Abstract
Cigarette smoking effects might correspond with paraoxonase 1 gene (PON1) single nucleotide variants (SNVs). We investigated the association of PON1 rs705379, rs854560, and rs662 with cardiovascular mortality in hemodialysis (HD) patients concerning conventional cigarette smoking. Cardiovascular, cardiac, coronary heart disease (CHD)- and non-CHD-related deaths were analyzed in 206 HD cigarette smokers and 659 HD non-smokers. P-values were adjusted for sex, age, and high-density lipoprotein cholesterol. Among all smokers, the rs705379 TT genotype was associated with cardiovascular (P = 0.028), cardiac (P = 0.046), and cardiac non-CHD-related (P = 0.001) mortality. Non-diabetic smokers showed similar qualitative significance to all smokers concerning mentioned death rates (P-values 0.011, 0.044, and 0.009, respectively). In diabetic non-smokers, the rs705379 T allele correlated with CHD-related deaths (P = 0.020). The rs854560 T allele was associated with lower cardiovascular mortality in non-diabetic smokers (P = 0.008). The rs854560 TT genotype showed a negative non-significant correlation with non-CHD-related cardiac death in all non-smokers (P = 0.079). In diabetic smokers, the rs662 G allele was associated with higher cardiac mortality (P = 0.005). In all non-smokers and non-diabetic non-smokers, the rs662 G correlated with cardiovascular deaths (P = 0.020 and P = 0.018, respectively). Genotyping PON1 SNVs may help argue HD smokers harboring the rs705379 TT genotype or T allele and non-smokers possessing the rs662 G allele for prevention against cardiovascular diseases. These groups are more burdened genetically for cardiovascular mortality.
Similar content being viewed by others
Introduction
Paraoxonase 1 gene (PON1) single nucleotide variants (SNVs) as influencing paraoxonase 1 (PON-1) activity and concentration are associated with the peroxidation rate of high-density and low-density lipoproteins (HDL and LDL, respectively)1,2,3. The glutamine-containing PON-1 Q192 isoform (associated with the rs662 A allele of rs662 Q192R, 575A > G) was shown to protect LDL against oxidative modification more effectively than the arginine-containing PON-1 R192 isoform (associated with the rs662 G allele)4,5. Opposite results have also been demonstrated6. Two mentioned papers4,6 showed that the PON-1 Q192 isoform hydrolyses less paraoxon. PON1 rs854560 (L55M) influences ribonucleic acid messenger (mRNA) levels. The PON1 rs854560 T allele transcript related to methionine-containing PON-1 isoform is less stable than the A allele transcript coding leucine-containing PON-1 isoform. The rs854560 T allele bearers show lower PON-1 concentration and activity and are more susceptible to atherosclerosis and diseases associated with oxidative stress7,8,9. PON1 rs705379 -108C > T expression is related to its impact on the formation of complexes between the specificity protein 1 (Sp1) transcription factor and the PON1 promoter10. The PON1 rs705379 CT and TT genotypes correspond with the lower PON-1 activity11.
PON1 SNVs were directly related to atherosclerotic diseases, including coronary heart disease (CHD)6,12,13. Moreover, the rs662 G allele enhanced susceptibility to CHD (particularly myocardial infarction—MI) in end-stage non-insulin-dependent diabetes mellitus (NIDDM) patients14, cigarette smokers15, and older subjects16. However, other investigations did not show the relationship between the PON1 SNVs and CHD17 or showing opposite results18.
Tobacco is responsible for 20% of CHD deaths19. Tobacco smoking is related to increased susceptibility to lipoprotein oxidation20. Cigarette smoke components, like acrolein, inhibit plasma PON-1 activity modifying its free thiols21 and decreasing HDL levels22. The cigarette packs smoked per year were associated with an increased MI risk in the rs662 AA homozygosity, related in this study to a low activity isoform of PON-115. On the other hand, smokers with the rs662 GG genotype showed a higher atherogenic index and Framingham risk score than smoking and non-smoking AA + AG carriers. The rs662 GG genotype was discussed as associated with low arylesterase activity23. As one can see, the mentioned above data also do not provide thoroughly consistent results.
The HD patients have similar PON1 SNVs distribution to a general population24, but their PON-1 activity is substantially lower24,25. A Japanese study showed that PON1 rs662 and rs854560 exerted no impact on cardiovascular disease (CVD) mortality in 81 HD patients26.
From 14 to 25% of prevalent HD patients smoke cigarettes27,28,29. Tobacco smoking increases susceptibility to hemoglobin glycation20,30. It is important because approximately 50% of the incident HD receivers show diabetes mellitus (DM)31. The PON1 rs705379 TT genotype was associated with NIDDM nephropathy, independently of demographic and clinical factors29.
This study planned to show differences between cigarette smokers and non-smokers among hemodialyzed patients who died from cardiovascular reasons. As cardiovascular effects of smoking might correspond to PON1 SNVs, we investigated the association of PON1 rs705379 (located in the PON1 promoter region), rs854560, and rs662 (both located in the PON1 coding region) with cardiovascular mortality in cigarette smokers and non-smokers. We assumed to document possible differences in the PON1 impact on cardiovascular mortality concerning smoking status for obtaining additional arguments in anti-tobacco strategy in genetically burdened HD groups.
Results
Patient characteristics
Among HD patients who died from cardiovascular reasons, there were 82 smokers and 239 non-smokers. Concerning cardiovascular mortality, the 80% power could be obtained at 1.5 OR in dominant and additive models for rs662 and rs854560, and an additive model for rs705379 (Supplementary Table 1).
Smokers’ group comprised younger individuals at RRT onset and death, more men as a percent of the total, and more frequent atherogenic pattern of serum lipid profile defined as triglyceride (TG)/HDL cholesterol ratio ≥ 3.8. Both groups did not differ significantly in RRT duration, frequency of MI, body mass index, and the type of lipid-modifying medicines used (Table 1).
Cardiovascular mortality
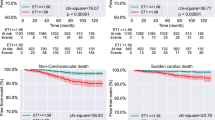
All cardiac deaths were more frequent in smokers (Fig. 1), but the Kaplan–Meier cumulative proportion surviving was not different between smokers and non-smokers concerning cardiovascular mortality. NIDDM nephropathy patients presented worse cardiovascular and CHD-related cardiac survival than non-DM subjects (Table 2).
Among HD cigarette smokers, the group showing NIDDM as a cause of end-stage renal disease (ESRD) did not differ in cardiovascular mortality from non-DM smokers (Supplementary Fig. 1). HD non-smokers demonstrated higher cardiovascular mortality if they had NIDDM nephropathy (Table 2). Cardiovascular mortality was not significantly different among NIDDM nephropathy patients or non-DM subjects categorized by smoking status (Supplementary Table 2).
PON1 SNVs
All selected patients (n = 865) underwent genotyping for PON1 SNVs. Successful results were obtained in 818 patients for rs705379, 843—for rs854560, and 821—for rs662. The tested SNVs were distributed according to the Hardy–Weinberg equilibrium (HWE). P-values for HWE were 0.600 for PON1 rs705379, 0.896 for PON1 rs854560, and 0.902 for PON1 rs662. PON1 SNVs demonstrated weak linkage disequilibrium (LD, r2 < 0.3).
PON1 SNVs and patients’ survival probability
Due to a high number of survival-related analyses concerning PON1 SNVs (Supplementary Tables 3–8), Table 3 shows only statistically significant (P < 0.05) results of these calculations. The Kaplan–Meier cumulative proportion surviving for analyses presented in Table 3 (for smokers and non-smokers) are shown in Supplementary Fig. 2A–L.
Among all smokers, the rs705379 TT genotype was associated with all cardiovascular (P = 0.028, Supplementary Fig. 2A), all cardiac (P = 0.046, Supplementary Fig. 2B), and cardiac non-related with CHD (P = 0.001, Supplementary Fig. 2C) mortality. The rs705379 TT genotype smokers, who died from cardiac reasons, showed a higher MI frequency than CC + CT bearers (66.7% vs. 29.2%, P = 0.047). Concerning the rs705379 TT genotype, non-DM smokers showed similar qualitative significance like all smokers for all cardiovascular, all cardiac, and cardiac non-related with CHD death rates (P = 0.011, Supplementary Fig. 2D; P = 0.044, Supplementary Fig. 2E; and P = 0.009, Supplementary Fig. 2F; respectively). The rs854560 TT + AT genotypes were inversely associated with cardiovascular deaths in non-DM smokers (P = 0.008, Supplementary Fig. 22). In DM smokers, the rs662 G allele was associated with a higher risk of cardiac mortality (P = 0.005, Supplementary Fig. 2H) (Table 3).
In all non-smokers and non-DM non-smokers, the rs662 G allele was associated with cardiovascular-related deaths (P = 0.020, Supplementary Fig. 2I and P = 0.018, Supplementary Fig. 2J, respectively). In DM non-smokers, the rs705379 T allele correlated with CHD's cardiac death (P = 0.020, Supplementary Fig. 2L) (Table 3).
Discussion
Our study indicates that cigarette smoking is associated with more unfavorable characteristics of HD patients who died from cardiovascular diseases than HD non-smokers who also died from cardiovascular reasons. HD cigarette smokers started RRT at a younger age, died at a younger age, and presented more frequently atherogenic dyslipidemia due to lower serum HDL-cholesterol and higher serum TG levels. Cardiac deaths were more frequent in HD smokers. As expected, smokers were predominantly men. Differences in cardiovascular longevity were present inside smoking or non-smoking groups if patients` data were analyzed concerning PON1 polymorphisms. Associations between PON1 SNVs and cardiovascular mortality were more frequent and stronger in smokers than non-smokers.
The PON1 rs705379 TT genotype was associated with higher cardiovascular and cardiac mortality, independently of age and NIDDM nephropathy32. The present study revealed that all HD smokers and non-DM smokers demonstrate increased cardiovascular, cardiac, and cardiac CHD non-related mortality if they possess the rs705379 TT genotype. In DM non-smokers, the rs705379 T allele correlated only with cardiac death related to CHD.
The PON1 rs705379 TT genotype is associated with attenuated antioxidant, anti-inflammatory, anti-thrombosis, and anti-adhesion activities1,33,34. It may predispose to cardiac diseases35,36. The mean activity of the rs705379 T allele is 0.67–0.77-fold lower than that attributed to the C allele37. Under hyperglycemic conditions, circulating PON-1 activity is additionally diminished due to glycation of HDL-PON-138. NIDDM patients have a decreased serum PON-1 activity39. The rs705379 TT genotype is associated with a 1.5-fold higher prevalence of end-stage NIDDM nephropathy than observed in the CT + CC genotype subjects, independently on smoking status29. All examined DM patients presented such type of nephropathy, and NIDDM nephropathy as a cause of ESRD was associated with higher cardiovascular mortality in subjects not categorized by smoking status. Therefore, in HD patients affected by end-stage NIDDM nephropathy, the rs705379 TT genotype could be at least partially related to cardiovascular deaths due to its association with this disease. PON-1 prevents or reduces cardiovascular complications in NIDDM patients, involving mechanisms such as decreasing plasma oxidized LDL concentrations, diminishing macrophage ability to uptake oxidized LDL and releasing reactive oxygen species, preventing macrophage proinflammatory responses, reducing foam cell generation, increasing macrophage cholesterol efflux, catabolizing homocysteine thiolactone, preventing oxidative inactivation of lecithin: cholesterol acyltransferase, inhibiting myeloperoxidase and monocyte chemotactic protein 1 activities, and preventing the glucose-induced glycoxidation40.
On the other hand, the rs705379 TT genotype relationship with cardiovascular mortality was revealed also in non-DM smokers, in whom it could not be related to NIDDM nephropathy. Non-DM current smokers are twice as likely as non-smokers to have increased glycated hemoglobin, but it remains in the pre-diabetes range30. Smoking itself enhances glycation to a lesser extent than DM does30. In HD non-DM smokers, decreased PON-1 activity in the TT genotype patients could be aggravated by glycation of HDL-PON-1, which is not present in HD non-smokers.
The current data showed that the rs662 G allele contributes to the higher susceptibility to cardiac deaths in HD smokers with NIDDM nephropathy, and cardiovascular mortality in all examined non-smokers and non-DM non-smokers. The rs662 GG genotype NIDDM nephropathy smokers and the rs662 G allele non-DM patients used a greater number of cigarettes or showed a higher frequency of smoking than those having the rs662 A allele or AA genotype29. Our results agree with meta-analyses indicating the association of the rs662 G allele with cardiovascular mortality in non-ESRD individuals12,13.
The PON1 rs854560 T allele corresponded with a higher risk of CHD-related death in non-DM men18, an increased risk of CHD in Iranian patients with atherosclerosis41, the higher prevalence of ischemic cerebral stroke in the entire HD population32 and end-stage NIDDM nephropathy individuals27, but not with cardiovascular, cardiac, or vascular mortality in the whole HD subjects32. Surprisingly, in this study, the rs854560 T allele was inversely associated with cardiovascular mortality in non-DM smokers. Also, the TT genotype showed a negative correlation with cardiac death non-related to CHD in all non-smokers (no significant association after adjustment). The TT genotype was related in HD smokers to the lower number of cigarettes smoked per day29. This finding could be some explanation for better survival in the smoking HD group.
Results of genotyping PON1 SNVs may help argue HD patients for prevention against cardiovascular diseases by rejecting or reducing cigarette smoking. According to the present findings, this conception is the most relevant in smokers harboring the rs705379 TT genotype or T allele. HD non-smokers possessing the rs662 G allele are also genetically burdened for cardiovascular mortality.
Strengths and limitations of the study
PON1 genetic variants determine serum PON-1 concentration and activity. Although serum PON-1 status influences oxidation, inflammation, and lipid properties, it is itself modified by several factors, which can be changing during a lifespan. Genetic variants are constant, so they may better predict mortality as a hard endpoint. Our study is the first one assessing PON1 SNVs concerning cardiovascular deaths in HD patients, whose cigarette smoking status and DM coexistence were also considered significant contributors to mortality.
This study's limitation is its retrospective, although longitudinal, design instead of prospective observation, and a small number of patients in subgroups. The latter is challenging to obtain even a conventional statistical significance. Using multiplicity adjustment as Bonferroni corrected thresholds leads to a severe power loss in empirical analyzes of population-based association studies42. It has also occurred in this study. For Table 3, the significance level after Bonferroni correction is at α < 0.001 for the log-rank test (36 analyses performed) and α < 0.004 for the Wald test (12 analyzes computed). However, a situation in which Bonferroni analyses are recommended is when searching for significant associations without pre-established hypotheses43. Associations between PON1 SNVs and cardiovascular death were documented already in CHD non-uremic patients18. In addition, our data show PON1 SNV associations with cardiovascular mortality in several subgroups who died from cardiovascular reasons, which suggests that they are not accidental. Therefore, using conventional P-values of < 0.05 as indicating significance seems to be justified in our study, although we are conscious that P-values are not impressive. According to the Better Associations for Disease and GEnes (BADGE) system44, our genetic associations shown in Table 3 would have to confirm first-class or second-class BADGE analyses in independent population samples to be recognized as undoubtedly associated. Such high P-values are observed rarely in specific clinical groups. Thus, replication analyses on other HD groups or functional validation assays are warranted highly in future investigations.
Materials and methods
Patients
We have used deoxyribonucleic acid (DNA) samples for PON1 genotyping from our DNA base. DNA probes were collected from January 2009 to June 2019 from Caucasian HD patients. At first, we selected subjects with known cigarette smoking status. HD patients were diagnosed as cigarette smokers if they smoked conventional cigarettes at least two years before RRT initiation and continued smoking through RRT. Non-smoking status was recognized if HD patients never smoked cigarettes or discontinued smoking at least five years before RRT started. Secondly, from DM patients identified in this primarily selected group, only NIDDM nephropathy subjects were enrolled. NIDDM nephropathy was diagnosed based on classical signs and symptoms. In the case of difficulties in NIDDM nephropathy's clinical diagnosis, a kidney biopsy confirmed or excluded this diagnosis. Among selected 865 HD subjects, there were 206 smokers and 659 non-smokers.
We analyzed demographic, clinical, and laboratory data collected during patients` lifespan and after that as needed and possible. Patients` outcome (survival on HD, death on HD, renal transplantation, a movement to not collaborating center) was checked in September 2020. In deceased individuals, death causes were registered based on medical documentation and categorized as cardiovascular, infection-related, cancer-related, and other or unknown. Among cardiovascular causes of death, we specified cardiac deaths related to CHD and those not associated with CHD.
PON1 genotyping
Three PON1 polymorphisms were genotyped: rs662 (Q192R, 575A>G), rs854560 (L55M, 163A>T), and rs705379 (− 108C>T) using previously described methods29,32.
Statistical analysis
Non-normally distributed variables by the Shapiro–Wilk test are presented as a median and range; data showing normal distribution are expressed as a mean ± standard deviation. Dichotomous variables are shown as a percentage of the total number. Mann–Whitney U test or Student's T-test were used to compare quantitative variables. Pearson's Chi-squared test or Fisher's exact test was applied to compare qualitative variables, as appropriate.
The power of the study was calculated using the Genetic Association Study (GAS) Power Calculator (http://csg.sph.umich.edu/abecasis/gas_power_calculator/index.html) with the following inputs: number of cases (HD smokers who died from cardiovascular reasons) = 100, number of controls = 240 (HD non-smokers who died from cardiovascular diseases), significance level = 0.05, prevalence = 55%45.
HWE was calculated by the Chi-squared test (df = 1, P > 0.05 for agreement). LD between PON1 SNVs was computed using the Haploview 4.2 software (http://www.broad.mit.edu/mpg/haploview/).
Survival analysis covered a period from the RRT start to death on regular HD treatment. CVD-related deaths were analyzed by the PON1 genotypes and in modes of inheritance using the Kaplan–Meier method with the log-rank test. Inheritance modes (dominant, recessive) were created concerning variant alleles as the risk alleles46. We have made three assumptions regarding the log-rank test, namely that the censoring is unrelated to the outcome, the survival probabilities are the same for participants recruited early and late in the study, and the events were occurred at the times specified. If the analyzes yielded the log-rank test P-values < 0.05, the Cox regression was applied. For the Cox regression model, we have tested two assumptions: the proportional hazards and the linear relationship between the log hazard and each covariate using graphical methods. We considered the significance in the Cox analyses if the Wald test P-value was < 0.05 (Supplementary Tables 3–8). Results, significant in the Cox regression, were adjusted for sex, age, and HDL-cholesterol. The latter was the serum lipid parameter, which yielded the most significant difference between smokers and non-smokers (Table 1). Hazard ratio (HR) with 95% confidence interval (CI) and P-values were computed. If the adjusted P-values were < 0.05, we considered a significant association between tested PON1 SNV and the defined cause of cardiovascular death.
All analyses we performed using Graph-Pad InStat 3.10, 32 bit for Windows, created July 9, 2009 (GraphPad Software, Inc., San Diego, California, United States), Statistica version 13, 2017 (TIBCO Software Inc., 3307 Hillview Avenue Palo Alto, CA 94,304 USA) and R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria)47.
Ethical issues
The Institutional Review Board of the Poznan University of Medical Sciences, Poland, approved our study's design. All methods we carried out by relevant guidelines and regulations (Declaration of Helsinki).
Consent to participate
The informed written consent was obtained at the blood collection for DNA extraction from all study participants or their parents if participants were under 18 years.
Conference presentation
Abstract of this paper has been accepted as a Mini-Oral at the 58th ERA-EDTA Congress, organized from June 5 to 8, 2021, in collaboration with the German Society of Nephrology (Deutsche Gesellschaft für Nephrologie).
Data availability
All data are available for any reasonable request from the first author.
References
Aviram, M. et al. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions: A possible peroxidative role for paraoxonase. J. Clin. Invest. 101, 1581–1590. https://doi.org/10.1172/JCI1649 (1998).
Mackness, M. I., Arrol, S. & Durrington, P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 286, 152–154. https://doi.org/10.1016/0014-5793(91)80962-3 (1991).
Mackness, M. I., Arrol, S., Abbott, C. & Durrington, P. N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 104, 129–135. https://doi.org/10.1016/0021-9150(93)90183-u (1993).
Mackness, B., Mackness, M. I., Arrol, S., Turkie, W. & Durrington, P. N. Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Lett. 423, 57–60. https://doi.org/10.1016/s0014-5793(98)00064-7 (1998).
Aviram, M. et al. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation 101, 2510–2517. https://doi.org/10.1161/01.cir.101.21.2510 (2000).
Bhattacharyya, T. et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 299, 1265–1276. https://doi.org/10.1001/jama.299.11.1265 (2008).
Garin, M. C. et al. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme: A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J. Clin. Invest. 99, 62–66. https://doi.org/10.1172/JCI119134 (1997).
Leviev, I., Negro, F. & James, R. W. Two alleles of the human paraoxonase gene produce different amounts of mRNA: An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler. Thromb. Vasc. Biol. 17, 2935–2939. https://doi.org/10.1161/01.atv.17.11.2935 (1997).
Tanhapour, M. et al. association between activity and genotypes of paraoxonase1 L55M (rs854560) increases the disease activity of rheumatoid arthritis through oxidative stress. Mol. Biol. Rep. 46, 741–749. https://doi.org/10.1007/s11033-018-4530-z (2019).
Deakin, S., Leviev, I., Brulhart-Meynet, M. C. & James, R. W. Paraoxonase-1 promoter haplotypes and serum paraoxonase: A predominant role for polymorphic position −107, implicating the Sp1 transcription factor. Biochem. J. 372, 643–649. https://doi.org/10.1042/bj20021670 (2003).
Gupta, N., Singh, S., Maturu, V. N., Sharma, Y. P. & Gill, K. D. Paraoxonase 1 (PON1) polymorphisms, haplotypes and activity in predicting CAD risk in North-West Indian Punjabis. PLoS ONE 6, e17805. https://doi.org/10.1371/journal.pone.0017805 (2011).
Durrington, P. N., Mackness, B. & Mackness, M. I. Paraoxonase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21, 473–480. https://doi.org/10.1161/01.atv.21.4.473 (2001).
Huo, X. et al. Paraoxonase 1 gene (Q192R) polymorphism confers susceptibility to coronary artery disease in type 2 diabetes patients: Evidence from case-control studies. Drug. Discov Ther. 13, 80–88. https://doi.org/10.5582/ddt.2019.01003 (2019).
Ruiz, J. et al. Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. Lancet 346, 869–872. https://doi.org/10.1016/s0140-6736(95)92709-3 (1995).
Sentí, M., Aubó, C. & Tomás, M. Differential effects of smoking on myocardial infarction risk according to the Gln/Arg 192 variants of the human paraoxonase gene. Metabolism 49, 557–559. https://doi.org/10.1016/s0026-0495(00)80026-8 (2000).
Sentí, M. et al. Relationship of age-related myocardial infarction risk and Gln/Arg 192 variants of the human paraoxonase1 gene: The REGICOR study. Atherosclerosis 156, 443–449. https://doi.org/10.1016/s0021-9150(00)00680-8 (2001).
Gardemann, A. et al. The paraoxonase Leu-Met54 and Gln-Arg191 gene polymorphisms are not associated with the risk of coronary heart disease. Atherosclerosis 152, 421–431. https://doi.org/10.1016/S0021-9150(99)00489-X (2000).
Regieli, J. J. et al. Paraoxonase variants relate to 10-year risk in coronary artery disease: Impact of a high-density lipoprotein-bound antioxidant in secondary prevention. J. Am. Coll. Cardiol. 54, 1238–1245. https://doi.org/10.1016/j.jacc.2009.05.061 (2009).
World Health Organization. Tobacco Responsible for 20% of Deaths From Coronary Heart Disease. https://www.who.int/news/item/22-09-2020-tobacco-responsible-for-20-of-deaths-from-coronary-heart-disease. Accessed 21 Nov 2021.
Asgary, S., Naderi, G. H., Sarrafzadegan, N. & Gharypur, M. In vitro effect of nicotine and cotinine on the susceptibility of LDL oxidation and hemoglobin glycosylation. Mol. Cell. Biochem. 246, 117–120 (2003).
Nishio, E. & Watanabe, Y. Cigarette smoke extract inhibits plasma paraoxonase activity by modification of the enzyme’s free thiols. Biochem. Biophys. Res. Commun. 236, 289–293. https://doi.org/10.1006/bbrc.1997.6961 (1997).
Milnerowicz, H., Kowalska, K. & Socha, E. Paraoxonase activity as a marker of exposure to xenobiotics in tobacco smoke. Int. J. Toxicol. 34, 224–232. https://doi.org/10.1177/1091581815584624 (2015).
Souza-Nogueira, A. et al. Paraoxonase 1 (PON1) Q192R genotypes and their interaction with smoking strongly increase atherogenicity and the Framingham risk score. Arch. Endocrinol. Metab. 60, 426–435. https://doi.org/10.1590/2359-3997000000184 (2016).
Baráth, A. et al. Roles of paraoxonase and oxidative stress in adolescents with uraemic, essential or obesity-induced hypertension. Kidney Blood Press Res. 29, 144–151. https://doi.org/10.1159/000095124 (2006).
Gbandjaba, N. Y. et al. Paraoxonase activity in healthy, diabetic, and hemodialysis patients. Clin. Biochem. 45, 470–474. https://doi.org/10.1016/j.clinbiochem.2012.01.005 (2012).
Ikeda, Y. et al. Human serum paraoxonase concentration predicts cardiovascular mortality in hemodialysis patients. Clin. Nephrol. 67, 358–365. https://doi.org/10.5414/cnp67358 (2007).
Foley, R. N., Herzog, C. A. & Collins, A. Smoking and cardiovascular outcomes in dialysis patients: The United States Renal Data System Wave 2 Study. Kidney Int. 63, 1462–1467. https://doi.org/10.1046/j.1523-1755.2003.00860.x (2003).
Tepel, M., Giet, M. V., Park, A. & Zidek, W. Association of calcium channel blockers and mortality in haemodialysis patients. Clin. Sci. 103, 511–515. https://doi.org/10.1042/cs1030511 (2002).
Grzegorzewska, A. E. et al. Paraoxonase 1 gene polymorphisms concerning non-insulin-dependent diabetes mellitus nephropathy in hemodialysis patients. J Diabetes Compl. 34, 107687. https://doi.org/10.1016/j.jdiacomp.2020.107687 (2020).
Vlassopoulos, A., Lean, M. E. & Combet, E. Influence of smoking and diet on glycated haemoglobin and “pre-diabetes” categorisation: A cross-sectional analysis. BMC Public Health 13, 1013. https://doi.org/10.1186/1471-2458-13-1013 (2013).
Lok, C. E., Oliver, M. J., Rothwell, D. M. & Hux, J. E. The growing volume of diabetes-related dialysis: A population based study. Nephrol. Dial. Transplant. 19, 3098–3103. https://doi.org/10.1093/ndt/gfh540 (2004).
Grzegorzewska, A. E. et al. Paraoxonase 1 concerning dyslipidaemia, cardiovascular diseases, and mortality in haemodialysis patients. Sci. Rep. 11, 6773 (2021).
Chistiakov, D. A., Melnichenko, A. A., Orekhov, A. N. & Bobryshev, Y. V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 132, 19–27. https://doi.org/10.1016/j.biochi.2016.10.010 (2017).
Jaouad, L. et al. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1’s free sulfhydryl groups. Atherosclerosis 185, 191–200. https://doi.org/10.1016/j.atherosclerosis.2005.06.012 (2006).
Mangge, H., Becker, K., Fuchs, D. & Gostner, J. M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 6, 462–477. https://doi.org/10.4330/wjc.v6.i6.462 (2014).
Nagareddy, P. & Smyth, S. S. Inflammation and thrombosis in cardiovascular disease. Curr. Opin. Hematol. 20, 457–463. https://doi.org/10.1097/MOH.0b013e328364219d (2013).
Suehiro, T. et al. A polymorphism upstream from the human paraoxonase (PON1) gene and its association with PON1 expression. Atherosclerosis 150, 295–298. https://doi.org/10.1016/s0021-9150(99)00379-2 (2000).
Mastorikou, M., Mackness, B., Liu, Y. & Mackness, M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabet. Med. 25, 1049–1055. https://doi.org/10.1111/j.1464-5491.2008.02546.x (2008).
Letellier, C. et al. Serum paraoxonase activity and paraoxonase gene polymorphism in type 2 diabetic patients with or without vascular complications. Diabetes Metab. 28, 297–304 (2002).
Shokri, Y. et al. Importance of paraoxonase 1 (PON1) as an antioxidant and antiatherogenic enzyme in the cardiovascular complications of type 2 diabetes: Genotypic and phenotypic evaluation. Diabetes Res. Clin. Pract. 161, 108067. https://doi.org/10.1016/j.diabres.2020.108067 (2020).
Shahsavari, G., Nouryazdan, N., Adibhesami, G. & Birjandi, M. Genetic associations and serum paraoxonase levels with atherosclerosis in western Iranian patients. Mol. Biol. Rep. 47, 5137–5144. https://doi.org/10.1007/s11033-020-05585-2 (2020).
Lettre, G., Lange, C. & Hirschhorn, J. N. Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet. Epidemiol. 31, 358–362. https://doi.org/10.1002/gepi.20217 (2007).
Perneger, T. V. What’s wrong with Bonferroni adjustments. BMJ 316, 1236–1238. https://doi.org/10.1136/bmj.316.7139.1236 (1998).
Manly, K. F. Reliability of statistical associations between genes and disease. Immunogenetics 57, 549–558. https://doi.org/10.1007/s00251-005-0025-x (2005).
United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020). https://adr.usrds.org/2020/end-stage-renal-disease/5-mortality.
Zhao, F., Song, M., Wang, Y. & Wang, W. Genetic model. J. Cell Mol. Med. 20, 765 (2016).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2019). https://www.R-project.org/.
Funding
The Poznan University of Medical Sciences, Poznań, Poland [grant number 502-01-01124182-07474] supported this work. The funder had no role in study design, data collection, data analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
A.E.G.: Conceptualization, Methodology, Investigation, Data Curation, Writing the manuscript, Project administration. K.O.: Investigation, Visualization. M.K.Ś.: Software, Validation, Formal analysis. P.A.: Investigation. A.M.: Investigation. P.P.J.: Resources, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grzegorzewska, A.E., Ostromecka, K., Świderska, M.K. et al. Paraoxonase 1 gene variants concerning cardiovascular mortality in conventional cigarette smokers and non-smokers treated with hemodialysis. Sci Rep 11, 19467 (2021). https://doi.org/10.1038/s41598-021-98923-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-98923-8