained from the ethics committee of the University Hospital Bonn (approval ID 047/18) and informed consent was obtained from all study participants. The study was conducted according to the tenets of the Declaration of Helsinki. Inclusion criteria were a diagnosis of PAD based on clinical staging according to Fontaine and characteristic changes of the arteries of the lower extremities in color-coded Doppler sonography or computer tomography angiography or pathologic ankle-brachial-pressure-index (ABI) according to the current classification of PAD18. We excluded all patients with diabetes mellitus (as this is known to have a profound effect on OCT-A parameters19) or any current ocular symptoms, a history of any ocular surgery (except cataract surgery) or a history of any ocular diseases, corneal, lens or vitreous opacities, poor image quality on OCT-A (signal-strength-index < 8 was excluded) or investigator-determined reduced image quality (in terms of image artifacts, distinguishability of the foveal avascular zone and vessel continuity). Every scan has been graded by two graders (MWMW and PF).
Angiologic examination and past medical history
ABI (the ratio between the systolic pressure in the lower and upper extremities20 was measured with Doppler-technique as recommended from the American Heart Association of the dorsal pedal and the posterior tibial artery21. All measurements were performed with the use of appropriately sized pneumatic cuffs for both the ankle and the arm. The systolic ankle pressures were recorded with a handheld 5 MHz bi-directional pocket Doppler instrument by continuous wave velocity detection (Bidop ES-100V3, HADECO, Kawasaki, Japan). The lowest ABI from all extremities was used for further analysis, as it has a higher sensitivity for detection of PAD and for estimating prognosis22,23. ABI < 0.9 was classified as PAD with intima calcification and ABI > 1.3 as PAD with media calcification, given high positive predictive values of these thresholds21,24. Considering that the Fontaine stage can decrease by local (i.e. lower extremities) surgery, the worst ever Fontaine stage (FS; stage I: defined as asymptomatic or effort pain; stage IIA/IIB: pain starting over a walking distance of > 200 m / < 200 m; stage III: pain in rest; stage IV: trophic lesions in the lower extremities18,25) was determined by a standardized self-report of possible walking distance26. Participants also self-reported medical history including risk factors such as smoking, arterial hypertension, hypercholesterolemia, diabetes mellitus and history of cardiovascular events. In all control-participants, the peripheral pulses of the lower extremities were detectable. Any reported PAD symptoms were an exclusion criteria for the controls.
Ocular image acquisition and analyses
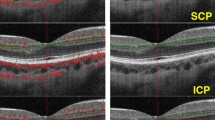
Optical coherence tomography is based on back-reflected light and generates structural images of the different retinal and choroidal layers. OCT-A has been developed for a no-injection, dye-free method for visualization of retinal and choroidal perfusion16. This technique detects blood flow from static tissue by analyzing the change in optical coherence tomography signal caused by the blood cells’ motion (Doppler shift and speckle variance/decorrelation) and allows for high-resolution visualization of perfusion stratified for different anatomical retinal and choroidal layers16. We imaged the study participants with swept-source OCT-A with 100.000 A-Scans/second (Zeiss PLEX Elite 9000; Carl Zeiss Meditec, Dublin, California, USA). A 3 × 3 mm scan of the macula was performed. Every 3 × 3 mm scan consisted of 300 A-scans per B-scan and four repetitions at 300 B-scans. Every A-line was acquired over a depth of 3 mm and contained 300 × 300 pixel (10 µm/pixel). OCT-A images were generated using an optical micro-angiography (OMAG) algorithm. Using the proprietary algorithm from the Zeiss OCT-A system, we analyzed the superficial (spanning from the inner limiting membrane to the inner plexiform layer) and the deep (spanning from the inner plexiform layer to the outer plexiform layer) retinal layers and the choriocapillaris (spanning from 29 to 49 µm below the retinal pigment epithelium). Before analyses, the deep vascular layer and the choriocapillaris were automatically corrected for projection artefacts by the proprietary software provided by the OCT-A device. Additionally we imaged all participants with conventional optical coherence tomography (Heidelberg Spectralis; Heidelberg Engineering, Heidelberg, Germany) to confirm the absence of any retinal pathologies. For quantitative analysis of the 3 × 3 mm macula-scans images were binarized and skeletonized with Fiji27 (an expanded version of ImageJ28) and analyzed for vessel density as previously described29. Exemplary unprocessed and binarized superficial retinal OCT-A images are provided in the supplemental figure. The choriocapillaris was evaluated for percentage of non-perfused choriocapillaris area as previously described30.
Statistical analysis
Student’s t-test, Wilcoxon rank sum test, Kruskal–Wallis test and Fisher’s exact test were used for descriptive statistical analyses as indicated. Statistical analyses were performed with R (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, v4.0.3, 2020). Differences were considered significant if they exceeded the 95% confidence level. Eyes with a maximum Fontaine stage > I were pooled in one category to increase statistical power. For univariate and multivariate regression analysis we used linear mixed models including a random intercept for each patient.
Results
Demographics and clinical characteristics
Ninety-seven eyes from 52 patients with PAD and 34 eyes from 23 healthy controls were included. Characteristics of the sample are shown in Table 1. 51 eyes (53% of the PAD group) were from patients with systemic media calcification and 51 eyes (53% of the PAD group) from patients with cerebrovascular disease. Ten eyes were from patients with a history of stroke (10% of the PAD group) and 25 (26% of the PAD group) from patients with a history of acute coronary syndrome.
Quantitative analysis of OCT-A images
Superficial retinal layer vessel density and choriocapillaris non-perfused area were significantly associated with Fontaine stage (Fig. 1, Table 2) and there was a significant correlation of all OCT-A parameters with ABI (Fig. 2, Table 2). For the deep retinal layer, there was no significant difference for vessel density and no association with Fontaine stage (Fig. 1, Table 2). Post-hoc analysis revealed significant differences for superficial retinal vessel density between controls and pooled Fontaine stages IIa, IIb, III (p = 0.0016) and Fontaine stage I and pooled Fontaine stages IIa, IIb, III group (p = 0.0051) and for choriocapillaris non-perfused area between controls and Fontaine stage I (p = 0.017) and controls and pooled Fontaine stages IIa, IIb, III (p = 0.00047).
Correlation of retinal and choriocapillaris perfusion with ankle-brachial-index. Vessel density on OCT-A in the superficial and deep retina and choriocapillaris non-perfused area were plotted against ankle-brachial-index. The blue line represents a linear regression model with the surrounding confidence interval in light grey.
Subgroup analysis excluding eyes from patients with media calcification
As intima and media calcification are thought to be two distinct pathologies24, as the validity of the ABI for diagnosing PAD is reduced when there is media calcification31 and as media calcification has probably no effect on capillary perfusion, we excluded eyes from patients with media calcification in a subgroup analysis (resulting in 40 eyes for analysis). There was a larger significant overall difference in superficial retinal layer vessel density between controls and eyes from patients with PAD (mean [control group] = 0.289 ± 0.022, mean [PAD group] = 0.270 ± 0.035, difference of the mean = 0.019, t-test p = 0.0086, 95% confidence interval = 0.0050–0.0330) and choriocapillaris perfusion was more severely impaired than in the analysis of the overall cohort (mean [control group] = 40.78 ± 5.44, mean [PAD group] = 46.44 ± 4.19, difference of the mean = − 5.66, t-test p < 0.0001, 95% confidence interval = − 8.12 till − 3.20). There was no significant difference in retinal vessel density in the deep retinal layer (mean [control group] = 0.056 ± 0.014, mean [PAD group] = 0.051 ± 0.018, difference of the mean = 0.0052, t-test p = 0.19, 95% confidence interval = − 0.0026 till 0.0130).
Regression analysis
In multivariate multiple regression analysis using linear mixed models including a random intercept for each patient with the OCT-A parameters as dependent variables and age, OCT-A signal strength index, maximum Fontaine stage, ABI, sex, history of smoking, hypercholesterolemia, and arterial hypertension as independent variables, ABI and OCT-A signal strength index were associated with all OCT-A parameters, maximum Fontaine stage with deep retinal vessel density and choriocapillaris non-perfused area, arterial hypertension with choriocapillaris non-perfused area, and age with superficial retinal layer vessel density and choriocapillaris non-perfused area (Supplemental Table).
Discussion
In this first study of retinal and choroidal perfusion in patients with PAD we found both retinal and choroidal perfusion to be significantly reduced. The results of this exploratory study imply an ocular asymptomatic involvement in PAD and retinal and choriocapillaris OCT-A parameters are potential candidates for non-invasive staging and monitoring of PAD and atherosclerosis in the future. This ocular asymptomatic involvement in PAD might be due to a common pathway with the general pathophysiology in PAD (that is atherosclerosis).
Associations of retinal perfusion with systemic cardiovascular disease have been described for acute coronary syndrome, stroke and heart failure9,10,11,12,13,14 and it has also been shown that PAD impacts microcirculation32. Furthermore, retinal microvascular findings on color fundus photographs (e.g. hemorrhages, microaneurysms, and exudates) can predict PAD progression in diabetes mellitus33, and it has been suggested that this also holds true beyond the potential confounding effect of diabetes mellitus15. As we excluded patients with diabetes mellitus in our study, our results support existing data that there is retinal microvascular involvement in PAD irrespective of presence of diabetes mellitus33.
Resolution of color fundus photography is inferior to OCT-A and stratification in different retinal layers and the choriocapillaris is impossible. In contrast, OCT-A allows to stratify between retinal and choriocapillaris perfusion and we also found microvascular abnormalities in the choriocapillaris of eyes from patients with PAD.
ABI was significantly associated with all OCT-A parameters and this association held true in multiple regression analyses. Interestingly, there also seems to be an association of ABI with optic nerve head circulation and development of diabetic retinopathy34,35,36. These findings might reflect a potential link of systemic atherosclerotic disease with ocular microcirculation. Furthermore, our results support existing literature on an association of arterial hypertension with impaired choriocapillaris perfusion37,38.
As intima and media calcification are thought to be two distinct pathologies24, as the validity of the ABI for diagnosing PAD is reduced when there is media calcification31 and as media calcification has probably no effect on capillary perfusion, we excluded patients with media calcification in a subgroup analysis. This revealed a greater difference in OCT-A perfusion parameters compared to the overall sample, which might reflect the different pathophysiological entities of media calcification and atherosclerotic intima calcification31. Hence, stratification of atherosclerotic intima and media calcification should be taken into account in future studies of microcirculation in atherosclerosis.
Our study demonstrates that OCT-A imaging biomarkers might be useful indirect measures for non-invasively staging and monitoring PAD. Although clinicians mainly rely on patient history and physical examination in guiding treatment, compared with the existing PAD outcome measures intra-arterial digital subtraction angiography and computer tomography angiography OCT-A is non-invasive, causes no radiation exposure and is less affected by intra- and inter-grader variation. Furthermore, currently used clinical measures such as ABI have several well-known limitations including low accuracy due to their subjective nature39. Against this, OCT-A offers an objective indirect parameter for PAD staging and monitoring which could aid in a study setting, e.g. for the development of novel interventions.
The strengths of our study are a comparison of OCT-A imaging biomarkers against the established clinical reference standards ABI and Fontaine stage, an objective automated image analysis approach, a reasonable sample size and stratification of our analysis in retinal and choriocapillaris perfusion. Our limitations include the cross-sectional nature of the study, therefore no causal relationships can be concluded. Furthermore, no assessment of ABI in the healthy controls has been done and the majority of our sample had only mild to moderate PAD. Another limitation is the different sex distribution between the control and patient group, as sex might have an effect on OCT-A parameters40,41. However, in our multivariate regression analysis sex was not significantly associated with any OCT-A parameter, so any present effect is likely very small. Owing to the cross-sectional design, our study cannot indicate whether observed changes are reversible or predictive of future disease progression and/or functional loss. As we did not investigate the effect of other reasons for atherosclerosis like diabetes and hypertension on the reported OCT-A parameters in our explorative study, our results cannot indicate the relationship. Furthermore, patients with diabetes are an important subpopulation in patients with PAD and can have falsely high ABI, which needs to be accounted for when using OCT-A for monitoring and staging PAD. Future, larger studies would benefit from including patients with diabetes and analyzing the confounding effect.
This is the first study assessing ocular microvascular perfusion in PAD, describing a previously unknown impairment and association of retinal and choroidal perfusion with ABI in PAD. OCT-A imaging parameters may aid as imaging-biomarkers for PAD staging and atherosclerosis monitoring. Further longitudinal studies on the prognostic value of OCT-A for cardiovascular disease and events are warranted.
References
Fowkes, F. G. et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet (London, England) 382, 1329–1340. https://doi.org/10.1016/s0140-6736(13)61249-0 (2013).
Ouriel, K. Peripheral arterial disease. Lancet (London, England) 358, 1257–1264. https://doi.org/10.1016/s0140-6736(01)06351-6 (2001).
Sartipy, F., Sigvant, B., Lundin, F. & Wahlberg, E. T. Year mortality in different peripheral arterial disease stages: a population based observational study on outcome. Eur. J. Vasc. Endovasc. Surg. 55, 529–536. https://doi.org/10.1016/j.ejvs.2018.01.019 (2018).
Criqui, M. H. & Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 116, 1509–1526. https://doi.org/10.1161/circresaha.116.303849 (2015).
Hiatt, W. R. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 344, 1608–1621. https://doi.org/10.1056/nejm200105243442108 (2001).
Hirsch, A. T. et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 286, 1317–1324. https://doi.org/10.1001/jama.286.11.1317 (2001).
Peach, G., Griffin, M., Jones, K. G., Thompson, M. M. & Hinchliffe, R. J. Diagnosis and management of peripheral arterial disease. BMJ (Clin. Res. Ed.) 345, e5208. https://doi.org/10.1136/bmj.e5208 (2012).
Olin, J. W. & Sealove, B. A. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin. Proc. 85, 678–692. https://doi.org/10.4065/mcp.2010.0133 (2010).
Wong, T. Y. et al. Retinal microvascular abnormalities and incident stroke: the atherosclerosis risk in communities study. Lancet (London, England) 358, 1134–1140. https://doi.org/10.1016/s0140-6736(01)06253-5 (2001).
Wong, T. Y. et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology 110, 933–940. https://doi.org/10.1016/s0161-6420(03)00084-8 (2003).
Hanff, T. C. et al. Retinal microvascular abnormalities predict progression of brain microvascular disease: an atherosclerosis risk in communities magnetic resonance imaging study. Stroke; J. Cereb. Circ. 45, 1012–1017. https://doi.org/10.1161/strokeaha.113.004166 (2014).
Arnould, L. et al. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 59, 4299–4306. https://doi.org/10.1167/iovs.18-24090 (2018).
Seidelmann, S. B. et al. Retinal vessel calibers in predicting long-term cardiovascular outcomes: the atherosclerosis risk in communities study. Circulation 134, 1328–1338. https://doi.org/10.1161/circulationaha.116.023425 (2016).
Arnould, L. et al. Association between the retinal vascular network with Singapore “I” Vessel Assessment (SIVA) software, cardiovascular history and risk factors in the elderly: the Montrachet study, population-based study. PLoS ONE 13, e0194694. https://doi.org/10.1371/journal.pone.0194694 (2018).
Yang, C. et al. Retinal microvascular findings and risk of incident peripheral artery disease: an analysis from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis https://doi.org/10.1016/j.atherosclerosis.2019.10.012 (2019).
Gao, S. S. et al. Optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 57, OCT27–OCT36. https://doi.org/10.1167/iovs.15-19043 (2016).
Jia, Y. et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. U.S.A. 112, E2395-2402. https://doi.org/10.1073/pnas.1500185112 (2015).
Hirsch, A. T. et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113, e463–e654. https://doi.org/10.1161/circulationaha.106.174526 (2006).
de Carlo, T. E. et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina (Philadelphia, Pa.) 35, 2364–2370. https://doi.org/10.1097/iae.0000000000000882 (2015).
Winsor, T. Influence of arterial disease on the systolic blood pressure gradients of the extremity. Am. J. Med. Sci. 220, 117–126. https://doi.org/10.1097/00000441-195008000-00001 (1950).
Aboyans, V. et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 126, 2890–2909. https://doi.org/10.1161/CIR.0b013e318276fbcb (2012).
Dachun, X. et al. Sensitivity and specificity of the ankle–brachial index to diagnose peripheral artery disease: a structured review. Vasc. Med. (London, England) 15, 361–369. https://doi.org/10.1177/1358863x10378376 (2010).
Lawall, H., Huppert, P., Espinola-Klein, C., Zemmrich, C. S. & Ruemenapf, G. German guideline on the diagnosis and treatment of peripheral artery disease—a comprehensive update 2016. VASA. Zeitschrift fur Gefasskrankheiten 46, 79–86. https://doi.org/10.1024/0301-1526/a000603 (2017).
Ix, J. H., Miller, R. G., Criqui, M. H. & Orchard, T. J. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J. Vasc. Surg. 56, 721–727. https://doi.org/10.1016/j.jvs.2012.02.042 (2012).
Novo, S., Coppola, G. & Milio, G. Critical limb ischemia: definition and natural history. Curr. Drug Targets. Cardiovasc. Haematol. Disord. 4, 219–225. https://doi.org/10.2174/1568006043335989 (2004).
Fontaine, R. et al. Long-term results of restorative arterial surgery in obstructive diseases of the arteries. J. Cardiovasc. Surg. 5, 463–472 (1964).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Terheyden, J. H. et al. Automated thresholding algorithms outperform manual thresholding in macular optical coherence tomography angiography image analysis. PLoS ONE 15, e0230260. https://doi.org/10.1371/journal.pone.0230260 (2020).
Wintergerst, M. W. M. et al. Optical coherence tomography angiography in intermediate uveitis. Am. J. Ophthalmol. 194, 35–45. https://doi.org/10.1016/j.ajo.2018.06.023 (2018).
Ho, C. Y. & Shanahan, C. M. Medial arterial calcification: an overlooked player in peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 36, 1475–1482. https://doi.org/10.1161/atvbaha.116.306717 (2016).
Cisek, P. L. et al. Microcirculatory compensation to progressive atherosclerotic disease. Ann. Vasc. Surg. 11, 49–53. https://doi.org/10.1007/s100169900009 (1997).
Hoe, J. et al. Predictors of decrease in ankle-brachial index among patients with diabetes mellitus. Diabet. Med.: J. Br. Diabet. Assoc. 29, e304-307. https://doi.org/10.1111/j.1464-5491.2012.03705.x (2012).
Chen, S. C. et al. Abnormally low or high ankle-brachial index is associated with proliferative diabetic retinopathy in type 2 diabetic mellitus patients. PLoS ONE 10, e0134718. https://doi.org/10.1371/journal.pone.0134718 (2015).
Lee, M. Y. et al. Abnormally low or high ankle-brachial index is associated with the development of diabetic retinopathy in type 2 diabetes mellitus. Sci. Rep. 8, 441. https://doi.org/10.1038/s41598-017-18882-x (2018).
Shiba, T., Takahashi, M., Hori, Y. & Maeno, T. Pulse-wave analysis of optic nerve head circulation is significantly correlated with brachial-ankle pulse-wave velocity, carotid intima-media thickness, and age. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 250, 1275–1281. https://doi.org/10.1007/s00417-012-1952-5 (2012).
Chua, J. et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 37, 572–580. https://doi.org/10.1097/hjh.0000000000001916 (2019).
Terheyden, J. H. et al. Retinal and choroidal capillary perfusion are reduced in hypertensive crisis irrespective of retinopathy. Transl. Vis. Sci. Technol. 9, 42. https://doi.org/10.1167/tvst.9.8.42 (2020).
Crawford, F., Welch, K., Andras, A. & Chappell, F. M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst. Rev. 9, Cd010680. https://doi.org/10.1002/14651858.CD010680.pub2 (2016).
You, Q. S. et al. Macular vessel density measured with optical coherence tomography angiography and its associations in a large population-based study. Invest. Ophthalmol. Vis. Sci. 60, 4830–4837. https://doi.org/10.1167/iovs.19-28137 (2019).
Brucher, V. C., Storp, J. J., Eter, N. & Alnawaiseh, M. Optical coherence tomography angiography-derived flow density: a review of the influencing factors. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie https://doi.org/10.1007/s00417-019-04553-2 (2019).
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding was provided by the Else Kroener-Fresenius Foundation and the German Scholars Organization (EKFS/GSO 16) to RPF, by the BONFOR GEROK Program, Faculty of Medicine, University of Bonn (Grant No O-137.0028) to MWMW and by the German Ophthalmic Society to PF.
Author information
Authors and Affiliations
Contributions
RPF., MWMW., NS. and CS. conceived the study and provided the scientific overview with FGH. PF. recruited and imaged the participant and prepared the data for analyses. MWMW. performed the statistical analyses and generated the figures and tables. MWMW., PF., RPF., NS., CS. and FGH. interpreted the data. The introduction was drafted by PF., MWMW., RPF. and the methods by PF. and MWMW. Results and discussion were drafted by MWMW. All authors critically reviewed the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The Department of Ophthalmology, University of Bonn received imaging devices from Heidelberg Engineering, Optos, Carl Zeiss Meditec, and CenterVue. Additional competing interests: Wintergerst MWM: Heine Optotechnik: Grant, personal fees and non-financial support, ASKIN & CO: personal fees and non-financial support, Berlin-Chemie AG: Grant and non-financial support, D-EYE: non-financial support, DigiSight Technologies: non-financial support, Eyenuk: non-financial support; Falahat P: no additional disclosures; Holz FG: Acucela: Grant, personal fees and non-financial support, Allergan: Grant and non-financial support, Appelis: personal fees and non-financial support, Bayer: Grant, personal fees and non-financial support, Boehringer-Ingelheim: personal fees, Bioeq/Formycon: personal fees and non-financial support, CenterVue: non-financial support, Ellex: non-financial support, Roche/Genetech: Grant and personal fees, Geuder: personal fees, Grayburg Vision: personal fees and non-financial support, Heidelberg Engineering: Grant, per-sonal fees and non-financial support, Kanghong: personal fees and non-financial sup-port, LinBioscience: personal fees and non-financial support, NightStarX: non-financial support, Novartis: Grant, personal fees and non-financial support, Optos: non-financial support, Pixium Vision: Grant, personal fees and non-financial support, Oxurion: personal fees and non-financial support, Stealth BioTherapeutics: personal fees and non-financial support, Zeiss Meditec: Grant and non-financial support; Schaefer C: no additional disclosures; Finger RP: Novartis: Grant, personal fees and non-financial support, Bayer: personal fees, Roche/Genetech: personal fees, Alimera: personal fees, Santhera: personal fees, Ellex: personal fees, Opthea: personal fees, Inositec: personal fees, CenterVue: non-financial support, Heidelberg Engineering: non-financial support, Optos: non-financial support, Zeiss Meditec: non-financial support; Schahab N: no additional disclosures; the funding organization had no role in design and conduct of the study, collection, management, analyses, and interpretation of the data, preparation, review, or approval of the manuscript and decision to submit the manuscript for publication. Data used in this study will be provided on reasonable request. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wintergerst, M.W.M., Falahat, P., Holz, F.G. et al. Retinal and choriocapillaris perfusion are associated with ankle-brachial-pressure-index and Fontaine stage in peripheral arterial disease. Sci Rep 11, 11458 (2021). https://doi.org/10.1038/s41598-021-90900-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-90900-5
This article is cited by
-
Retinal optical coherence tomography angiography (OCTA) biomarkers of cardiovascular disease: a review article
Eye (2025)
-
The Use of Retinal Imaging Including Fundoscopy, OCT, and OCTA for Cardiovascular Risk Stratification and the Detection of Subclinical Atherosclerosis
Current Atherosclerosis Reports (2024)
-
Retinale optische Kohärenztomographie-Biomarker bei demenziellen Erkrankungen
Die Ophthalmologie (2024)
-
Modifications in ocular microperfusion after transcatheter aortic valve implantation
Scientific Reports (2023)
-
Ankle-brachial index and ocular diseases in a Russian population
Eye (2022)